Abstract
There have been a number of recent advances in photodetector technology, notably in photomultiplier tubes with high quantum efficiency (up to ~50%), hybrid photodetectors, and silicon-based Geiger-mode photodetectors. This paper looks at the potential benefits that these technologies can bring to nuclear medicine, notably SPECT and PET. We find that while the potential benefits to SPECT are relatively small, they can bring performance improvements in many areas for PET.
Introduction
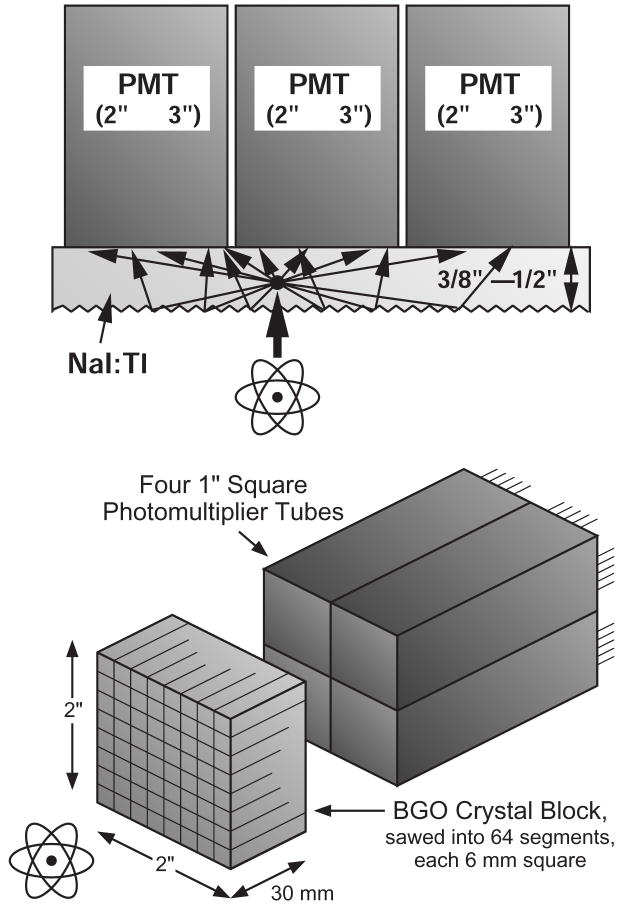
Photodetectors are an important component in the radiation sensors used in nuclear medical imaging, which includes SPECT (single photon emission computed tomography) and PET (positron emission tomography). Virtually all commercial SPECT and PET cameras use radiation imagers based on the Anger principle, where a large volume of scintillator is read out by a relatively small number of photodetectors. As shown in figure 1a, the interaction position within the scintillator volume is determined by the analog ratio of the signals in each photodetector, while the deposited energy is measured by the sum of the signals. Using this technique, spatial resolutions significantly smaller than the photodetector size are achieved. For example, a SPECT camera can locate a 140 keV gamma ray to ~4 mm fwhm accuracy using an array of 75 mm diameter photomultiplier tubes (PMTs). Similarly, a PET detector module, shown in figure 1b, can decode which of ~64 BGO crystals or ~169 LSO crystals a 511 keV gamma ray interacted in using a 2×2 array of 25 mm diameter PMTs.
Figure 1.
a) Diagram of a gamma camera used for SPECT, known as an Anger camera. Scintillation light from gamma ray interactions is detected by multiple photomultiplier tubes. The interaction position is determined by the ratio of the analog signals, and the energy by the analog sum of the signals. b) Diagram of a PET detector module. A block of scintillator crystal is sawed into 64 segments, each 6 mm × 6 mm × 30 mm deep. When a 511 keV photon interacts in any of the segments, the scintillation light is distributed across the back face of the BGO crystal, where it is simultaneously measured by four 1” square PMTs. The sum of the four output signals is used to derive both a timing signal and a signal proportional to the energy deposit. Anger logic (i.e. the ratio of the light observed in each of the four PMTs) is then used to determine the segment of interaction.
This presentation describes the advantages that novel photodetector technologies can bring to nuclear medical imaging, the disadvantages that have prevented them from dominating this field, and the challenges that must be overcome to make them commonly used.
Photodetector Advances
While there have been a number of recent advances in photodetector technology, there are three that stand out. The first is that conventional photomultiplier tubes have been developed that have substantially higher quantum efficiency (QE) than the ~25% efficiency that is typical of “standard” bialkali PMTs [1, 2]. Several commercial PMT manufacturers are capable of reliably producing PMTs with ~35% QE and have produced individual PMTs with quantum efficiencies as high as 50%. With the exception of the quantum efficiency and dark current (which is perhaps a factor of two higher in the high QE PMTs), the performance of these high QE PMTs is virtually identical to that of “standard” PMTs. At present only a few PMT models are available with high QE photocathodes, but there does not appear to be a fundamental reason that prevents every model of bialkali PMT from being made with a high QE photocathode—the main impediments are development time and cost (both for production and development).
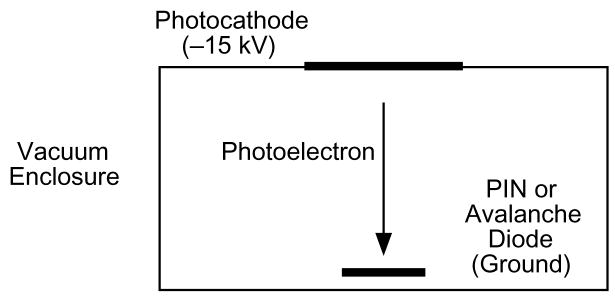
The second advance is a type of photomultiplier known as a hybrid photodetector [3–8]. These devices are similar to conventional PMTs, in that they are vacuum devices where an incident photon results in an electron being liberated from a photocathode. However, this electron is amplified not by a dynode chain, but by accelerating the electron to ~10 keV and then bombarding a silicon detector with this electron, as shown in figure 2. The silicon detector can be either a PIN diode, in which case the gain is from converting the kinetic energy of the electron into electron-hole pairs in the silicon (3.64 eV/eh pair [9]), or an avalanche diode, in which case further multiplication (~102 ) occurs in the silicon. Many of the characteristics of these devices (gain, QE, size, time response, etc.) are similar to conventional PMTs. However, there are two performance characteristics that are substantially different—the pulse height resolution for a single photoelectron is excellent (~20% fwhm) and the gain is exceptionally stable, as the gain is controlled by the stability of the accelerating voltage and the mean ionization energy in silicon. The largest drawback of these devices is that there is typically a large insensitive region around the perimeter of the device, which makes it difficult to use them to create close-packed detector arrays.
Figure 2.
Diagram of a hybird photodetector. A photon incident on the photocathode liberates a photoelectron. The photocathode is biased 10–15 kV negative with respect to a solid state detector (either a PIN diode or an avalanche diode), which accelerates the electron and causes it to collide with the solid state detector. The electron kinetic energy creates gain, and additional gain can be created in the solid state detector (in the case of the avalanche detector).
The final technology is silicon-based Geiger-mode photodetectors [1, 10–17]. This class of detector contains a number of designs and the devices are known by a number of acronyms, such as SiPM, SSPM, and MAPD. However, this paper will refer to them as GAPDs (Geiger avalanche photodetectors). With this type of devices, a small photodiode (tens of microns square) is biased several volts above breakdown voltage through a resistor. If a photon produces an electron hole pair in this “micropixel” photodiode, the junction becomes conductive and current flows through the diode until the bias voltage equals the breakdown voltage, at which point the current stops. The diode then recharges through the biasing resistor and the amount of charge released is given by the diode capacitance times the difference between the bias and breakdown voltages. Although each micropixel operates in Geiger mode, where the amount of charge produced is independent of the number of photons that impinges on it, a quasi-linear device is obtained by connecting hundreds to thousands of micropixels together in parallel to make a larger pixel. If the number of photons impinging on this larger pixel is much less than the number of micropixels, the probability that two photons interact in the same pixel is small enough that the pixel response is reasonably linear.
GAPDs have reasonably high gain and are silicon devices and so are physically very compact, can be made as monolithic arrays with small (mm) pixels, are insensitive to magnetic fields, and hold the promise of being manufactured relatively inexpensively. Their signal to noise ratio is excellent, as they can easily resolve single photoelectrons. Their main drawbacks are that they have very high capacitance (~1 nF/mm2), are prone to saturation at fairly low signal levels (each micropixel has a recovery time of tens of nanoseconds after an avalanche and there only ~102–103 micropixels per pixel), and have a dead area around the perimeter of each micropixel (to limit crosstalk due to infrared photons generated by the avalanche) that limits the theoretical maximum quantum efficiency to ~40% (as opposed to nearly 100% for avalanche photodiodes). In practice, the quantum efficiency that is achieved is significantly lower than this theoretical maximum, and is often below 10%.
Nuclear Medical Imaging Needs
Before we can assess how new photodetector technology can improve nuclear medical imaging, we must identify the improvements that are desired for SPECT and PET and determine whether the limitations that block these improvement are caused by the photodetectors. In general, there are five main physical performance parameters for both PET and SPECT (spatial resolution, energy resolution, efficiency, timing resolution, and dead time) plus two “commercial” considerations (cost and stability).
SPECT
For SPECT, the physical performance parameters are all limited by either the collimator or the scintillator, and so new photodetector technologies can only improve the commercial aspects. Photomultiplier tubes with high quantum efficiency can potentially reduce the cost of SPECT cameras. Although the spatial resolution is determined by the collimator, the spatial resolution of the camera is the convolution of the collimator resolution and the “intrinsic resolution,” which is the resolution of the Anger camera. However, in SPECT cameras the intrinsic resolution is typically 4 mm fwhm or less, which is small compared to the resolution of the collimator (typically >10 mm) and so contributes very little to the camera resolution. The intrinsic resolution is proportional to the diameter of the photomultiplier and inversely proportional to the square root of the number of photoelectrons detected. Thus, increasing the quantum efficiency from 25% to 50% implies that the PMTs can be 1.4 times larger in diameter and still give the same intrinsic resolution. Thus, an Anger camera could be constructed with a small number of larger diameter, high quantum efficiency PMTs without degrading the spatial resolution. This would reduce the cost provided that the cost per unit area of the high quantum efficiency PMTs was lower than conventional PMTs.
Gain stability is highly desired for Anger cameras, as the event positioning depends on the analog ratio of the PMT outputs and so changes in PMT gain will affect the positioning accuracy. Conventional PMTs are notorious for gain drift, and so frequent (daily) calibration of PMT gain is required maintain the accuracy of the position (and energy) measurement. Hybrid photomultipliers, with their exceptional gain stability, could eliminate frequent calibrations and allow the gain to be calibrated once at the factory. However, they must first significantly reduce the relatively large non-photosensitive area around the perimeter, and as cost is a strong driver in medical imaging instrumentation, they are unlikely to be used unless their cost per unit area is similar to that of conventional PMTs.
PET
Like SPECT, virtually all PET detector modules use Anger logic, and so novel photodetectors can bring PET the same potential benefits that they can give SPECT. High quantum efficiency PMTs can reduce the cost, while hybrid photodetectors could improve the stability. However, dead time is considerably more important for PET than SPECT, which will reduce the attractiveness of using larger diameter, high quantum efficiency PMTs to reduce cost. Unlike SPECT, there are several areas where new photodetector technology can improve the physical performance parameters in PET cameras. In fact, the only physical performance parameter that cannot be improved with new photodetector technology is the energy resolution, as this is limited by the scintillator.
Improving the coincidence timing resolution has become important for PET in recent years, as time-of-flight PET is experiencing a rebirth [18–20]. By accurately measuring the difference in arrival time, the position of the positron annihilation can be confined to a region along the line connecting the two detectors. The size of this region is generally 5–10 cm in length, which is too large to improve the spatial resolution. However, if this length is smaller than the object being imaged, this extra information can be used by the reconstruction algorithm to reduce the statistical noise, with the noise variance reduction being proportional to the factor by which the timing resolution is improved. As timing resolution is inversely proportional to the square root of the number of photoelectrons detected, substituting PMTs with 50% quantum efficiency for conventional 25% QE PMTs will improve the timing resolution by a factor of 1.4, which should lead to a reduction in the statistical noise variance by a factor of 1.4. GAPDs have single photoelectron timing jitter that is comparable to that of a high-performance PMT, so they can be used in TOF PET cameras and could improve their performance provided that they achieve their theoretical maximum quantum efficiency (~40%).
Improved spatial resolution is also possible, and is especially important for small animal PET cameras, which are usually used to image mice and so have spatial resolutions that are near 1 mm fwhm. The small pixel size of GAPDs (millimeter dimensions as opposed for centimeter dimensions for PMTs) can be used to improve the spatial resolution. Smaller photodetector pixel sizes enable smaller scintillator crystals to be decoded, and the crystal size is one of the main factors that influences spatial resolution. However, for whole body PET cameras the limiting factor is the fact that the annihilation photons are not perfectly collinear, and so smaller scintillator crystal size will not improve the spatial resolution of whole body cameras.
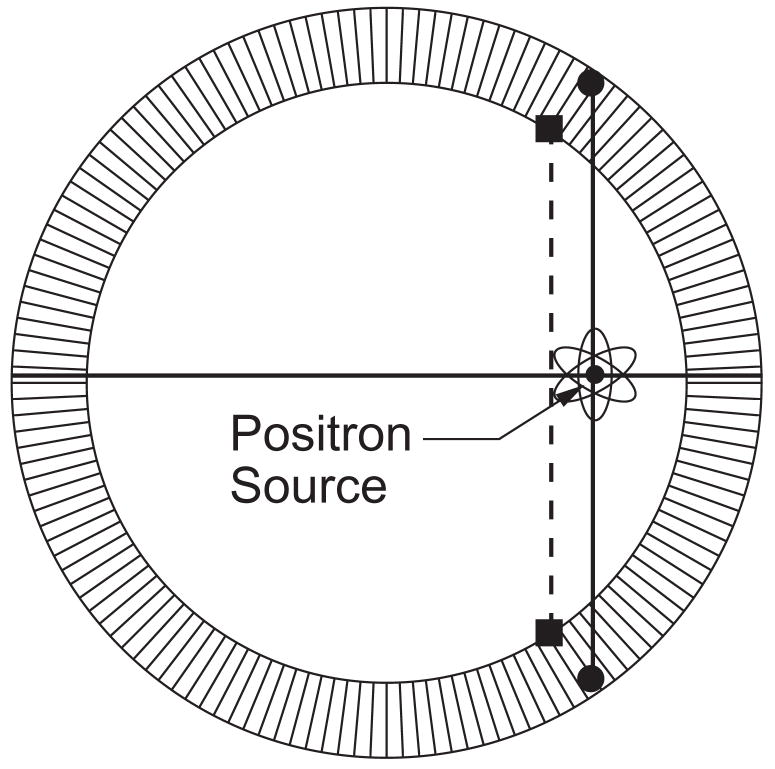
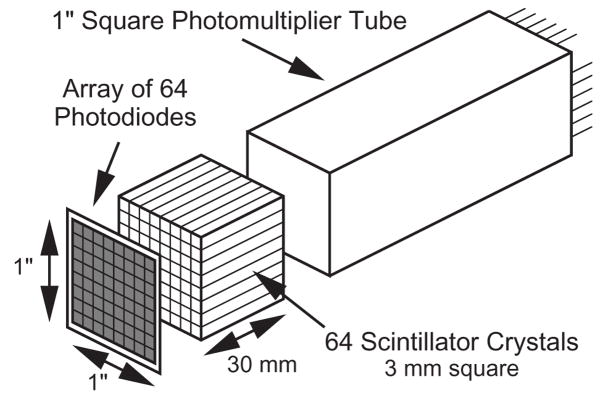
The spatial resolution in both small animal and whole body PET cameras is also degraded by the fact that the 511 keV gamma rays usually penetrate a nontrivial distance into the scintillator crystal before they interact and are detected. As shown in figure 3, This leads to an effect known as “radial elongation”, where the spatial resolution worsens with increasing radial distance from the center of the camera. This degradation can be eliminated if the detector module measures not only which crystal the interaction occurred in (which is what virtually all PET detector modules do), but also the interaction depth within the crystal. Numerous designs for such “depth-of-interaction” (DOI) detectors have been proposed and numerous prototypes evaluated, but commercially viable designs remain elusive [21]. A common concept is attaching photodetectors to either end of the scintillator crystal (such as in figure 4), with the ratio of the signals used to measure the interaction depth. While effective, this type of design requires that photodetectors be placed between the scintillator crystal and the patient, and so must be very compact mechanically (and low mass) in addition to meeting the rest of the performance requirements. The combination of the small pixel size, good signal to noise ratio, and “compactness” of GAPDs make them very attractive for DOI detectors.
Figure 3.
Origin of ragial elongation. Gamma rays can penetrate a significant distance into the scintillator crystal ring before they interact (solid circles). Most PET detector modules only identify which crystal the interaction took place in, and so locate the event position at the front face of the crystals (solid squares). The positron annihilation is incorrectly assumed to lie on the line connecting the interaction positions (the dashed line), which leads to a distorted image. By measuring the depth of interaction within the scintillator crystals, the positron annihilation is correctly assumed to lie on the line connecting the solid circles, which eliminates this distortion.
Figure 4.
One design for a PET detector capable of measuring depth of interaction. When the 511 keV gamma interacts in any of the scintillator crystals, some light is detected by the common photomultiplier tube, which provides a timing signal. Some light is detected by one of the elements of the photodiode array, which determines the crystal of interaction. The sums of the two signals provides the energy, while the ratio gives the depth of interaction.
A final potential improvement for PET is for dual-modality PET/MRI imaging. Detector modules for these cameras must fit inside the MRI magnet be non-magnetic, insensitive to magnetic fields, and very compact. While avalanche photodiodes have been used in this application, there is also potential here for GAPDs.
GAPD Requirements for Success
It is clear that GAPDs show considerable potential for improving PET, but they are not presently being used, even though there are a number of GAPD manufacturers. This final section will describe, in decreasing order of importance, the improvements in GAPD performance that are desired, emphasizing the challenges that GAPDs mst be overcome before they are commonly used in commercial PET cameras.
Scale Up Area. The biggest challenges for the use of GAPDs in PET lie in the scale-up needed to cover the large area of scintillator in a PET camera. The performance of an individual, small-area GAPD already meets the requirements for PET, so if PET cameras consisted of a single pair of scintillator crystals, GAPDs would probably be used already. A clinical, whole-body PET camera consists of approximately 50,000 scintillator crystals, each with a 4 mm × 4 mm surface area that couples to the photodetector. The typical GAPD has a surface area of 1 mm2 and is individually packaged. The area of the individual GAPD pixels must increase to cover a reasonable fraction of the individual scintillator crystal area, with 3 mm × 3 mm being a reasonable minimum pixel dimension. In addition, PET detector modules have a surface area of roughly 5 cm × 5 cm. Coupling hundreds of individual 4 mm × 4 mm GAPD devices to this detector module requires a lot of assembly. Manufacturing would be greatly simplified if multiple GAPD pixels were in a single package. Whether this is a monolithc array or a hybrid is immaterial, but the area of the package should be greater than 2 cm × 2 cm.
Practical Readout Electronics. Having a separate electronics readout channel (amplifier, timing discriminator, ADC, etc.) for each individual scintillator crystal in a PET camera is impractical. The power consumption for a complete electronics channel is a typically more than 100 mW, so 50,000 individual channels would consume over 5 kW. In addition, packaging, interconnects, and physical volume would be significant issues. This is why current PET cameras use Anger logic, which allows an entire detector module consisting of ~100 scintillator crystals to be read out using only four electronics channels. Detector module designs that incorporate a larger number of individual photodetector pixels (such as multi-anode PMTs) usually couple a resistor array to the photodetector pixels and use current division to mimic Anger logic (and thus still use only four electronics channels to read out the module). This is not a reasonable approach for GAPD arrays, as the large capacitance of each GAPD pixel, coupled with the resistance of the resistor array, produces an RC filter that reduces the bandwidth of the output signal to an unacceptable level. There are some potential solutions to this problem, such as using an analog buffer circuit (essentially a voltage follower) between the GAPD pixel and the resistor array. This circuit could either be implemented in a custom ASIC or as part of the GAPD (i.e., on the same piece of silicon as the GAPD).
Stabilize Gain. The amount of charge produced by a single GAPD micro-cell is given by the difference between the bias and breakdown voltages multiplied by the micro-cell capacitance. Although the capacitance and bias voltages are quite stable, the breakdown voltage depends strongly on temperature, and so the GAPD gain typically has a temperature coefficient of a few percent per degree Celsius. Such variations would cause considerable problems in commercial PET cameras, and so stabilizing the GAPD gain is very much desired. While it may be possible to design a GAPD microcell with a reduced temperature coefficient, some form of active electronic gain control seems more likely. For example, the breakdown voltage could be monitored, and the bias voltage actively controlled to be a fixed voltage above breakdown. Another alternative would be to monitor the charge produced when a single micropixel fires and to adjust the bias voltage to keep this charge constant. In either case, the necessary circuitry could either be implemented in a custom ASIC or as part of the GAPD.
-
Reduce Cost. Cost is an important driver in commercial products, and the cost per unit area of GAPDs must become similar to that of conventional PMTs (~$15/cm2) before they gain widespread acceptance.
I believe that the four items above are necessary—that commercial PET cameras will not utilize GAPDs unless improvements are made in all of these items. I believe that the three items that follow are optional—while they are desireable, they do not affect PET performance enough to prevent the use of GAPDs in commercial PET.
Increase Photon Detection Efficiency. While increasing the photon detection efficiency from its present value of ~10% to its practical maximum of ~40% would be nice, prototype PET modules utilizing GAPDs with 10% photon detection efficiency already meet the PET performance requirements (incluing energy, timing, and spatial resolution). However, increased QE might allow detectors with significantly improved performance.
Reduce Dark Count Rate. Present dark count rates are ~1 MHz/mm2, or 16 million micro-pixels discharging per second in a 4 mm square pixel. However, a 511 keV interaction results in ~1000 micro-cells discharging in a 100 ns time window. Thus, during this 100 ns about 16 dark counts will be added to a signal of ~1000 counts, which is completely negligible. Reducing this dark count rate becomes more important when detector modules consisting of ~100 scintillator crystals are constructed, but even modest reductions in the dark count rate or the use of techniques such as thresholding or shortening the time window will again make the contribution due to dark counts negligible.
Reduce Saturation. Because the response of a micropixel is independent of the number of photons that impinge on it, GAPDs are non-linear devices whose output saturates at higher light intensities. This distorts the pulse height spectrum and artificially narrows the width of the 511 keV photopeak. The distortion can be corrected by calibration, but even this is unnecessary, as the measurement of energy is used only to reject photons that Compton scatter in the patient. PET only requires that 511 keV photons can be distinguished from scattered scattered photons (usually done by requiring that the measured energy be within an energy window placed around the photopeak), and for this task, it does not matter whether the energy spectrum is distorted or perfectly linear.
Conclusion
Although conventional PMTs are almost exclusively used as the photosensor in these detectors, novel photodetectors could potentially have great advantages in these systems. Conventional PMTs with higher quantum efficiency (35%–50%) are becoming available, and these could be used to improve the spatial resolution without changing the detector geometry. Hybrid photodetectors (HPDs), which have a conventional photocathode but replace the PMT dynode chain with silicon detectors (either low-gain PIN diodes or high-gain APDs) allow smaller pixel sizes and much improved temperature/gain stability, especially those that utilize PIN diodes. The smaller pixel size can improve the spatial resolution, but only if the electronics cost is also increased. The improved stability is extremely important, as it would eliminate the need to have frequent (daily) calibrations performed by relatively unskilled operators. However, the relative large dead area around the perimeter of HPDs causes difficulties. Avalanche photodiodes (APDs), and especially APD arrays, combine moderate gain, small pixel size, and high quantum efficiency, and are also very compact and insensitive to magnetic fields. These properties can be used for a variety of purposes, but the most attractive ones are to improve the spatial resolution, to build detectors that localize the interaction point in 3-dimensions within the scintillator crystal (which further improves the spatial resolution), and to build detectors that can be used in magnetic fields (such as are needed for PET/MRI systems). However, designers must overcome the drawbacks of larger electronics challenges and temperature dependence. Silicon photomultipliers (SiPMs) share many of the advantages and promises of APD arrays. The biggest challenges for SiPMs probably lie in the scale-up. Current SiPMs are limited to areas of only a few square millimeters, where nuclear medicine cameras require photodetector areas of 0.1–0.5 square meters. Finally, cost cannot be ignored in any nuclear medical imaging application.
Acknowledgments
This work is supported in part by the National Nuclear Security Administration, Office of Defense Nuclear Nonproliferation, Office of Nuclear Nonproliferation Research and Engineering (NA-22) of the U.S. Department of Energy under contract No. DE-AC02-05CH11231, grant number NNSA LB06-316-PD05/NN2001000, and in part under the auspices of the U.S. Department of Energy Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344 and by the Domestic Nuclear Detection Office of the Department of Homeland Security.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suyama M. Latest status of PMTs and related sensors. Proceedings of Science. 2007;PD07:018. [Google Scholar]
- 2.Kapusta M, Lavoute P, Lherbet F, Rossignol E, Moussant C, et al. In: Yu B, editor. Breakthrough in quantum efficiency of bi-alkali photocathodes PMTs; Proceedings of The IEEE Nuclear Science Symposium and Medical Imaging Conference; Honolulu, HI. 2007. p. N06-2. [Google Scholar]
- 3.Datema CP. Hybrid photodiodes in scintillation counter applications. Nucl Instr Meth. 1997 Mar;A-387:100–103. [Google Scholar]
- 4.DeSalvo R. Why people like the hybrid photodiode. Nucl Instr Meth. 1997 Mar;A-387:92–96. [Google Scholar]
- 5.D’Ambrosio C, Leutz H. Hybrid photon detectors. Nucl Instr Meth. 2003 Apr;A-501:463–498. [Google Scholar]
- 6.D’Ambrosio C, De Notaristefani F, Leutz H, Puertols D, Rosso E. X-ray detection with a scintillating YAP-window hybrid photomultiplier tube. IEEE Trans Nucl Sci. 2000 Feb;NS-47:6–12. [Google Scholar]
- 7.D’Ambrosio C, Ercoli C, Jaaskelainen S, Lecoeur G, Leutz H, et al. A HPMT based set-up to characterize scintillating crystals. Nucl Instr Meth. 1999 Sept;A-434:387–398. [Google Scholar]
- 8.Moszynski M, Klamra W, Wolski D, Czarnacki W, Kapusta M, et al. Comparative study of PP0275C hybrid photodetector and XP2020Q photomultiplier in scintillation detection. J Inst. 2006 May;1:P05001. [Google Scholar]
- 9.Scholze F, Rabus H, Ulm G. Spectral responsivity of silicon photodiodes: high-accuracy measurement and improved self-calibration in the soft x-ray spectral range. Proc SPIE. 1996;2808:534–543. [Google Scholar]
- 10.Bondarenko G, Buzhan P, Dolgoshein B, Golovin V, Guschin E, et al. Limited Geiger-mode microcell silicon photodiode: new results. Nucl Instr Meth. 2000;A-442:187–192. [Google Scholar]
- 11.Buzhan P, Dolgoshein B, Filatov L, Ilyin A, Kantzerov V, et al. Silicon photomultiplier and its possible applications. Nucl Instr Meth. 2003;A-504:48–52. [Google Scholar]
- 12.Popova E. In: Siebert A, editor. Silicon photomultiplier: application for high-granularity scintillator calorimeters; Proceedings of The IEEE 2004 Nuclear Science Symposium; Rome, Italy. 2004. pp. 102–106. [Google Scholar]
- 13.Otte AN, Barral J, Dolgoshein B, Hose J, Klemin S, et al. In: Siebert A, editor. New results from a test of silicon photomultiplier as readout for PET; Proceedings of The IEEE 2004 Nuclear Science Symposium; Rome, Italy. 2004. pp. 3738–3742. [Google Scholar]
- 14.Dolgoshein B, Balagura V, Buzhan P, Danilov M, Filatov L, et al. Status report on silicon photomultiplier development and its applications. Nucl Instr Meth. 2006;A-563:368–376. [Google Scholar]
- 15.Herbert DJ, Saveliev V, Belcari N, D’Ascenzo N, Del Guerra A, et al. First results of scintillator readout with silicon photomultiplier. IEEE Trans Nucl Sci. 2006;NS-53:389–394. [Google Scholar]
- 16.Otte AN, Dolgoshein B, Hose J, Klemin S, Lorenz E, et al. Prospects of using silicon photomultipliers for the astroparticle physics experiments EUSO and MAGIC. IEEE Trans Nucl Sci. 2006;NS-53:636–640. [Google Scholar]
- 17.Dinu N, Battiston R, Boscardin M, Collazuol G, Corsi F. Development of the first prototypes of silicon photomultiplier (SiPM) at ITC-irst. Nucl Instr Meth. 2007;A-572:422–426. [Google Scholar]
- 18.Moses WW, Derenzo SE. Prospects for time-of-flight PET using LSO scintillator. IEEE Trans Nucl Sci. 1999;NS-46:474–478. [Google Scholar]
- 19.Moses WW. Time of flight in PET revisited. IEEE Trans Nucl Sci. 2003;NS-50:1325–1330. [Google Scholar]
- 20.Moses WW. Recent and future advances in time-of-flight PET. Nucl Instr Meth. 2007;A-580:919–924. doi: 10.1016/j.nima.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewellen TK. Recent developments in PET detector technology. Phys Med Biol. 2008;53:R287–R317. doi: 10.1088/0031-9155/53/17/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]