Abstract
Background
Each winter respiratory viruses account for a significant proportion of serious respiratory illness, including hospitalization, in older adults and those with underlying medical conditions. We describe the incidence and clinical impact of human Metapneumovirus (hMPV), a newly identified virus, in adults.
Methods
hMPV infection was identified in three prospectively enrolled adult cohorts (young persons age 19-40, healthy adults ≥ 65 years, and high-risk adults) and a hospitalized cohort over 4 consecutive winters. The incidence and clinical impact was compared to influenza A and Respiratory Syncytial Virus infection in the same groups.
Results
Using reverse transcriptase-polymerase chain reaction (RT-PCR) and serology hMPV infection was identified in 2.2-10.5% of the three prospectively followed outpatient cohorts annually. Asymptomatic infection was common, accounting for at least 40% of infections in each of the cohorts. Symptoms, when they did occur, were typical of an upper respiratory illness although a few high-risk persons required hospitalization. Among 1386 hospitalized subjects, hMPV was identified in 8.5%, ranging from 4.3% to 13.2% depending upon the year. Dual viral infection was identified in 22.9%. Wheezing was frequent (80%) and more common than with influenza. Twelve percent required intensive care unit admission and 11% ventilatory support, rates similar to influenza and RSV infection.
Conclusion
hMPV is a common infection in adults of all ages, and although often asymptomatic, can result in serious infection requiring hospitalization. Like influenza A and RSV, hMPV is also a major contributor to the burden of winter-time respiratory illnesses in older adults.
Introduction
Viral respiratory tract infections are common among adults at all ages and, although they generally represent reinfection with common childhood viruses, may cause severe disease among the elderly and persons with underlying cardiopulmonary disease. 1 Influenza A and Respiratory Syncytial Virus (RSV) account for a substantial proportion of these illnesses and their impact in adults is relatively well described. 2-3 Other agents, such as parainfluenza viruses (PIV), coronaviruses, rhinoviruses and adenovirus, also contribute to a lesser extent to the burden of respiratory illnesses in these populations. 4,5 In addition, human Metapneumovirus (hMPV), a recently identified cause of respiratory illness in children, has also been linked to respiratory illness in adult, although its overall clinical significance has yet to be fully elucidated.6
Human Metapneumovirus was first identified in 2001 in the Netherlands from archived respiratory cultures collected from infants and young children in whom other pathogens could not be isolated. 7 It is an enveloped RNA virus classified in the Paramyxovirus family (pneumoviridae subfamily) and closely related to RSV and PIV. Two major strains, designated A and B each with two subtypes, have been identified by antigenic and genetic analysis.8,9 Since its discovery, infection has been widely reported each winter in young infants with an illness similar to RSV and characterized by wheezing and bronchiolitis. 10,11,12 However, as with many pediatric respiratory viral pathogens, hMPV infection induces incomplete immunity and reinfections occur later in life at all ages. 13,14 Although nursing home outbreaks and severe disease in hospitalized older persons have been reported, the complete epidemiology and full clinical spectrum of hMPV disease in adult populations has not yet been established.
In this report we describe the incidence and clinical impact of hMPV infection during 4 consecutive winters in young and older adults in both inpatient and outpatient settings. Infection with hMPV was identified in healthy young and elderly persons, in frail high-risk adults, and among persons hospitalized with acute respiratory symptoms who were prospectively evaluated for respiratory tract infections.
Methods
Study Design
Infections were identified by analysis of serum and respiratory secretion samples collected from volunteers participating in a study of RSV and influenza infections as previously described in detail. 2 The study encompassed four consecutive winters from 1999 through 2003 in Rochester, New York. Four groups were studied: three prospective cohorts (young adults ages 19-40, healthy adults ≥ 65 years, and high-risk adults) and a hospitalized cohort. High-risk adults were considered those persons with symptomatic lung disease, primarily chronic obstructive lung disease (COPD) or congestive heart failure (CHF). The prospective cohorts were recruited and enrolled in the late summer-early fall and were followed for a maximum of two consecutive winters. We used a rolling enrollment scheme to ensure that one-third to one-half of the subjects were new each season. Upon enrollment, demographic, medical history and functional performance were recorded, a directed respiratory exam performed, and a serum specimen collected.
Prospective volunteers notified study personnel of any respiratory symptoms (cough, sore throat, sputum production, nasal congestion, dyspnea, wheezing) or change in baseline respiratory symptoms for high-risk individuals, from November 15 through April 15 each winter. Illnesses were evaluated by study personnel, either in the study clinic or during home visits. Evaluation included a directed respiratory exam including measurement of SaO2, and collection of nasal swab and serum specimens. Four to six-weeks later a convalescent serum specimen was collected during a follow-up visit during which symptom resolution and medical care use was assessed.
The hospitalized cohort was recruited from persons with admission diagnoses consistent with an acute cardiopulmonary illness. Eligible subjects included those with admission diagnoses of community or nursing home acquired pneumonia, acute bronchitis, acute exacerbations of COPD or CHF, upper respiratory illness, viral or influenza syndrome, asthma, or respiratory failure. Patients with acute coronary syndrome, myocardial infarction or documented pulmonary embolism were excluded. Acute illness and follow-up evaluations were identical to that used for the prospective cohorts except that hospital records were also reviewed. The University of Rochester Research Subjects Review Board and the Clinical Investigation Committee of Rochester General Hospital approved this study. All subjects or their legal guardians signed informed consent prior to enrollment.
Laboratory Diagnostics
Nasopharyngeal swab specimens were stored at -80°C and later analyzed for hMPV RNA by real time reverse transcriptase polymerase chain reaction (RT-PCR). Conserved forward and reverse primers and a FAM-labeled probe were selected from hMPV N gene sequences available in Genbank. Briefly, RNA was extracted from 250 uL aliqots of sample using LStat according to the manufacturer's instructions, resuspended in water and subjected to reverse transcription using 200 nM of forward primer (5′CATCGTATATTAAAAGAGTCTCA3′). The resulting DNA was subjected to 42 cycles of PCR reaction (5 seconds at 95°C, 40 seconds at 55°C and 15 seconds at 68°C) using the forward primer and reverse primer (5′TCTGCAGCATATTTGTAATCAG3′) each at 300nM and a probe (FAM-TGCATTGATGAGGGTGTCACTGCGGTTG-BHQ). The RT-PCR has a sensitivity of 1 plaque forming unit of virus.
Serology for hMPV was performed using an enzyme immunoassay (EIA) in which purified virus was used in the solid phase. Briefly, the CAN 97-83 strain (a group A virus) was obtained from Dr. Guy Boivin (Laval University, Quebec, Canada) and grown in media containing 0.1 % porcine pancreatic trypsin and 1% albumen on LLC-MK2 monolayers as described. 15 After cytopathic effect was evident the supernatant was harvested and clarified at low speed for 10 minutes. The virus was then pelleted followed by banding on a 60/30% sucrose gradient. The purified virus was diluted in bicarbonate buffer and coated overnight on EIA microtiter plates. Serum dilutions were incubated in plates and developed using alkaline phosphatase conjugated goat anti-human IgG followed by substrate.
Laboratory diagnostic assays for additional respiratory viruses
Although the study was initially designed to evaluate RSV and influenza A infections (diagnosed by culture, RT-PCR and serology), other viruses were also identified either concurrently or retrospectively. These include influenza B (culture and serology), Parainfluenza viruses (culture only), adenovirus (culture only), and coronaviruses 229E and OC43 (serology and RT-PCR).
Definition of infection
Symptomatic hMPV infection was defined as a respiratory illness associated with a positive RT-PCR collected at the time of symptoms, or a ≥ 4-fold rise in serum hMPV-specific IgG titer between acute and convalescent serum. Asymptomatic infection was defined as a ≥ 4-fold rise in hMPV-specific IgG in serum samples bracketing a time frame in which no illnesses were reported. For example, a rise in titer from pre- to post-season serum in persons who did not report an illness during the observation period of November 15 to April 15 was considered evidence of asymptomatic infection. Incompletely evaluated illnesses were those respiratory illnesses for which study subjects were either out of town or failed to report during the winter, but did report to the study staff at the final spring interview and demonstrated a rise in hMPV antibody titer during the study period. Thus, respiratory samples for RT-PCR were not available for these illnesses, and most did not have tightly bracketed serum samples.
Statistics
Differences between groups were first analyzed by ANOVA and if significant differences were noted, comparisons between specific groups were calculated using the chi-square test of independence for dichotomous variables and t-tests for continuous variables.
Results
Populations Studied
One thousand four hundred thirty-nine persons were enrolled in the prospective cohorts (611 healthy elderly, 537 high-risk and 291 young persons) and 1386 hospitalized subjects were recruited during the four winters of study. The demographic and baseline clinical characteristics of each cohort are shown in Table 1. All except the young subjects have previously been described in detail. 2 The latter group had a mean age of 33 years, was predominantly female and non-smokers, and had daily exposure to children. These characteristics differ from the healthy elderly group who had a mean age of 75 years and rarely lived with children, and from the older high-risk group who had high rates of underlying heart and pulmonary disease. The hospitalized cohort was slightly older and frailer than the high-risk group (reflected by worse functional scores), but was similar in other respects with a high incidence of underlying cardiopulmonary conditions and smoking history.
Table 1.
Demographic and Clinical Characteristics of Cohorts
| Characteristic | Young (N=291) | Healthy elderly (N=611) | High-Risk (N=537) | Hospitalized (N=1386) |
|---|---|---|---|---|
| Age, yr (mean ± SD) | 33±5 | 75±6 | 70±11 | 75±12 |
| % Female | 84 | 58 | 46# | 55 |
| Race | ||||
| White | 82 | 97 | 94 | 85# |
| Black | 11 | 2 | 5 | 10 |
| Hispanic | 5 | <1 | <1 | 5 |
| Living situation | ||||
| Alone | 2 | 26 | 27 | 28 |
| With adults only | 11 | 72 | 67 | 65 |
| With children | 88 | 2 | 6 | 7 |
| Chronic illnesses | ||||
| Any cardiac disease | 0 | 17 | 48 | 55 |
| Lung disease | 10 | 2 | 65 | 59 |
| Diabetes | 1 | 10 | 16 | 29 |
| Smoking (current or past) | 33 | 55 | 82 | 74 |
| Influenza Vaccine | 38 | 90 | 90 | 78# |
| IADL score * | 0.03±0.4 | 0.31±1.1 | 1.2±2.2 | 3.8±4.1 |
| No. of illnesses | 314 | 525 | 519 | 1471 |
Instrumental Activities of Daily Living (IADL) are functional assessments based on a 12-point scale, with 0 representing total independence and 12 total dependence.
significantly different compared to the other groups of older subjects (P < .001)
Incidence of hMPV infection in the Prospective Cohorts
The healthy elderly and high-risk subjects reported, on average, slightly less than one illness each and the young cohort slightly greater than one illness per subject, during the two-year period that most were under observation (table 2). Overall, there were 36, 48 and 38 hMPV infections documented by RT-PCR and/or serology in the healthy elderly, high-risk and young cohorts, respectively. The percentage of subjects under surveillance that were infected with hMPV each year varied considerably, from 2.2 % to 10.5%, with the highest number and rate of infections in the second and fourth winters. It was striking that a significant proportion of infections were asymptomatic, being detected only by serology during intervals when no respiratory illness symptoms were reported. Among the healthy elderly group, 16 of the 36 infections (44%) were asymptomatic, while 19 of 49 infections (39%) in the high-risk group were asymptomatic. The percentage of asymptomatic infection was even greater in the young group (27 of 38 infections [71%]).
Table 2.
Incidence of hMPV infection by year
| Year | Healthy Elderly Subjects | High-Risk Subjects | Young Subjects | Hospitalized Subjects |
|---|---|---|---|---|
| 1999-2000 | ||||
| No. in cohort | 212 | 206 | 103 | 274 |
| No. of illnesses | 123 | 146 | 78 | 289 |
| No. hMPV infections (% of cohort) | 5 (2.3) | 6 (2.9) | 6 (5.8) | 12 (4.3) |
| No. asymptomatic hMPV infections | 1 | 2 | 3 | NA |
| 2000-2001 | ||||
| No. in cohort | 280 | 271 | 180 | 296 |
| No. of illnesses | 159 | 160 | 113 | 307 |
| No. hMPV infections (% of cohort) | 8 (2.9) | 17 (6.3) | 19 (10.5) | 39 (13.2) |
| No. asymptomatic hMPV infections | 3 | 5 | 13 | NA |
| 2001-2002 | ||||
| No. in cohort | 180 | 195 | 164 | 434 |
| No. of illnesses | 102 | 115 | 101 | 465 |
| No. hMPV infections (% of cohort) | 4 (2.2) | 8 (4.1) | 7 (4.2) | 30 (6.9) |
| No. asymptomatic hMPV infections | 2 | 6 | 7 | NA |
| 2002-2003 | ||||
| No. in cohort | 295 | 210 | 90 | 384 |
| No. of illnesses | 135 | 103 | 37 | 410 |
| No. hMPV infections (% of cohort) | 19 (6.4) | 18 (8.6) | 6 (6.7) | 37 (9.6) |
| No. asymptomatic hMPV infections | 10 | 6 | 4 | NA |
| All years combined | ||||
| No. in cohort | 611 | 537 | 291 | 1386 |
| No. illnesses | 519 | 524 | 329 | 1471 |
| No. hMPV infections | 36 | 49 | 38 | 118 |
| No. asymptomatic hMPV infections | 16 | 19 | 27 | NA |
Among subjects with symptomatic infection, in whom both RT-PCR and serology were available, there was evidence of co-infection with other viruses in 26% and 14% of the healthy elderly and high-risk groups, and in none of the young persons. Co-infecting viruses included influenza A (2 cases; one culture positive and one seropositive), coronaviruses 229E (5 cases; 2 RT-PCR positive and 3 seropositive) and OC43 (1 case; RT-PCR positive).
Incidence of hPMV infection in hospitalized subjects
One thousand three hundred eighty-six subjects had 1471 hospitalizations evaluated over the four winters of study. Overall, 118 hMPV infections, were identified, representing 8.5% of the cohort and 8.0% of the illnesses evaluated (table 2). The incidence of infection varied from year to year, paralleling the rates noted in the prospective groups, ranging from 4.3% to 13.2 % of illnesses each winter. Twenty-seven of the 118 hMPV infections (22.9%) in this group had evidence of dual infection with other viruses, a rate similar to that observed in the elderly and high-risk prospective cohorts. The most frequent co-infecting viruses were RSV (13 cases), coronavirus 229E (6 cases) and influenza A (4 cases).
Temporal distribution of hMPV infections
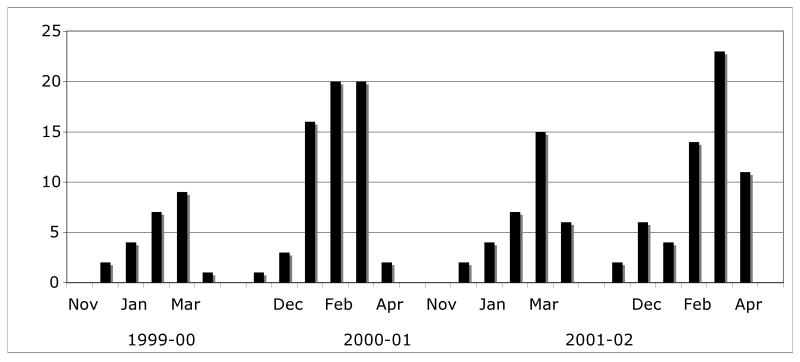
Human Metapneumovirus infections were detected during each of the 4 winter seasons of the study (figure 1). The number of symptomatic illnesses attributable to hMPV was 23, 62, 34, and 60 indicating variable activity from year to year. Infections were detected during most months studied, although heaviest activity was in late winter-early spring.
Figure 1.
Epidemic pattern of symptomatic hMPV infections in combined prospective and hospitalized cohorts during four consecutive winters.
Diagnostic virology
Of the total of 241 hMPV infections identified, 179 were considered symptomatic (51% of the prospective and all of the hospitalized infections), of whom 122 (67%) had both RT-PCR and tightly bracketed serology available. Of these, 46 (38%) were RT-PCR positive/seropositive, 14 (11%) were RT-PCR positive/seronegative, and 63 (52%) were RT-PCR negative/seropositive. Assuming serology provides the most sensitive assay for hMPV diagnosis, RT-PCR had a sensitivity of 42% (46/109). Conversely, using RT-PCR as the standard for diagnosis, serology was 78% sensitive (46/59).
Clinical characteristics of hMPV infection in prospective cohorts
In order to characterize the clinical syndrome associated with hMPV infection in each of the three prospective groups, only symptomatic fully evaluated illnesses not associated with other viruses were analyzed (Table 3). The symptoms were typical of upper respiratory tract virus infection, with the majority of subjects complaining of nasal congestion and cough, and rhinorrhea was present in approximately 75%. The younger group had significantly greater complaints of hoarseness but was less dyspneic than the other groups. Roughly one-third of the healthy elderly and high-risk group also complained of wheezing, although observed wheezing on examination was less common. Although feverishness was reported in 31-55%, recorded temperatures were generally normal. The outcome of hMPV infection varied according to group (Table 4). The duration of illness ranged from a mean of 10 days in the young group to 16 days in the high-risk group, although some remained ill for as long as 34 days. Utilization of medical care services was most notable for the high-risk group in which more than half made a physician office visit, one used the emergency room, and 3 were hospitalized during the illness. Treatment primarily consisted of symptom relief, although the majority of high-risk subjects and several from the other two groups were prescribed antibiotics.
Table 3.
Clinical characteristics of symptomatic hMPV infections in subjects, exclusive of mixed viral infections.
| Healthy elderly (N=13) | High-Risk (N= 17) | Young (N=11) | Hospitalized (N=91) | |
|---|---|---|---|---|
| Symptoms (%) | ||||
| Congestion | 92 | 71 | 100 | 49* |
| Sore throat | 58 | 41 | 64 | 27 |
| Hoarse | 38 | 29 | 91* | 29 |
| Cough | 100 | 100 | 91 | 94 |
| Sputum | 62 | 65 | 45 | 74 |
| Dyspnea | 31 | 76 | 18 | 98* |
| Wheeze | 31 | 41 | 9 | 79* |
| Constitutional | 92 | 72 | 64 | 33* |
| Feverish | 31 | 35 | 55 | 53 |
| Signs (%) | ||||
| Rhinorrhea | 85 | 65 | 73 | 9* |
| Wheezing | 15 | 12 | 0 | 82* |
| Rales | 23 | 24 | 0 | 54 |
| Temp, °C; (mean ± SD) | 36.4 ± 0.9 | 36.8 ± 1.1 | 36.7 ± 0.8 | 37.8 ± 1.0* |
| SaO2 on room air (mean ± SD) | 96.2 ± 2.9 | 95.4 ± 2.3 | 97.8 ± 1.2 | 88.4 ± 9.6* |
P< .01 compared to other groups
Table 4.
Outcomes in hMPV infected subjects in prospective cohorts
| Healthy elderly (N=13) | High-Risk (N= 17) | Young (N=11) | |
|---|---|---|---|
| Outcome of illness | |||
| Days of illness; mean (range) | 12 (5-30) | 16 (5-34) | 10 (3-21) |
| Days house bound (mean ± SD) | 1.8 ± 3.1 | 3.9 ± 6.6 | 0.6 ± 1.1 |
| Telephone call to MD (%) | 23 | 53 | 36 |
| Office visit (%) | 38 | 67* | 9 |
| Emergency room visit (%) | 0 | 7 | 0 |
| Hospitalizations (%) | 0 | 7 | 0 |
| Medications (%) | |||
| Antipyretics | 69 | 27 | 73 |
| Cough suppressants | 69 | 60 | 36 |
| Decongestants | 38 | 0# | 55 |
| Bronchodilators | 0 | 35# | 0 |
| Systemic glucocorticosteroids | 0 | 35# | 0 |
| Antibiotics | 23 | 82# | 18 |
P < .05 compared to young group
P < .05 compared to other two groups
Clinical characteristics and outcome of hMPV infection in hospitalized subjects
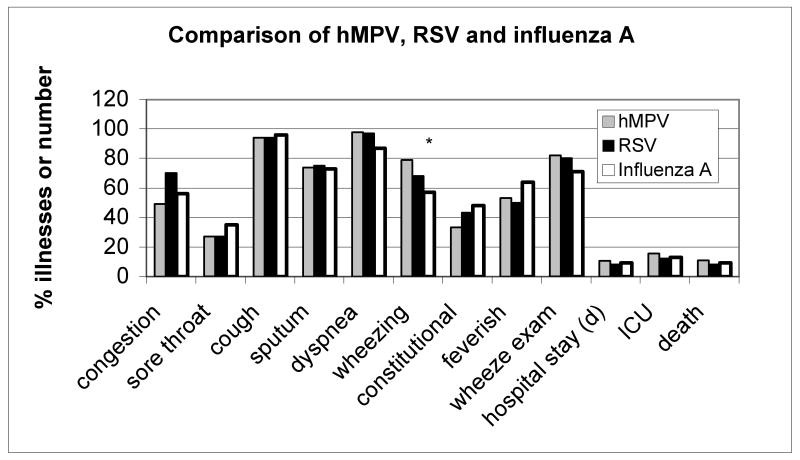
The clinical characteristics of the 91 hospitalized subjects with hMPV infections (excluding those with dual virus infection) are shown in Table 3 and Figure 2. Upper respiratory tract symptoms such as nasal congestion were present in about half of the patients although rhinorrhea was rarely observed on examination. Cough was nearly universal, as in the prospective cohorts, and the majority complained of shortness of breath on admission consistent with an average room air SaO2 of 87%. Wheezing was frequent; elicited on history in 80% and confirmed on chest examination in an equal number. Half of the subjects complained of feverishness although the mean temperature was only 37.8°C. The average duration of symptoms prior to hospitalization was 5 days. The most frequent admission diagnoses were acute bronchitis or exacerbation of COPD (38%), pneumonia (25%) and congestive heart failure (15%). Admission chest radiographs were completely normal in 37% and showed an infiltrate in 27%. Sputum was obtained in 43% of admitted persons, but grew a pathogen in only one. Blood cultures were obtained in a similar proportion of subjects, and grew S. pneumoniae in one subject. Systemic glucocorticosteroids were administered in 71%, bronchodilators in 86% and antibiotics in 93% of cases. Twelve subjects (13%) required ICU care and 11 (11%) required ventilatory support. The mean length of hospitalization was 9 ± 7 days (range 2-42 days) and six (6.6 %) subjects died during hospitalization.
Figure 2.
Comparison of clinical presentation for hMPV (n=91), RSV (n=109) and influenza A (n=138) in hospitalized patients, exclusive of mixed viral infections. RSV and influenza data from reference 2. *, P=.006 for hMPV compared to influenza A; †, P=.06 for hMPV compared to influenza A
Discussion
Despite its discovery only six years ago, a large body of information has already accumulated about hMPV. Published epidemiologic data indicate that it accounts for 5-15% of respiratory disease among hospitalized infants and causes a clinical syndrome very similar to RSV. 10-12,16 Like RSV, hMPV induces incomplete immunity and reinfection later in life is well documented among adults of all ages. 14 Infection has been associated with febrile respiratory illnesses in young and older adults, asthma and COPD exacerbations, and associated with fatal diffuse pneumonia in immunocompromised patients. 13, 19, 20 Although these reports provide information on the clinical spectrum of disease in adults, none present a comprehensive picture of annual attack rates or the full burden of hMPV disease in community dwelling adults over an extended time frame. As most published studies utilized RT-PCR and/or culture for diagnosis, the prevalence of asymptomatic or minimally symptomatic infection has not been determined. Thus, we took advantage of a recently completed prospective study of acute respiratory illness in several large adult cohorts, including ∼1400 hospitalized persons, over four consecutive winters, to assess the incidence and clinical impact of hMPV infection in this population.
We found that the proportion of the combined prospective cohorts with evidence of hMPV infection varied each winter, ranging from 3.0-3.3% in years one and three to 6.0-7.1% in years two and four. This variable pattern of virus activity is consistent with the small number of published studies that report hMPV infections over more than a single year. 11, 21-24 Notably, the incidence of hMPV infection was similar to the 5.5% annual average infection rate for RSV and greater than that of influenza A (2.4%) in these cohorts during the same time frame. 2 The low infection rate for influenza may reflect the high uptake of influenza vaccination. This differs from estimates in infants, in whom the relative activity of hMPV is generally 2-3 fold less than RSV. 12, 25 This apparent difference in adults may be due to the relatively high frequency of serologically diagnosed asymptomatic or unreported illnesses found in the outpatient cohorts. Although most evident in the young healthy adult, it also was relatively common even among frail elderly subjects with underlying cardiopulmonary disease. This is distinctly different from RSV or influenza A infection in which asymptomatic infection is relatively uncommon (∼10% or less). 2 In contrast to the high frequency of asymptomatic rises in serum antibody to hMPV adults, we had previously found that randomly selected asymptomatic persons do not have hMPV RNA detectable in their respiratory secretions. 26 Nevertheless, it appears that mild infection characterized by a serological response is relatively common. Thus, determining causality with an acute illness solely based on antibody response may be difficult. It is notable that the only description of asymptomatic infection in adults, detected by RT-PCR or culture, is from a survey study in severely immunocompromised bone marrow transplant recipients. 27
Among the symptomatic outpatients, typical upper and lower respiratory tract signs and symptoms characterized illness and were similar to illness caused by the other winter respiratory viruses. Given the high asymptomatic infection rate, one might expect minor symptoms if they occurred. However, when symptoms occurred, illness was not trivial as 38% and 67% of the healthy elderly and high-risk group visited their doctor, and even one-third of the young group called their physician's office. Symptom duration, on average, was about 2 weeks and symptomatic treatment with antipyretics and cough suppressants frequent. Across all cohorts, antibiotics use was common, especially among the high-risk patients.
Perhaps the most significant of our findings is the frequency that hMPV infection was associated with hospitalization for acute respiratory symptoms in elderly adults. During the four-year period, hMPV infection was identified in 118 of 1471 (7.3%) illnesses, 56% of which were RT-PCR positive. In comparison, we had previously reported the incidence of influenza A and RSV in this group at 10.5% and 9.6%, respectively. 2 Presenting signs and symptoms were also similar to these other viruses, although like RSV, wheezing was more common in hMPV infection than in influenza A infection (figure 2). This latter finding is consistent with the similarity of RSV and hMPV in infants in which wheezing is characteristic. The average length of hospitalization for hMPV infected adults was 9 days, with 13% requiring ICU care, and the mortality was slightly less than influenza A and RSV. It is important to recognize the relatively high frequency of hMPV infection, which similar to RSV, can clinically be mistaken for influenza during winter months when documented influenza circulates.
Of the 118 hMPV infections, co-infection with another virus was noted in 27 (23%). Due to the high rate of asymptomatic infection in the outpatient cohorts, it is possible that some subjects whose diagnosis was made by serology only may have been hospitalized for reasons other than hMPV infection. Interestingly a high rate of dual virus infection has also been reported in infants with hMPV diagnosed by RT-PCR. 21, 28 We did not note more severe disease to be associated with the dual infections, as reported by some investigators in infants with RSV and hMPV infection.28, 29
In conclusion, as with other respiratory viruses common in childhood, we find that hMPV is a relatively frequent infection in adults of all ages. The spectrum of disease is wide, ranging from asymptomatic to severe respiratory failure. Overall hMPV has a substantial impact, although less than influenza A and RSV infection, especially in the frail older person with underlying heart or lung disease. Collectively these three viruses were associated with nearly 30% of hospitalizations for acute respiratory illness during the winter. Development of a vaccine for hMPV for use in high-risk adults should be considered.
Acknowledgments
We thank Patricia Hennessey and Mary Criddle for enrollment and subject surveillance, and Maryanne Formica, Ben Korones and Gloria Andolina for technical support, and Christine Brower for organizing and maintaining subject records and reports.
Funding support: This work was supported by grants (AI055861 and AI045465) from the National Institutes of Allergy Immunology and Infectious Diseases.
References
- 1.Falsey AR, Walsh EE. Viral pneumonia in older adults. Clin Infect Dis. 2006;42:518–524. doi: 10.1086/499955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Dowell SF, Anderson LJ, Gary HE, Jr, et al. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174:456–462. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 4.Glezen PW, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 5.El-Sahly HM, Atmar RL, Glezen WP, Greenberg SB. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Hoogen BG. Respiratory tract infection due to human metapneumovirus among elderly patients. Clin Infect Dis. 2007;44:1159–1160. doi: 10.1086/513295. [DOI] [PubMed] [Google Scholar]
- 7.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biacchesi S, Skiadopoulos MH, Boivin G, et al. Genetic diversity between human metapneumovirus subgroups. Virology. 2003;315:1–9. doi: 10.1016/s0042-6822(03)00528-2. [DOI] [PubMed] [Google Scholar]
- 9.van den Hoogen BG, Besterbroer TM, Osterhaus AD, Fouchier RA. Analysis of the genomic sequence of a human metapneumovirus. Virology. 2002;295:119–132. doi: 10.1006/viro.2001.1355. [DOI] [PubMed] [Google Scholar]
- 10.Boivin G, Abed Y, Pelletier G, et al. Virological features and clinical manifestations associated with human metapneumovirus: A new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 11.Williams JV, Wang CK, Yang CF, et al. The role of human metapneumovirus in upper respiratory tract infections in children: A 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullins JA, Erdman DD, Weinberg GA, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boivin G, De Serres G, Hamelin ME, et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. 2007;44:1152–1158. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 14.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 15.Peret TC, Boivin G, Li Y, et al. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002;185:1660–1663. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Hoogen BG, van Doornum GJ, Fockens JC, et al. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- 17.Freymuth F, Vabret A, Legrand L, et al. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr Infect Dis J. 2003;22:92–94. doi: 10.1097/00006454-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Peiris JSM, Tang W, Chan K, et al. Children with respiratory disease associated with metapneumovirus in hong kong. Emerging Infect Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamelin ME, Cote S, Laforge J, et al. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis. 2005;41:498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- 20.Englund JA, Boeckh M, Kuypers J, et al. Brief communication: Fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144:344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Garcia ML, Calvo C, Perez-Brena P, De Cea JM, Acosta B, Casas I. Prevalence and clinical characteristics of human metapneumovirus infections in hospitalized infants in Spain. Pediatr Pulmonol. 2006;41:863–871. doi: 10.1002/ppul.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sloots TP, Mackay IM, Bialasiewicz S, et al. Human metapneumovirus, Australia, 2001-2004. Emerg Infect Dis. 2006;12:1263–1266. doi: 10.3201/eid1208.051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madhi SA, Ludewick H, Kuwanda L, van Niekerk N, Cutland C, Klugman KP. Seasonality, incidence, and repeat human metapneumovirus lower respiratory tract infections in an area with a high prevalence of human immunodeficiency virus type-1 infection. Pediatr Infect Dis J. 2007;26:693–699. doi: 10.1097/INF.0b013e3180621192. [DOI] [PubMed] [Google Scholar]
- 25.Bosis S, Esposito S, Niesters HG, Crovari P, Osterhaus AD, Principi N. Impact of human metapneumovirus in childhood: Comparison with respiratory syncytial virus and influenza viruses. J Med Virol. 2005;75:101–104. doi: 10.1002/jmv.20243. [DOI] [PubMed] [Google Scholar]
- 26.Falsey AR, Criddle MC, Walsh EE. Detection of respiratory syncytial virus and human metapneumovirus by reverse transcription polymerase chain reaction in adults with and without respiratory illness. J Clin Virol. 2006;35:46–50. doi: 10.1016/j.jcv.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Debiaggi M, Canducci F, Sampaolo M, et al. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J Infect Dis. 2006;194:474–478. doi: 10.1086/505881. [DOI] [PubMed] [Google Scholar]
- 28.Maggi F, Pifferi M, Vatteroni M, et al. Human metapneumovirus associated with respiratory tract infections in a 3-year study of nasal swabs from infants in Italy. J Clin Microbiol. 2003;41:2987–2991. doi: 10.1128/JCM.41.7.2987-2991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semple MG, Cowell A, Dove W, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]