Abstract
The oxygen distribution in the microcirculation of the piglet’s brain and striatal extracellular dopamine were determined during repetitive apnea and resuscitation with 21% or 100% oxygen. Pre-apnea cortical oxygen was 49.5 ± 10.4 mm Hg and during each apnea decreased to 8 ± 0.9 mm Hg. After ten apneic episodes followed by resuscitation with 21% or 100% oxygen, 7.48 ± 1.6% or 2.6 ± 0.5% of the tissue volume was below 10 mm Hg, respectively. Extracellular dopamine increased progressively with an increase in the number of apneas with resuscitation of 21% oxygen and at the end of ten apneic episodes it was up to 59,500 ± 11,320% of control. There was no increase in extracellular dopamine during apnea resuscitated with 100% oxygen. Repetitive apnea caused progressive increase in fraction of hypoxic brain tissue in newborn. The magnitude of the increase is dependent on whether the animals were resuscitated with room air or 100% oxygen.
Keywords: Brain, dopamine, newborn, oxygen, repetitive apnea
INTRODUCTION
Neonatal apnea may trigger cellular and molecular changes that can lead to metabolic disturbance and/or cell death. In spite of the vast literature on the pathophysiology of neonatal apnea, the mechanism(s) by which repetitive apnea affects the metabolic pathways that sustain the normal processes of cell survival and cell death in the brain are not very well known. Parameters that, alone or in combination, can determine the pathogenic outcome of repetitive apnea in newborn remain to be elucidated. These parameters include the length and frequency of apneic episodes, the time and extent of the intervening recovery period as well as the oxygen concentration used for resuscitation. In the clinical setting, the oxygen pressure used for resuscitation ranges from 21% to 100%. How these differences in oxygen concentrations during resuscitation effects brain oxygenation and metabolism and later neurodevelopment prognosis is not yet understood due, in part, to the lack of reliable measurements of oxygen in brain tissue (1,2).
Our hypothesis was that changes in brain oxygenation and extracellular dopamine during repetitive apnea are dependent on % of oxygen given to the animals during each postapneic resuscitation. To test this, we examined the oxygen distribution in the microcirculation of brain tissue during repetitive apnea and resuscitation with two oxygen concentrations, 21% and 100%, using oxygen dependent quenching of phosphorescence. Brain oxygenation was correlated with the levels of extracellular dopamine in the striatum. Dopamine was chosen, because the dopaminergic system of the striatum of a newborn piglet’s brain has been shown to be very sensitive to the local oxygen pressure. Dopamine can also be a mediator of neuronal injury, particularly within the striatum.
EXPERIMENTAL PROCEDURES
Animal Model
Twenty-one newborn piglets, 2–4 days of age (1.4–2.5 kg) were assigned to one of 3 groups: (1) repetitive apnea with resuscitation of 21% oxygen, (2) repetitive apnea with resuscitation of 100% oxygen, (3) sham operated. Anesthesia was induced with halothane (4% mixed with 96% oxygen), and 1.5% lidocaine-HCl was used as a local anesthetic. Halothane was withdrawn entirely after the tracheotomy, and pancuronium was used for neuromuscular blockade and mechanical ventilation (1.5 mg/kg). Fentanyl-citrate (30 µg/kg) was intravenously injected, and the animals were mechanically ventilated with a mixture of oxygen (FiO2 21%) and 0.5% isoflurane. The femoral artery and femoral vein were then cannulated and anesthesia was maintained with isoflurane 0.5%. Muscle relaxation was achieved with a bolus of pancuronium followed by continuous infusion at 1 mg/kg/h. The head was placed in a Kopf stereotaxic holder and an incision was made along the circumference of the scalp. The scalp was removed to expose the skull and a hole approximately 5 mm in diameter was made in the skull over the right parietal hemisphere for measuring the oxygen pressure. A small hole was drilled over the left parietal hemisphere for implantation of a microdialysis probe into the left striatum. Following apnea, the animals recovered for 2 h while anesthetized and then were euthanized with 4 M KCl.
In all experiments, the systolic, diastolic and mean blood pressures, hemoglobin oxygen saturation, end tidal CO2 pressure, EKG, and body temperature were monitored. The blood pH, PaCO2, and PaO2 were measured using a Chiron/Diagnostics Rapidlab 800 blood gas machine.
All animal procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and have been approved by the local Animal Care Committee.
Repetitive Apnea Model
The animals underwent 10 episodes of apnea and resuscitation. The resuscitation time between each apneic episode was 15 min. Apnea was initiated by disconnecting the animal from the ventilator and completed by reconnecting it to the ventilator. The apneic episodes were terminated at 3.5 min, or 0.5 min after the heart rate reached the bradycardic threshold of 60 beats/min. The resuscitation was with 21% of oxygen or with 100% oxygen for 10 min and then the FiO2 of the latter was lowered to 21% for 5 min before the next apnea cycle. Blood gases were measured between 6 and 8 min of the cycle, the microdialysis samples were collected from beginning of each apnea up to 1 min of post-apneic resuscitation and histograms were obtained at 14 min of the cycle, just before initiation of the next apnea.
Measurements of Oxygen Pressure and Oxygen Distribution by the Oxygen Dependent Quenching of Phosphorescence
Cortical oxygen pressure was measured using oxygen dependent quenching of phosphorescence (3–7). The technical basis for measuring oxygen in the microcirculation of tissue using phosphorescence quenching, including determining the oxygen histogram from the distribution of phosphorescence lifetimes, has been described in detail (8). Briefly, a near infrared oxygen sensitive phosphor, (Oxyphor G2) was injected i.v. at approximately 1.5 mg/kg. The measurements were made using a multi-frequency phosphorescence lifetime instrument (PMOD 5000) and the data analyzed using algorithms and software developed by Vinogradov and coworkers (4). The light was carried to the tissue through a 3 mm light guide. Phosphorescence (λmax=790 nm) emitted from the tissue was collected through a second light guide placed against the tissue at approximately 8 mm (center to center) from the excitation light-guide. This positioning of the light-guides allowed effective sampling of brain tissue oxygenation down to about 6 mm under the neocortical surface. The mean oxygen pressure was measured repetitively (each 5 s) from shortly before beginning each apnea until 1 min before the beginning the next apnea. The excitation light (635 nm) was modulated at two frequencies, one about 36 kHz and the other dynamically adjusted to giving a phase shift of 28 ± 2 degrees (between 100 and 2,000 Hz). The higher frequency measurement was used to determine the contribution of scattered light/fluorescence and the lower frequency, after correction, to calculate the mean lifetime and oxygen pressure. Oxygen histograms were measured about 1 min before the beginning of each apnea. The excitation light was modulated as the sum of 38 sinusoids with frequencies spaced between 100 Hz and 40 kHz. The phosphorescence was analyzed to give the phase and amplitude relationship throughout the frequency range. A maximal entropy algorithm was used to convert into distribution of lifetimes and then the oxygen histogram calculated using the Stern-Volmer relationship and the appropriate quenching constant and lifetime at zero oxygen.
Measurement of Extracellular Dopamine by In vivo Micro-dialysis
The extracellular level of dopamine in striatum was measured as described in earlier publications (6,9–11). The microdialysis samples were collected from the beginning of apnea until 1 min into the recovery period. Five µl dialysate was injected into HPLC microbore column for analysis of dopamine. The detection limit of the assay was 5–10 fmole/sample. Identification and quantitation of dopamine was done by comparison with chromatograms of standard solutions. The efficiency of the microdialysis probe was determined in vitro at 36°C and the relative recoveries were 16 ± 2% (means ± SD for 5 experiments). The values for the level of dopamine in the dialysate are presented after correction for relative recovery by the microdialysis probe.
Statistical Analysis
All values are expressed as means ± SD. Statistical significance was determined using one-way analysis of variance with repeated measures by Wilcoxon signed-rank test, P < 0.05 was considered statistically significant.
RESULTS
Physiological Parameters of the Piglets During Repetitive Apnea
The effects of repetitive apnea with resuscitation of 21% oxygen on the physiological parameters of newborn piglets are shown in Table I. As can be seen, during 10 episodes of apnea, there were no significant differences in PaCO2, PaO2 and blood pressure. However, pH decreased progressively with increasing number of apnea and at the end of the last apnea, was 7.19 ± 0.04 (P < 0.005). Similar changes in pH was observed during repetitive apnea with resuscitation of 100% oxygen (decrease from control 7.47 ± 0.01 to 7.22 ± 0.02, P < 0.005). The only significant difference between apnea with resuscitation with 21% oxygen and apnea with resuscitation with 100% oxygen was in level of PaO2 (about 5 times higher in 100% oxygen resuscitation) (Table II).
Table I.
Physiological Parameters in Newborn Piglets During Repetitive Apnea with Resuscitation of 21% and Recovery Periods
| Conditions | PaO2 (mm Hg) | PaCO2 (mm Hg) | pH | Blood pressure (mm Hg) |
|---|---|---|---|---|
| Control | 82.3 ± 3.7 | 31.8 ± 1.4 | 7.48 ± 0.02 | 91.5 ± 5.1 |
| Apnea 1 | 94.1 ± 5.7 | 32.4 ± 3.2 | 7.41 ± 0.02a | 92.4 ± 4.7 |
| Apnea 2 | 90.2 ± 6.1 | 32.9 ± 3.7 | 7.32 ± 0.04a | 98.6 ± 5.7 |
| Apnea 3 | 92.1 ± 5.6 | 31.9 ± 3.4 | 7.28 ± 0.03b | 97.2 ± 4.3 |
| Apnea 4 | 91.8 ± 6.3 | 30.9 ± 3.1 | 7.25 ± 0.03b | 97.1 ± 5.1 |
| Apnea 5 | 100.1 ± 7.8 | 30.1 ± 3.5 | 7.25 ± 0.04b | 90.2 ± 3.4 |
| Apnea 6 | 100.9 ± 6.4 | 29.7 ± 2.7 | 7.24 ± 0.03b | 91.3 ± 5.2 |
| Apnea 7 | 100.3 ± 4.9 | 29.1 ± 3.1 | 7.22 ± 0.03b | 90.1 ± 5.1 |
| Apnea 8 | 104.5 ± 5.7 | 29.8 ± 2.8 | 7.21 ± 0.04b | 89.5 ± 6.7 |
| Apnea 9 | 101.8 ± 3.1 | 30.4 ± 2.7 | 7.19 ± 0.02b | 88.1 ± 5.1 |
| Apnea 10 | 99.7 ± 6.9 | 29.1 ± 1.9 | 7.19 ± 0.04b | 84.6 ± 4.8 |
| Recovery, 1 h | 87.1 ± 5.9 | 30.9 ± 4.2 | 7.36 ± 0.04a | 84.0 ± 4.1 |
| Recovery, 2 h | 81.2 ± 4.3 | 34.7 ± 3.1 | 7.40 ± 0.04a | 82.2 ± 5.6 |
The values are the means ± SD for n = 7 experiments.
P < 0.05;
P < 0.005 for difference from pre-apnea conditions as determined by one-way analysis of variance with repeated measures by Wilcoxon signed-rank test.
Table II.
Physiological Parameters in Newborn Piglets During Repetitive Apnea with Resuscitation of 100% and Recovery Periods
| Conditions | PaO2 (mm Hg) | PaCO2 (mm Hg) | pH | Blood Pressure (mm Hg) |
|---|---|---|---|---|
| Control | 87 ± 2.5 | 36.7 ± 1.8 | 7.47 ± 0.01 | 91.8 ± 2 |
| Apnea 1 | 451.5 ± 43.4b | 42 ± 1.1 | 7.34 ± 0.02a | 87.5 ± 2.6 |
| Apnea 2 | 504.1 ± 13.5b | 42.6 ± 3.1 | 7.29 ± 0.02a | 85.2 ± 4.1 |
| Apnea 3 | 491.1 ± 16.8b | 40.5 ± 2.9 | 7.28 ± 0.03a | 82.7 ± 3.3 |
| Apnea 4 | 496.7 ± 12.6b | 39.2 ± 2 | 7.27 ± 0.04b | 85.7 ± 4.2 |
| Apnea 5 | 489.8 ± 22.6b | 39.1 ± 1.5 | 7.25 ± 0.03b | 83.8 ± 4.8 |
| Apnea 6 | 515.2 ± 12.3b | 37 ± 1.7 | 7.26 ± 0.03b | 83.2 ± 3.9 |
| Apnea 7 | 507.4 ± 15.3b | 36.5 ± 2 | 7.24 ± 0.03b | 83.4 ± 4.3 |
| Apnea 8 | 512.2 ± 17.5b | 37.4 ± 1.8 | 7.22 ± 0.04b | 85.8 ± 4.5 |
| Apnea 9 | 501.4 ± 12.6b | 36 ± 1.3 | 7.22 ± 0.03b | 85.7 ± 4 |
| Apnea 10 | 503.3 ± 16.4b | 35.4 ± 1 | 7.22 ± 0.02b | 82.5 ± 3.9 |
| Recovery, 1 h | 79.1 ± 2.6 | 34.8 ± 1.2 | 7.44 ± 0.01 | 75.7 ± 3.4 |
| Recovery, 2 h | 80.2 ± 2.9 | 35.5 ± 0.6 | 7.49 ± 0.01 | 78.1 ± 2.8 |
The values are the means ± SD for n = 7 experiments.
P < 0.05;
P < 0.005 for difference from pre-apnea conditions as determined by one-way analysis of variance with repeated measures by Wilcoxon signed-rank test.
Oxygen Distribution in Brain Tissue During Repetitive Apnea
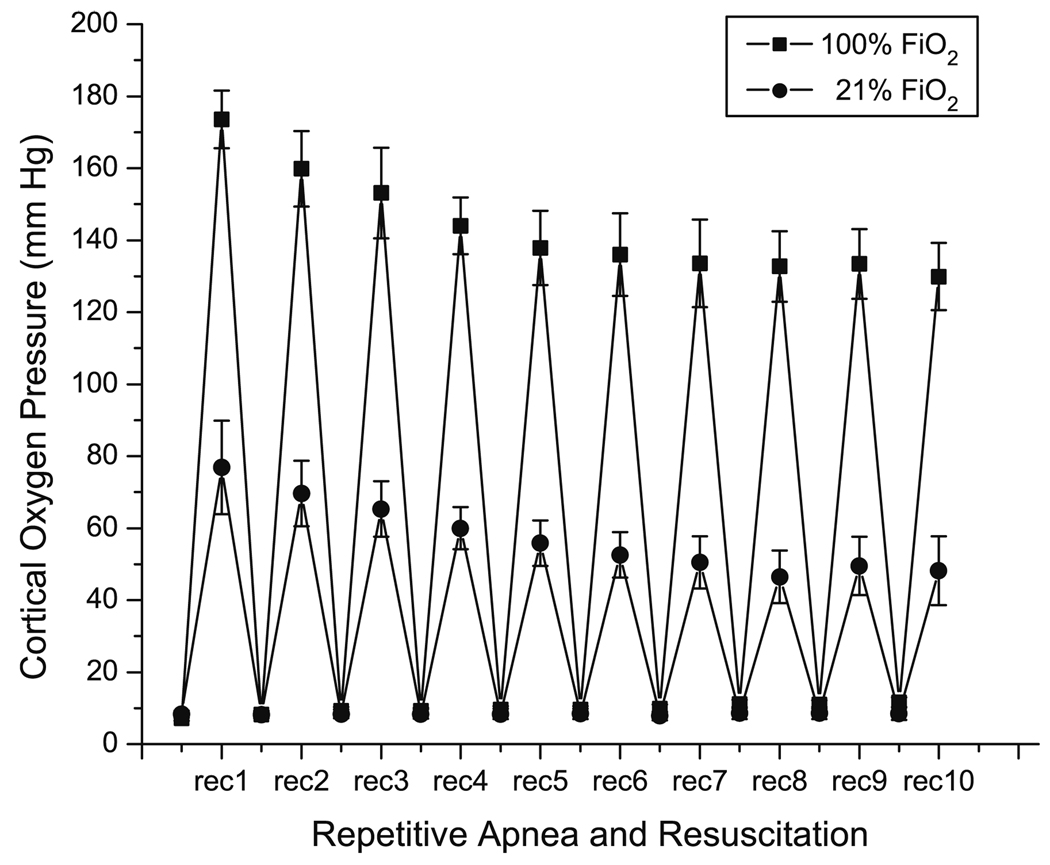
The values for the mean cortical oxygen pressure were measured throughout each apnea and 14 min of the recovery period. The data for 21% and 100% oxygen are shown in Fig. 1. When apnea was initiated, there was a delay of a few seconds and then the oxygen pressure in the cortex fell rapidly, attaining a minimal value of 8 ± 0.9 mm Hg. This was an averaged value, however, and includes surface vessels and any exudate containing phosphor that may be present under the dura. Histograms of the oxygen distribution taken at the minimum showed a peak near 1 mm Hg and indistinguishable from zero (data not shown). During post-apneic resuscitation with 21% and 100% oxygen, the mean oxygen pressures rises rapidly. The maximum value that occurs during the each post-apneic resuscitation period using either 21% or 100% oxygen decreased with increasing numbers of apnea. During resuscitation with 21% oxygen, following the first and second apneas upon reoxygenation the oxygen levels transiently rose above pre-apnea levels (reactive hyperemia). The peak value reached 78 ± 7 mm Hg after the first apnea but was markedly attenuated with increasing numbers of apnea. After six apneic episodes, the maximum oxygen tension decreased to 50 ± 6 mm Hg and then remained nearly constant during rest of the post-apneic resuscitation periods. During resuscitation with 100% oxygen the maximal cortical oxygen pressure following an apnea also decreased with the increasing number of apneas. After the first apnea, the value of oxygen was 173 ± 9 mm Hg and this decreased progressively to 137 ± 8 mm Hg through the first 6 apneas and then remained at the same level through the 10th apnea.
Fig. 1.
Cortical oxygen pressure as a function of repeated numbers of apneic episodes. Maxima represent the peak oxygen values during resuscitation periods with 21% and 100% oxygen and the minima (A1–A10) represent the lowest oxygen values during apneic periods. The values are the means ± SD for n = 7 experiments.
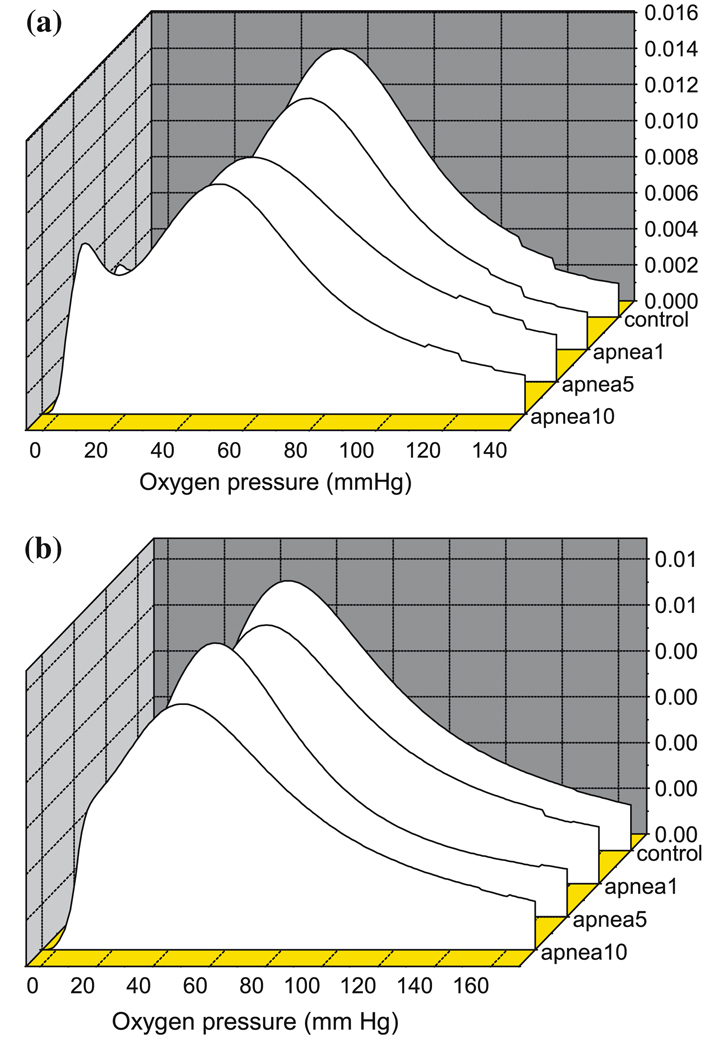
Histograms of the oxygen distributions in the cortical tissue were measured approximately 1 min prior to each apnea. At that time, the animals resuscitated with 100% oxygen had been breathing air for 4–5 min. Representative histograms are shown in Fig. 2a and b. The peak values of the histograms showed a slight decrease with increasing numbers of apnea. The most marked change, however, was that the distribution became bimodal with the oxygen pressures in a substantial fraction of the microcirculatory volume remaining below control level. During resuscitation with 21% oxygen, following 1st, 5th and 10th apnea the peak values for the histograms were 55 ± 5.5, 51 ± 6.9, 49 ± 6.3 mm Hg, respectively. For resuscitation with 100% oxygen, peak values were 52 ± 5.5, 46 ± 4.8, 45 ± 5.4 mm Hg, respectively.
Fig. 2.
Oxygenation of piglet brain during repetitive apnea and resuscitation with 21% oxygen (a) and 100% oxygen (b). Oxygen histograms were determined by deconvolution of the distribution of phosphoresence lifetimes for Oxyphor G2 in the microcirculation. The histogram was taken during resuscitation period, at 1 min prior to each apneic episodes.
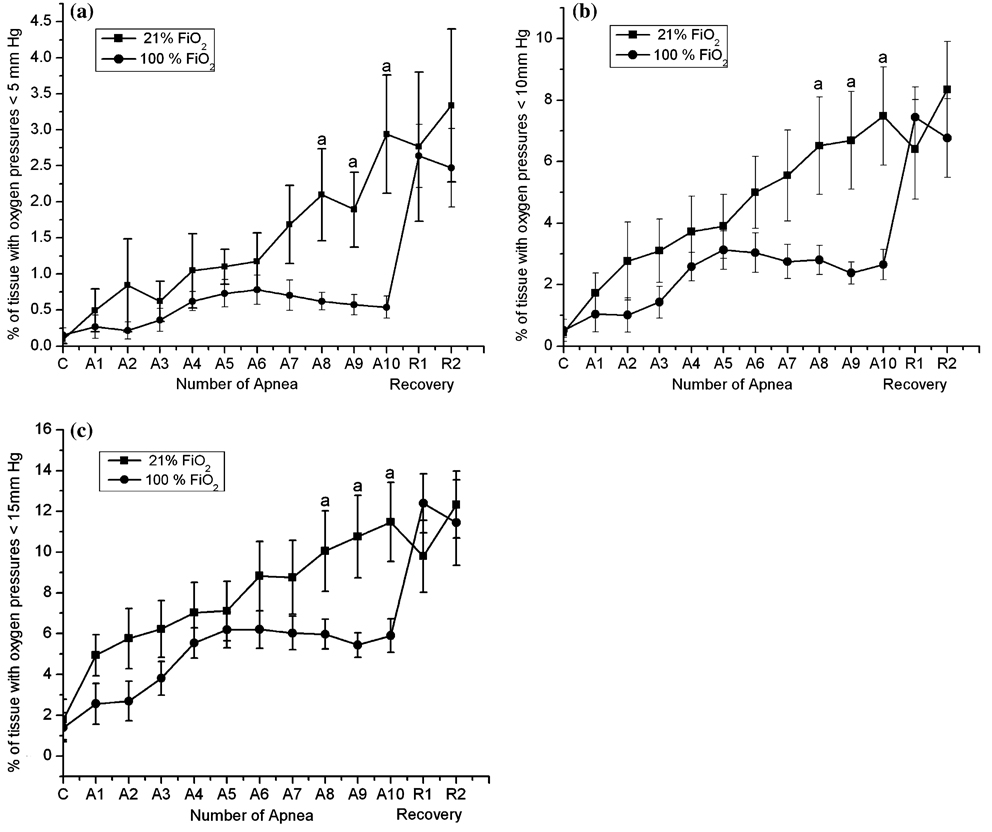
When the volume fractions of the cortical microcirculation with oxygen pressures less than 5, 10 or 15 mm Hg were calculated and plotted as the function of number of apnea, the values increased progressively with increased numbers of apnea (Fig. 3a–c). When resuscitation was with 21% oxygen, after the 10th apnea, volume fractions of 2.9 ± 0.8%, 7.48 ± 1.6% and 11.5 ± 1.9% of the microcirculation was below 5, 10 and 15 mm Hg, respectively. When resuscitating with 100% oxygen, after the 10th apnea these values were 0.53 ± 0.15%, 2.6 ± 0.5% and 5.9 ± 0.8%, respectively. The pre-apnea (control) values were 0.14 ± 0.1%, 0.51 ± 0.35% and 1.4 ± 0.7%, respectively.
Fig. 3.
Effect of repetitive apnea and resuscitation with 21% oxygen and 100% oxygen on fraction of the measured histogram that has oxygen pressures less than (a) 5 mm Hg; (b) 10 mm Hg and (c) 15 mm Hg. Oxygen histograms were determined by deconvolution of the distribution of phosphorescence lifetimes for Oxyphor G2 in the microcirculation. The results are means from 7 experiments ± SD. aP < 0.05 for significant difference between 21% and 100% oxygen values as determined by one-way analysis of variance, followed by the Wilcoxon signed-rank test.
By 1 h into the recovery period following the last apnea the oxygen histograms for resuscitation with 21% and 100% oxygen were not significantly different, indicating that improvement in oxygenation by the latter was restricted to immediately after the individual apnea. In both cases, the histograms remained bimodal throughout the 2 h post-apneic recovery period and a substantial fraction of the microcirculatory volume remained hypoxic throughout the recovery period.
Extracellular Dopamine in the Striatum During Repetitive Apnea with Resuscitation of 21% and 100% Oxygen
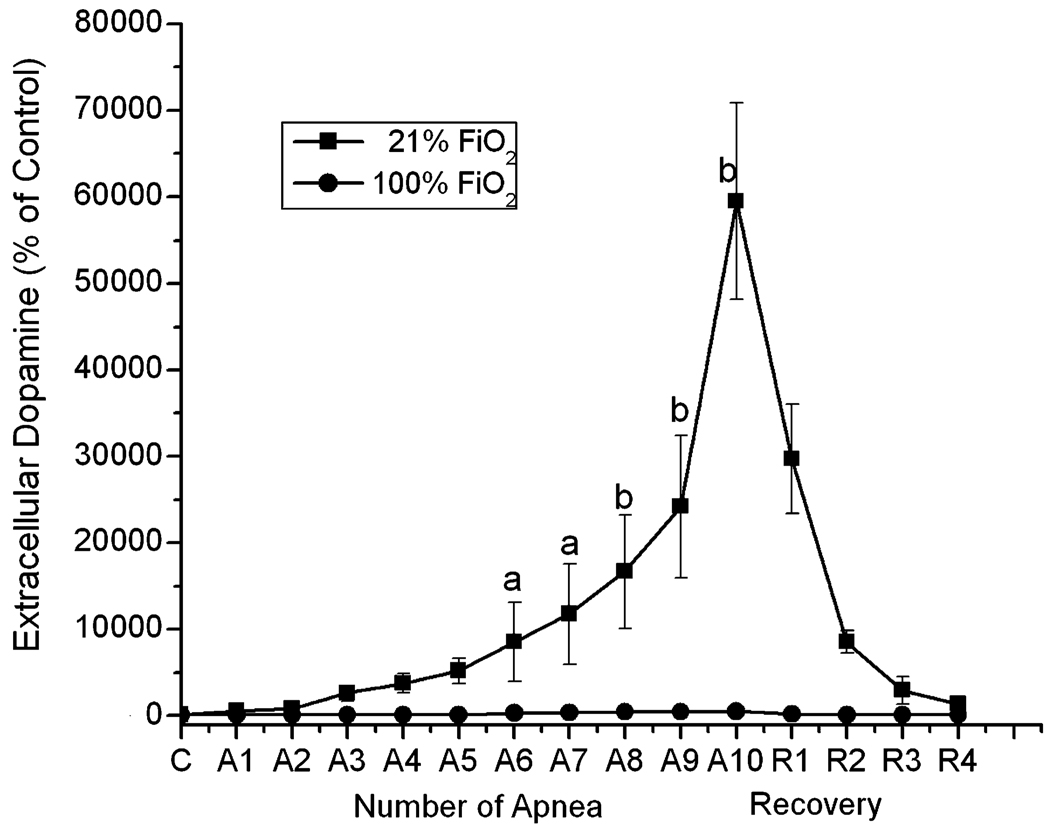
The control value of dopamine was stable prior to apnea with absolute levels of dopamine below 1 pmole/ml. Repetitive apnea with resuscitation of 21% and 100% oxygen on the extracellular levels of dopamine are shown in Fig. 4. During repetitive apnea with resuscitation using 21% oxygen, extracellular dopamine levels increased progressively with increase in number of apneas. After 10 apneic episodes, dopamine had increased to 59,500 ± 11,320% of control. This is in contrast to resuscitation using 100% oxygen, where even after the 10th apnea the increase in extracellular dopamine was insignificant when compared to sham operated animals.
Fig. 4.
The effect of repetitive apnea and resuscitation with 21% oxygen and 100% oxygen on the extracellular concentrations of dopamine in striatum of newborn piglets. The microdialysis probe was implanted in the striatum of newborn piglet and perfused with Ringer solution at 1 µl/min. Collection of the microdialysis samples was initiated 2 h after the probe was inserted and the samples start to be collected from beginning of apnea and ended 1 min after each apneic episodes. R1 and R2 represented 1 h and 2 h of recovery following last apnea. The level of dopamine was analyzed by HPLC with electrochemical detection. The 3 measurements of the dopamine during the pre-apnea period were averaged and the value considered as the baseline (100%). The results are means for 7 experiments in each group ± SD. aP < 0.05; bP < 0.005 for significant difference from control values as determined by one-way analysis of variance, followed by the Wilcoxon signed-rank test.
DISCUSSION
Our study describes the effects of repetitive apnea with resuscitation of 21% oxygen and 100% oxygen on cortical oxygen pressure and extracellular dopamine. Cortical oxygen pressure was measured by oxygen dependent quenching of phosphorescence. This method allows quantitative measurement of oxygen distribution in the microcirculation and small veins of the brain cortex in vivo and, therefore, correlation of tissue oxygenation with neuronal function. Each animal underwent 10 episodes of apnea and recovery, based on our experience that this number produces measurable brain injury and yet cardiovascular function remains sufficient (12). A repetitive apnea model was chosen because the cumulative effect of brief but repetitive asphyxial events, as may occur in the human newborn with severe apnea, may result in clinically significant deficits in long term neuropsychological function. Study of bicuculline-induced seizures in rats (13) revealed that systemic and cerebral vascular responses and the associated changes in cerebral oxygenation were better maintained during long-duration seizures than during shorter, repeated seizures. In a gerbil model of repeated ischemic events secondary to bilateral carotid occlusion, a series of three 5 min occlusions at various intervals produced more cerebral edema and neuronal loss than a single 15 min occlusion (14). In fetal sheep, 3 episodes of 10 min ischemia were reported to be more deleterious than an isolated 30 min ischemia (15), and in particular there was increased neuronal loss in the striatum.
The goal of the present study was to compare brain oxygenation following repetitive apneic episodes when two different concentration of oxygen, 21% and 100%, were given during a brief post-apneic resuscitation period. There were significant differences in fraction of the microcirculatory volume in the cortex that was significantly hypoxic at the 14 min resuscitation period following each apnea. For both repetitive apneas, with 21% oxygen resuscitation and with 100% resuscitation, the volume of hypoxic microcirculatory volume increased progressively with increasing the number of apneas. When resuscitation was with 100% oxygen, the hypoxic volume was much smaller than when resuscitation was with room air. However, measurements made at 1 and 2 h post-apneic recovery from repetitive apnea with 21% and 100% oxygen resuscitation, showed there was no longer a difference in hypoxic volume between both groups, although a substantial fraction of the microcirculation remained hypoxic.
The progressive increase in hypoxic microcirculation is consistent with the progressive increase in extracellular dopamine during repetitive apnea when resuscitation was with 21% oxygen. This was suppressed when 100% oxygen was used, where the increase in extracellular striatal dopamine was not significantly different from sham operated animals. The observed changes in oxygen pressure and increase in extracellular dopamine during repetitive apnea with 21% of resuscitation are very similar to those in our early studies (12,16). In the earlier study, the same numbers of apnea episodes were used as in the present study, but the resuscitation time was shorter (10 min vs. 15 min in present paper). This suggests that the decrease in brain oxygenation and increase in extracellular dopamine levels in the striatum are less dependent on the time between apnea than on the oxygen pressure in the inspired gas during resuscitation.
Extracellular dopamine was determined in the striatum. This ‘‘selectively vulnerable’’ dopaminergic region of the brain has been shown to be uniquely susceptible to recurrent hypoxic events. Our earlier studies showed that the dopaminergic system of the striatum of a newborn piglet’s brain is very sensitive to the local oxygen pressure (6,9–11). Therefore, resuscitation with 100% oxygen, by improving oxygen levels in the microcirculation, may eliminate the increase in extracellular dopamine in the striatum.
The increase in extracellular dopamine observed during apnea with resuscitation of 21% oxygen could have major importance in development of short and long-term neuropsychologic function. Dopamine is widely believed to play a critical role in the pathophysiology of brain function. The release of dopamine during ischemic/hypoxic conditions may play a major role in mediating neuronal damage, particularly in the striatum. Marie et al. (17) evaluated rat brain 72 h after ischemia (four vessel occlusion) and reported that alpha-methyl-para-tyrosine (AMT, inhibitor of dopamine synthesis) treatment significantly decreased neuronal necrosis in the striatum but had no cytoprotective effect in the CA1 section of the hippocampus or of the neocortex. They suggested that the striatal cytoprotective effect of AMT is linked to cerebral dopamine depletion and that excessive dopamine release during ischemia plays a detrimental role in the development of ischemic cell damage in the striatum. A beneficial effect of AMT pretreatment has also been reported for gerbils subjected to unilateral carotid artery ligation (18).
One of the possible mechanisms for the neurotoxic effect of dopamine is through increase in the production of free radicals. There is evidence that increase in extracellular dopamine may promote or accentuate brain damage during reoxygenation by increase of level of free radicals. Both spin trapping (19,20) and electron paramagnetic resonance (21) measurements have indicated that reactive oxygen metabolites are formed in the first minutes of reperfusion after cerebral ischemia. Free radicals are probably the major cause of endothelial damage and brain edema after asphyxia. The newborn infant, particularly the preterm infant, is thought to be prone to tissue damage from oxidative stress because of reduced total antioxidant capacity (22). Our earlier studies have shown an increase in level striatal ortho-tyrosine following repetitive apnea with resuscitation of 21% of oxygen (12). The level of ortho-tyrosine in the tissue can be used as a reliable measurement of in vivo hydroxyl radical production (23). This compound is not formed by metabolism, and results only from OH radical attack on the ortho position of free and bound phenylalanine.
The increase of hydroxyl radicals in the striatum during apnea with 21% oxygen resuscitation may be directly related to the increase in extracellular dopamine. Olano et al. (24) showed that inhibition of dopamine synthesis could completely abolishes the ischemia dependent increase of striatal hydroxyl radicals in newborn piglets. This increase in extracellular dopamine and of hydroxyl radicals in striatum of newborn brain is likely to be at least partly responsible for the neuronal injury and neurological side effects of repetitive apnea with 21% resuscitation (12). The latter study showed significant increases in positive label Fluoro-Jade cells, marker of neuronal injury following repetitive apnea with resuscitation of 21% of oxygen. Fluoro Jade sensitively and reliably stains the cell bodies, dendrites, axons and axon terminals of degenerating neurons, but does not stain healthy neurons, myelin or vascular elements of neuropil (25).
Further studies are needed to determine the effects of apnea with resuscitation of 100% oxygen on level of free radicals in striatum. Several studies have suggested that central nervous system is very sensitivity to hyperoxia and this sensitivity is related to increases generation of oxygen derived free radicals (26–30). Agardh et al. (31) reported that the oxygen tensions during postischemic recovery would determine the rates of hydrogen peroxide production. However, the authors postulated that generation of free radicals during reoxygenation is dependent on the extent and period of ischemia but not on the different oxygen tensions. In our present study, the decrease in extracellular dopamine (major source of hydroxyl radicals production in striatum) and lower volume of severe hypoxic striatal tissue observed after resuscitation with 100% oxygen suggests the striatal injury will be decreased. The effect of apnea with resuscitation of 100% oxygen on level of free radicals and neuronal injury needs to be determined in other regions of brain, where mostly other pathways of free radical production can be activated by high oxygen concentrations. Consideration also should be given to whether an intermediate oxygen pressure, such as 40%, might not give a better outcome in recovery of brain from repetitive apneic episodes.
CONCLUSION
In a newborn piglet model, repetitive apnea caused progressive increase in fraction of hypoxic tissue in the brain and cumulative increase in the extracellular dopamine in the striatum. When repetitive apnea with resuscitation of 100% oxygen were compared to those using 21% oxygen, the former suppress formation of local regions of tissue hypoxia and the decreased extracellular dopamine, possible marker of striatal injury.
ACKNOWLEDGMENTS
Supported by NIH Grants HD041484, HL-58669 and NS-31465
REFERENCES
- 1.Jonesand RA, Lukeman D. Apnea of immaturity. 2. Mortality and handicap. Arch. Dis. Child. 1982;57:766–768. doi: 10.1136/adc.57.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levitt GA, Mushin A, Bellman S, Harvey DR. Outcome of preterm infants who suffered neonatal apneic attack. Early Human Dev. 1988;16:235–243. doi: 10.1016/0378-3782(88)90104-1. [DOI] [PubMed] [Google Scholar]
- 3.Wilson DF, Rumsey W, Green TJ, Vanderkooi JM. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J. Biol. Chem. 1988;263:2712–2718. [PubMed] [Google Scholar]
- 4.Vinogradov SA, Fernandez-Seara MA, Dugan BW, Wilson DF. Frequency domain instrument for measuring phosphorescence lifetime distributions in heterogeneous samples. Rev. Sci. Instrum. 2001;72:3396–3406. [Google Scholar]
- 5.Vinogradov SA, Fernandez-Seara MA, Biruski D, Wilson DF. A method for measuring oxygen distributions in tissue using frequency domain phosphorometry. Comp. Biochem. Physiol. 2002;32:147–152. doi: 10.1016/s1095-6433(01)00541-4. [DOI] [PubMed] [Google Scholar]
- 6.Pastuszko A, Lajevardi SN, Chen J, Tammela O, Wilson DF, Delivoria-Papadopoulos M. Effects of graded levels of tissue oxygen pressure on dopamine metabolism in striatum of newborn piglets. J. Neurochem. 1993;60:161–166. doi: 10.1111/j.1471-4159.1993.tb05834.x. [DOI] [PubMed] [Google Scholar]
- 7.Schears G, Shen J, Creed J, Zaitseva T, Wilson DF, Pastuszko A. Brain oxygenation during cardiopulmonary bypass and circulatory arrest. Adv. Exptl. Med. Biol. 2003;510:325–330. doi: 10.1007/978-1-4615-0205-0_53. [DOI] [PubMed] [Google Scholar]
- 8.Dunphy I, Vinogradov SA, Wilson DF. Oxyphor R2 and G2: Phosphors for measuring oxygen by oxygen dependent quenching of phosphorescence. Anal. Biochem. 2002;310:191–198. doi: 10.1016/s0003-2697(02)00384-6. [DOI] [PubMed] [Google Scholar]
- 9.Pastuszko A. Metabolic responses of the dopaminergic system during hypoxia in newborn brain. Biochem. Med. Metab. Biol. 1994;51:1–15. doi: 10.1006/bmmb.1994.1001. [DOI] [PubMed] [Google Scholar]
- 10.Huang Ch-Ch, Lajevardi NS, Tammela O, Pastuszko A, Delivoria-Papadopoulos M, Wilson DF. Relationship of extracellular dopamine in striatum of newborn piglets to cortical oxygen pressure. Neurochem. Res. 1994;19:649–655. doi: 10.1007/BF00967702. [DOI] [PubMed] [Google Scholar]
- 11.Yonetani M, Huang Ch-Ch, Lajevardi NS, Pastuszko A, Delivoria-Papadopoulos M, Wilson DF. Effect of hemorrhagic hypotension on extracellular level of dopamine, cortical oxygen pressure and blood flow in brain of newborn piglets. Neurosci. Lett. 1994;180:247–252. doi: 10.1016/0304-3940(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 12.Schears G, Creed J, Antoni D, Zaitseva T, Greeley W, Wilson DF, Pastuszko A. Brain injury following repetitive apnea in newborn piglets. Adv. Exptl. Med. Biol. 2005 doi: 10.1007/0-387-29540-2_51. in press. [DOI] [PubMed] [Google Scholar]
- 13.Kreisman NR, Sick TJ, Rosenthal M. Importance of vascular responses in determining cortical oxygenation during recurrent paroxysmal events of varying duration and frequency of repetition. J. Cereb. Blood Flow Metab. 1983;3:330–338. doi: 10.1038/jcbfm.1983.48. [DOI] [PubMed] [Google Scholar]
- 14.Tomida S, Nowak TS, Vass K, Lohr JM, Klatzo I. Experimental model of repetitive ischemic attacks in the gerbil. J. Cereb. Blood Flow Metab. 1987;7:773–782. doi: 10.1038/jcbfm.1987.133. [DOI] [PubMed] [Google Scholar]
- 15.Mallard EC, Williams CE, Gunn AJ, Gunning MI, Gluckman PD. Frequent episodes of brief ischemia sensitize the fetal sheep brain to neuronal loss and induce striatal injury. Ped. Res. 1993;33:61–65. doi: 10.1203/00006450-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Schears G, Creed J, Zaitseva T, Schultz S, Wilson DF, Pastuszko A. Cerebral oxygenation during repetitive apnea in newborn piglets. Adv. Exptl. Med. Biol. 2005 doi: 10.1007/0-387-26206-7_1. in press. [DOI] [PubMed] [Google Scholar]
- 17.Marie C, Mossiat C, Beley A, Bralet J. Alpha-methyl-para-tyrosine pretreatment protects from striatal neuronal death induced by four-vessel occlusion in the rat. Neurochem. Res. 1992;17:961–965. doi: 10.1007/BF00966821. [DOI] [PubMed] [Google Scholar]
- 18.Weinberger J, Nieves-Rosa J, Cohen G. Nerve terminal damage in cerebral ischemia: protective effect of alpha-methyl-para-tyrosine. Stroke. 1985;16:864–870. doi: 10.1161/01.str.16.5.864. [DOI] [PubMed] [Google Scholar]
- 19.Zini I, Tomasi A, Grimaldi R, Vannini V, Agnati LF. Detection of free radicals during brain ischemia and reperfusion by spin trapping and microdialysis. Neurosci. Lett. 1992;138:279–282. doi: 10.1016/0304-3940(92)90933-x. [DOI] [PubMed] [Google Scholar]
- 20.Phillis JW, Sen S. Oxypurinol attenuates hydroxyl radicals production during ischemia/reperfusion injury of the rat cerebral cortex: an ESR study. Brain Res. 1993;628:309–312. doi: 10.1016/0006-8993(93)90970-x. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa K, Yoshioka H, Sawada T, Nishikawa H. Direct measurement of free radicals in the neonatal mouse brain subjected to hypoxia: an electron spin resonance spectroscopic study. Brain Res. 1993;607:161–166. doi: 10.1016/0006-8993(93)91502-j. [DOI] [PubMed] [Google Scholar]
- 22.Taylor DL, Edwards D, Mehmet H. Oxidative metabolism, apoptosis and perinatal brain injury. Brain Pathol. 1999;9:93–117. doi: 10.1111/j.1750-3639.1999.tb00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karam LR, Bergtold DS, Simic MG. Biomarkers of OH radical damage in vivo, (review) Free Radic. Res. Comm. 1991;12:11–16. doi: 10.3109/10715769109145762. [DOI] [PubMed] [Google Scholar]
- 24.Olano M, Song D, Murphy S, Wilson DF, Pastuszko A. Relationships of dopamine, cortical oxygen pressure, and hydroxyl radicals in brain of newborn piglets during hypoxia and posthypoxic recovery. J. Neurochem. 1995;65:1205–1212. doi: 10.1046/j.1471-4159.1995.65031205.x. [DOI] [PubMed] [Google Scholar]
- 25.Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 26.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab. Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- 27.Fridovich I. The biology of oxygen radicals. Science. 1978;209:875–877. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 28.Halliwell B, Gutteridge JMC. Oxygen radicals and the nervous system. Trends Neurosci. 1985;8:22–26. [Google Scholar]
- 29.Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 30.Yusa T, Beckman JS, Crapo JD, Freeman BA. Hyperoxia increases H2O2 production by brain in vivo. J. Appl. Physiol. 1987;63:353–358. doi: 10.1152/jappl.1987.63.1.353. [DOI] [PubMed] [Google Scholar]
- 31.Agardh CD, Zhang H, Smith ML, Siesjo BK. Free radical production and ischemic brain damage: influance of postischemic oxygen tension. Int. J. Dev. Neurosci. 1991;9:127–138. doi: 10.1016/0736-5748(91)90003-5. [DOI] [PubMed] [Google Scholar]