Abstract
4-(Dimethylamino)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)benzamide (WC-10), a N-phenyl piperazine analog, displays high affinity and moderate selectivity for dopamine D3 receptors versus dopamine D2 receptors (Chu et al. [2005] Bioorg. Med. Chem. 13; 77–87). In this study, WC-10 was radiolabeled with tritium (specific activity = 80 Ci/mmol) and quantitative autoradiography studies were conducted using rhesus monkey and Sprague-Dawley rat brain sections. Kd values for the binding of [3H]WC-10 to D3 receptors obtained from quantitative autoradiography with rhesus monkey and rat brain sections are in agreement with Kd values obtained from cloned human and rat receptors (Xu et al., 2009). The D2 selective antagonist [3H]raclopride binds with 11-fold higher affinity to human HEK D2L (Kd = 1.6 nM) than HEK D3 (Kd = 18 nM) receptors; and [3H]raclopride binds to rat Sf9 rD2L receptors with a Kd of 6.79 nM, a value that is 4-fold lower than binding to human HEK D2L receptors and 2.5-fold higher than binding to rat Sf9 rD3 receptors. In vitro quantitative autoradiography studies with [3H]WC-10 and [3H]raclopride were conducted on adult rat and rhesus monkey brain sections. A mathematical model for calculating the absolute densities of dopamine D2 and D3 receptors based on the in vitro receptor binding data of [3H]WC-10 and [3H]raclopride was developed.
Keywords: dopamine D3/D2 receptors, quantitative autoradiography
INTRODUCTION
D1-like and D2-like receptor subtypes are the two major classes of dopamine receptors. The D1-like receptor subtypes include the D1 (D1a) and D5 (D1b) receptors and stimulation of D1-like receptors activates adenylate cyclase. The D2-like receptor subtypes consist of the D2 (both long and short isoforms), D3, and D4 receptors. Agonist stimulation of D2-like receptors a) inhibits adenylate cyclase activity and b) increases the release of arachadonic acid and phosphatidylinositol hydrolysis (Luedtke and Mach, 2003; Neve et al., 2004).
D2-like receptors exist in two interconvertible affinity states for their natural agonist dopamine: a high-affinity state and a low-affinity state (Sibley et al., 1982). Under physiological conditions, dopamine binds predominantly to the high affinity state and mediates the activation of the second-messenger cascade. Although autoradiography studies using the D2/D3 agonists, [3H]7-OH-DPAT and [3H]quinpirole, under conditions minimizing binding to the D2 receptor, suggest that dopamine D3 receptors are localized in the ventral striatum and the Islands of Calleja (Gehlert et al., 1992; Kaichi et al., 2000; Levesque et al., 1992), there are data indicating that the density of dopamine D3 receptors measured with agonists [3H]7-OH-DPAT and [3H]PD128907 is higher in the adult rat caudate–putamen than in the islands of Calleja (Hillefors and von Euler, 2001; Hillefors et al., 1999). The high affinity state is believed to be the functionally important state (George et al., 1985; Leff, 1995). Nevertheless, the low affinity state of D2-like receptors, and its conversion to a high affinity state, needs to be further investigated in evaluating their regulatory functions in both diseased and healthy individuals (Briand et al., 2008; Graff-Guerrero et al., 2009; King et al., 2009; Skinbjerg et al., 2009).
It has been difficult to obtain ligands specifically selective for D2 or D3 receptors due to the high degree of amino acid homology in the helical transmembrane spanning regions of those receptors. Some selective dopamine D3 agonists (7-OH-DPAT and PD128947) and dopamine D2 agonists (PHNO) are available, but selective D2 or D3 receptor antagonists are not well documented (Ginovart et al., 2006; Luedtke and Mach, 2003; Vasdev et al., 2007). The development of high affinity D3 and D2 receptor antagonists would be valuable for studying the regulating mechanism of these receptors in neurological disorders.
A N-phenyl piperazine analog, 4-(dimethylamino)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)benzamide (WC-10), displays higher affinity and binding selectivity for dopamine D3 receptors versus dopamine D2 receptors (Chu et al., 2005). In a previous publication, WC-10 was radiolabeled with tritium (specific activity = 80 Ci/mmol) and the binding of [3H]WC-10 to genetically cloned human and rat dopamine D2L and D3 receptors was evaluated in vitro (Xu et al., 2009). In vitro autoradiography studies showed that [3H]WC-10 specifically labeled the D3 sites of striatum in adult Sprague-Dawley rat and rhesus monkey brains. In this paper, saturation binding with [3H]WC-10 was carried out to evaluate the binding affinity (Kd) with quantitative autoradiography. Comparatively, we characterized D2 selective antagonist [3H]raclopride binding properties at D2 or D3 receptors. In vitro quantitative autoradiography studies combining the D3/D2 ligand, [3H]WC-10 and the D2/D3 ligand, [3H]raclopride, were also conducted on adult rat and rhesus monkey brain sections. A mathematical model for calculating the absolute densities of dopamine D2 and D3 receptors based on the in vitro binding data obtained from [3H]WC-10 and [3H]raclopride was also developed and used to determine the density of D2 and D3 dopamine receptors.
MATERIALS AND METHODS
Precursor synthesis and radiolabeling
[3H]WC-10 (Fig. 1) was synthesized by American Radiolabeled Chemicals, Inc. (St. Louis, MO, U.S.A.) by alkylation of the des-methyl precursor with [3H]methyl iodide. The specific activity of the radioligand was 80 Ci/mmol. The detailed synthesis scheme for [3H]WC-10 was reported (Xu et al., 2009).
Fig. 1. Chemical structure of [3H]WC-10.
The structure of [3H]WC-10 are shown, detailed synthesis scheme was reported previously (Xu et al., 2009).
Drugs and preparation
Chemical reagents and the standard compounds were purchased from Sigma (St. Louis, MO) and Tocris (Ellisville, MO). Novel compounds used in this study were synthesized by our group. N,N-Dimethylformamide (DMF), dimethyl sulfoxide (DMSO) or ethanol were used to dissolve the various compounds to 3 mM as stock solutions. Different concentrations were then achieved by diluting stock solutions with a solution containing 50 mM Tris-HCl, 150 mM NaCl and 100 mM EDTA at pH 7.4. The final solvent volume in the dilutions was less than 0.1% of the total volume. [3H]raclopride (60.1 Ci/mmol) was purchased from Pekin Elmer Life Sciences (Boston, MA).
Membrane homogenate preparation
HEK or Sf9 cells expressing human or rat D2 and D3 receptors were harvested by centrifugation at 6,000g for 10 min (Luedtke et al., 2000). The cell pellet was resuspended in cold (4°C) homogenization buffer (50 mM Hepes, pH 7.4, 0.1 mM EDTA, 1 mM DTT) by vigorous vortexing and then homogenized using an Ultra-Turrax T8 polython homogenizer (IKA Works, Inc, Wilmington, NC). The homogenate was centrifuged at 40,000g for 10 min at 4°C and the resulting crude membrane pellet was resuspended in homogenization buffer. Aliquots were stored at −80°C until used. The protein concentration of the suspension was determined using the DC protein assay (Bio-Rad, Hercules, CA) and averaged 1~2 mg of protein/ml of stock solution.
Radioligand binding assay
Scatchard analysis of [3H]raclopride binding to receptor membrane homogenates
Membrane homogenates were diluted and incubated for 60 min with the radioligand [3H]raclopride in a total volume of 150 μl at 25°C in 96-well polypropylene plates (Fisher Scientific, Pittsburgh, PA). The amount of protein added to each well was 10~20 μg for genetically cloned D2L and D3 receptors. The concentrations of the radioligand ranged from 0.1 to 30.0 nM, reactions were terminated by the addition of 150 μl of cold wash buffer (10 mM Tris-HCl, 150 mM NaCl, pH 7.4, at 4°C) using a 96-channel transfer pipette (Fisher Scientific, Pittsburgh, PA), and the samples were harvested and filtered rapidly to 96-well fiberglass filter plate (Millipore, Billerica, MA) that had been presoaked with 100 μl of 50 mM Tris-HCl buffer, pH 8.0 for 1 hour. Each filter was washed with 200 μl of ice-cold wash buffer for a total of three washes and 150 μl of scintillation fluid was added in each well. A Wallac 1450 MicroBeta liquid scintillation counter (Perkin Elmer, Boston, MA) was used to quantitate the bound radioactivity. Nonspecific binding was determined from samples which contained 10 μM haloperidol.
The equilibrium dissociation constant (Kd) and maximum number of binding sites (Bmax) were determined by a linear regression analysis of the transformed data using the method of Scatchard (Scatchard, 1949).
Data from saturation radioligand binding studies were transformed to determine the Hill coefficient, nH, defined as:
| (1) |
(Hill 1910; McGonigle and Molinoff, 1989). L is the concentration of radioligand. nH, Hill slope, was determined from a Hill plot of versus log L.
Scatchard analysis of autoradiography binding assay with [3H]WC-10 to rhesus monkey and rat brain sections
Rhesus monkey and rat brain adjacent sections were incubated with [3H]WC-10, with concentration ranging from 0.5 to 16.0 nM. Quantitative imaging analysis for the anatomical regions of interest (ROI), caudate and putamen, was performed using a Beta Imager 2000Z Digital Beta Imaging System. Scatchard binding analysis were carried out as described in the previous section. Nonspecific bond activity at each concentration was defined from slides using 1 uM S(−)-Eticlopride.
Quantitative autoradiography
Intact brains from ~500 g male Sprague-Dawley rats at age of 5 months and a 5 kg male rhesus monkey at age of 6 years (euthanized due to a pancreatic tumor) were flash frozen in dry ice, pre-cooled in isopentane and stored at −80°C until used. Coronal sections (20 μm) were cut with a Microm cryotome and mounted on superfrost plus glass slides (Fisher Scientific, Pittsburgh, PA). Sections were incubated with 4 nM of [3H]WC-10 or 10 nM of [3H]raclopride in the same buffer used for binding assays, in the presence or absence of 1 μM cold S(−)-Eticlopride (to define nonspecific binding) for 30 minutes, and then rinsed 5 times at one minute intervals each time with ice-cold buffer. Slides were incubated in the wide open staining jar, and the free radioligand concentration loss was less than 5% after ligands bound to brain sections. Slides were dried and made conductive by coating the free side with a copper foil tape. Slides were then placed in the gas chamber [mixture of argon and triethylamine (Sigma–Aldrich, USA)] of a gaseous detector system, the Beta Imager 2000Z Digital Beta Imaging System (Biospace, France). After the gas was well mixed and a homogenous state was reached, further exposure for 24 hours yielded high quality images. [3H]Microscale (American Radiolabeled Chemicals, Inc., St. Louis, MO) was counted at the same time as a reference for total radioactivity quantitative analysis. Quantitative analysis was performed with the program Beta-Vision Plus (BioSpace, France) for the anatomical regions of interest (ROI).
In comparison with digital Beta Imager, the dried slides with brains sections were also exposed to a 3H-sensitive film (3H-Hyperfilm, Amersham, Buckinghamshire, UK) for 1 or 3 months, and the film was developed with a Kodak X-OMAT 2000A processor.
Determination of absolute densities ratio of D2 and D3 receptors from autoradiography studies
[3H]WC-10 and [3H]raclopride bind to dopamine D2 and D3 receptors with different labeling proportions. Based upon receptor homogenates binding studies, 4 nM [3H]WC-10 will label 2% of D2 receptors and 50% of D3 receptors in rat striatum, and 5% of D2 receptors and 78% of D3 receptors in monkey striatum. These values can be derived from the saturation binding isotherm (i.e. Michaelis-Menten equation):
| (2) |
where B is the specific receptor bound radioligand, Bmax is the receptor density, L is the radioligand concentration, and Kd is the equilibrium dissociation constant. In this study, the L value was 4 nM and Kd values were 1.16 nM for D3 and 76 nM for D2L receptors in the monkey study, which were determined using binding data with cloned human receptors (Table I). In contrast, Kd values were 3.94 nM for D3 and 158 nM for D2 receptors in the rat brain study, which were determined from binding study with cloned rat receptors (Table I). With the same analysis and the binding data (Table I), 10 nM [3H]raclopride will label a) 60% of D2 receptors and 37% of D3 receptors in rat striatum, and b) 86% of D2 receptors and 36% of D3 receptors in monkey striatum.
TABLE I.
Dissociation constants (Kd values) of [3H]WC-10 and [3H]raclopride binding to dopamine D2 and D3 receptors
| Receptors | Radioligand |
|
|---|---|---|
| [3H]WC-10* | [3H]raclopride | |
| hD2L | 76 ± 4 | 1.6 ± 0.1 |
| hD3 | 1.16 ± 0.1 | 18 ± 1 |
| D2L/D3 | 65.5 | 0.09 |
| rD2L | 158 ± 8.3 | 6.79 ± 0.6 |
| rD3 | 3.94 ± 0.19 | 17.1 ± 1.7 |
| D2L/D3 | 40.1 | 0.4 |
Kd values (nM), presented as mean value ± S.E.M for n=3, were obtained through saturation binding of [3H]WC-10 and [3H]raclopride to a: cloned human D3 and D2L receptors expressed in HEK cells and b: rat D3, D2L receptors expressed in Sf9 cells.
Data were taken from Xu et. al., 2009
The total bound amount of receptors of 4.0 nM [3H]WC-10 or 10 nM [3H]raclopride binding can be expressed by the formulas:
| (3) |
| (4) |
Where a1 and b1 are the fractional occupancies of 4nM of [3H]WC-10 to D2 and D3 receptors; B1 is the apparent receptor binding density (D2+D3) directly measured from autoradiography studies of 4.0 nM of [3H]WC-10; a2, b2, and B2 are the same parameters for 10nM of [3H]raclopride; D2, D3 are the absolute densities of D2 and D3 receptors. The absolute densities of D2 and D3 receptors were calculated by solving the simultaneous equations:
| (5) |
| (6) |
It’s assumed that a) law of mass actions applies in this study; b) all receptors are equally accessible to both radioligands [3H]WC-10 and 10 nM [3H]raclopride; c) both radioligands are antagonists and label high and low affinity sites of dopamine D2 or D3 receptors with equal affinity; d) neither receptor nor ligand are altered by binding; e) free radioligand concentration remains unchanged after binding.
RESULTS
Saturation experiments
Saturation experiments with genetically cloned receptors
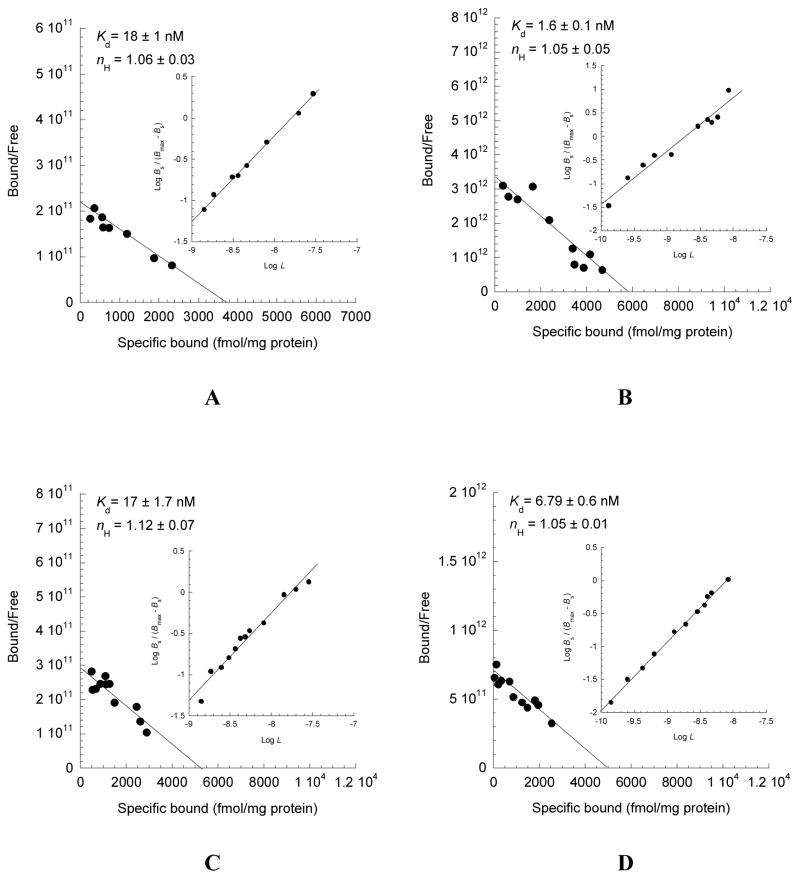
Direct saturation binding studies were carried out using [3H]WC-10 and [3H]raclopride with cloned human hD3, hD2L receptors on HEK cell membranes and rat rD3, rD2L receptors on Sf9 cell membranes. Detailed descriptions and data for [3H]WC-10 binding were reported previously (Xu et al., 2009). The Scatchard plots and Kd values of [3H]raclopride binding to human hD3, hD2L receptors expressed in HEK cells (Fig. 2A, B) and rat rD3, rD2L receptors expressed in Sf9 cell membranes (Fig. 2C, D) are shown in Figure 2. The Kd values of the receptor-radioligand binding of [3H]WC-10 and [3H]raclopride to human and rat D2L, D3 receptors and their binding selectivity ratios are summarized (Table I). [3H]WC-10 binds with a 66-fold higher affinity to human HEK D3 than to HEK D2L receptors, with a dissociation constant (Kd) of 1.2 nM. [3H]WC-10 binds to rat Sf9 D3 receptors with a Kd of 3.9 nM, demonstrating a 3-fold lower affinity for human HEK D3 receptors and a 40-fold higher affinity for rat Sf9 D2L receptors. [3H]raclopride binds with a 11-fold higher affinity to human HEK D2L (Kd = 1.6 nM) than to HEK D3 (Kd = 18 nM) receptors; and [3H]raclopride binds to rat Sf9 rD2L receptors with a Kd of 6.79 nM, a value that is 4-fold lower than binding to human HEK D2L receptors and 2.5-fold higher than binding to rat Sf9 rD3 receptors. Because the average nH values are close to unity, results indicate that receptor binding data fit well to one site model where the binding is non-cooperative.
Fig. 2. Saturation analysis of the binding of [3H]raclopride to cloned D2-like dopamine receptors.
Direct binding analysis was performed to determine the equilibrium binding affinity of [3H]raclopride for human hD3 and hD2 (A, B) or rat rD3 and rD2 (C, D) receptors. Human receptors were expressed in HEK 293 cells and the rat receptors were expressed in Sf9 cells. Scatchard plots were used to determine the dissociation constants (Kd values). The inset graphs are the Hill plots for determining the Hill coefficient (nH values). Kd and nH are presented as mean values ± S.E.M for n = 3.
Saturation experiments with rhesus monkey and rat brain sections
Direct saturation binding studies were conducted using [3H]WC-10 with rhesus monkey caudate (Fig. 3A), putamen (Fig. 3B) and rat caudate-putamen (Fig. 3C). The Kd and nH values of the receptor-radioligand binding from Scatchard plots of [3H]WC-10 to rhesus monkey and rat caudate-putamen are shown in Figure 3. nH values were close to equity, which suggest that the binding of [3H]WC-10 is to single binding site. [3H]WC-10 binds with dissociation constants (Kd s) of 1.3 and 1.1 nM to rhesus monkey caudate and putamen respectively. [3H]WC-10 binds to Sprague-Dawley rat striatum with dissociation constant (Kd) of 3.4 nM. Similar results of dissociation constants (Kd s) were also observed in Fisher 344 and Wistar rat brain (data not shown). These Kd values are in good accordance with the Kd values obtained from [3H]WC-10 binding to cloned human or rat receptors (Xu, et al., 2009). Therefore, the Kd values obtained from cloned receptors are suitable values for receptor occupancy estimates for the in vitro autoradiography of this study.
Fig. 3. Saturation analysis of the binding of [3H]WC-10 to rhesus monkey and rat brain.
Direct binding analysis was performed to determine the equilibrium binding affinity of [3H]WC-10 binding sites in the rhesus monkey caudate (graph A), putamen (graph B) and rat striatum (graph C). Nonspecific binding was determined from samples which contained 1 uM S(−)-Eticlopride. Scatchard plots were used to determine the dissociation constants (Kd values). The inset graphs are the Hill plots for determining the Hill coefficient (nH values). Kd and nH are presented as mean values ± S.E.M for n = 3.
Quantitative autoradiography
Autoradiography showed that [3H]WC-10 and [3H]raclopride labeled the D2/D3 sites in monkey striatal caudate and putamen, as well as the D2/D3 sites in rat striatum (Fig. 4 A-D). The sensitivity limit of Beta Imager 2000Z Digital Beta Imaging System was 0.07 dpm/mm2. A tritium standard [3H]Microscale (Figure 4E) with a series sections of known amount of radioactivity (ranging from 0.14 to 63.1 nCi/mg) was counted, and quantitative data analysis with Beta-Vision Plus yielded a ROI activity number ranging from 0.06 to 21.2 cpm/mm2. These data were linearly fitted with a coefficient (R) greater than 0.99 (Fig. 5). This standard curve was used for calibration of tritium autoradiography of tissue sections, (i.e., converting cpm/mm into nCi/mg tissue) and the receptor bond radioligand densities were readily calculated utilizing the specific activities of radioligands.
Fig. 4. Quantitative autoradiographic analysis of the binding of [3H]WC-10 and [3H]raclopride to rat and rhesus monkey brain.
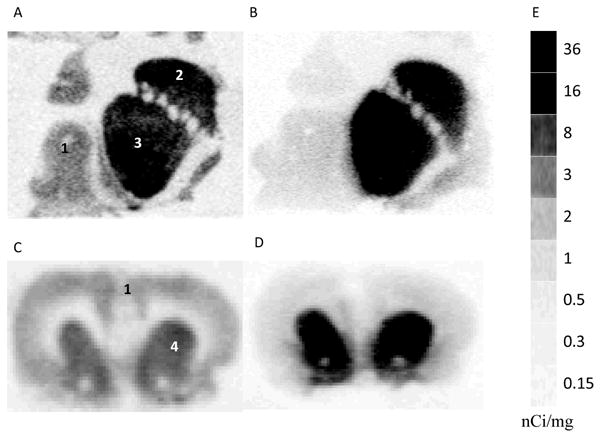
Autoradiograms show neuroanatomical localization of [3H]WC-10 and [3H]raclopride binding sites in rhesus monkey (A, B) and Sprague-Dawley rat (C, D) brain sections. For this study [3H]WC-10 was used at a concentration of 4 nM (A, C) and [3H]raclopride was used at a concentration of 10 nM (B, D). The numbers 1 through 4 in panels A and C designate the following CNS anatomical regions: 1) cortex, 2) primate caudate, 3) primate putamen and 4) rat striatum. Panel E shows autoradiographic image of a [3H]Microscale, which was counted for 24 hours along with the brain sections for the purpose of quantitative analysis.
Fig. 5. Quantitative calibration of in vitro autoradiography.
Calibrated autoradiography standard typical curve obtained by counting a series of tritium standards of a [3H]Microscale, digitalized image was used to analyze the region of interest. This curve was used to convert cpm/mm2 to nCi/mg to quantify autoradiography.
Absolute dopamine D2 and D3 receptors densities from in vitro quantitative autoradiography
Based on the saturation binding analysis and the in vitro binding data of [3H]WC-10 and [3H]raclopride to cloned human and rat D2 and D3 receptors, fractions of D2 and D3 receptor occupancies with 4 nM [3H]WC-10 and 10nM [3H]raclopride binding in monkey and rat caudate-putamen are readily determined. The values of a1, b1, a2, b2, are summarized in Table II. With quantitative autoradiographic analysis, we measured the apparent receptor binding density (B1s) of D2 plus D3 receptors using 4 nM [3H]WC-10 in monkey anterior caudate, putamen and rat striatum to be 125, 141 and 38 fmol/mg tissue respectively. The apparent receptor binding density (B2s) of D2 plus D3 receptors in monkey caudate, putamen and rat striatum using 10 nM [3H]raclopride are found to be 318, 345 and 145 fmol/mg tissue respectively. With these receptor occupancy fraction numbers and the apparent receptor binding densities (B1 and B2 values), we obtained the absolute D2 and D3 receptors densities from equations (5) and (6). D2 density were found to be about 310, 307 and 143 fmol/mg tissue for monkey caudate, putamen and rat striatum respectively, about 2.1~2.4 fold of D3 densities. The apparent receptor binding densities of 4 nM [3H]WC-10 and 10 nM [3H]raclopride, absolute D2 and D3 receptor densities (D2 and D3 values) and their density ratios (D2/D3) in rhesus monkey anterior caudate-putamen and rat striatum are summarized in Table III. With the same method, we calculated the absolute D2 and D3 receptor densities in monkey post anterior caudate-putamen where globus pallidus was present. We found that D3 receptor density in post anterior striatum was lower than in anterior striatum and about one-fourth of D2 receptor density (data not shown).
TABLE II.
Fractions of D2 and D3 receptors occupancy
| a1 | b1 | a2 | b2 | |
|---|---|---|---|---|
| Monkey | 0.05 | 0.78 | 0.86 | 0.36 |
| Rat | 0.02 | 0.5 | 0.6 | 0.37 |
a1, b1, a2, and b2 represents the receptors occupancy fractions of 4 nM [3H]WC-10 and 10 nM [3H]raclopride binding to dopamine D2 and D3 receptors in the monkey and rat striatum. These values were derived from the saturation binding isotherm, equation (2), using the Kd values in Table I.
TABLE III.
Absolute D2 and D3 receptor densities density ratios in rhesus monkey caudate, putamen and rat striatum
| B1 fmol/mg Tissue | B2 fmol/mg Tissue | D2 fmol/mg Tissue | D3 fmol/mg Tissue | D2:D3 | |
|---|---|---|---|---|---|
| Rhesus caudate | 125 ± 3 | 318 ± 17 | 310 ± 21 | 141 ± 2 | 2.20 ± 0.16 |
| Rhesus putamen | 141 ± 4 | 345 ± 23 | 307 ± 30 | 130 ± 5 | 2.36 ± 0.2 |
| Rat striatum | 38 ± 2 | 145 ± 4 | 143 ± 7 | 68 ± 3 | 2.11 ± 0.23 |
Apparent binding densities (B1 and B2) of 4 nM [3H]WC-10 and 10 nM [3H]raclopride were directly measured with quantitative autoradiography; absolute D2 and D3 receptor densities and the receptor density ratios D2:D3 were calculated from the equations (5) and (6) using the a1, b1, a2, and b2 values in the Table II and the B1, B2 values in this Table. Data were presented as mean value ± S.E.M, n=8 for rhesus monkey caudate-putamen and n=30 for rat striatum.
Comparison of digital Beta Imager autoradiography and film autoradiography
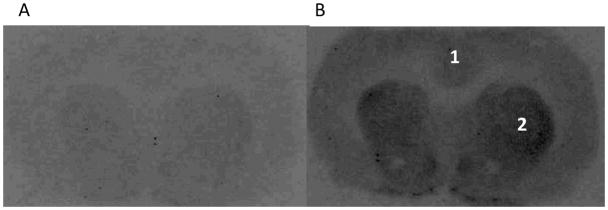
Autoradiograms of 4nM [3H]WC-10 incubated brain slides obtained with the traditional 3H-Hyperfilm exposure techniques with different exposure period, 1 and 3 months, are shown in Figure 6. Although the image of 3-month exposure had a clear view of striatum and islands of Calleja, the image of 1-month exposure didn’t show enough signal for visualization. While the digital Beta Imager can produce decent images for quantitative analysis after 24-hour acquisition (Figure 4), compared to traditional film autoradiography, the Biospace Beta Imager 2000Z Digital Beta Imaging System is a more sensitive, quantitative and higher-throughput tool for receptor autoradiography binding assays with tritium.
Fig. 6. Film autoradiography of the binding of [3H]WC-10 to rat brain.
Autoradiograms obtained with the traditional film exposure techniques with different exposure time, A. 1 month; B, 3 months. The numbers 1 and 2 designate the following CNS anatomical regions: 1) cortex, 2) rat striatum. For this study [3H]WC-10 was used at a concentration of 4 nM.
DISCUSSION
In a previous report (Xu et al., 2009), we have characterized D3 receptor antagonist [3H]WC-10 and demonstrated it to be a high affinity and moderately selective radioligand for D3 versus D2L receptors using in vitro radioligand binding and autoradiography studies. WC-10 was radiolabeled with tritium, and the in vitro binding of [3H]WC-10 to cloned dopamine D2L and D3 receptors was evaluated. [3H]WC-10 binds with a 66-fold higher affinity to human HEK D3 than to HEK D2L receptors, with a dissociation constant (Kd) of 1.2 nM. However, [3H]WC-10 binds to rat Sf9 rD3 receptors with a Kd of 3.9 nM, a value that is 3-fold lower than binding to human HEK D3 receptors and a 40-fold value higher than binding to rat Sf9 rD2L receptors. In vitro autoradiography studies show that [3H]WC-10 labeled the majority of D3 sites in the caudate and putamen for both adult rats and monkey brains.
In this comparative study, we characterized D2 selective antagonist [3H]raclopride binding properties to genetically cloned dopamine D2L and D3 receptors. [3H]raclopride binds with a 11-fold higher affinity to human HEK D2L (Kd = 1.6 nM) than to HEK D3 (Kd = 18 nM) receptors, and [3H]raclopride binds to rat Sf9 rD2L receptors with a Kd of 6.79 nM, a value that is 4-fold lower than its binding to human HEK D2L receptors and 2.5-fold higher than its binding to rat Sf9 rD3 receptors. In vitro quantitative autoradiography studies with the combination of D3/D2 ligand, [3H]WC-10 and the D2/D3 ligand, [3H]raclopride, were also conducted on adult rat and rhesus monkey brain sections. A mathematical model for calculating the absolute densities of dopamine D2 and D3 receptors based on the in vitro binding data obtained from [3H]WC-10 and [3H]raclopride was also developed. Although it would be ideal to develop a more selective dopamine D2 or D3 receptor ligand for direct measurements of D2 and D3 receptors, the assay we presented in this paper permits an indirect evaluation of D2 and D3 receptor density ratios.
Although the D3 receptor density was found to be lower (2.1–2.4-fold) than D2 receptor density, and much lower (7–8-fold) than the dopamine D1 receptor or vesicular monoamine transporter 2 (VMAT-2) density in the caudate putamen (unpublished data from our group), the results presented here clearly show extensive binding of [3H]WC-10 in the caudate-putamen, which we believe represents binding to D3 receptors. These results contradict the binding data obtained with the D2/D3 agonist, [3H]7-OH-DPAT, but they are consistent with immunoprecipitation and immunofluoresence studies with antibodies demonstrating D1 and D3 heterodimerization, D2 and D3 receptors co-expression in the striatum (Ariano and Sibley, 1994; Boundy et al., 1993; Fauchey et al., 2000; Fiorentini et al., 2008; Marcellino et al., 2008; Schwartz et al., 1998). They are also in agreement with in situ hybridization studies showing extensive distribution of D3 mRNA in the striatal tissue (Deransart et al., 2001; Joyce et al., 2004; Mihara et al., 2003; Quik et al., 2000). A D3 knock-out mice binding study using the D2/D3 agonist [3H]-(+)-PHNO (Rabiner et al., 2007) demonstrated a 50% reduction in ligand binding in the striatum of knock-out versus wild type mice, suggesting that D2 and D3 receptors are expressed with similar densities in the striatum (Yaroslavsky et al., 2006). Furthermore, in vitro autoradiography, radioligand binding and PET imaging studies also indicate that D3 receptors are extensively expressed in the rat, cat, monkey and human striatums (Camacho-Ochoa et al., 1995; Joyce et al., 1998; Narendran et al., 2006; Neisewander et al., 2004; Rabiner et al., 2009; Silvers et al., 2006; Sweet et al., 2001; Wade et al., 2001; Wallace and Booze, 1995).
The D2-like dopamine receptors (D2, D3, and D4 receptors) are believed to be involved in the pathogenesis of several psychiatric and neurological disorders and they may play important roles in schizophrenia, Parkinson’s disease and cocaine addiction. D2 and D3 receptors are regulated differently in these central nervous system (CNS) disorders (Abi-Dargham et al., 2000; Martinez et al., 2007; Ryoo et al., 1998; Staley and Mash, 1996). Due to the high amino acid homology and binding domain similarity of D2 and D3 receptors, development of highly selective ligands either to D2 or D3 receptors for direct evaluation of D2 or D3 receptors has been challenging. We have developed a unique procedure for determining absolute D2 and D3 receptor densities; this novel assay requires low radioactivity. Compared to traditional Scatchard saturation binding with multiple concentrations of single radioligand for measuring the receptor density, the method described in this paper took advantage of the less D2 selective D2/D3 ligand [3H]raclopride and novel moderately D3 selective D3/D2 ligand [3H]WC-10. This method represent a novel assay to indirectly evaluate D2 and D3 receptor densities with single concentrations of each radioligand. Our in vitro characterization and in vivo study showed that WC-10 is a useful probe for investigating the role of dopamine D3 receptors in behavioral pharmacology. The procedure generated here for measuring changes in absolute densities of dopamine D3 and D2 receptors can be utilized generally for the neuroscience community in investigating pathological and biological roles of D3 and D2 receptors in the variety of CNS disorders known to have an alteration in dopamine receptors.
With agonist binding studies, aging related or species related D3 and D2 receptor densities’ dynamic changes were also reported (Flores et al., 1998; Levant, 1998). It’s not well known if these alterations come from low and high affinity states exchanging or fresh receptor expressing. In the previous study (Xu et al., 2009), we demonstrated that WC-10 can displace agonist [3H]7-OH-DPAT binding in caudate and putamen. With the currently available agonist radiotracers and the novel antagonist binding assays we have described in this study, we would be able to quantitatively investigate the high and low affinity states of Dopamine D2 and D3 receptors for a better understanding of the alterations of these receptors and their significance in both health and disease. (Gonzalez and Sibley, 1995; Levant et al., 1993; Mukherjee et al., 2004; Sibley et al., 1982; Stanwood et al., 2000)
In summary, results from our studies indicate that [3H]WC-10 is an excellent radioligand for dopamine D3 receptors in vitro. Its weak partial agonist properties make this a unique radiotracer for studying the anatomical distribution and the functional and biological roles of dopamine D3 receptors in neurological systems. Comparative studies with [3H]WC-10 and [3H]raclopride using the mathematical model described here enable the indirect measurement of the absolute densities of D2 and D3 receptors. The mathematical model provided an excellent method for quantifying the D2 and D3 receptor density ratio, which would be valuable for general use for further investigating the pathological and pharmacological roles of dopamine receptors in neurological regulation.
Acknowledgments
We thank Terry Anderson and Deborah Carter for help with brain section preparation.
Contract grant sponsor: NIH; Contract grant numbers: DA12647, DA16181, NS048056 DA13584-03S1; Contract grant sponsor: NIH Shared Instrumentation Grant (The Beta Imager 2000Z Digital Beta Imaging System); Contract grant numbers: S10 RR021007).
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano MA, Sibley DR. Dopamine receptor distribution in the rat CNS: elucidation using anti-peptide antisera directed against D1A and D3 subtypes. Brain Research. 1994;649:95–110. doi: 10.1016/0006-8993(94)91052-9. [DOI] [PubMed] [Google Scholar]
- Boundy VA, Luedtke RR, Gallitano AL, Smith JE, Filtz TM, Kallen RG, Molinoff PB. Expression and characterization of the rat D3 dopamine receptor: pharmacologic properties and development of antibodies. J Pharmacol Exp Ther. 1993;264:1002–1011. [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Seeman P, Robinson TE. Cocaine self-administration produces a persistent increase in dopamine D2 High receptors. Eur Neuropsychopharmacol. 2008;18:551–556. doi: 10.1016/j.euroneuro.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Ochoa M, Walker EL, Evans DL, Piercey MF. Rat brain binding sites for pramipexole, a clinically useful D3-preferring dopamine agonist. Neurosci Lett. 1995;196:97–100. doi: 10.1016/0304-3940(95)11857-s. [DOI] [PubMed] [Google Scholar]
- Chu W, Tu Z, McElveen E, Xu J, Taylor M, Luedtke RR, Mach RH. Synthesis and in vitro binding of N-phenyl piperazine analogs as potential dopamine D3 receptor ligands. Bioorg Med Chem. 2005;13:77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Deransart C, Landwehrmeyer GB, Feuerstein TJ, Lucking CH. Up-regulation of D3 dopaminergic receptor mRNA in the core of the nucleus accumbens accompanies the development of seizures in a genetic model of absence-epilepsy in the rat. Brain Res Mol Brain Res. 2001;94:166–177. doi: 10.1016/s0169-328x(01)00240-6. [DOI] [PubMed] [Google Scholar]
- Fauchey V, Jaber M, Caron MG, Bloch B, Le Moine C. Differential regulation of the dopamine D1, D2 and D3 receptor gene expression and changes in the phenotype of the striatal neurons in mice lacking the dopamine transporter. Eur J Neurosci. 2000;12:19–26. doi: 10.1046/j.1460-9568.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK. Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brain Res. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Gackenheimer SL, Seeman P, Schaus J. Autoradiographic localization of [3H]quinpirole binding to dopamine D2 and D3 receptors in rat brain. Eur J Pharmacol. 1992;211:189–194. doi: 10.1016/0014-2999(92)90528-c. [DOI] [PubMed] [Google Scholar]
- George SR, Watanabe M, Di Paolo T, Falardeau P, Labrie F, Seeman P. The functional state of the dopamine receptor in the anterior pituitary is in the high affinity form. Endocrinology. 1985;117:690–697. doi: 10.1210/endo-117-2-690. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Houle S, Kapur S, Wilson AA. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97:1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Sibley DR. [3H]7-OH-DPAT is capable of labeling dopamine D2 as well as D3 receptors. European Journal of Pharmacology. 1995;272:R1–R3. doi: 10.1016/0014-2999(94)00738-s. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, Wilson AA, Zipursky R, Kapur S. The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology. 2009;34:1078–1086. doi: 10.1038/npp.2008.199. [DOI] [PubMed] [Google Scholar]
- Hillefors M, von Euler G. Pharmacology of [3H]R(+)-7-OH-DPAT binding in the rat caudate-putamen. Neurochem Int. 2001;38:31–42. doi: 10.1016/s0197-0186(00)00047-4. [DOI] [PubMed] [Google Scholar]
- Hillefors M, von Euler M, Hedlund PB, von Euler G. Prominent binding of the dopamine D3 agonist [3H]PD 128907 in the caudate-putamen of the adult rat. Brain Res. 1999;822:126–131. doi: 10.1016/s0006-8993(99)01138-5. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Myers AJ, Gurevich E. Dopamine D2 receptor bands in normal human temporal cortex are absent in Alzheimer’s disease. Brain Res. 1998;784:7–17. doi: 10.1016/s0006-8993(97)01005-6. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Woolsey C, Ryoo H, Borwege S, Hagner D. Low dose pramipexole is neuroprotective in the MPTP mouse model of Parkinson’s disease, and downregulates the dopamine transporter via the D3 receptor. BMC Biol. 2004;2:22. doi: 10.1186/1741-7007-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaichi Y, Nonaka R, Hagino Y, Watanabe M. Dopamine D3 receptor binding by D3 agonist 7-OH-DPAT (7-hydroxy-dipropylaminotetralin) and antipsychotic drugs measured ex vivo by quantitative autoradiography. Can J Physiol Pharmacol. 2000;78:7–11. doi: 10.1139/cjpp-78-1-7. [DOI] [PubMed] [Google Scholar]
- King MV, Seeman P, Marsden CA, Fone KC. Increased dopamine D2High receptors in rats reared in social isolation. Synapse. 2009;63:476–483. doi: 10.1002/syn.20624. [DOI] [PubMed] [Google Scholar]
- Leff P. The two-state model of receptor activation. Trends Pharmacol Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- Levant B. Differential distribution of D3 dopamine receptors in the brains of several mammalian species. Brain Res. 1998;800:269–274. doi: 10.1016/s0006-8993(98)00529-0. [DOI] [PubMed] [Google Scholar]
- Levant B, Grigoriadis DE, DeSouza EB. [3H]quinpirole binding to putative D2 and D3 dopamine receptors in rat brain and pituitary gland: a quantitative autoradiographic study. J Pharmacol Exp Ther. 1993;264:991–1001. [PubMed] [Google Scholar]
- Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedtke RR, Freeman RA, Boundy VA, Martin MW, Huang Y, Mach RH. Characterization of 125I-IABN, a novel azabicyclononane benzamide selective for D2-like dopamine receptors. Synapse. 2000;38:438–449. doi: 10.1002/1098-2396(20001215)38:4<438::AID-SYN9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Mach RH. Progress in developing D3 dopamine receptor ligands as potential therapeutic agents for neurological and neuropsychiatric disorders. Current Pharmaceutical Design. 2003;9:643–671. doi: 10.2174/1381612033391199. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J Biol Chem. 2008;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K, Kondo T, Suzuki A, Yasui-Furukori N, Ono S, Sano A, Koshiro K, Otani K, Kaneko S. Relationship between functional dopamine D2 and D3 receptors gene polymorphisms and neuroleptic malignant syndrome. Am J Med Genet B Neuropsychiatr Genet. 2003;117B:57–60. doi: 10.1002/ajmg.b.10025. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Narayanan TK, Christian BT, Shi B, Yang ZY. Binding characteristics of high-affinity dopamine D2/D3 receptor agonists, 11C-PPHT and 11C-ZYY-339 in rodents and imaging in non-human primates by PET. Synapse. 2004;54:83–91. doi: 10.1002/syn.20068. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, Vora S, Litschge M, Kendro S, Cooper TB, Mathis CA, Laruelle M. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63:447–461. doi: 10.1002/syn.20628. [DOI] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Quik M, Police S, He L, Di Monte DA, Langston JW. Expression of D3 receptor messenger RNA and binding sites in monkey striatum and substantia nigra after nigrostriatal degeneration: effect of levodopa treatment. Neuroscience. 2000;98:263–273. doi: 10.1016/s0306-4522(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Rabiner E, Raymond R, Diwan M, McCormick P, Wilson A, Nobrega J. D3 and D2 components of ex vivo regional (+)-PHNO brain binding in wild-type and knock-out mice. J nucl med meeting abstracts. 2007;48:113P-b. [Google Scholar]
- Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, Diwan M, Wilson AA, McCormick P, Gentile G, Gunn RN, Laruelle MA. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 2009;63:782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- Ryoo HL, Pierrotti D, Joyce JN. Dopamine D3 receptor is decreased and D2 receptor is elevated in the striatum of Parkinson’s disease. Mov Disord. 1998;13:788–797. doi: 10.1002/mds.870130506. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Diaz J, Bordet R, Griffon N, Perachon S, Pilon C, Ridray S, Sokoloff P. Functional implications of multiple dopamine receptor subtypes: the D1/D3 receptor coexistence. Brain Res Brain Res Rev. 1998;26:236–242. doi: 10.1016/s0165-0173(97)00046-5. [DOI] [PubMed] [Google Scholar]
- Sibley DR, De Lean A, Creese I. Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D2 dopamine receptor. J Biol Chem. 1982;257:6351–6361. [PubMed] [Google Scholar]
- Silvers JM, Wallace DR, Harrod SB, Mactutus CF, Booze RM. Prenatal cocaine alters dopamine and sigma receptor binding in nucleus accumbens and striatum in dams and adolescent offspring. Neurotoxicol Teratol. 2006;28:173–180. doi: 10.1016/j.ntt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Skinbjerg M, Namkung Y, Halldin C, Innis RB, Sibley DR. Pharmacological characterization of 2-methoxy-N-propylnorapomorphine’s interactions with D2 and D3 dopamine receptors. Synapse. 2009;63:462–475. doi: 10.1002/syn.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [125I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000;295:1223–1231. [PubMed] [Google Scholar]
- Sweet RA, Hamilton RL, Healy MT, Wisniewski SR, Henteleff R, Pollock BG, Lewis DA, DeKosky ST. Alterations of striatal dopamine receptor binding in Alzheimer disease are associated with Lewy body pathology and antemortem psychosis. Arch Neurol. 2001;58:466–472. doi: 10.1001/archneur.58.3.466. [DOI] [PubMed] [Google Scholar]
- Vasdev N, Seeman P, Garcia A, Stableford WT, Nobrega JN, Houle S, Wilson AA. Syntheses and in vitro evaluation of fluorinated naphthoxazines as dopamine D2/D3 receptor agonists: radiosynthesis, ex vivo biodistribution and autoradiography of [18F]F-PHNO. Nucl Med Biol. 2007;34:195–203. doi: 10.1016/j.nucmedbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Wade TV, Rothblat DS, Schneider JS. Changes in striatal dopamine D3 receptor regulation during expression of and recovery from MPTP-induced parkinsonism. Brain Research. 2001;905:111–119. doi: 10.1016/s0006-8993(01)02513-6. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Booze RM. Identification of D3 and sigma receptors in the rat striatum and nucleus accumbens using (+/−)-7-hydroxy-N,N-di-n-[3H]propyl-2-aminotetralin and carbetapentane. J Neurochem. 1995;64:700–710. doi: 10.1046/j.1471-4159.1995.64020700.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH. [(3)H]4-(Dimethylamino)-N-[4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl]benzamide, a selective radioligand for dopamine D3 receptors. I. In vitro characterization. Synapse. 2009;63:717–728. doi: 10.1002/syn.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Colletti M, Jiao X, Tejani-Butt S. Strain differences in the distribution of dopamine (DA2 and DA3) receptor sites in rat brain. Life Sci. 2006;79:772–776. doi: 10.1016/j.lfs.2006.02.030. [DOI] [PubMed] [Google Scholar]