Abstract
The kinase Akt plays a central role as a regulator of multiple growth factor input signals, making it an attractive anti-cancer drug target. A-443654 is an ATP-competitive Akt inhibitor. Unexpectedly, treatment of cells with A-443654 causes paradoxical hyperphosphorylation of Akt at its two regulatory sites (Thr308 and Ser473). We explore whether inhibitor-induced hyperphosphorylation of Akt by A-443654 is a consequence of disrupted feedback regulation at a pathway level or whether it is a direct consequence of inhibitor binding to the ATP binding site of Akt. Catalytically inactive mutants of Akt reveal that binding of an inhibitor to the ATP site of Akt is sufficient to directly cause hyperphosphorylation of the kinase in the absence of any pathway feedback effects. We conclude that ATP-competitive Akt inhibitors impart regulatory phosphorylation of their target kinase Akt providing new insights into both natural regulation of Akt activation and Akt inhibitors entering the clinic.
Introduction
Akt (also called protein kinase B or PKB) is a member of the serine/threonine protein kinase AGC family and has three isoforms (Akt1, 2 and 3). Akt is a positive regulator of growth factor signaling processes including proliferation and survival1–3. As a central node in growth factor signaling Akt activity is subject to multiple regulatory inputs1–3. In the absence of growth factors, Akt is cytoplasmic and inactive. Upon growth factor stimulation of PI3K activity, Akt is recruited to the plasma membrane through binding of its plekstrin homology (PH) domain to PIP3 which is produced by PI3K. Translocation of Akt enables phosphorylation of residue Thr308 on its activation loop by membrane localized phosphoinositide-dependent kinase 1 (PDK1) (see Fig. 1a)4,5. Further activation of Akt requires phosphorylation on Ser473 which lies in a C-terminal hydrophobic motif (HM) of Akt by the rapamycin insensitive mTORC2 complex6–8. Aberrant activation of Akt has been observed in a variety of human cancers through multiple mutations including PI3K activating mutations, PTEN phosphatase inactivation, Akt overexpression, Akt point mutations in the PH domain which lead to constitutive membrane localization, and others1,3,9. The frequent mutational activation of the PI3K/Akt/mTORC1 pathway in cancer has led to the development of numerous inhibitors of kinases in the pathway including growth factor tyrosine kinase10,11, PI3K3,11–13, PDK13,11,12, Akt3,12, and mTORC1 inhibitors3,11,14.
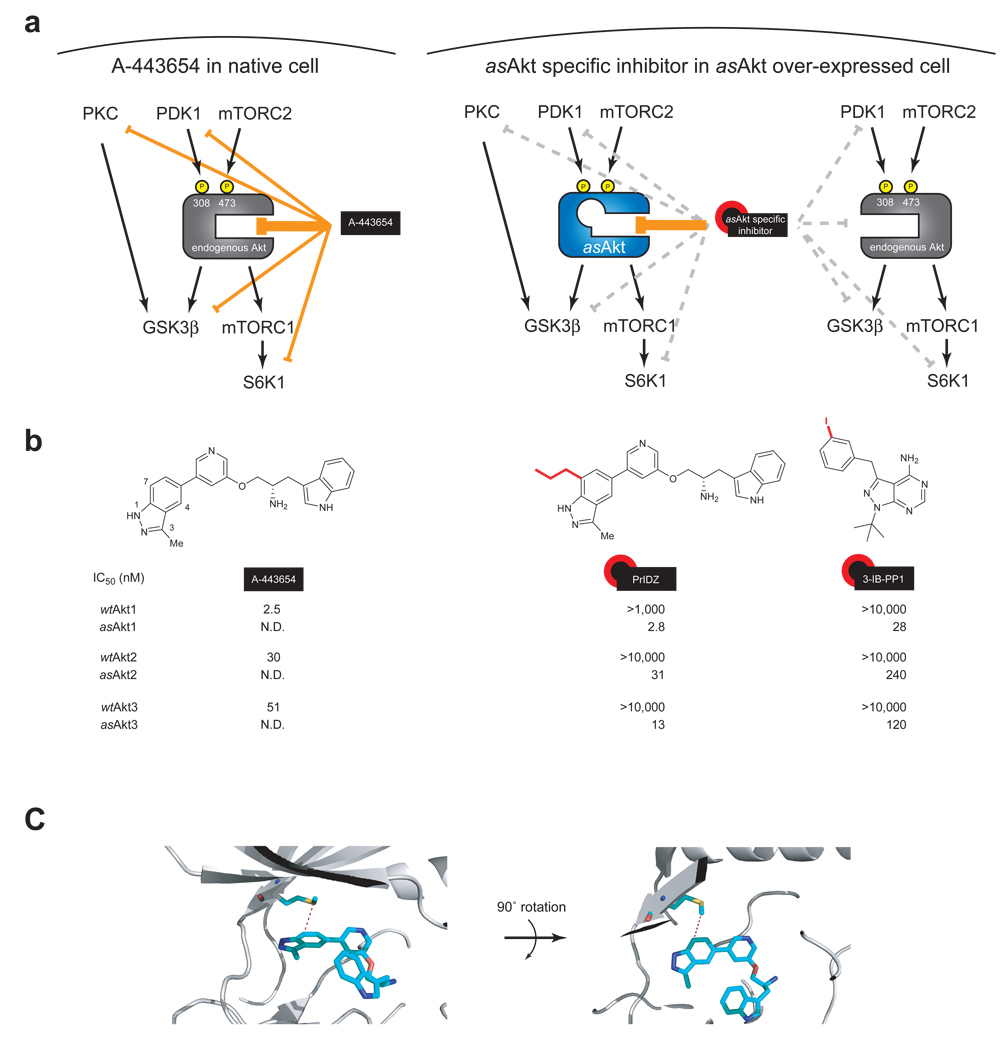
Figure 1. Chemical genetic strategy for achieving Akt-specific inhibition.
(a) Schematic representation of wild type Akt inhibition versus asAkt inhibition. A-443654 inhibits all three isoforms of endogenous Akt (left), while asAkt specific inhibitors such as PrIDZ and 3-IB-PP1 inhibit only the corresponding asAkt isoform, which can be overexpressed in a cell (right). (b) Chemical structures and in vitro inhibitory activity of Akt inhibitors against all three Akt isoforms. The asAkt inhibitors specifically block asAkt activity. IC50 values were determined by an in vitro IP kinase assay for myr-HA-wtAkt1/2/3 and myr-HA-asAkt1/2/3 expressed in HEK293T cells. (C) Co-crystal structure of Akt2 with A-443654 (PDB: 2JDR)28. The gatekeeper methionine in Akt2 and A-443654 are shown in stick representation. Colors are as follows: light blue, carbon; red, oxygen; blue, nitrogen; yellow, sulfur.
Not all of the inhibitors of the PI3K/Akt/mTORC1 pathway antagonize the pathway. Surprisingly, in some patients, the mTORC1 inhibitor rapamycin caused completely unanticipated upstream activation, leading to increased Akt activity in tumor tissues15. Several groups have shown that rapamycin induced feedback activation of Akt is a result from the loss of S6K (p70S6K) destabilization of the scaffolding protein insulin receptor substrate-1 (IRS-1)16–19. To develop the most effective PI3K/Akt/mTORC1 pathway antagonists, it is important to understand the architecture of negative feedback loops in this pathway.
Like rapamycin, another PI3K/Akt/mTORC1 pathway inhibitor, the ATP-competitive inhibitor A-443654 (1), has been reported to cause aberrant Akt phosphorylation. A-443654 was discovered at Abbott laboratories and shown to inhibit the growth of PC-3, MiaPaCa-2, and 3T3-Akt1 tumor growth in xenograft animal models20. At the doses required to inhibit tumor growth, potent inhibition of downstream Akt signaling was observed. Paradoxically however, Akt hyperphosphorylation at Thr308 and Ser473 was induced. The induction of Akt hyperphosphorylation by A-443654 was observed in multiple cancer cell lines, and thus appears to be a general phenomenon regardless of cell type21. Although hyperphosphorylation was initially thought to be caused through Akt/mTORC1/S6K negative feedback similar to that described previously for rapamycin, a subsequent study indicated that the hyperphosphorylation by A-443654 was observed even in TSC2−/− MEF cells21. Since TSC2 is a direct downstream target of Akt and is an inhibitor of mTORC1 activation, the result suggested that hyperphosphorylation is independent of Akt/mTORC1/S6K pathway inhibition. However, it is unclear whether Akt controls mTORC1 activation solely by phosphorylating TSC222,23 and whether TSC2−/− MEF cells possess a canonical PI3K/Akt/mTORC1 pathway.
Since the PI3K/Akt/mTORC1 pathway is central to cancer cell survival and because several inhibitors of the pathway have been shown to trigger Akt phosphorylation, we focused on understanding the mechanism of Akt hyperphosphorylation by the Akt inhibitor A-443654. Using chemical genetics we explore two distinct mechanistic possibilities for how A-443654 causes Akt hyperphosphorylation. In the first mechanism, A-443654 inhibits a kinase which reduces feedback inhibition of Akt phosphorylation. This mechanism is conceptually similar to the feedback induced by rapamycin inhibition of mTORC1, which we term extrinsic feedback since it involves a signaling cascade. The second possible mechanism of hyperphosphorylation we consider is intrinsic to the kinase and relies solely on drug binding to Akt. Importantly, the intrinsic model does not involve a pathway mediated feedback control mechanism. To distinguish between these potential mechanisms we use a combination of Akt chemical genetics, Akt mutations, synthesis of A-443654 analogs, fluorescence microscopy and pathway analysis with phosphospecific antibodies.
Results
A-443654 profiling reveals a spectrum of kinase targets
Abbott laboratories reported the ATP-competitive Akt inhibitor A-443654 (in vitro Akt1 Ki = 160 pM)20. A-443654 inhibits all three Akt isoforms in FL5.12 cells stably transfected with constitutively active myristoylated Akt1/2/3, and showed moderate selectivity when screened against related kinases in the AGC family, such as PKA and PKC20. To obtain a more complete view of A-443654’s cellular targets we tested it against a larger panel of kinases. Of the 220 purified kinases tested, A-443654 inhibited 47 kinases (>90% inhibition at 1 µM), including kinases that potentially impinge on the PI3K/Akt pathway such as PDK1, S6K, PKA, PKC and GSK3β (Supplementary Table 1 online). The spectrum of kinases inhibited by A-443654, especially the targeting of multiple members of the PI3K/Akt pathway make deciphering the cellular response to this compound extremely challenging.
Design of analog sensitive alleles of Akt isoforms
ATP-competitive kinase inhibitors such as A-443654 often inhibit related protein kinases owing to the conserved nature of ATP binding sites across the kinome. To circumvent the natural degeneracy in the kinase family we employed a chemical genetic approach to create a selective Akt inhibitor. This technique employs the combination of an analogue sensitive (as) kinase allele with an as allele specific inhibitor to achieve selective inhibition of Akt as shown in Fig. 1a24. The approach exploits a conserved, large hydrophobic residue in the kinase active site (termed the gatekeeper), which is in direct contact with the N6 amino group of ATP.
To establish this system for all Akt isoforms, mutations enlarging the size of the ATP-binding pocket were introduced by substituting the gatekeeper methionine with glycine (i.e. M227G/M225G/M229G, respectively for asAkt1/2/3). The mutants were expressed in a myristoylated form to provide constitutive kinase activation when expressed in HEK293T cells. In vitro immunoprecipitation kinase assays revealed that all three isoforms of asAkt retained approximately 30% of the activity of the corresponding wtAkt isoforms (Supplementary Fig. 1 online).
Design and synthesis of asAkt specific inhibitors
We next screened inhibitor analogs for potent and selective inhibition of asAkt isoforms. The pyrazolopyrimidine1 (PP1) scaffold has proven to be a versatile starting point for development of many analog sensitive kinase inhibitors24,25. A structurally diverse series of PP1 analogues were screened against asAkt1/2/3 leading to the identification of the 3-iodobenzyl analogue, 3-IB-PP1 (2)26, inhibiting asAkt1/2/3 with good potency, and without inhibition of wtAkt1/2/3 (Fig. 1b). The in vitro potency and selectivity of 3-IB-PP1 for asAkt1 (IC50 = 28 nM) vs. wtAkt1 (IC50 >10,000 nM) provides a valuable tool for cellular studies of asAkt1 specific functions. In contrast, the potency of 3-IB-PP1 for asAkt2 (IC50 = 240 nM) and asAkt3 (IC50 = 120 nM) is low for an ATP-competitive kinase inhibitor27. Thus, although the availability of a structurally distinct chemical series of selective Akt inhibitors afforded by 3-IB-PP1 provides a critical tool for assessing the effects of asAkt1 inhibition we were concerned about the weak affinity for the asAkt2 and asAkt3 targets.
We therefore sought to design an analog of A-443654 which targets asAkt isoforms but does not bind to wtAkt isoforms. Evaluation of the co-crystal structure28 of Akt2 with A-443654 suggested the C7 position on the indazole ring of A-443654 to be a promising position for introducing large substituents which would clash with the gatekeeper methionine of wtAkt (Fig. 1c). Extensive SAR studies of various C7-alkyl substituted A-443654 analogues revealed the 7-n-propylindazole analogue PrINZ (3) as a potent inhibitor (low nanomolar range IC50 values against all asAkt isoforms, see Fig. 1b). As predicted, PrINZ did not inhibit wtAkt1/2/3.
Cellular effects of asAkt specific inhibitors
We next proceeded to validate the use of 3-IB-PP1 and PrINZ in cells. To test the orthogonality of 3-IB-PP1 and PrINZ, we studied the IGF-1 stimulated activation of Akt in non-transfected HEK293 cells. HEK293 cells were treated with A-442654, PrINZ and 3-IB-PP1, and phosphorylation on Akt and GSK3β, an immediate downstream target of Akt, was measured (Fig. 2a). Treatment with A-443654 potently inhibited phosphorylation on GSK3β at Ser9 while it induced Akt phosphorylation at Thr308 and Ser473 as reported20. In contrast, the phosphorylation level of Ser9 on GSK3β and the two Akt sites was unperturbed after treatment with PrINZ and 3-IB-PP1. Collectively, these data suggest that inhibitors PrINZ and 3-IB-PP1 are sufficiently selective against wtAkt and potential off-target effects of these compounds, if any, do not have observable effects on the upstream and downstream signaling of Akt.
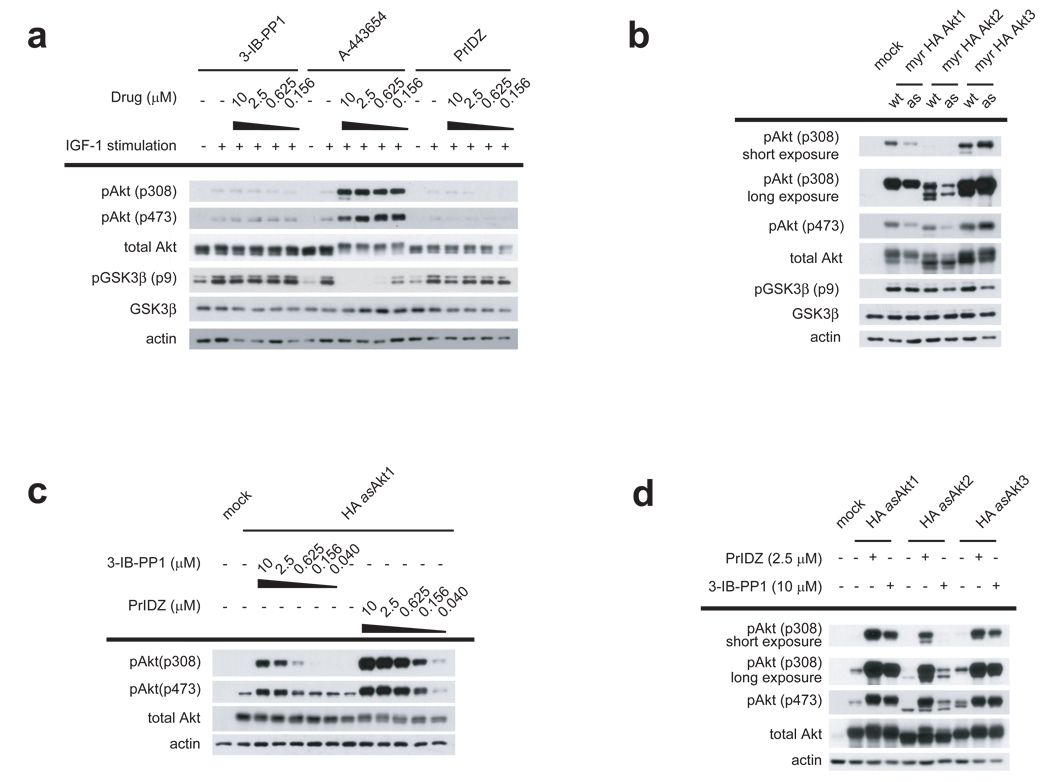
Figure 2. Cellular effects of asAkt transfection and asAkt specific inhibitor treatment.
(a) HEK293 cells were serum-starved overnight and then treated with PrINZ, A-443654 or 3-IB-PP1 for 20 min prior to stimulation with 50 ng/ml IGF-1 for an additional 10 min. Cell lysates were analyzed for Akt (Thr308, Ser473) and GSK3β (Ser9) phosphorylation by immunoblotting. (b) HEK293 cells were transfected with myr-HA-wtAkt1/2/3 or myr-HA-asAkt1/2/3. Cell lysates were analyzed for Akt (Ser473 and Thr308) and GSK3β (Ser9) phosphorylation by immunoblotting. (c) HEK293 cells transfected with HA-asAkt1 were treated for 30 min with serially diluted PrINZ or 3-IB-PP1. Cell lysates were analyzed for Akt (Thr308, Ser473) phosphorylation by immunoblotting. (d) HEK293 cells transfected with HA-asAkt1/2/3 were treated for 30 min with 2.5 µM PrINZ or 10 µM 3-IB-PP1. Cell lysates were analyzed for Akt (Thr308, Ser473) phosphorylation by immunoblotting.
We next tested the effect of 3-IB-PP1 and PrINZ on asAkt function in cells to assess whether the specific inhibition of Akt downstream signaling and/or specific binding of the Akt inhibitors would result in Akt hyperphosphorylation on Thr308 and Ser473. Accordingly, the level of asAkt1/2/3 activity in cells was first determined. Akt constructs containing a c-Src myristoylation recognition sequence (myr-HA-asAkt) are constituitively membrane localized and thus constitutively active without growth factor stimulation29,30. As expected, expression of myr-HA-asAkt1/2/3 and myr-HA-wtAkt1/2/3 in HEK293 cells resulted in elevated phosphorylation of GSK3β at Ser9 (Fig. 2b). Elevation of GSK3β phosphorylation by myr-HA-asAkt1/2/3 transfection was comparable to that by myr-HA-wtAkt1/2/3 transfection, confirming the cellular activity of each asAkt isoforms is similar to the corresponding activity of wtAkt isoforms.
To determine the effects of the inhibitors in vivo, HEK293 cells were next transfected with HA-asAkt1 and treated with serially diluted 3-IB-PP1 or PrINZ (Fig. 2c). HA-asAkt1 hyperphosphorylation was induced by 3-IB-PP1 and PrINZ in a dose-dependent manner, strongly suggesting that induction of phosphorylation results from specific inhibition of Akt downstream signaling and/or specific binding of the Akt inhibitors to the kinase and not from off-target kinase inhibitory activity as is clearly possible with A-443654. The fact that two structurally distinct Akt inhibitors induced Akt hyperphosphorylation indicates that Akt hyperphosphorylation is likely a general phenomenon for multiple classes of ATP-competitive Akt inhibitors.
We then assessed the generality of the phenomenon across the remaining asAkt2 and asAkt3 isoforms and again observed hyperphosphorylation of these isoforms, demonstrating that hyperphosphorylation is consistently induced on all the isoforms of Akt by ATP-competitive Akt inhibitors (Fig. 2d).
The downstream consequences of 3-IB-PP1 and PrINZ induced Akt hyperphosphorylation were assessed in HEK293 cells transfected with the constituitively activated myr-HA-asAkt1. Both inhibitors decreased the phosphorylation level of Ser9 on GSK3β in an inverse dose-dependent manner to the induction of Akt hyperphosphorylation suggesting that PrINZ and 3-IB-PP1 block downstream signaling of Akt while concomitantly inducing Akt hyperphosphorylation (Supplementary Fig. 2 online).
Upstream regulators of Akt phosphorylation
Physiological Akt activation is regulated by three upstream kinases1–3: 1) PI3K which produces PIP3 for PH domain recruitment of Akt to the membrane; 2) PDK1 phosphorylation of activation loop Thr308; and 3) mTORC2 phosphorylation of the HM Ser473 (Fig. 3a). We asked whether each of these kinase inputs to Akt still regulated inhibitor-induced hyperphosphorylation. The role of each upstream kinase was explored using both inhibitors of the upstream kinases and mutational analysis of Akt.
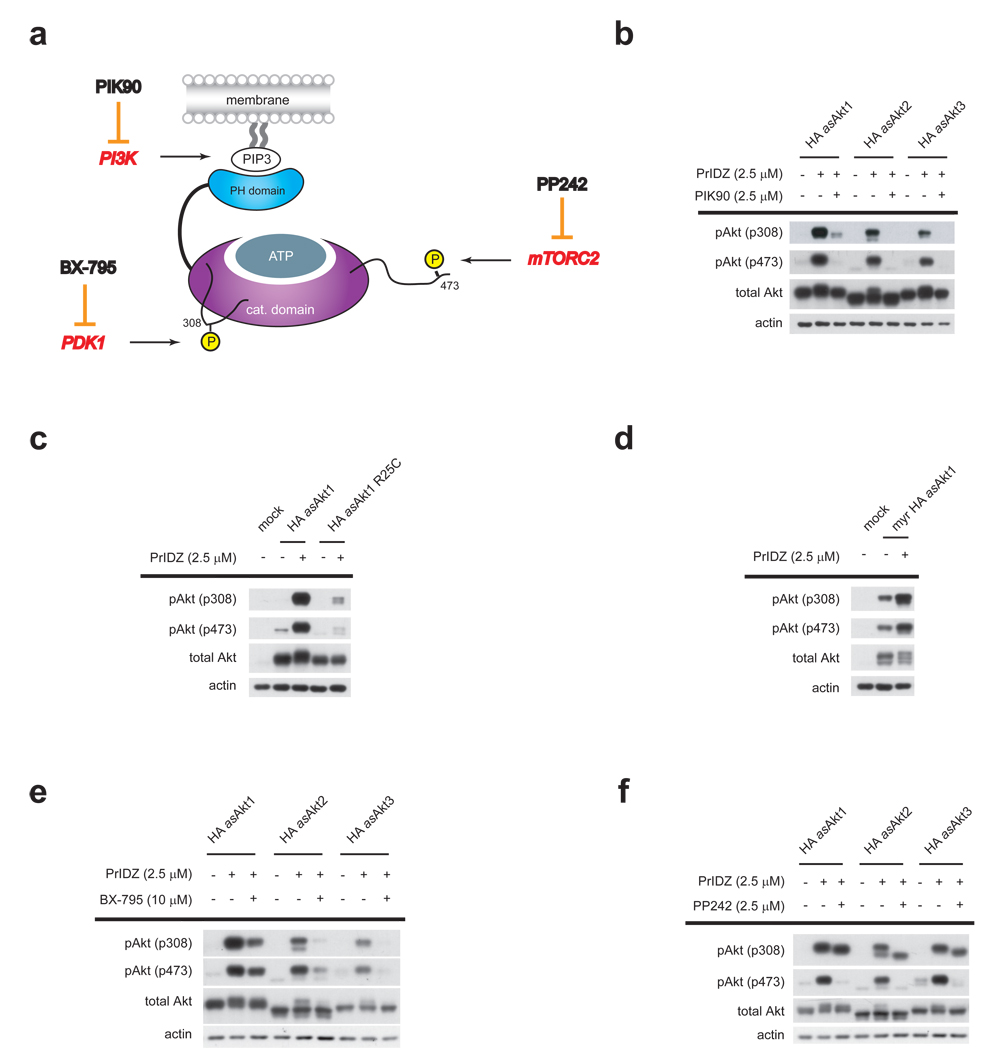
Figure 3. Pharmacological and genetic dissection of upstream regulators of Akt inhibitor-induced Akt hyperphosphorylation.
(a) Analyzed regulators of physiological Akt activation include: 1) PI3K, which produces PIP3 for PH domain recruitment of Akt to the membrane; 2) PDK1, which phosphorylates Thr308; and 3) mTORC2, which phosphorylates Ser473. PI3K, PDK1 and mTORC2 are inhibited: PIK90, BX-795 and PP242, respectively. (b) HEK293 cells transfected with HA-asAkt1/2/3 were treated with 2.5 µM PIK90 for 10 min prior to the addition of 2.5 µM PrINZ for an additional 30 min. Cell lysates were analyzed for Akt (Thr308, Ser473) phosphorylation by immunoblotting. (c) HEK293 cells transfected with either HA-asAkt1 or HA-asAkt1R25C were treated with 2.5 µM PrINZ for 30 min. Cell lysates were analyzed for Akt (Thr308, Ser473) phosphorylation by immunoblotting. (d) HEK293 cells transfected with myr-HA-asAkt1 were treated with 2.5 µM PrINZ for 30 min. Cell lysates were analyzed for Akt (Thr308, Ser473) phosphorylation by immunoblotting. (e) HEK293 cells transfected with HA-asAkt1/2/3 were treated with 10 µM BX-795 for 10 min prior to the addition of 2.5 µM PrINZ for an additional 30 min. Cell lysates were analyzed for Akt (Thr308, Ser473) phosphorylation by immunoblotting. (f) HEK293 cells transfected with HA-asAkt1/2/3 were treated with 2.5 µM PP242 for 10 min prior to the addition of 2.5 µM PrINZ for an additional 30 min. Cell lysates were analyzed for Akt (Thr308, Ser473) phosphorylation by immunoblotting.
Role of membrane localization in hyperphosphorylation
To assess the requirement for Akt membrane translocation in Akt hyperphosphorylation, we used the inhibitor PIK90 (4), a selective pan-PI3K inhibitor31. Pre-treatment of HA-asAkt1/2/3 transfected HEK293 cells with PIK90 significantly attenuated hyperphosphorylation of all three asAkt isoforms induced by PrINZ (Fig. 3b). These results are consistent with previous studies of the role of PIP3 in both canonical Akt activation1 and A-443654 induced Akt hyperphosphorylation21.
The pharmacological blockade of PI3K may influence multiple downstream pathways complicating interpretation of the requirement for PI3K activity in inhibitor-induced hyperphosphorylation. As a direct test of the requirement for PIP3 binding by Akt we utilized an Akt mutant (R25C), which exhibits significantly decreased affinity for PIP3 (Fig. 3c)32. Transfection of HA-asAkt1 and HA-asAkt1R25C into HEK293 cells, followed by treatment with PrINZ, showed that the R25C mutation greatly reduced the PrINZ induced phosphorylation levels on both Thr308 and Ser473 confirming the requirement of Akt membrane translocation through Akt binding to PIP3 to achieve hyperphosphorylation.
We next asked if membrane localization was sufficient to cause Akt hyperphosphorylation. In cells transfected with constituitively membrane localized myr-HA-asAkt1, treatment with PrINZ resulted in hyperphosphorylation of myr-HA-asAkt1 (Fig. 3d). These data suggest that membrane localization of Akt is not sufficient to produce hyperphosphorylation of the kinase and that Akt localized to the membrane is still subject to drug-induced regulation of Thr308 and Ser473 phosphorylation.
We wondered if the constitutively membrane localized construct, myr-HA-asAkt1/2 still requires PIP3 binding to be hyperphosphorylated. In other words, Akt hyperphosphorylation may require Akt binding to PIP3 but membrane localization itself would not be essential. We investigated whether treatment with PIK90 or introduction of the R25C mutation in the PH domain affected hyperphosphorylation on myr-HA-asAkt1. Pre-treatment with PIK90 reduces hyperphosphorylation on HA-asAkt1 induced by PrIDZ (Fig. 3b) while hyperphosphorylation on myr-HA-asAkt1 was not inhibited by PIK90 (Supplementary Fig. 3a online). The constituitively membrane localized myr-HA-asAkt combined with the R25C mutation was also studied, with similar results (Supplementary Fig. 3b online). These results reveal that hyperphosphorylation of myr-HA-asAkt1 does not require PH domain binding to PIP3.
PDK1 and mTORC2 are responsible for phosphorylation
We next explored the mechanistic basis for the regulation by asking whether the upstream kinases (PDK1 and mTORC2) are required for drug-induced Akt hyperphosphorylation. The phosphorylation of Akt has been the subject of intense study in part because of the fact that full activation requires phosphorylation by two kinases on two sites at distant segments of the polypeptide. The kinase PDK1 is responsible for phosphorylation at Thr308 during normal growth factor stimulation4,5. The kinase responsible for Ser473 phosphorylation has been the subject of significant controversy, although it now seems clear that the rapamycin insensitive mTOR complex, mTORC2, is the Ser473 kinase7,8. We asked if Akt inhibitor-induced hyperphosphorylation also relied on these upstream kinases in a cell.
To assess the relevance of PDK1, we used an inhibitor reported by Berlex Biosciences, BX-795 (5)33. Screening of BX-795 against a panel of 220 kinases revealed that BX-795 was selective for only PDK1 within the PI3K-mTORC1 pathway (Supplementary Table 1 online). HEK293 cells transfected with HA-asAkt1 were pre-treated with BX-795 before addition of PrINZ (Fig. 3e). A significant decrease in PrINZ induced Thr308 phosphorylation was observed, confirming that PDK1 is involved in Akt hyperphosphorylation. Interestingly, BX-795 also reduced drug-induced hyperphosphorylation at Ser473 as well. Although the mechanistic basis for the BX-795 effect on Ser473 status is not clear at this point, the same treatment of a non-phosphorylatable Thr308 form of Akt, HA-asAktT308A revealed that BX-795 does not affect Ser473 phosphorylation status directly (Supplementary Fig. 4 online).
We next investigated the role of mTORC2 using PP242 (6), an ATP-competitive mTOR kinase inhibitor, which inhibits both mTORC1 and mTORC2, and does not inhibit any PI3Ks or protein kinases in the PI3K-mTORC1 pathway8. When HEK293 cells transfected with HA-asAkt1/2/3 were treated with PP242 prior to treatment with PrINZ, hyperphosphorylation on Ser473 was completely inhibited (Fig. 3f). The induction of phosphorylation at Thr308 was unaffected under these conditions. These results suggest that the mTORC2 complex is the kinase responsible for drug-induced Akt hyperphosphorylation at Ser473.
Hyperphosphorylation is independent of Akt signaling
Having determined that the same upstream kinases lead to both Akt activation in growth factor signaling and inhibitor-induced Akt hyperphosphorylation, we sought to understand how Akt inhibitors could lead to its hyperphosphorylation. We consider two broad categories of mechanisms—kinase extrinsic and kinase intrinsic. A kinase extrinsic mechanism of inhibitor-induced hyperphosphorylation encompasses any form of inhibitor-induced pathway feedback, which causes the loss of pathway inhibition leading to hyperphosphorylation of Akt. A kinase intrinsic mechanism encompasses any drug-induced change to the kinase itself which either makes it a better substrate for upstream activators or a worse substrate for deactivating phosphatases.
The possibilities for kinase extrinsic forms of inhibitor-induced Akt hyperphosphorylation are numerous since so many downstream substrates1–3 are candidates for being in known or unknown feedback loops. The most probable extrinsic mechanism for Akt hyperphosphorylation is mTORC1/S6K mediated feedback, as has been reported for rapamycin15–19. Previous work revealed that hyperphosphorylation by A-443654 occurred in TSC2−/− cells, which are defective in activating mTORC1 via Akt and TSC221. However, it is possible that mTORC1 activity is controlled by Akt in a TSC2 independent fashion. In fact, mTORC1 kinase activity was recently revealed to also be regulated by PRAS40 which is a direct target of Akt22,23. In addition, it is unclear whether TSC2−/− cells maintain the normal PI3K/Akt/mTORC1 pathway or have compensated in some unknown way for the loss of TSC2.
Our studies using DG2 (7), a new selective S6K inhibitor34 however revealed that inhibition of S6K does not induce Akt phosphorylation at Thr308 and Ser473 when compared to the hyperphosphorylation induced by Akt inhibitors (Supplementary Fig. 5 online). Therefore it appears that S6K inhibition is insufficient to cause the large induction of phosphorylation seen with direct Akt inhibitors.
Since testing of kinase extrinsic pathways of inhibitor-induced Akt hyperphosphorylation requires development of new pharmacological tools for each candidate pathway, we sought to rule out the kinase intrinsic model before further investigating the extrinsic model. We took advantage of a mutation to Akt which destroys its catalytic activity. Such a mutant is incapable of activating any downstream signals via substrate phosphorylation and thus should not induce hyperphosphorylation in the presence or absence of the inhibitor if a block of downstream signaling is required to trigger Akt hyperphosphorylation.
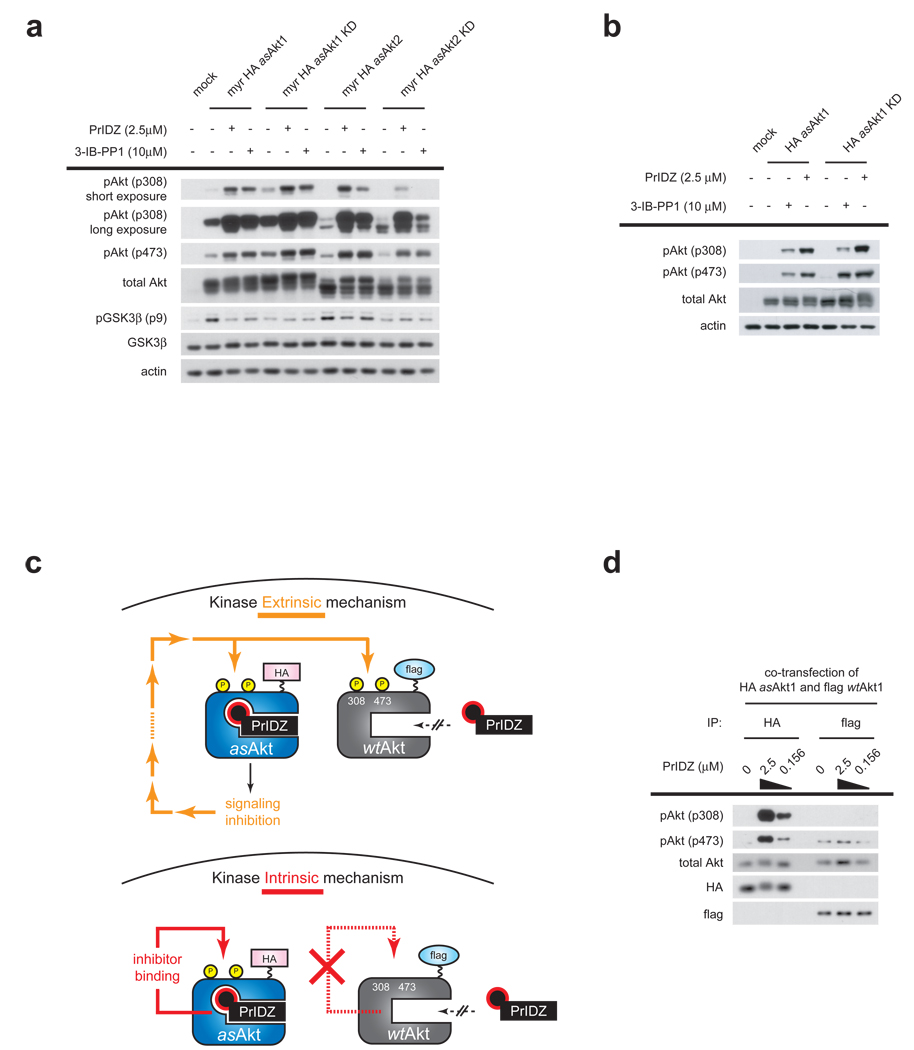
Double mutant constructs combining the gatekeeper mutation (e.g. M227G/ M225G for asAkt1/2) with mutations that abrogate kinase activity, D292A/D289A for Akt1/2, lacking the active-site Asp residue of the DFG motif35 which is required for chelation of catalytically essential Mg2+ were prepared and transfected into HEK293 cells. Treatment of cells expressing the kinase dead (KD) mutants, myr-HA-asAkt1-KD or myr-HA-asAkt2-KD with PrINZ or 3-IB-PP1 induced striking hyperphosphorylation on Thr308 and Ser473. The drug-induced hyperphosphorylation on the KD mutants was comparable in magnitude to the catalytically active variants, myr-HA-asAkt1 or myr-HA-asAkt2 (Fig. 4a). The non-myristoyl HA-asAkt1-KD was evaluated as well, with similar results (Fig. 4b). The drug induced hyperphosphorylation of the KD variants was further confirmed in multiple cell lines (HEK293, MiaPaCa-2, MCF-7 and PC-3), including both transformed and non-transformed cells (Supplementary Fig. 6 online). These results validate the hypothesis that inhibition of Akt signaling is not involved in hyperphosphorylation, and supports the kinase intrinsic model in which inhibitor binding to the ATP site triggers hyperphosphorylation.
Figure 4. Hyperphosphorylation is independent of Akt signaling and results from inhibitor binding to Akt.
(a) HEK293 cells transfected with either kinase active myr-HA-asAkt1/2 or kinase dead (KD) form of myr-HA-asAkt1/2 were treated for 30 min with 2.5 µM PrINZ or 10 µM 3-IB-PP1. Cell lysates were analyzed for Akt (Thr308, Ser473) and GSK3β (Ser9) phosphorylation by immunoblotting. (b) HEK293 cells transfected with either kinase active HA-asAkt1 or kinase dead (KD) form of HA-asAkt1 were treated for 30 min with 2.5 µM PrINZ or 10 µM 3-IB-PP1. Cell lysates were analyzed for Akt (Thr308, Ser473) and GSK3β (Ser9) phosphorylation by immunoblotting. (c) Schematic representation of the expected outcomes due to extrinsic and intrinsic regulation of Akt upon treatment with PrIDZ and co-transfection of HA-asAkt1 and flag-wtAkt1. If pathway mediated feedback is causing Akt hyperphosphorylation (kinase extrinsic) then all Akt molecules in the same cell including both HA-asAkt1 and flag-wtAkt1 should be hyperphosphorylated equally. In contrast, if the occupancy of the ATP site was the only determinant of hyperphosphorylation (kinase intrinsic), then only the Akt capable of drug binding (HA-asAkt1) should be hyperphosphorylated. (d) HEK293 cells co-transfected with HA-asAkt1 and flag-wtAkt1 were treated for 30 min with various concentration of PrINZ. Cell lysates were immunoprecipitated by using either anti-HA or anti-flag antibody and then the immunoprecipitates were analyzed for Akt (Thr308, Ser473) phosphorylation by immunoblotting.
Drug-induced intrinsic kinase regulatory phosphorylation is unprecedented. Hundreds of protein kinase inhibitors have been developed which do not trigger their target kinases to become hyperphosphorylated on the activating sites. As a further test of this model and to rule out any non-catalytic activity mediated signals from Akt we carried out a double Akt transfection experiment. The experiment relies on the co-transfection of HA-asAkt1 and flag-wtAkt1 (Fig. 4c). If the occupancy of the ATP site was the only determinant of hyperphosphorylation (kinase intrinsic), then only the Akt capable of drug binding (HA-asAkt1) should be hyperphosphorylated. In cells co-transfected with HA-asAkt1 and flag-wtAkt1, treatment with PrIDZ revealed Thr308 and Ser473 phosphorylation is induced only on HA-asAkt1 and not on drug insensitive flag-wtAkt1 after immunoprecipitation (Fig. 4d). The finding demonstrates that feedback mediated by downstream signaling of Akt is not involved in hyperphosphorylation of Akt (Fig. 4c). The ability of flag-tagged Akt1 to become hyperphosphorylated by Akt inhibitors was confirmed separately (Supplementary Fig. 7 online). A second tagged construct of asAkt1 containing mCherry, which exhibits a large MW gel shift from endogenous Akt was also studied, with similar results (Supplementary Fig. 8 online).
Akt inhibitor induces Akt membrane localization
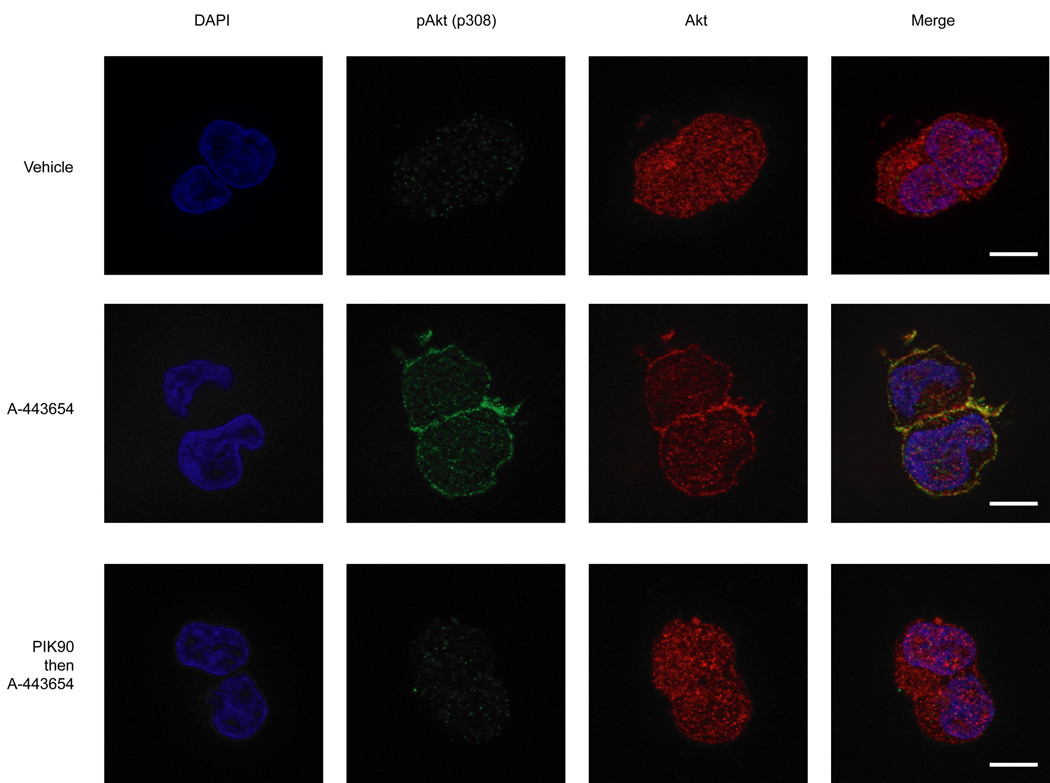
The finding that drug binding to Akt results in Akt hyperphosphorylation mediated by a kinase intrinsic mechanism was particularly surprising in light of our early finding that both membrane localization of Akt (Fig. 3b,c and Supplementary Fig. 3 online) and drug binding were required for the hyperphosphorylation. One prediction of the kinase intrinsic model of inhibitor-induced Akt hyperphosphorylation is that drug binding should cause relocalization of Akt from the cytoplasm to the membrane. No known kinase inhibitors that we are aware of induce cellular translocation of their target kinase upon binding. To determine whether such a drug-induced cellular relocalization was in fact occurring, we carried out immunofluorescence studies of Akt. We chose to utilize untransfected HEK293 cells and A-443654, instead of asAkt transfected cells and PrIDZ, to avoid overexpression of the kinase. In particular, the untransfected cells maintain the physiological stoichiometry between PIP3 and Akt whereas excess asAkt molecules might be mislocalized in asAkt overexpressed cells due to insufficient PIP3. After HEK293 cells were treated with A-443654, fixed cells were stained with anti-Akt and anti-pThr308 to determine the location of Akt and pAkt. In the absence of any growth factor stimulation, treatment with A-443654 resulted in translocation of Akt to the plasma membrane (Fig. 5). Moreover, the membrane localized Akt was phosphorylated at Thr308. In addition, both the translocation and the phosphorylation events were inhibited by pre-treatment with PIK90.
Figure 5. The Akt inhibitor A-443654 induces Akt membrane localization.
After treatment of HEK293 cells with various conditions described below, cells were fixed and stained with rabbit anti-Akt or mouse anti-pAkt (p308) followed by Alexa 488-conjugated goat anti-rabbit and Alexa 568-conjugated goat anti-mouse, and examined by fluorescence microscopy. Cells were treated with vehicle (DMSO) for 15min (top panel), treated with 2.5 µM A-443654 for 15 min (middle panel), or treated with 2.5 µM PIK90 for 10 min prior to the addition of 2.5 µM A-443654 for further 15 min (bottom panel). Scale bar 10 µm.
Hyperphosphorylation is inhibited by Akti-1,2
Merck has reported an allosteric Akt inhibitor, Akti-1,2 (8), which binds outside of the active site and inhibits in vitro kinase activity. Interestingly, in cells Akti-1,2 also inhibits growth factor-stimulated activation of Akt by preventing phosphorylation at Thr308 and Ser473 in a PH-domain dependent fashion36,37. Although it is still controversial whether Akti-1,2 prevents Akt translocation induced by growth factor stimulation36,37, we asked if Akti-1,2 inhibits hyperphosphorylation induced by the ATP-competitive inhibitor, PrIDZ. In HEK293 cells transfected with HA-asAkt1, treatment with Akti-1,2 prior to induction of hyperphosphorylation by PrIDZ resulted in dose-dependent inhibition of hyperphosphorylation (Supplementary Fig. 9 online). Akti-1,2 thus inhibits both physiological activation of Akt and drug induced Akt hyperphosphorylation. These results further support the idea that the upstream regulation of Akt hyperphosphorylation is similar for physiological phosphorylation since both exhibit the same pharmacological sensitivity to Akti-1,2.
Catalytic activity of hyperphosphorylated Akt
One pharmacologically important question about the drug induced hyperphosphorylation of Akt is whether hyperphosphorylated Akt is more catalytically active if the inhibitor were to dissociate after Akt is hyperphosphorylated. We measured the in vitro kinase activity of HA-asAkt1 after inducing hyperphosphorylation by PrIDZ in cells (Fig. 6). HEK293 cells transfected with HA-asAkt1 were treated with PrIDZ and hyperphosphorylated HA-asAkt1 was immunoprecipitated. An in vitro IP kinase assay was carried out after thorough washing of the immunoprecipitate to ensure that PrIDZ would dissociate. Hyperphosphorylated asAkt1 is revealed to be approximately 10-fold more active than asAkt1 immunoprecipitated from cells not treated with the active site Akt inhibitor, as anticipated based on the phosphorylation status of the two regulatory sites.
Figure 6. Hyperphosphorylated Akt is hyper-active in vitro after dissociation of Akt inhibitor.
HEK293 cells transfected with HA-asAkt1 were cultured either with or without serum overnight and then treated with 2.5 µM PrIDZ for 30 min before stimulation with IGF-1 (50 ng/ml) for 10 min. HA-asAkt1 was immunoprecipitated from cell lysates and assayed for Akt activity. Data are represented as mean ± SEM (n=8) and as relative values to IGF-1 stimulated HA-asAkt1 activity. The immunoprecipitates were analyzed for Akt (Thr308, Ser473) phosphorylation by immunoblotting.
Discussion
The widespread involvement of aberrant protein kinase signaling in disease has made the development of protein kinase inhibitors a major focus of pharmaceutical research for the last ten years. The majority of kinase inhibitors have been shown to inhibit kinase signaling pathways through blocking the target kinases’ substrate phosphorylation and subsequent downstream pathway components. Paradoxically however, several kinase inhibitors such as the mTORC1 inhibitor, rapamycin activate the target pathway due to inhibition of a negative feedback loop16–19. Since the pathways targeted in cancer are growth promoting, it is critical to understand which pathways may have active feedback loops and which kinases are responsible for their control, in order to avoid inhibitor-induced pathway activation in patients15. Other kinase inhibitors including the p38 inhibitor SB20358038, a Raf inhibitor ZM33637239, and the Akt inhibitor A-443654 studied here21 induce phosphorylation of pathway components. We reasoned that elucidation of the mechanism of inhibitor induced phosphorylation of these kinases could influence the development of next generation agents.
Unlike rapamycin, the majority of kinase inhibitors are ATP-competitive making the dissection of their effects more difficult because of off-target effects. The first reported Akt inhibitor, A-443654 is a case in point. We thus turned to a chemical genetic approach to develop highly selective Akt inhibitors. Mutation of the gatekeeper in Akt from methionine to glycine enabled selective inhibition by two inhibitors (3-IB-PP1 and PrINZ) which do not have effects on kinases which lie upstream or downstream of Akt. All three ATP-competitive inhibitors induce the same hyperphosphorylation of their target, suggesting that A-443654 induced effects will be representative of other Akt inhibitors as well. Indeed, Glaxo-Smith Klein discovered another ATP-competitive Akt inhibitor, GSK690693, possessing a completely different structure from A-443654, which also induces Akt hyperphosphorylation40,41. The chemical genetic inhibitors additionally demonstrated that all Akt isoforms (1, 2, and 3) are subject to the same inhibitor-induced hyperphosphorylation.
Having conclusive evidence of the class specific nature of Akt hyperphosphorylation induced by ATP-competitive inhibitors we turned to dissection of the mechanism. Our studies with a new S6K inhibitor revealed that inhibition of S6K, a key mediator of rapamycin-driven feedback, is insufficient to cause the large induction of phosphorylation seen with direct Akt inhibitors.
The inability to induce Akt hyperphosphorylation through inhibition of downstream components of the Akt pathway led us to investigate a non-pathway based mechanism of drug-induced Akt hyperphosphorylation. Indeed we observed indistinguishable drug-induced Akt hyperphosphorylation whether the kinase was active and able to transduce signals downstream in the pathway or whether it was inactive.
The central result that the ATP-competitive inhibitor binding is sufficient to induce hyperphosphorylation while loss of Akt-downstream signaling inhibition is not, is quite surprising. This form of drug-induced kinase regulation is unprecedented to our knowledge. We refer to this new form of kinase regulation as “inhibitor hijacking of kinase activation” or intrinsic to distinguish it from a loss of negative feedback regulation at a pathway level as has been described for rapamycin inhibition of mTORC115–19.
How does drug binding to a kinase induce its hyperphosphorylation in the absence of any stimulation of the Akt pathway? Our studies reveal that binding of Akt ligands in the ATP pocket template two alterations in the susceptibility of Akt to become phosphorylated. The first effect is through drug-induced potentiation of the binding of the Akt PH domain to basal levels of PIP3 which promotes membrane location of Akt. If membrane localization is disrupted by pharmacological or genetic means, the drug-induced hyperphosphorylation of Akt does not occur. How does drug binding to the catalytic domain of Akt influence PH domain binding to PIP3? The results here suggest that the Akt inhibitor sensitizes the PH domain to bind basal levels of PIP3 to facilitate membrane location perhaps through a conformational change templated by the inhibitor. Recent FRET studies of Akt dynamics suggested that the PH domain of Akt is sequestered in the cytoplasm by its interaction with Akt kinase domain and is induced to become available to bind PIP337,42.
Our studies with constituitively membrane localized Akt reveal that membrane localization alone is not sufficient to induce Akt hyperphosphorylation. Thus, a second drug dependent change to Akt in addition to membrane localization is required for hyperphosphorylation to occur. This second step involves alteration of the reactivity of the two phosphorylation sites (Thr308 and Ser473). The two most easily envisioned mechanisms responsible are either an effect on the conformation of Akt to make it more susceptible to kinase phosphorylation or a conformational change which makes it less susceptible to phosphatase dephosphorylation. Either mechanism alone or a combination of effects could lead to drug-induced Akt hyperphosphorylation. However, such regulation is perhaps not surprising given the fact that dual phosphorylation of Akt is known to increase its catalytic activity by several orders of magnitude, suggesting a means of communication between Thr308-P/Ser-473-P and the ATP active site.
Recent FRET studies of Akt suggested that intramolecular interaction between the PH domain and kinase domain in the cytoplasm prevents Thr308 phosphorylation by PDK137,42. Our results with a constituitively membrane localized Akt construct lacking the PH domain, which would be predicted to be constituitively phosphorylated, by analogy to the FRET based model, show that hyperphosphorylation was still induced by A-443654 (Supplementary Fig. 10 online). Thus, it appears that disruption of the PH-kinase domain interface is not sufficient alone to induce T308 phosphorylation.
Additional mechanisms for intrinsic activation can be envisioned. Akt associated protein partners could be responsible for the drug-induced regulation as seen in some kinases regulated by protein-protein association43. Indeed, a number of proteins have been suggested to be involved in Akt regulation, including CTMP and Cdc37/HSP9044. A drug-induced conformational change to Akt which subsequently induces a change in protein-protein association would be similar to the mechanism observed in regulation of small GTP-binding protein (small GTPases) such as Ras and Rho45,46. Small GTPases are triggered by GTP binding to modulate protein-protein interactions.
In the case of small GTPases, ligand structure (GTP vs. GDP) controls different outputs of the protein (GTP-on/GDP-off). Traditionally, kinases have been assumed to use ATP as a phosphodonor rather than a regulator of kinase function. Recently however, chemical genetic studies of the unfolded protein response regulator, Ire1 have revealed that Ire1 kinase inhibitors can bypass the need for Ire1 kinase activity to trigger the unfolded protein response47,48. Structural studies of the Ire1/kinase inhibitor complex reveal that drug binding induces a conformational change in the kinase which triggers oligomerization and activation of the RNAse domain of Ire149. This precedent suggests that kinases can be regulated by ligand binding to the ATP binding site in ways independent of the canonical ATP dependent phosphotransfer reaction. As more kinases are shown to exhibit catalytic activity-independent functions that can be controlled by “inhibitor” binding perhaps it will be possible to uncover the function of pseudokinases, the 10% of human kinases which naturally lack catalytic activity50.
What do our findings mean for development of kinase inhibitor based therapeutics? Our studies revealed that inhibitor induced hyperphosphorylated Akt was extremely active after dissociation of ATP-competitive Akt inhibitor. These observations suggest that following in vivo treatment with an ATP-competitive Akt inhibitor, if the drug dissociates from Akt, the enzyme would be hyper-active and phosphorylate downstream targets, potentially promoting oncogenesis. It is important however to realize that our enhanced activity of Akt was only observed following isolation of the kinase and that in cells, we never observed increased Akt substrate phosphorylation (Fig. 6 and data not shown). Perhaps the phosphatases for T308P and S473P are highly active and there is sufficiently rapid dephosphorylation, or our washout studies never adequately removed the drug from Akt. Our findings do add to the number of studies revealing the importance of various forms of kinase inhibitor induced feedback activation observed in cells thus warranting further study of feedback networks, both extrinsic (rapamycin-like) and intrinsic (Akt-inhibitor like).
Methods
Chemical synthesis
All compounds except Akti-1/2 were synthesized from commercially available starting materials and purified by RP-HPLC. See Supplementary Methods online for complete details. Akti-1/2 was purchased from Calbiochem (Akt Inhibitor VIII).
Buffer solutions
Buffer A: 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X, 2.5 mM Sodium Pyrophosphate, 1 mM β-glycerophosphate, Complete™ Protease Inhibitor Cocktail (Roche Applied Science), Phosphatase Inhibitor Cocktail 1 (Sigma-Aldrich), Phosphatase Inhibitor Cocktail 2 (Sigma-Aldrich) and 20 nM Microcystin LR. Buffer B: 25 mM Tris (pH 7.5), 10 mM Magnesium Chloride, 5 mM β-glycerophosphate, 0.1 mM Sodium Orthovanadate and 2 mM DTT.
Cell-based assay
We used HEK293 cells for cell-based assay in preference to HEK293T line employed for in vitro IP kinase assay, because the latter shows constitutive activation of PI3K/Akt signaling, as indicated by high level of phosphorylation on Thr308 and Ser473 of Akt, and Ser9 of GSK3β (Supplementary Fig. 11 online). In contrast, HEK293 cells show only basal PI3K/Akt activity, and are markedly activated by stimulation with IGF-1. Cells were plated in six-well dishes and were transfected at 80–90% confluence with a variety of plasmids by using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s instructions. Unless otherwise noted, drug treatments of these Akt-expressing HEK293 cells were carried out in growth factor containing normal media as shown in Cell culture section (Supplementary Methods online). In all cases, DMSO inhibitor stocks were used at 1:1000.
Cell lysis
Following drug treatment and/or stimulation, cells were detached with ice cold Ca2+, Mg2+ free PBS containing 0.04% EDTA (for HEK293 and HEK293T) or washed with PBS (for MCF7, PC-3, MiaPaCa-2 and L6), and then lysed in Buffer A (for HEK293, HEK293T, MCF7, PC-3, MiaPaCa-2) or RIPA buffer (L6). Whole-cell lysates were centrifuged (14000 g at 4°C for 20 min) and then protein amount in supernatants was quantified by using Bradford assay (Protein Assay kit, Bio-Rad Laboratories).
Immunoblotting
Cell lysate samples were subjected to SDS/PAGE and proteins were transferred onto nitrocellulose membranes (Bio-Rad Laboratories) and blocked with 5% skim milk in 0.1% Tween 20/Tris Buffered Saline (TBST). The nitrocellulose membranes were probed with various antibodies in 5% BSA/TBST described in the figure legends (all primary antibodies from Cell Signaling Technology). Detection of primary antibodies was performed using appropriate peroxidase-conjugated IgGs (Pierce Biotechnology or Santa Cruz Biotechnology) in 5% BSA/TBST and protein signals were visualized using enhanced chemiluminescence (Pierce Biotechnology) by exposure to CL-X Posure™ film (Pierce Biotechnology).
Immunoprecipitation
After cell lysis in Buffer A, protein amount of each sample was adjusted to the same. Each sample was immunoprecipitated over night at 4°C with either Anti-HA Affinity Matrix (Roche Applied Science) or Anti-Flag® M2 Agarose (Sigma-Aldrich) each blocked in advance with 1% BSA in PBS for 3 hrs at 4°C. After washing three times with Buffer A, the immunoprecipitates were denatured by boiling with loading buffer, and subjected to immunoblotting.
Immunofluorescence
HEK293 cells were cultured on cover slips coated with poly-L-lysine (Aldrich). After treatment with drugs described in the figure legends, cells were washed once with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS for 15 min at room temperature (rt). After washing three times with PBS, cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min and then washed three times with PBS. After blocking with 5% BSA/PBS for 1 h, cells were incubated over night at 4°C with mouse monoclonal anti-Akt antibody and rabbit monoclonal anti-pAkt (p308) antibody (Both from Cell Signaling Technology) in 2% BSA/PBS. After washing three times with PBS, cells were further incubated for 1 h at rt with Alexa Fluor® 488-conjugated goat anti-rabbit IgG and Alexa Fluor® 568-conjugated goat anti-mouse IgG1 (Both from Invitrogen). After washing three times with PBS and once with water, cover slips were mounted on cover slides with VECTASHIELD® mounting medium (Vector Labs) containing 4',6-diamino-2-phenylinodole (DAPI). Fluorescent images were obtained with a Zeiss Axiovert 200M florescence microscope equipped with an apotome (which permits optical sectioning of cells) using AxioVision Rel. 4.6 software. Images shown in figure were taken with a Zeiss Plan-Apochromat 63×/1.4 Oil Dic objective.
Supplementary Material
ACKNOWLEDGEMENTS
We thank B. Houseman (University of California, San Francisco) for PIK90 synthesis. We thank A. Dar, M. Feldman, A. Garske and A. Statsuk for helpful comments on the manuscript. T.O. was supported by Ajinomoto Co., Inc. D.G. thanks the GREAT Fellowship program from the University of California for funding and the NIH Chemistry and Chemical Biology Training Grant. D.F. thanks the D.F.G. for funding.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
REFERENCES
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 4.Alessi DR, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 5.Stokoe D, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 6.Alessi DR, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 8.Apsel B, et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat. Chem. Biol. 2008;4:691–699. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpten JD, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 10.Shor AC, Agresta SV, D'Amato GZ, Sondak VK. Therapeutic potential of directed tyrosine kinase inhibitor therapy in sarcomas. Cancer Control. 2008;15:47–54. doi: 10.1177/107327480801500106. [DOI] [PubMed] [Google Scholar]
- 11.Fresno Vara JA, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–7492. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- 13.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim. Biophys. Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6Kcassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Manning BD, et al. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 19.Harrington LS, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y, et al. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol. Cancer Ther. 2005;4:977–986. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 21.Han EK, et al. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007;26:5655–5661. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J. Biol. Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 23.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 24.Bishop AC, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 25.Bishop AC, et al. Generation of Monospecific Nanomolar Tyrosine Kinase Inhibitors via a Chemical Genetic Approach. J. Am. Chem. Soc. 1999;121:627–631. [Google Scholar]
- 26.Miller AL, Zhang C, Shokat KM, Lowell CA. Generation of a novel system for studying spleen tyrosine kinase function in macrophages and B cells. J. Immunol. 2009;182:988–998. doi: 10.4049/jimmunol.182.2.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight ZA, Shokat KM. Features of selective kinase inhibitors. Chem. Biol. 2005;12:621–637. doi: 10.1016/j.chembiol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Davies TG, et al. A structural comparison of inhibitor binding to PKB, PKA and PKA-PKB chimera. J. Mol. Biol. 2007;367:882–894. doi: 10.1016/j.jmb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Andjelkovic M, et al. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 30.Meier R, Alessi DR, Cron P, Andjelkovic M, Hemmings BA. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J. Biol. Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 31.Knight ZA, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 33.Feldman RI, et al. Novel small molecule inhibitors of 3-phosphoinositide-dependent kinase-1. J. Biol. Chem. 2005;280:19867–19874. doi: 10.1074/jbc.M501367200. [DOI] [PubMed] [Google Scholar]
- 34.Anand NK, et al. Preparation and structure activity of pyrazolo-pyrimidine derivatives as antitumor agents and kinase modulators. PCT application: WO2005117909. [Google Scholar]
- 35.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–282. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 36.Green CJ, et al. Use of Akt inhibitor and a drug-resistant mutant validates a critical role for protein kinase B/Akt in the insulin-dependent regulation of glucose and system A amino acid uptake. J. Biol. Chem. 2008;283:27653–27667. doi: 10.1074/jbc.M802623200. [DOI] [PubMed] [Google Scholar]
- 37.Calleja V, Laguerre M, Parker PJ, Larijani B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS Biol. 2009;7:e17. doi: 10.1371/journal.pbio.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S, Jiang MS, Adams JL, Lee JC. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 1999;263:825–831. doi: 10.1006/bbrc.1999.1454. [DOI] [PubMed] [Google Scholar]
- 39.Hall-Jackson CA, et al. Paradoxical activation of Raf by a novel Raf inhibitor. Chem. Biol. 1999;6:559–568. doi: 10.1016/s1074-5521(99)80088-x. [DOI] [PubMed] [Google Scholar]
- 40.Levy DS, Kahana JA, Kumar R. AKT inhibitor, GSK690693, induces growth inhibition and apoptosis in acute lymphoblastic leukemia cell lines. Blood. 2009;113:1723–1729. doi: 10.1182/blood-2008-02-137737. [DOI] [PubMed] [Google Scholar]
- 41.Heerding DA, et al. Identification of 4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7- {[(3S)-3-piperidinylmethyl]oxy}-1H-imidazo[4,5-c]pyridin-4-yl)-2-methyl-3-butyn-2-ol (GSK690693), a novel inhibitor of AKT kinase. J. Med. Chem. 2008;51:5663–5679. doi: 10.1021/jm8004527. [DOI] [PubMed] [Google Scholar]
- 42.Calleja V, et al. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 2007;5:e95. doi: 10.1371/journal.pbio.0050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levinson NM, Seeliger MA, Cole PA, Kuriyan J. Structural basis for the recognition of c-Src by its inactivator Csk. Cell. 2008;134:124–134. doi: 10.1016/j.cell.2008.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du K, Tsichlis PN. Regulation of the Akt kinase by interacting proteins. Oncogene. 2005;24:7401–7409. doi: 10.1038/sj.onc.1209099. [DOI] [PubMed] [Google Scholar]
- 45.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 46.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papa FR, Zhang C, Shokat K, Walter P. Bypassing a kinase activity with an ATP-competitive drug. Science. 2003;302:1533–1537. doi: 10.1126/science.1090031. [DOI] [PubMed] [Google Scholar]
- 48.Lee KP, et al. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell. 2008;132:89–100. doi: 10.1016/j.cell.2007.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korennykh AV, et al. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2008 doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443–452. doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.