Abstract
We have examined induction of neuropeptide expression in adrenal medulla after treatment of mice with lipopolysaccharide (LPS), a model for septic shock, which activates both immune and stress responses in vivo. Messenger RNAs encoding vasoactive intestinal polyeptide (VIP) and galanin, both modulators of steroidogenesis in neighboring adrenal cortex, are up-regulated at 24 hours (eight-fold for VIP and two-fold for galanin) after LPS injection, and remain elevated for the following 24 hours. Up-regulation of VIP and galanin by LPS is abrogated in pituitary adenylate cyclase-activating polypeptide (PACAP)-deficient mice, suggesting an interaction between LPS, or LPS-induced cytokines, and PACAP released in adrenal medulla from the splanchnic nerve. Treatment of cultured chromaffin cells with 100 nM PACAP and 10 nM tumor necrosis factor-alpha (TNF-α), a cytokine whose production is elevated by LPS, results in long-term synergistic up-regulation of VIP and galanin mRNA. PACAP blocks the earlier induction by TNF-α of mRNA encoding inhibitor of NF-κB alpha (IκBα, normally a negative autoregulator of TNF-α signaling through nuclear factor-κB (NF-κB), without affecting the induction of TNF-α–induced protein 3 (TNFAIP3), another NF-κB-dependent gene induced by TNF-α in chromaffin cells. By acting downstream of NF-κB to inhibit IκBα gene induction by TNF-α, PACAP may block IκBα-dependent negative autoregulation of TNF-α-signaling through NF-κB, prolonging TNF-α-dependent signaling to neuropeptide-encoding genes in chromaffin cells. This mechanism may also underlie PACAP-dependent neuropeptide gene induction by LPS in vivo.
Keywords: PACAP, VIP, galanin, septic shock, stress, LPS, TNF-alpha, adrenal medulla, adrenal cortex
1. Introduction
The adrenal medulla produces and secretes both catecholamines and neuropeptides in response to metabolic and psychogenic stress (Wong and Tank, 2007). Catecholamines released by both acetylcholine and PACAP secreted from the splanchnic nerve play an important role in glucohomeostasis during metabolic stress (Hamelink et al., 2002). Catecholamines, released from both adrenal medulla and sympathetic nerves, can directly affect both the balance between humoral and cellular immune response, and overall immune activation (Elenkov et al., 2000). Several neuropeptides are also stored in and secreted from chromaffin cells, and their expression is increased by splanchnic nerve firing during metabolic stress (Fischer-Colbrie et al., 1992; Stroth et al., 2007). Two of these, galanin and VIP, are reported to affect steroid production by adrenocortical cells (Andreis et al., 2007; Bornstein et al., 1996; Ehrhart-Bornstein et al., 1991). This raises the question of whether the expression of adrenomedullary neuropeptides is regulated under conditions of septic shock, and could modulate the inflammatory response by enhancing steroidogenesis in the neighboring adrenal cortex, or altering the function of immune cells trafficking the gland (Fischer-Colbrie et al., 2005; Goetzl et al., 2008; Yadav and Goetzl, 2008).
The regulation of biosynthesis of galanin and VIP by metabolic stress in adrenal medulla requires the presence of the slow transmitter PACAP (Stroth et al., 2007), released along with acetylcholine during high-frequency firing of the splanchnic nerve. Thus trans-synaptic signaling through PACAP could potentially provide a permissive signal for cytokine-specific regulation of neuropeptide biosynthesis under inflammatory conditions, especially as prolonged cytokine elevation is known to elicit, over time, a hypoglycemia which could trigger a delayed rise in PACAP and acetylcholine release from the splanchnic nerve (Oguri et al., 2002). To address this question, we assessed the interplay between cytokine production and PACAP-mediated neurotransmission in neuropeptide production by the adrenal medulla. We examined neuropeptide mRNA induction in the adrenal gland in wild-type and PACAP-deficient mice, 24–48 hours after administration of LPS, at a dose chosen to mimic an episode of septic shock (Ulloa and Tracey, 2005; Zacharowski et al., 2006). Our findings indicate that PACAP innervation is required for activation of VIP and galanin biosynthesis in this model for septic shock. These results reveal a potential locus of steroid regulation by adrenomedullary neuropeptides during systemic inflammation. Synergistic induction of VIP and galanin by TNF-α and PACAP was examined in chromaffin cells in vitro, along with modulation of induction of TNF-α-responsive genes in the presence of PACAP. Results obtained suggest that PACAP augments TNF-α-dependent neuropeptide gene induction, and that this occurs through suppression of IκBα transcription, attenuating autoinhibitory mechanisms involved in TNF-α signaling through NF-κB.
2. Materials and methods
2.1. Animals
Male mice (age 6–9 weeks) were used in the present study. Animals harboring the PACAP knock-out allele (Hamelink et al., 2002) were fully backcrossed onto the C57BL/6N strain background, and homozygous PACAP-deficient (KO) mice were age-matched with wild-type (WT) controls for all treatment groups. Animals were housed in a temperature- and humidity-controlled facility with 12 h light/dark cycle (lights off at 3:00 p.m.) and had access to chow and water ad libitum. Treatments were performed between 10:00–11:30 a.m. All experiments were approved by the NIMH Animal Care and Use Committee.
2.2. LPS treatments
Animals were randomized (n=8 per group) and treated where indicated with LPS (i.p.; 1 mg/kg of a 0.25 mg/ml solution in saline, of Escherichia coli, serotype 0.111:B4, Sigma–Aldrich, St. Louis, MO), or saline (i.p.) for 24 or 48 h, after which animals were euthanized by cervical dislocation followed by decapitation, and the adrenal glands collected.
2.3. Bovine chromaffin cells (BCCs) culture and drug treatments
Primary cultures of BCCs were obtained after retrograde perfusion of bovine adrenal glands with 0.1% collagenase (Worthington Biochemical, Lakewood, NJ) and 30 U/ml DNAse (Sigma-Aldrich), followed by dissociation of the digested adrenal medulla. The cells were cultured in DMEM (Invitrogen, Grand Island, NY) supplemented with 5% fetal calf serum and 100 U/ml penicillin-streptomycin, 2 mM glutamine, 10 μg/ml cytosine β-D-arabinofuranoside, and 100 U/ml nystatin. Chromaffin cells were purified by differential plating as described previously (Anouar et al., 1994). Cells were then plated in the same medium as above at a density of 1.5 × 106 cells/ml, 2 ml/well and a density of 0.75 × 106, 1.5 ml/well, in poly-D-lysine-coated 6-well and 12-well plates respectively and treated with LPS (10 μg/ml, Sigma-Aldrich) TNF-α (10 nM, PeproTech, Rocky Hill, NJ) and/or PACAP-38 (100 nM, Phoenix Pharmaceuticals, Burlingame, CA).
2.4. RNA extraction and Q-RT-PCR
Total RNA was harvested and extracted from individual BCCs culture wells or two adrenal glands by adding TRIzol-Reagent (Invitrogen, Carlsbad, CA) using the method of Chomczynski and Sacchi (Chomczynski and Sacchi, 1987), or by using the RNeasy kit (Qiagen, Maryland) and approximately 2 μg of total RNA was submitted to DNase I (RNase-free; Invitrogen) digestion and reverse transcribed using random hexamers pdN6 (Invitrogen) and SuperScript II RNase H− reverse transcriptase (Invitrogen). Gene-specific forward and reverse primers were chosen using Primer 3 Input (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), to amplify in each case across an exon-exon junction in order to avoid generation of same-sized amplicons from contaminating genomic DNA. Mouse primers: 5′-CTGAGCGACCACCATGTCTA-3′ and 5′-ACAGGAAAGTGACCCCAGTG-3′ for aldolase A (ALDOA); 5′-CACAGCAGCTTTGAGGATGA-3′ and 5′-ATGGGGGACTCTTGGTTAGG-3′ for chromogranin A (CgA); 5′-AGTGTGCTGTTCTCTCAGTCG-3′ and 5′-GCCATTTTCTGCTAAGGGATTCT-3′ for VIP; 5′-GGCAGCGTTATCCTGCTAGG-3′ and 5′-CTGTTCAGGGTCCAACCTCT-3′ for galanin; bovine primers: 5′-TCCTCTCAATCCTGCGACATC-3′ and 5′-TCCTCGTAACTGCTGTGCTTCTT-3′ for CgA; 5′-GACAGCCACAGGTCATTTCAA-3′ and 5′-GCCGGGCTTCGTCTTCA-3′ for galanin; 5′-TTGAGTCCCTTATTGGAAAACGA-3′ and 5′-AGCATCTGAGTGGCGTTTGA-3′ for VIP; 5′-ACCTGATGGTTACATGTCACTCA-3′ and 5′-GGGAAGGTTATTCAAAATTTGATGA-3′ for IκBα 5′-TGCTACGACACTCGGAACTG-3′ and 5′-GGAGCAAAATTGGAACCAGA-3′ for TNFAIP3. Real time PCR (Q-RT-PCR) was performed in a premade reaction mix in the presence of the transcribed cDNA and 10 nM of specific primers, using the SYBR green chemistry and an iCycler real-time detection system (Bio-Rad, Hercules, CA). Relative amounts of mRNA were determined from standard curve generated using dilutions of the cDNA and by normalizing against a non-variable control transcript, ALDOA or CgA, that was analyzed in parallel on the same Q-RT-PCR.
2.5. Statistical analysis
For comparison among treatment groups, a one-way ANOVA was performed, and Tukey or Dunnett multiple comparisons post-tests were used when comparing multiple treatment groups, using GraphPad Prism 4.0. For comparisons between two groups a t-test was performed. A p value of <0.05 was taken as statistically significant.
3. Results
3.1. Up-regulation of VIP and galanin in mouse adrenal gland after LPS challenge
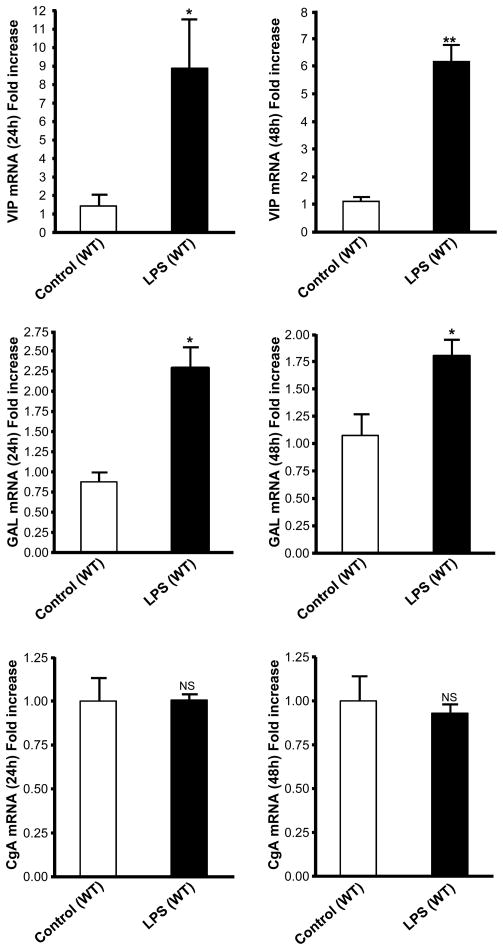
To determine if adrenomedullary peptides are regulated under conditions of septic shock, we measured the level of VIP, galanin and CgA mRNA in adrenal gland in response to LPS challenge. Studies were performed by injecting C57BL/6 male mice with LPS (1 mg/kg) or saline solution. Adrenal glands were collected after 24 and 48 h, and the expression of VIP, galanin and CgA was analyzed by Q-RT-PCR. LPS induced a significant stimulation, ranging from 6- to 8-fold, of VIP mRNA levels at 24 h and 48 h (Fig. 1, upper panel). In parallel, galanin mRNA is up-regulated by LPS treatment at 24 and 48 h (Fig. 1, middle panel). Chromogranins, including CgA, represent the main secretory granule component of chromaffin cells. LPS treatment did not affect CgA mRNA levels (Fig. 1, lower panel), suggesting a specific regulatory effect rather than a general up-regulation of the secretory machinery of the chromaffin cell.
Fig. 1.
VIP and galanin, but not CgA mRNA, are upregulated in adrenal gland after 24 and 48 h LPS challenge. C57BL/6 males were injected with LPS (1 mg/kg) or saline solution. After 24 h and 48 h, adrenal glands were collected and the expression of VIP (upper panel), galanin (middle panel) and CgA (lower panel) was analyzed by Q-RT-PCR. Results are expressed as fold increase over corresponding control values and represent means ± SEM of four or five determinations for each condition. NS, P>0.05; *, P<0.05; **, P<0.01 vs. the corresponding control (Student’s t test).
3.2. Up-regulation of VIP and galanin mRNA in adrenal gland does not occur in PACAP-deficient mice
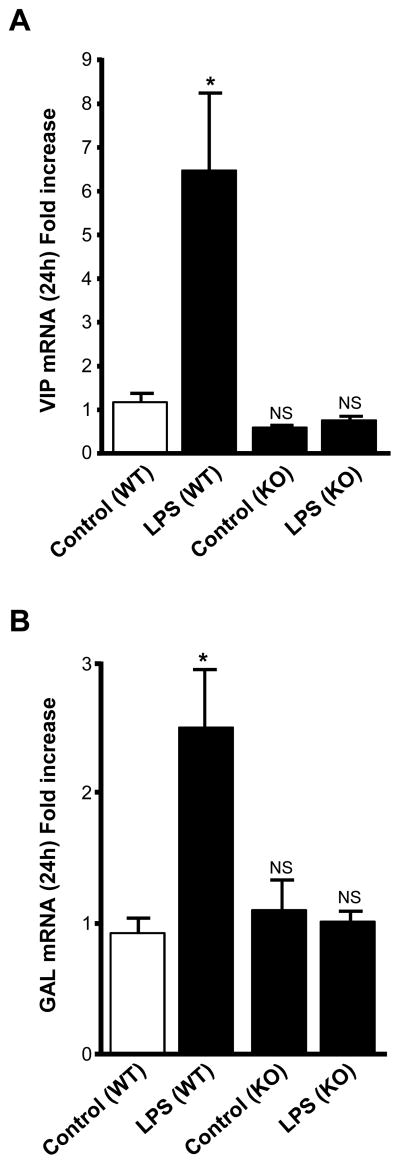
In vivo studies were performed by injecting LPS (1 mg/kg) into PACAP knock-out (KO) and wild-type (WT) mice. After 24 h, adrenal glands were collected and the expression of galanin and VIP mRNA was analyzed by Q-RT-PCR. Our results show that galanin and VIP mRNA levels do not increase after LPS treatment in PACAP KO mice (Fig. 2A, B), indicating that LPS-induced elevation of the mRNAs encoding these neuropeptides occurs via a PACAP-dependent mechanism.
Fig. 2.
LPS significantly increases galanin and VIP mRNA levels, by a PACAP-dependent mechanism. PACAP KO and WT mice were injected with LPS (1 mg/kg) or saline solution and adrenal glands were collected after 24 h. VIP (A) and galanin (B) mRNA levels, determined by Q-RT-PCR, are expressed as fold increase over control wild type mice values and represent means ± SEM of four or five determinations for each condition from the mean of two different experiments. NS, P>0.05; *, P<0.05 vs. the corresponding control (one-way ANOVA test, Tukey posttest).
3.3. TNF-α but not LPS stimulates the expression of neuropeptides in cultured primary chromaffin cells
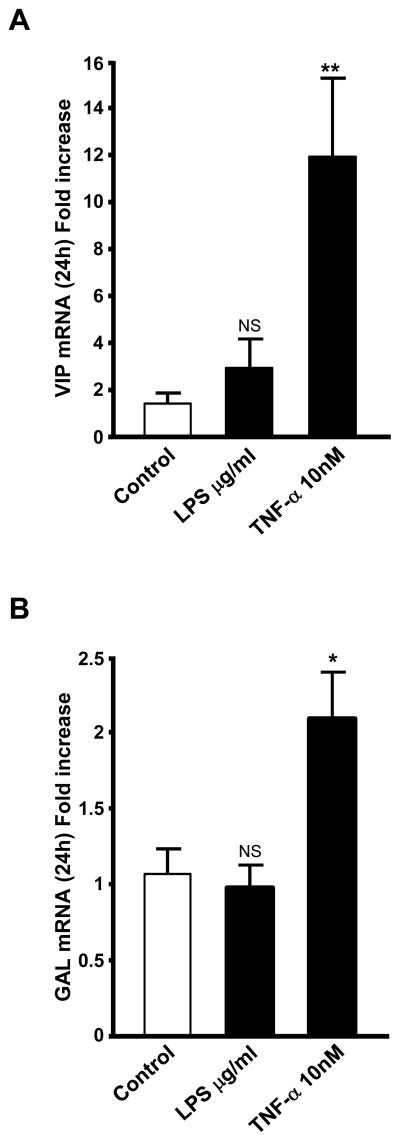
We have previously shown that TNF-α increases VIP and galanin mRNA levels in chromaffin cells in vitro (Ait-Ali et al., 2004). Elevation of serum TNF-α biosynthesis is known to occur after LPS treatment in vivo and in cultured immune cells exposed to LPS (Papadakis and Targan, 2000; Ulloa and Tracey, 2005). Chromaffin cells also respond to treatment with LPS by induction of iNOS and eNOS (Perez-Rodriguez et al., 2009). TNF-α as well as LPS itself are therefore reasonable candidates for neuropeptide regulation in the adrenal gland in vivo after LPS injection. We measured the induction of VIP and galanin mRNA in cultured BCCs after treatment with either LPS (10 μg/ml), or TNF-α (10 nM), doses similar to those administered in vivo (for LPS), and expected to occur after LPS administration in vivo(for TNF-α. Our results show that TNF-α, but not LPS, increases the expression of both VIP and galanin mRNA after 24 h of treatment (Fig. 3A, B).
Fig 3.
VIP and galanin mRNA are stimulated by TNF-α but not by LPS in BCCs after 24 h. BCCs were exposed to either TNF- α (10 nM), or LPS (10 μg/ml) for 24 hours. VIP (A) and galanin (B) mRNA levels, determined by Q-RT-PCR, are expressed as fold increase over corresponding control values and represent means ± SEM of three determinations for each condition. NS, P>0.05; *, P<0.05 vs. the corresponding control (one-way ANOVA test, Dunnett post test).
3.4. TNF-α and PACAP synergistic up-regulation of VIP and galanin in BCCs
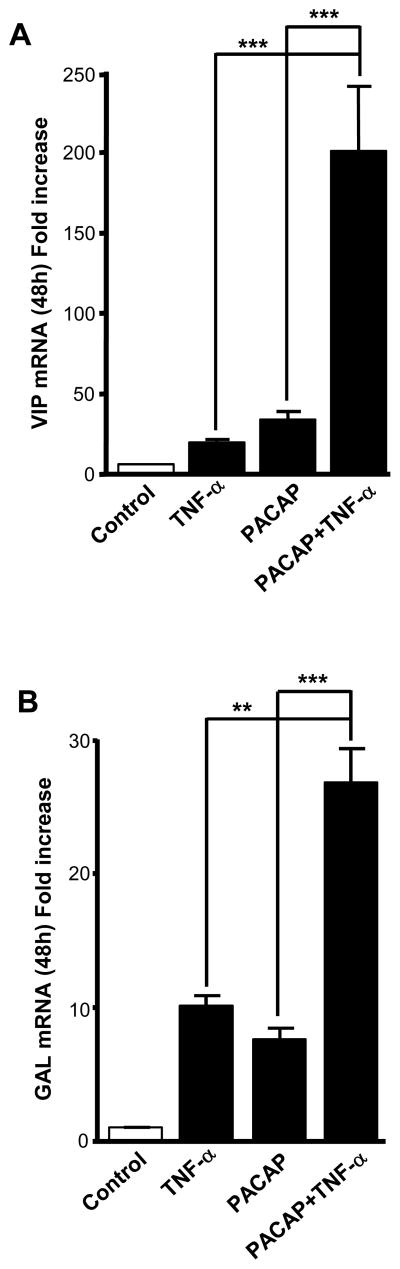
To determine whether an interaction between TNF-α and PACAP regulation of neuropeptide biosynthesis occurs at the level of the chromaffin cell itself, we measured induction of neuropeptide mRNA levels in BCCs 48 h after treatment with PACAP, TNF-α, or a combination of both first messengers (Fig. 4A, B). Treatment of chromaffin cells with a combination of PACAP (100 nM) and TNF-α (10 nM) revealed a synergistic increase in both VIP and galanin mRNA levels after 48 h (Fig. 4A, B), suggesting that maximal stimulation of VIP and galanin mRNA in vivo after LPS may be a result of simultaneous exposure of chromaffin cells to both the slow transmitter PACAP, and the proinflammatory cytokine TNF-α.
Fig. 4.
PACAP and TNF-α synergistically up-regulate neuropeptide gene expression. BCCs were exposed to either TNF-α (10 nM), PACAP (100 nM) or both agents, for 48 hours. VIP (A) and galanin (B) mRNA levels, determined by Q-RT-PCR, are expressed as fold increase over corresponding control values and represent means ± SEM of four determinations for each condition from one experiment representative of three different experiments. **, P<0.01; ***, P<0.001 combined treatment (PACAP plus TNF-α)vs. individual treatment (PACAP or TNF-α) using one-way ANOVA test, with Tukey posttest.
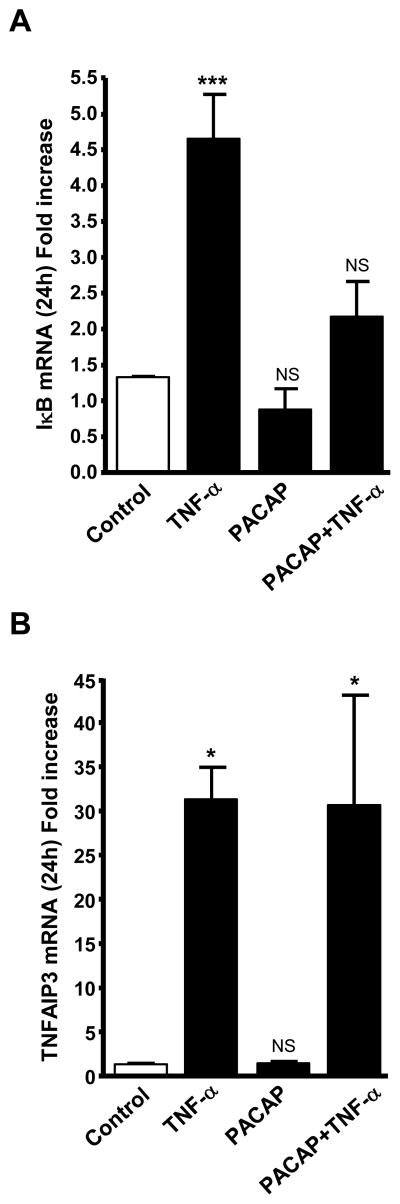
3.5. PACAP-dependent attenuation of TNF-α induction of IκBα mRNA
Treatment with TNF-α (10 nM, 24 h) stimulates a number of NF-κB-dependent genes including IκBα and TNFAIP3 mRNA (Ait-Ali et al., 2008) in chromaffin cells. We confirmed here regulation of these two genes by TNF-α, and then examined the effects of co-treatment with PACAP on induction of each of these two transcripts by TNF-α. PACAP inhibited TNF-αinduction of IκBα mRNA (Fig. 5A), but had no effect on TNF-α elevation of TNFAIP3 mRNA (Fig. 5B). These findings suggest that PACAP enhances NF-κB-dependent induction of neuropeptide gene transcription by TNF-α by inhibiting the transcription of IκBα at a locus downstream of the activation of NF-κB itself.
Fig. 5.
PACAP attenuates TNF-α induction of IκBα but not TNFAIP3 mRNA. BCCs were exposed to either TNF-α (10 nM), PACAP (100 nM) or both agents, for 24 hours. IκBα (A) and TNFAIP3 (B) mRNA levels, determined by Q-RT-PCR, are expressed as fold increase over corresponding control values and represent means ± SEM of four determinations for each condition from one experiment representative of three different experiments. NS, P>0.05; *, P<0.05; ***, P<0.001 vs. the corresponding control (one-way ANOVA test, Tukey posttest).
4. Discussion
In this report we have shown that VIP and galanin mRNA are induced by LPS administration in vivo, and that this induction does not occur in mice deficient in PACAP expression. PACAP is co-released, with acetylcholine, from the splanchnic nerve innervating the adrenal medulla. It has previously been demonstrated to be required for regulation of tyrosine hydroxylase enzymatic activity, which helps support sustained catecholamine secretion, without depletion of catecholamine stores, from the adrenal medulla during episodes of prolonged metabolic stress (Hamelink et al., 2002). Tai et al. have recently shown that restraint stress, although eliciting massive release of adrenomedullary catecholamines, does not result in epinephrine depletion from the adrenal gland (Tai et al., 2007). These workers postulated that induction of phenylethanolamine N-methyltransferase could be responsible for the compensatory increase in catecholamine biosynthesis that prevents exhaustion of epinephrine stores. It is not yet known if PACAP is required for PNMT induction in stress, although PACAP induces PNMT biosynthesis in cultured BCCs (Tonshoff et al., 1997).
Biosynthesis of neuropeptides present in adrenal chromaffin cells is also increased after reflex stimulation of the splanchnic nerve induced by hypoglycemia (Fischer-Colbrie et al., 1992; Kanamatsu et al., 1986). Physiological role(s) suggested for neuropeptide secretion from chromaffin cells include enhancement of adrenocortical steroidogenesis, of particular importance in down-regulating the inflammatory response (Andreis et al., 2007; Bornstein et al., 1996; Ehrhart-Bornstein et al., 1991) and autocrine regulation of catecholamine secretion itself (Mahata et al., 2000; Zhou and Livett, 1990). Local paracrine neuropeptide functions, particularly for VIP and galanin, include protection by VIP, at very low concentrations, of neuroendocrine cells from immunocytotoxicity elicited by LPS (Brenneman, 2007; Li et al., 2005), and regulation of monocyte/macrophage and lymphocyte function during inflammation (Arranz et al., 2008; Fischer-Colbrie et al., 2005; Goetzl et al., 2008; Su et al., 2003). VIP in particular can elicit potent effects on macrophage function, including down-regulation of TNF-α secretion, through induction of astrocyte-derived neurotrophic factor (ADNF) (Quintana et al., 2006).
PACAP-dependent regulation of VIP and galanin biosynthesis in septic shock is predicted from the observation that hypoglycemia occurs after endotoxin administration (Oguri et al., 2002). This would be expected to cause reflex splanchnic nerve stimulation and subsequent PACAP release in the adrenal medulla, leading to induction of biosynthesis of VIP and galanin as observed following insulin shock in rodents (Fischer-Colbrie et al., 1992; Stroth et al., 2007). Epinephrine secretion is in fact increased in septic shock (Qi et al., 1991), but is not as prolonged or intense as that occurring after insulin administration. Thus, secretion of PACAP alone, although clearly required, may be insufficient on its own for the pronounced and prolonged up-regulation of neuropeptide mRNAs observed here after LPS. The much greater effects of TNF-α plus PACAP, in comparison to either TNF-α or PACAP alone, on both VIP and galanin induction in culture chromaffin cells, suggests to us that PACAP by itself is not mediating neuropeptide induction in vivo. This is especially the case for VIP mRNA, for which induction by insulin shock is considerably lower (2–2.5 fold; N. Stroth and C. Hamelink, unpublished observations) than induction by LPS (8 fold; this report) in adrenal gland in vivo.
LPS exerts its primary effects via activation of Toll like receptors (TLRs), transmembrane proteins that function as pattern-recognition in detection of and response to microbial ligands (Imler and Zheng, 2004; Takeda et al., 2003). TLR-2 and TLR-4 are the most common members of the TLR family (Misch and Hawn, 2008), with TLR-4 generally implicated in LPS signalling, innate immunity, and inflammation, and TLR-2 is involved in the recognition of Gram-positive bacteria (Takeda and Akira, 2007). Exposure of chromaffin cells to LPS results in iNOS and nNOS induction through activation of NF-κB, but the nature of the TLR mediating these effects is unknown (Perez-Rodriguez et al., 2009). In any event, LPS itself does not appear to be directly involved, at the level of the chromaffin cell, in LPS-mediated adrenal neuropeptide induction, because direct exposure of chromaffin cells to LPS did not elicit induction of galanin mRNA.
We have previously shown that proinflammatory cytokines such as IL-1 and TNF-α significantly increase the biosynthesis of neuropeptides in isolated chromaffin cells (Ait-Ali et al., 2004; Eskay and Eiden, 1992), and appear to do so through activation of NF-κB via TNFR2 receptor signaling (Ait-Ali et al., 2008). We therefore examined the interaction between TNF-α and PACAP in stimulation of VIP and galanin biosynthesis in isolated BCCs, and found that PACAP and TNF-α exert strongly synergistic effects on both galanin and VIP biosynthesis. It is therefore quite plausible that LPS signals to the cells of the adrenal medulla indirectly through cytokines such as TNF-α, synergizing with PACAP released from the splanchnic nerve in response to hypoglycemia that develops several hours after administration of LPS (Oguri et al., 2002). Here we have directly demonstrated the requirement for PACAP in this regulation. Similar experiments to demonstrate that TNF-α is the cytokine that acts with PACAP, i.e. blockade of neuropeptide induction by LPS after inhibition of TNF-α biosynthesis or receptor action in vivo, would be highly informative for establishing this mechanism in vivo.
PACAP-dependent induction of VIP and galanin mRNA after LPS in vivo is prolonged, similar to TNF-α induction of these peptides, and PACAP synergy with TNF-α, in bovine chromaffin cells in vitro. Synergy between PACAP and TNF-α signaling could be the result of PACAP augmentation of TNF-α or TNF-α potentiation of PACAP signaling mechanisms, since both independently stimulate induction of both VIP and galanin biosynthesis. Our data suggest a mechanism for TNF-α/PACAP synergism that involves PACAP modulation of TNF-α signaling auto-inhibition through IκBα In chromaffin cells, delayed and sustained activation of neuropeptide gene expression by TNF-α appears to depend upon a unique mode of persistent activation of NF-κB and NF-κB-dependent genes encoding the NF-κB inhibitory proteins, IκBα and TNFAIP3 (Ait-Ali et al., 2008). These proteins exert strong auto-inhibitory effects on NF-κB trans-activation via binding and nuclear export of NF-κB (Sun et al., 1993), or through inhibition of ongoing IκBα ubiquitination/degradation (Lee et al., 2000). Co-treatment with PACAP and TNF-α strongly attenuated IκBα mRNA induction by TNF-α at 24 h, while PACAP was without effect on TNFAIP3 induction. It is not clear how PACAP could differentially affect these two NF-κB targets. Since both genes respond strongly to NF-κB through NF-κB binding sites in their promoters (Ito et al., 1994; Lipniacki et al., 2004), it seems unlikely that PACAP acts via blockade of NF-κB-dependent induction of IκBα. Rather, the differential effects of PACAP on induction of these two strongly NF-κB-dependent genes by TNF-α suggests that PACAP decreases IκBα transcription, and thereby prolongs TNF-α action on neuropeptide gene induction, through actions elsewhere in the TNF-α/NF-κB/IκBα signalling pathway, such as modulation of activity of a co-activation unique to the IκBα promoter.
Acknowledgments
The authors wish to thank Ms. Chang-Mei Hsu for expert technical assistance in preparation of chromaffin cell cultures, and the NIMH Intramural Research Program for support of Project 1ZO1-MH002386-21.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ait-Ali D, Turquier V, Grumolato L, Yon L, Jourdain M, Alexandre D, Eiden LE, Vaudry H, Anouar Y. The proinflammatory cytokines tumor necrosis factor-alpha and interleukin-1 stimulate neuropeptide gene transcription and secretion in adrenochromaffin cells via activation of extracellularly regulated kinase 1/2 and p38 protein kinases, and activator protein-1 transcription factors. Mol Endocrinol. 2004;18:1721–1739. doi: 10.1210/me.2003-0129. [DOI] [PubMed] [Google Scholar]
- Ait-Ali D, Turquier V, Tanguy Y, Thouennon E, Ghzili H, Mounien L, Derambure C, Jegou S, Salier JP, Vaudry H, Eiden LE, Anouar Y. Tumor necrosis factor (TNF)-alpha persistently activates nuclear factor-kappaB signaling through the type 2 TNF receptor in chromaffin cells: implications for long-term regulation of neuropeptide gene expression in inflammation. Endocrinology. 2008;149:2840–2852. doi: 10.1210/en.2007-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreis PG, Tortorella C, Ziolkowska A, Spinazzi R, Malendowicz LK, Neri G, Nussdorfer GG. Evidence for a paracrine role of endogenous adrenomedullary galanin in the regulation of glucocorticoid secretion in the rat adrenal gland. Int J Mol Med. 2007;19:511–515. [PubMed] [Google Scholar]
- Anouar Y, MacArthur L, Cohen J, Iacangelo AL, Eiden LE. Identification of a TPA-responsive element mediating preferential transactivation of the galanin gene promoter in chromaffin cells. J Biol Chem. 1994;269:6823–6831. [PubMed] [Google Scholar]
- Arranz A, Androulidaki A, Zacharioudaki V, Martinez C, Margioris AN, Gomariz RP, Tsatsanis C. Vasoactive intestinal peptide suppresses toll-like receptor 4 expression in macrophages via Akt1 reducing their responsiveness to lipopolysaccharide. Mol Immunol. 2008;45:2970–2980. doi: 10.1016/j.molimm.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Haidan A, Ehrhart-Bornstein M. Cellular communication in the neuro-adrenocortical axis: role of vasoactive intestinal polypeptide (VIP) Endocr Res. 1996;22:819–829. doi: 10.1080/07435809609043781. [DOI] [PubMed] [Google Scholar]
- Brenneman DE. Neuroprotection: a comparative view of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Peptides. 2007;28:1720–1726. doi: 10.1016/j.peptides.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Bornstein SR, Scherbaum WA, Pfeiffer EF, Holst JJ. Role of the vasoactive intestinal peptide in a neuroendocrine regulation of the adrenal cortex. Neuroendocrinology. 1991;54:623–628. doi: 10.1159/000125969. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP, Wilder RL. Neuroendocrine regulation of IL-12 and TNF-alpha/IL-10 balance. Clinical implications. Ann N Y Acad Sci. 2000;917:94–105. doi: 10.1111/j.1749-6632.2000.tb05374.x. [DOI] [PubMed] [Google Scholar]
- Eskay RL, Eiden LE. Interleukin-1 alpha and tumor necrosis factor-alpha differentially regulate enkephalin, vasoactive intestinal polypeptide, neurotensin, and substance P biosynthesis in chromaffin cells. Endocrinology. 1992;130:2252–2258. doi: 10.1210/endo.130.4.1372239. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R, Eskay RL, Eiden LE, Maas D. Transsynaptic regulation of galanin, neurotensin, and substance P in the adrenal medulla: combinatorial control by second-messenger signaling pathways. J Neurochem. 1992;59:780–783. doi: 10.1111/j.1471-4159.1992.tb09440.x. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R, Kirchmair R, Kahler CM, Wiedermann CJ, Saria A. Secretoneurin: a new player in angiogenesis and chemotaxis linking nerves, blood vessels and the immune system. Curr Protein Pept Sci. 2005;6:373–385. doi: 10.2174/1389203054546334. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Chan RC, Yadav M. Diverse mechanisms and consequences of immunoadoption of neuromediator systems. Ann N Y Acad Sci. 2008;1144:56–60. doi: 10.1196/annals.1418.008. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, Eiden LE. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler JL, Zheng L. Biology of Toll receptors: lessons from insects and mammals. J Leukoc Biol. 2004;75:18–26. doi: 10.1189/jlb.0403160. [DOI] [PubMed] [Google Scholar]
- Ito CY, Kazantsev AG, Baldwin AS., Jr Three NF-kappa B sites in the I kappa B-alpha promoter are required for induction of gene expression by TNF alpha. Nucleic Acids Res. 1994;22:3787–3792. doi: 10.1093/nar/22.18.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamatsu T, Unsworth CD, Diliberto EJ, Jr, Viveros OH, Hong JS. Reflex splanchnic nerve stimulation increases levels of proenkephalin A mRNA and proenkephalin A-related peptides in the rat adrenal medulla. Proc Natl Acad Sci U S A. 1986;83:9245–9249. doi: 10.1073/pnas.83.23.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, David C, Kikuta T, Somogyvari-Vigh A, Arimura A. Signaling cascades involved in neuroprotection by subpicomolar pituitary adenylate cyclase-activating polypeptide 38. J Mol Neurosci. 2005;27:91–105. doi: 10.1385/JMN:27:1:091. [DOI] [PubMed] [Google Scholar]
- Lipniacki T, Paszek P, Brasier AR, Luxon B, Kimmel M. Mathematical model of NF-kappaB regulatory module. J Theor Biol. 2004;228:195–215. doi: 10.1016/j.jtbi.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Mahata SK, Mahata M, Livsey Taylor CV, Taupenot L, Parmer RJ, O’Connor DT. The novel catecholamine release-inhibitory peptide catestatin (chromogranin A344–364). Properties and function. Adv Exp Med Biol. 2000;482:263–277. doi: 10.1007/0-306-46837-9_21. [DOI] [PubMed] [Google Scholar]
- Misch EA, Hawn TR. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 2008;114:347–360. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- Oguri S, Motegi K, Iwakura Y, Endo Y. Primary role of interleukin-1 alpha and interleukin-1 beta in lipopolysaccharide-induced hypoglycemia in mice. Clin Diagn Lab Immunol. 2002;9:1307–1312. doi: 10.1128/CDLI.9.6.1307-1312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis KA, Targan SR. Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology. 2000;119:1148–1157. doi: 10.1053/gast.2000.18160. [DOI] [PubMed] [Google Scholar]
- Perez-Rodriguez R, Roncero C, Olivan AM, Gonzalez MP, Oset-Gasque MJ. Signaling mechanisms of interferon gamma induced apoptosis in chromaffin cells: involvement of nNOS, iNOS, and NFkappaB. J Neurochem. 2009;108:1083–1096. doi: 10.1111/j.1471-4159.2008.05862.x. [DOI] [PubMed] [Google Scholar]
- Qi M, Zhou ZZ, Wurster RD, Jones SB. Mechanisms involved in the rapid dissipation of plasma epinephrine response to bacterial endotoxin in conscious rats. Am J Physiol. 1991;261:R1431–1437. doi: 10.1152/ajpregu.1991.261.6.R1431. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Zaltzman R, Fernandez-Montesinos R, Herrera JL, Gozes I, Cohen IR, Pozo D. NAP, a peptide derived from the activity-dependent neuroprotective protein, modulates macrophage function. Ann N Y Acad Sci. 2006;1070:500–506. doi: 10.1196/annals.1317.069. [DOI] [PubMed] [Google Scholar]
- Stroth N, Hamelink C, Eiden LE. PACAP-dependent cellular plasticity in the mouse adrenal gland. FASEB J. 2007;21:A1249–1250. [Google Scholar]
- Su Y, Ganea D, Peng X, Jonakait GM. Galanin down-regulates microglial tumor necrosis factor-alpha production by a post-transcriptional mechanism. J Neuroimmunol. 2003;134:52–60. doi: 10.1016/s0165-5728(02)00397-1. [DOI] [PubMed] [Google Scholar]
- Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Tai TC, Claycomb R, Siddall BJ, Bell RA, Kvetnansky R, Wong DL. Stress-induced changes in epinephrine expression in the adrenal medulla in vivo. J Neurochem. 2007;101:1108–1118. doi: 10.1111/j.1471-4159.2007.04484.x. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2007;Chapter 14(Unit 14 12) doi: 10.1002/0471142735.im1412s77. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tonshoff C, Hemmick L, Evinger MJ. Pituitary adenylate cyclase activating polypeptide (PACAP) regulates expression of catecholamine biosynthetic enzyme genes in bovine adrenal chromaffin cells. J Mol Neurosci. 1997;9:127–140. doi: 10.1007/BF02736856. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Tracey KJ. The "cytokine profile": a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Wong DL, Tank AW. Stress-induced catecholaminergic function: transcriptional and post-transcriptional control. Stress. 2007;10:121–130. doi: 10.1080/10253890701393529. [DOI] [PubMed] [Google Scholar]
- Yadav M, Goetzl EJ. Vasoactive intestinal peptide-mediated Th17 differentiation: an expanding spectrum of vasoactive intestinal peptide effects in immunity and autoimmunity. Ann N Y Acad Sci. 2008;1144:83–89. doi: 10.1196/annals.1418.020. [DOI] [PubMed] [Google Scholar]
- Zacharowski K, Zacharowski PA, Koch A, Baban A, Tran N, Berkels R, Papewalis C, Schulze-Osthoff K, Knuefermann P, Zahringer U, Schumann RR, Rettori V, McCann SM, Bornstein SR. Toll-like receptor 4 plays a crucial role in the immune-adrenal response to systemic inflammatory response syndrome. Proc Natl Acad Sci U S A. 2006;103:6392–6397. doi: 10.1073/pnas.0601527103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XF, Livett BG. Substance P has biphasic effects on catecholamine secretion evoked by electrical stimulation of perfused rat adrenal glands in vitro. J Auton Nerv Syst. 1990;31:31–39. doi: 10.1016/0165-1838(90)90169-j. [DOI] [PubMed] [Google Scholar]