Abstract
Background
Most HIV-infected persons in the US present to care with advanced disease and many discontinue therapy prematurely. We sought to evaluate gender and racial/ethnic disparities in life-years lost due to risk behavior, late presentation and early discontinuation of HIV care, and to compare these survival losses in HIV-infected persons with losses from high-risk behavior and HIV disease itself.
Methods
Using a state-transition model of HIV disease, we simulated cohorts of HIV-infected persons and compared them to non-infected individuals with similar demographic characteristics. We estimated non-HIV-related mortality using risk-adjusted standardized mortality ratios as well as years of life lost due to late presentation and early discontinuation of antiretroviral therapy (ART) for HIV infection. Data from the national HIV Research Network, stratified by gender and race/ethnicity, were used for estimating CD4 counts at ART initiation.
Results
In HIV-uninfected persons in the US with risk profiles similar to those with HIV, the projected life expectancy starting at age 33 was 34.58 years, compared to 42.91 years for the general US population. Those with HIV lost an additional 11.92 years if they received HIV care concordant with guidelines; late treatment initiation resulted in 2.60 additional years of life lost, while premature ART discontinuation led to 0.70 more years of life lost. Losses from late initiation and early discontinuation were greatest for Hispanics (3.90 years).
Conclusions
The high-risk profile of HIV-infected persons, HIV infection itself, as well as late initiation and early discontinuation of care, all lead to substantial decreases in life expectancy. Survival disparities from late initiation and early discontinuation are most pronounced for Hispanic HIV-infected men and women. Interventions focused on risk behaviors as well as earlier linkage and better retention in care will lead to improved survival of HIV-infected persons in the US.
Keywords: HIV/AIDS, disparities, gender, race and ethnicity, access to care
Introduction
Despite the remarkable success of antiretroviral therapy (ART) in improving the survival of patients with HIV disease, challenges of competing risk and access to care persist. Persons at risk for HIV are also at an increased mortality risk from non-HIV-related causes, including substance and alcohol abuse [1, 2]. Late initiation of care, inconsistent adherence, and premature discontinuation of therapy continue to exert a substantial impact on survival [3-9]. Gender and racial/ethnic disparities in access, adherence, and risk further compound the difficulty of addressing these problems [3-5, 7-17].
Racial and ethnic disparities in access to care have been well documented [18, 19]. It is less clear, however, how to measure the impact of these disparities on mortality or how to evaluate the improvements in survival by interventions designed to address these disparities. We sought to define and evaluate three categories of survival losses associated with HIV: first, the years of life lost due to non-HIV-related causes in persons at risk for HIV in the US; second, the years of life lost due to HIV infection itself, under conditions of guideline-concordant care; and third, the additional life-year losses due to late initiation and premature discontinuation of ART.
Methods
Overview
We used a state-transition model of HIV disease to project the life expectancy of HIV-uninfected persons with a demographic and risk profile similar to HIV-infected persons in the US and compared the life expectancy to that of HIV-infected persons under a range of scenarios regarding ART use [20-22]. Cohort characteristics were derived from the HIV Research Network, a consortium of primary care sites for HIV-infected patients in four regions of the US.
The Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model
The Cost-Effectiveness of Preventing AIDS Complications model is a widely published computer simulation model of HIV disease [20-22]. In the model, each simulated person transitioned among health states defined by CD4 count, HIV RNA, history of opportunistic infection (OI), and ART use. Monthly probabilities of CD4 count decline, OIs, and death in the absence of treatment were derived from secondary data analyses from the Multicenter AIDS Cohort Study [20, 23, 24]. ART efficacy and OI prophylaxis data were from published clinical trials and meta-analyses [25-31]. Further details of the model have been described elsewhere [20-22], and are in the Technical Appendix.
Cohort characteristics: The HIV Research Network
Gender and race/ethnicity distribution
The demographic characteristics of a cohort of HIV-infected individuals were obtained from new enrollees at seven HIV Research Network sites in the US (Table 1). There were six academic sites and one community-based site from the Eastern (2), Southern (2), Mid-Western (2), and Western (1) regions of the US. Further details on the HIV Research Network are published elsewhere [13, 32]. Respondents reported their main racial/ethnic group as White (non-Hispanic), Black (non-Hispanic), Hispanic, or Other (American Indian or Alaskan Native, Asian or Pacific Islander). The gender and racial/ethnic distribution among enrollees in the HIV Research Network sites were similar to that reported by the Center for Disease Control and Prevention (CDC) (Technical Appendix, Table A1).
Table 1. Baseline input parameters for a simulation study of the years of life lost due to suboptimal HIV care in the United States.
| Variable | Baseline input | Source | |||
|---|---|---|---|---|---|
| Age at seroconversion, mean years (SD) | 33.0 (7.5) | Multicenter AIDS Cohort Study [23] | |||
| HIV RNA, mean log10 copies/ml (SD) | 4.8 (0.6) | Staszewski, et al. [63] | |||
| CD4 count, mean cells/μl (SD) † | 534 (164) | Walensky, et al. [33] | |||
| Antiretroviral regimens | % suppressed at 48 weeks | CD4 increase at 48 weeks (cells/μl) | Source | ||
| 1. Efavirenz + tenofovir DF + emtricitabine | 84 | 190 | Gallant, et al. [64] | ||
| 2. Atazanavir/ritonavir + 2 NRTIs | 71 | 110 | Johnson, et al. [29] | ||
| 3. Regimen 3* | 66 | 121 | Johnson, et al. [29] | ||
| 4. Regimen 4* | 65 (24 weeks) | 102 (24 weeks) | Grinsztejn, et al. [65] | ||
| 5. Regimen 5* | 40 (24 weeks) | 121 | Nelson, et al. [31] Lalezari, et al. [66] |
||
| Discontinuation rates for ART | White | Black | Hispanic | ||
| Women | 5% | 10% | 14% | Anastos, et al. [3] Ahdieh-Grant, et al. [37] |
|
| Men | 5% | 5% | 5% | Li, et al. [9] | |
| Variable | Baseline input | ||||
| CD4 count at ART initiation (%) ‡ | |||||
| Subgroup | Percent of cohort (%) | ≥ 200 cells/μl | 50 – 199 cells/μl | < 50 cells/μl | |
| Overall | Total | 100.0 | 57.9 | 22.6 | 19.5 |
| Women | 25.0 | 62.9 | 20.2 | 16.9 | |
| Men | 75.0 | 56.3 | 23.4 | 20.3 | |
| Black | Total | 49.6 ¶ | 55.8 | 21.9 | 22.3 |
| Women | 16.9 | 61.3 | 20.8 | 17.9 | |
| Male | 32.6 | 53.0 | 22.4 | 24.6 | |
| Hispanic | Total | 20.6 ¶ | 54.4 | 24.8 | 20.7 |
| Women | 4.3 | 63.0 | 19.4 | 17.6 | |
| Men | 16.2 | 52.1 | 26.3 | 21.5 | |
| White | Total | 27.2 ¶ | 63.6 | 22.8 | 13.6 |
| Women | 3.5 | 70.6 | 19.0 | 10.4 | |
| Men | 23.6 | 62.7 | 23.3 | 14.1 | |
ART: antiretroviral therapy; SD: standard deviation; NRTI: nucleoside reverse transcriptase inhibitor.
Regimen selection is based on results of genotype analysis.
CD4 count at time of seroconversion
Data from HIV Research Network [13].
Data do not sum to 100%, since 3% were identified as “Other” and excluded from the analysis.
Table A1. Cohort composition stratified by gender and race/ethnicity.
| HIV Research Network (%) | CDC (%) [8] | |
|---|---|---|
| Gender | ||
| Women | 25.0 | 25.8 |
| Men | 75.0 | 74.2 |
| Race/Ethnicity* | ||
| Black (non-Hispanic) | 49.6 | 49.8 |
| Hispanic | 20.6 | 10.1 |
| White (non-Hispanic) | 27.2 | 38.4 |
CDC: Center for Disease Control and Prevention
Data do not sum to 100% since patients identified as “Other” were excluded from the analysis.
Patient characteristics at presentation to care
Patients entered the model at the time of HIV-infection with a mean age of 33.0 years and mean CD4 count of 534/μl [23, 33]. CD4 counts at treatment initiation were stratified by gender and race/ethnicity based on HIV Research Network data (Table 1). “Late” presentation to care, with a CD4 count <200/μl, occurred in 42.1% of patients. “Very late” presentation, with a CD4 count <50/μl, occurred in 19.5% of patients. Blacks and Hispanics were significantly more likely to initiate ART very late compared to Whites (22.3% and 20.7% vs. 13.6%, p<0.001, Table 1). Because similar data on CD4 count at presentation at a national level were not available from the CDC, we performed a sensitivity analysis on this parameter using data from a second study [15].
Antiretroviral therapy regimens and strategies
In the model, HIV-infected patients presenting to care and eligible for ART had the opportunity to receive five sequential ART regimens, based on current US guidelines [6, 34]. Patients remained on a fifth and final ART regimen until death, maintaining some of the benefits of ART even after virologic failure [35, 36].
Initiation
To represent the range of CD4 counts at which patients might initiate ART, we considered three initiation scenarios: 1) current US treatment guidelines – immediately for patients who presented for care with CD4 counts 200-350/μl, or when CD4 counts dropped to <350/μl if in care, or with the diagnosis of an OI even with CD4 counts ≥350/μl [6, 34]; 2) late presentation – immediately (CD4 50-199/μl); and 3) very late presentation – immediately (CD4 <50/μl).
Discontinuation
We defined premature ART discontinuation of ART as not continuing to the next available regimen after failing a current regimen. For example, a 5% discontinuation rate implies that 5% of patients discontinue therapy after the first regimen, 5% of the remaining patients discontinue after the second regimen, etc. Discontinuation data were derived from the Multicenter AIDS Cohort Study and the Women's Interagency HIV Study, ranged from 5% to 14%, and were highest in Black and Hispanic women (Table 1) [3, 9, 37].
Decreased survival with HIV
Loss of life expectancy was calculated in three sequential components: 1) loss of life-years due to non-HIV risk factors, 2) additional loss attributable to HIV disease, and 3) additional loss attributable to late initiation and/or early discontinuation of ART.
1) Risk adjustment for high-risk behavior
Persons with risk profiles similar to those with HIV infection in the US have a higher mortality rate, aside from HIV causes [1, 38, 39]. This is due to multiple risks, including substance abuse and alcohol abuse [40, 41]. To adjust for this excess mortality, we derived overall and gender/race/ethnicity-specific standardized mortality ratios (SMRs) by using published risk-group specific SMRs, weighted by the distribution of risk factors among persons living with HIV from CDC surveillance reports (Technical Appendix, Table A2) [42, 43]. High-risk behavior for the SMR derivations was defined as men who have sex with men, injection drug use, multiple sex partners, commercial sex workers, and a history of sexually transmitted infections [42]. All analyses were performed both adjusted and unadjusted for this excess mortality. Results in the manuscript are reported for the adjusted analyses. (For unadjusted analyses, see Technical Appendix.)
Table A2. Risk-adjusting standardized mortality ratios, stratified by gender and race/ethnicity.
| Race/ethnicity | Risk-adjusted standardized mortality ratio | |
|---|---|---|
| Male | Female | |
| White | 1.706 | 8.085 |
| Black | 2.778 | 6.683 |
| Hispanic | 2.565 | 7.348 |
| Overall | 2.307 | 7.059 |
2) Years of life lost due to HIV infection
We calculated years of life lost due to HIV infection itself as the difference between the life expectancy of HIV-infected persons receiving guideline-concordant HIV care in the US and the life expectancy of HIV-uninfected persons with similar demographic and risk profiles.
3) Years of life lost due to late initiation and/or early discontinuation of ART
To examine the individual and aggregate role of late treatment initiation and early discontinuation, we considered three possible scenarios: 1) late initiation of ART alone, 2) early discontinuation of ART alone, and 3) both late initiation and early discontinuation of ART. All analyses were performed for the overall cohort; then separately for Hispanics, Blacks and Whites; and then for each of the three race/ethnicity groups stratified by gender. For each analysis, we estimated both the absolute and relative reduction in life expectancy. The relative reduction was calculated as the number of years of life lost due to both late presentation and early discontinuation, divided by the life expectancy under a guideline-concordant scenario with no discontinuation. Details of years of life lost calculations are presented in the Technical Appendix.
Secondary analyses
We also performed a ‘what-if’ policy analysis to estimate the potential lifesaving impact of a program designed specifically to improve HIV care among Black and Hispanic women. We examined scenarios where women of all racial/ethnic backgrounds presented to care earlier (i.e., with the same CD4 count distribution and ART discontinuation rates as White women). We also performed a sensitivity analysis varying ART discontinuation rates, from 0% to 20%, examining the impact of CD4 count at the time of ART initiation. We also conducted an additional sensitivity analysis assuming lower rates of treatment discontinuation (3%) in subsequent ART regimens after the initial regimen. Recognizing that the efficacies observed in clinical trial settings may be different than in clinical practice, we conducted a sensitivity analysis on the ART efficacy assumption, using a range of values extending both 15% above and 15% below the published data.
Results
Years of life lost from high-risk behaviors and from HIV infection
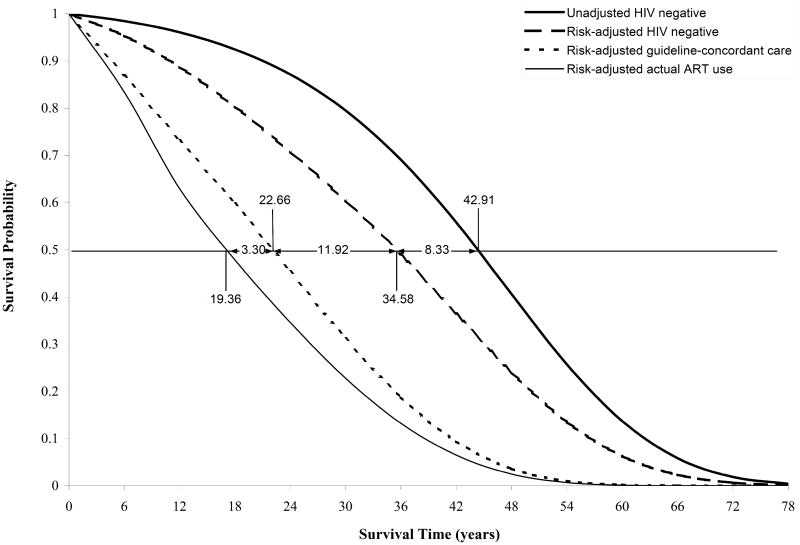
The estimated life expectancy in the general, HIV-uninfected US population from age 33.0 was estimated at 42.91 years. A simulated cohort of HIV-negative people in the US with a similar demographic and risk profile to the HIV-infected population had an estimated life expectancy from age 33.0 of 34.58 years. The difference resulted in an estimated 8.33 year reduction in life expectancy (Figure 1).
Figure 1.
Model-generated survival curves from seroconversion for four different population groups: 1) HIV-infected patients receiving “actual use” ART; 2) HIV-infected patients receiving guideline-concordant care; 3) HIV-uninfected persons with risk profiles similar to HIV-infected persons; 4) a general population of HIV-uninfected persons. Time denotes years from the time of seroconversion; mean age at seroconversion is 33.0 years [23]. See Methods. ART: antiretroviral therapy
If all HIV-infected individuals initiated ART according to current US treatment guidelines and without discontinuation, the estimated life expectancy of HIV-infected persons infected at age 33.0 would be 22.66 years, accounting for the excess non-HIV mortality. Thus, with the current standard of HIV care, estimated life lost due to HIV infection was 11.92 years (34.58 – 22.66; Figure 1).
Losses due to late initiation and early ART discontinuation
Late presentation (CD4 <200/μl) resulted in an estimated life expectancy of 18.75 years from age 33.0, representing an additional 3.90 years of life expectancy lost compared to initiating ART with CD4 ≥200/μl; life expectancy for those initiating ART very late (CD4 <50/μl) was estimated at 13.82 years, resulting in an additional 8.83 years of life lost compared to initiating ART with CD4 ≥200/μl (Table 2). We also estimated the life expectancy of HIV-infected persons stratified by the time ART was initiated as well as the number of regimens taken prior to ART discontinuation (Table 2). For example, among HIV-infected individuals with CD4 ≥200/μl who initiated treatment according to guidelines, life expectancy for those who discontinued treatment after four regimens compared to those who did not discontinue ART was reduced from 22.66 to 21.92 years.
Table 2. Risk-adjusted impact on overall cohort of premature treatment discontinuation, stratified by timing of antiretroviral therapy initiation.
| Life expectancy (years) | |||||
|---|---|---|---|---|---|
| Number of ART regimens received prior to discontinuation | |||||
| CD4 count at ART initiation | No discontinuation* | 4 | 3 | 2 | 1 |
| ≥ 200/μl | 22.66 | 21.92 | 20.29 | 18.32 | 15.79 |
| 50 – 199/μl | 18.75 | 18.20 | 16.99 | 15.57 | 13.71 |
| < 50/μl | 13.82 | 13.52 | 12.92 | 12.20 | 11.20 |
ART: antiretroviral therapy
Patients in this category received all 5 regimens.
Regimen selection is based on results of genotype analysis
The combined impact of late presentation and early treatment discontinuation
The estimated life expectancy of HIV-infected patients in the cohort with the characteristics of the HIV Research Network participants decreased by 2.60 years due to the late or very late presentation of a substantial proportion of patients (Table 3). Early discontinuation alone decreased overall life expectancy by 0.70 years.
Table 3. Risk-adjusted life expectancy and years of life lost due to suboptimal HIV care stratified by gender and race/ethnicity.
| Race/ethnicity | Gender | Life expectancy (years) | Years of life lost (vs. guideline-concordant) | |||
|---|---|---|---|---|---|---|
| Guideline-concordant treatment | Actual treatment use in the HRN | Late ART initiation | Early ART discontinuation | Late ART initiation and Early ART discontinuation | ||
| Overall | Total | 22.66 | 19.36 | 2.60 | 0.70 | 3.30 |
| Women | 19.80 | 17.14 | 1.86 | 0.80 | 2.66 | |
| Men | 23.56 | 20.09 | 2.86 | 0.60 | 3.46 | |
| Black | Total | 20.47 | 17.46 | 2.43 | 0.58 | 3.01 |
| Women | 19.11 | 16.52 | 1.85 | 0.73 | 2.59 | |
| Men | 21.16 | 17.94 | 2.75 | 0.46 | 3.22 | |
| Hispanic | Total | 24.08 | 20.18 | 3.06 | 0.85 | 3.90 |
| Women | 22.63 | 18.87 | 2.30 | 1.46 | 3.76 | |
| Men | 24.48 | 20.57 | 3.27 | 0.64 | 3.91 | |
| White | Total | 25.41 | 22.19 | 2.46 | 0.76 | 3.22 |
| Women | 21.62 | 19.56 | 1.53 | 0.53 | 2.06 | |
| Men | 25.94 | 22.54 | 2.61 | 0.78 | 3.39 | |
ART: antiretroviral therapy; HRN: HIV Research Network
The combined effects of late initiation and premature discontinuation led to life expectancy losses of 3.30 years (Table 3; Figure 1). The combined impact of late presentation and early discontinuation resulted in higher survival losses for Hispanics compared to Blacks and Whites. Hispanic men had the highest life expectancy losses (3.91 years), followed by Hispanic women (3.76 years; Table 3). Among women, racial/ethnic minorities had greater survival losses compared to their White counterparts.
Secondary analyses
The impact of decreasing disparities in presentation to and discontinuation of HIV care
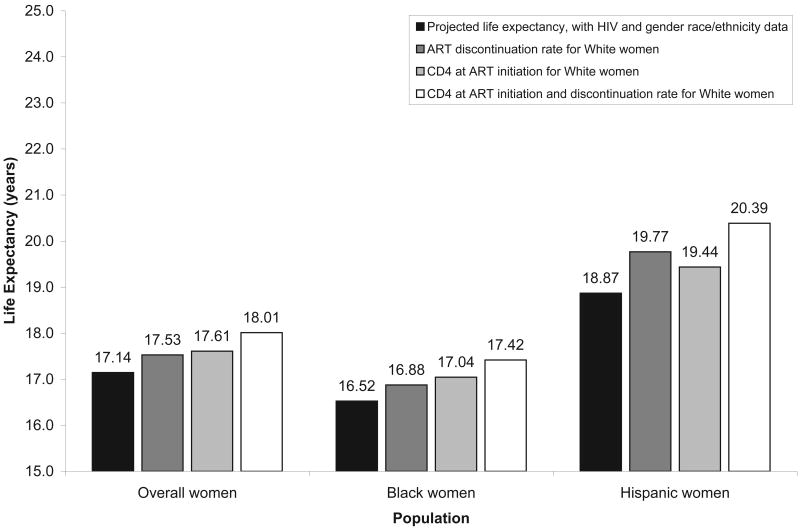
If Black and Hispanic women were to initiate and discontinue ART at rates similar to those of White women, life expectancy after risk adjustment would increase by 0.90 years for Black women (16.52 to 17.42 years) and by 1.52 years for Hispanic women (18.87 to 20.39 years). The estimated overall life expectancy for all HIV-infected women in the US would increase by 0.87 years (17.14 to 18.01 years; Figure 2).
Figure 2.
Change in potential life expectancy for women, depending on the CD4 count at the time of antiretroviral therapy initiation and rate of discontinuation associated with White women. Estimated life expectancy for White women (from age 33.0) after adjusting for excess risk due to risk behavior prevalent for HIV-infected persons is 19.56 years.
Treatment discontinuation and timing of presentation to care
We also performed sensitivity analyses to estimate the survival impact of early treatment discontinuation rates, ranging from 0% to 20%. A decrease in discontinuation rates from 10% to 5% increased life expectancy by 0.43 years in Black men; a decrease in discontinuation from 5% to 0% increased life expectancy by 0.53 years in White women. A sensitivity analysis using gender and race/ethnicity data on CD4 counts at the time of presentation as reported by Swindells et al. [15], rather than from the HIV Research Network, revealed similar trends in survival, with even greater life expectancy losses for Hispanic men and Hispanic women. Results from a sensitivity analysis showed that decreasing the rates of premature treatment discontinuation to 3% reduced life expectancy losses due to suboptimal access to care by 0.16 years. A sensitivity analysis increasing and decreasing ART efficacy by 15% resulted in an increase in life expectancy losses of 0.21 years and a decrease in life expectancy losses of 0.18 years, respectively. The relative impact of gender and race/ethnicity did not change meaningfully.
Discussion
Despite the tremendous success of HIV treatment in the US over the past 15 years, substantial avoidable losses in life expectancy persist due to non-HIV-related risk, as well as late presentation and early discontinuation of care. We estimated that the increased mortality due to substance abuse and other high-risk behaviors led to mean per-person survival losses of 8.33 years, even in the absence of HIV disease. Since SMRs for women (7.06) were greater than SMRs for men (2.31; Technical Appendix, Table A2), losses in life expectancy due to high-risk behavior were greater in women than in men across all races/ethnicities. These losses were comparable to those due to HIV infection itself and underscore the critical importance of interventions focused on reducing substance abuse and other high-risk behaviors. These findings support previous research highlighting the risk of premature death and mortality attributable to substance abuse [44]. This non-HIV premature mortality includes drug overdose, as well as homicide and suicide [1, 38, 39].
We estimated that HIV infection acquired on average at age 33.0 years, and treated according to current US guidelines with current regimens, led to 11.92 years of life lost. We found that 3.30 more years were lost per-person on average due to late presentation and early discontinuation of ART. Survival losses from late presentation and early discontinuation were greater for Hispanics than for Whites or Blacks, regardless of gender, with overall survival losses of 3.90 (21% and 30% more than Whites and Blacks). Among all HIV-infected patient subgroups, Hispanic men had the greatest losses in life expectancy, averaging 3.91 years per-person. Racial/ethnic minority women had greater survival losses compared to their White counterparts (26% and 83% more in Blacks and Hispanics). Data on delayed presentation to care for Hispanic and Black women have repeatedly shown that rates of HIV-related OIs are disproportionately high in both groups [7, 11, 13, 15, 45]. These differences remain, despite recent data suggesting a narrowing of the survival gap between White and Black HIV-infected persons in the US over the past decade [46].
The estimated life expectancy for HIV-infected individuals in the US who initiate ART very late (CD4 <50/μl) was 8.83 years lower than for patients who initiate ART according to current guidelines [6, 34]. Major survival losses were also due to inadequate retention in care. For White women, the group that presented to care earliest, the survival losses due to late presentation and early discontinuation averaged 2.06 years. These results further emphasize the gap between guideline-concordant and actual care [47].
This study underscores the importance of developing interventions focused on better linkage to and retention in care, especially for racial/ethnic minorities. The US Department of Health and Human Services has implemented several HIV prevention programs that target minority women [48]. Recent efforts to expand routine HIV testing in the US may also begin to address the problem of earlier diagnosis [49-51]. Special attention to linkage to care after HIV testing will be critical [52].
This study also demonstrates that treatment discontinuation adds substantially to survival losses from HIV. Findings from two large European cohorts report discontinuation rates ranging from 7% at 1 year to 18% at 2 years [8, 53]. Data from the US show even higher treatment discontinuation rates, ranging from 7% to 40% over 2 to 7 months [9, 54], with the highest rates among racial/ethnic minority women [3, 37, 54-56]. Since average ART initiation occurs at lower CD4 counts for men, especially among minorities, late initiation results in larger life expectancy losses for Hispanic and Black men than women. These results are consistent with the findings of Giordano et al., showing clear survival benefits for HIV-infected patients receiving consistent care, compared to irregular care, despite the observation that those who received consistent care presented with more advanced disease [57, 58].
This study has several limitations. First, patients who entered the HIV Research Network with HIV RNA levels >400 copies/ml and not on ART were assumed to be initiating therapy for the first time, since information on previous ART was unavailable. However, a sensitivity analysis using gender and race/ethnicity data on CD4 count at the time of presentation from another study [15] showed similar results, with even greater survival losses for Hispanics. Second, while we used data from only seven sites in the HIV Research Network, the demographic characteristics of persons receiving care in those sites were similar to those from CDC HIV surveillance reports [43]. Third, the high-risk definition included in our SMR calculations did not explicitly include tobacco use, but since a majority of HIV-infected individuals smoke at least one cigarette per day, this has been indirectly accounted for in the SMR calculations [59]. Fourth, we estimated the survival losses due to high-risk behavior and HIV-infection independently; however, the biological and social impact of HIV disease may amplify survival losses due to high-risk behavior.
Using currently available data on HIV care in the US, mean survival losses in HIV-infected patients were 23.55 years per-person compared to the general US population and 15.22 years compared to those without HIV infection but with a similar risk profile. Of these 15.22 years, 11.92 years were attributable to HIV infection itself; an additional 3.30 years were lost due to late presentation and early discontinuation of care. Improving access to medical and social services could address the additional risk factors for early mortality [60-62]. Survival losses for racial/ethnic minority women with HIV were higher than for Whites by as much as 83%, and these disparities were greatest for Hispanics. Future studies should focus on earlier testing, linkage to and retention in care for all HIV-infected persons, with an emphasis on women as well as racial and ethnic minorities.
Table A3. Unadjusted impact on overall cohort of premature treatment discontinuation, stratified by timing of antiretroviral therapy initiation.
| Life expectancy (years) | |||||
|---|---|---|---|---|---|
| Number of ART regimens received prior to discontinuation | |||||
| CD4 count at ART initiation |
No discontinuation* |
4 | 3 | 2 | 1 |
| ≥ 200/μl | 25.96 | 24.92 | 22.76 | 20.22 | 17.08 |
| 50 – 199/μl | 21.25 | 20.49 | 18.86 | 17.02 | 14.72 |
| < 50/μl | 15.25 | 14.86 | 14.04 | 13.07 | 8.72 |
ART: antiretroviral therapy
Patients in this category received all 5 regimens.
Table A4. Unadjusted life expectancy and years of life lost due to suboptimal HIV care stratified by gender and race/ethnicity.
| Race/ethnicity | Gender | Life expectancy (years) | Years of life lost (versus guideline-concordant) | |||
|---|---|---|---|---|---|---|
| Guideline- concordant treatment |
Actual treatment use in HRN |
Late ART initiation |
Early ART discontinuation |
Late ART initiation and Early ART discontinuation |
||
| Overall | Total | 25.96 | 21.89 | 3.15 | 0.92 | 4.07 |
| Women | 26.81 | 22.34 | 2.89 | 1.57 | 4.46 | |
| Men | 25.68 | 21.73 | 3.22 | 0.72 | 3.94 | |
| Black | Total | 25.34 | 21.12 | 3.30 | 0.91 | 4.22 |
| Women | 26.47 | 21.95 | 2.99 | 1.52 | 4.51 | |
| Men | 24.78 | 20.67 | 3.47 | 0.64 | 4.11 | |
| Hispanic | Total | 26.77 | 22.16 | 3.55 | 1.05 | 4.61 |
| Women | 27.78 | 22.39 | 3.09 | 2.30 | 5.39 | |
| Men | 26.52 | 22.11 | 3.66 | 0.75 | 4.41 | |
| White | Total | 26.64 | 23.19 | 2.63 | 0.82 | 3.45 |
| Women | 27.61 | 24.50 | 2.19 | 0.92 | 3.11 | |
| Men | 26.49 | 23.01 | 2.67 | 0.81 | 3.48 | |
ART: antiretroviral therapy; HRN: HIV Research Network
Acknowledgments
Elena Losina had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We would like to thank Anjali Saxena and Lindsey Wolf for technical assistance and other members of the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) group for their contributions, including Sue J. Goldie, MD, MPH, Paul E. Sax, MD, and George R. Seage, III, DSc, MPH.
Financial support. National Institute of Allergy and Infectious Diseases (K23 AI01794, K24 AI062476, K25 AI50436, and R37 AI42006), National Institute on Drug Abuse (K23 DA000523, K01 DA017179, and K24 DA00432), National Institute of Mental Health (MH065879), Agency for Healthcare Research and Quality (290-01-012), Doris Duke Charitable Foundation (Clinical Scientist Development Award), and Johns Hopkins University (Richard S. Ross Clinical Scientist Award).
Funding/Support: Supported in part by the National Institute of Allergy and Infectious Diseases (K23AI01794, K24AI062476, K25AI50436, R37AI42006), the National Institute on Drug Abuse (K23DA000523, K01DA017179, K24DA00432), National Institute of Mental Health (MH065879), the Agency for Healthcare Research and Quality (290-01-012), the Doris Duke Charitable Foundation, Clinical Scientist Development Award to Dr. Walensky, and the Johns Hopkins University, Richard S. Ross Clinical Scientist Award to Dr. Gebo.
Technical Appendix
Assumptions used in the analysis
The analysis included five assumptions:
Presentation to care includes the initiation of antiretroviral therapy (ART) and prophylaxis for opportunistic infections (OIs), when eligible, according to US guidelines.
All patients receiving ART also receive guideline-concordant OI prophylaxis.
The maximum duration of efficacy for each ART regimen is assumed to be 10 years.
Patients who discontinue ART resume and continue therapy if they develop an OI.
OI prophylaxis is not discontinued when ART is discontinued.
Each of the assumptions was tested in a sensitivity analysis.
The Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model: modeling the ART effect on HIV natural history
Prior to ART initiation, the natural history of HIV disease was defined by CD4 cell count decline, which in turn was a function of HIV RNA, and by CD4-specific rates of HIV morbidity and mortality [1]. ART reduced HIV RNA levels, increased CD4 counts, and conferred a decrease in OI incidence and HIV-related mortality [2]. ART regimen failure occurred when viral load increased one half-log or when CD4 count fell below a particular threshold. ART failures resulted in switching to the next ART regimen, until the last available regimen was utilized. Patients remained on the last regimen until death. ART-related drug related toxicity led to quality of life decrements and switches of individual drugs within a given regimen. Results from large numbers of these simulations were aggregated to develop stable population estimates [3-5].
Application of standardized mortality ratios (SMRs) to US Life Tables
We applied the corresponding gender- and race/ethnicity-stratified standardized mortality ratios (SMRs) to gender- and race/ethnicity-stratified US Life Tables to adjust the mortality rates in the model for those aged 33-55 years. We reduced the SMRs by half for all age groups above 55 to reflect two factors: 1) the data used to derive risk-adjusted SMRs were based on individuals ≤55 years old, and 2) as the population ages, the likelihood of engaging in risky behavior such as substance abuse decreases, although the impact of a history of high-risk behavior leads to an increase in mortality; for example, due to hepatic damage from a history of alcohol abuse or HCV [6, 7].
Algorithms for estimating the years of life lost
Years of life lost due to only late ART initiation
We subtracted the weighted average of the life expectancies under different initiation scenarios (based on CD4 cell count at ART initiation) from the life expectancy derived from the guideline-concordant scenario.
Years of life lost due to only early discontinuation
We estimated the life expectancies associated with receiving only 1, 2, 3, 4 or all 5 sequential regimens under each ART initiation scenario. We then calculated the weighted average life expectancy under each initiation scenario, assuming a specified percentage of the cohort does not continue to the next regimen (Table 1). Finally, we subtracted the weighted average life expectancy, assuming no discontinuation, from the weighted average life expectancy using the specified discontinuation rates.
Years of life lost due to both late initiation and early discontinuation
This was calculated as the difference between life expectancy under the guideline-concordant initiation scenario assuming no discontinuation, and the weighted average life expectancy estimated using actual CD4 distributions at the time of ART initiation and the reported discontinuation rates.
Literature Cited
- 1.Enger C, Graham N, Peng Y, et al. Survival from early, intermediate, and late stages of HIV infection. JAMA. 1996;275:1329–34. [PubMed] [Google Scholar]
- 2.Cole SR, Hernan MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158:687–94. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 3.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 4.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein MC, Goldie SJ, Losina E, et al. Use of genotypic resistance testing to guide HIV therapy: clinical impact and cost-effectiveness. Ann Intern Med. 2001;134:440–50. doi: 10.7326/0003-4819-134-6-200103200-00008. [DOI] [PubMed] [Google Scholar]
- 6.Diehl A. Liver disease in alcohol abusers: clinical perspective. Alcohol. 2002;27:7–11. doi: 10.1016/s0741-8329(02)00204-5. [DOI] [PubMed] [Google Scholar]
- 7.Wise M, Bialek S, Finelli L, Bell B, Sorvillo F. Changing trends in hepatitis C-related mortality in the United States, 1995-2004. Hepatology. 2008;47:1128–35. doi: 10.1002/hep.22165. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Report No.: Volume 14 2002. HIV/AIDS Surveillance Report: Cases of HIV infection and AIDS in the United States, 2002. [Google Scholar]
Footnotes
Financial Disclosures: None reported.
Potential conflicts of interest. All authors: no conflicts.
Literature Cited
- 1.Miller C, Kerr T, Strathdee A, Li K, Wood E. Factors associated with premature mortality among young injection drug users in Vancouver. Harm Reduct J. 2007;4:1. doi: 10.1186/1477-7517-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control. Surveillance and assessment of alcohol-related mortality--United States, 1980. MMWR. 1985;34:161–3. [PubMed] [Google Scholar]
- 3.Anastos K, Schneider MF, Gange SJ, et al. The association of race, sociodemographic, and behavioral characteristics with response to highly active antiretroviral therapy in women. J Acquir Immune Defic Syndr. 2005;39:537–44. [PubMed] [Google Scholar]
- 4.Anderson KH, Mitchell JM. Differential access in the receipt of antiretroviral drugs for the treatment of AIDS and its implications for survival. Arch Intern Med. 2000;160:3114–20. doi: 10.1001/archinte.160.20.3114. [DOI] [PubMed] [Google Scholar]
- 5.Wood E, Montaner JS, Bangsberg DR, et al. Expanding access to HIV antiretroviral therapy among marginalized populations in the developed world. AIDS. 2003;17:2419–27. doi: 10.1097/00002030-200311210-00003. [DOI] [PubMed] [Google Scholar]
- 6.Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. November 3, 2008. Department of Health and Human Services. [June 15 2009];2008 Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 7.Sabin CA, Smith CJ, Gumley H, et al. Late presenters in the era of highly active antiretroviral therapy: uptake of and responses to antiretroviral therapy. AIDS. 2004;18:2145–51. doi: 10.1097/00002030-200411050-00006. [DOI] [PubMed] [Google Scholar]
- 8.Touloumi G, Pantazis N, Stirnadel HA, Porter K. Seventh International Congress on Drug Therapy in HIV Infection. Glasgow, UK: 2004. Discontinuation of HAART: predictive value of and impact on immunological changes [Abstract P20] [Google Scholar]
- 9.Li X, Margolick JB, Conover CS, et al. Interruption and discontinuation of highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2005;38:320–8. [PubMed] [Google Scholar]
- 10.McNaghten AD, Hanson DL, Dworkin MS, Jones JL. Differences in prescription of antiretroviral therapy in a large cohort of HIV-infected patients. J Acquir Immune Defic Syndr. 2003;32:499–505. doi: 10.1097/00126334-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 11.Andersen R, Bozzette S, Shapiro M, et al. Access of vulnerable groups to antiretroviral therapy among persons in care for HIV disease in the United States. HCSUS Consortium. HIV Cost and Services Utilization Study. Health Serv Res. 2000;35:389–416. [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MH, Cook JA, Grey D, et al. Medically eligible women who do not use HAART: the importance of abuse, drug use, and race. Am J Public Health. 2004;94:1147–51. doi: 10.2105/ajph.94.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–15. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 15.Swindells S, Cobos DG, Lee N, et al. Racial/ethnic differences in CD4 T cell count and viral load at presentation for medical care and in follow-up after HIV-1 infection. AIDS. 2002;16:1832–4. doi: 10.1097/00002030-200209060-00020. [DOI] [PubMed] [Google Scholar]
- 16.Samet JH, Freedberg KA, Stein MD, et al. Trillion virion delay: time from testing positive for HIV to presentation for primary care. Arch Intern Med. 1998;158:734–40. doi: 10.1001/archinte.158.7.734. [DOI] [PubMed] [Google Scholar]
- 17.Wood E, Montaner JS, Tyndall MW, Schechter MT, O'Shaughnessy MV, Hogg RS. Prevalence and correlates of untreated human immunodeficiency virus type 1 infection among persons who have died in the era of modern antiretroviral therapy. J Infect Dis. 2003;188:1164–70. doi: 10.1086/378703. [DOI] [PubMed] [Google Scholar]
- 18.Stone V. Optimizing the care of minority patients with HIV/AIDS. Clin Infect Dis. 2004;38:400–4. doi: 10.1086/380969. [DOI] [PubMed] [Google Scholar]
- 19.Thrasher A, Earp J, Golin C, Zimmer C. Discrimination, distrust, and racial/ethnic disparities in antiretroviral therapy adherence among a national sample of HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49:84–93. doi: 10.1097/QAI.0b013e3181845589. [DOI] [PubMed] [Google Scholar]
- 20.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 21.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein MC, Goldie SJ, Losina E, et al. Use of genotypic resistance testing to guide HIV therapy: clinical impact and cost-effectiveness. Ann Intern Med. 2001;134:440–50. doi: 10.7326/0003-4819-134-6-200103200-00008. [DOI] [PubMed] [Google Scholar]
- 23.Multicenter AIDS Cohort Study (MACS) Public Dataset: Release PO4. Springfield, VA: National Technical Information Service; 1995. [Google Scholar]
- 24.Freedberg KA, Scharfstein JA, Seage GR, 3rd, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279:130–6. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 25.Benson CA, Williams PL, Cohn DL, et al. Clarithromycin or rifabutin alone or in combination for primary prophylaxis of Mycobacterium avium complex disease in patients with AIDS: A randomized, double-blind, placebo-controlled trial. The AIDS Clinical Trials Group 196/Terry Beirn Community Programs for Clinical Research on AIDS 009 Protocol Team. J Infect Dis. 2000;181:1289–97. doi: 10.1086/315380. [DOI] [PubMed] [Google Scholar]
- 26.Cohen C, Nieto-Cisneros L, Zala C, et al. Comparison of atazanavir with lopinavir/ritonavir in patients with prior protease inhibitor failure: a randomized multinational trial. Curr Med Res Opin. 2005;21:1683–92. doi: 10.1185/030079905x65439. [DOI] [PubMed] [Google Scholar]
- 27.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 28.Ioannidis JP, Cappelleri JC, Skolnik PR, Lau J, Sacks HS. A meta-analysis of the relative efficacy and toxicity of Pneumocystis carinii prophylactic regimens. Arch Intern Med. 1996;156:177–88. [PubMed] [Google Scholar]
- 29.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 30.Katlama C, Berger D, Bellos N, et al. Efficacy of TMC114/r in 3-class experienced patients with limited treatment options: 24-week planned interim analysis of 2 96-week multinational dose-finding trials. [abstract 164LB]. 12th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2005. [Google Scholar]
- 31.Nelson M, Arasteh K, Clotet B, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. J Acquir Immune Defic Syndr. 2005;40:404–12. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 32.Gebo KA, Moore RD, Fleishman JA. The HIV Research Network: a unique opportunity for real time clinical utilization analysis in HIV. Hopkins HIV Rep. 2003;15:5–6. [PubMed] [Google Scholar]
- 33.Walensky RP, Goldie SJ, Sax PE, et al. Treatment for primary HIV infection: projecting outcomes of immediate, interrupted, or delayed therapy. J Acquir Immune Defic Syndr. 2002;31:27–37. doi: 10.1097/00126334-200209010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 35.Cole SR, Hernan MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158:687–94. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 36.Losina E, Wolfe MI, Sax PE, et al. Does highly active antiretroviral therapy (HAART) reduce rates of opportunistic infections (OIs) independent of CD4 cell count? Results for the Adolescent and Adult Spectrum of HIV Disease (ASD) Project. XV International AIDS Conference; Bangkok, Thailand. 2004. [Google Scholar]
- 37.Ahdieh-Grant L, Tarwater PM, Schneider MF, et al. Factors and temporal trends associated with highly active antiretroviral therapy discontinuation in the Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2005;38:500–3. doi: 10.1097/01.qai.0000138160.91568.19. [DOI] [PubMed] [Google Scholar]
- 38.Hickman M, Carnwath Z, Madden P, et al. Drug-related mortality and fetal overdose risk: pilot cohort study of heroin users recruited from specialist drug treatment sites in London. J Urban Health. 2003;80:274–87. doi: 10.1093/jurban/jtg030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaccarelli M, Gattari P, Rezza G, et al. Impact of HIV infection on non-AIDS mortality among Italian injection drug users. AIDS. 1994;8:345–50. doi: 10.1097/00002030-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Kohli R, Lo Y, Howard A, et al. Mortality in an urban cohort of HIV-infected and at-risk drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41:864–72. doi: 10.1086/432883. [DOI] [PubMed] [Google Scholar]
- 41.Tyndall M, Craib K, Currie S, Li K, O'Shaughnessy M, Schechter M. Impact of HIV infection on mortality in a cohort of injection drug users. J Acquir Immune Defic Syndr. 2001;28:351–7. doi: 10.1097/00126334-200112010-00008. [DOI] [PubMed] [Google Scholar]
- 42.Seage GR, 3rd, Holte S, Metzger D, et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol. 2001;153:619–27. doi: 10.1093/aje/153.7.619. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. Report No.: Volume 14 2002. HIV/AIDS Surveillance Report: Cases of HIV infection and AIDS in the United States, 2002. [Google Scholar]
- 44.Degenhardt L, Hall W, Warner-Smith M. Using cohort studies to estimate mortality among injecting drug users that is not attributable to AIDS. Sex Transm Infect. 2006;82(Suppl 3):iii56–63. doi: 10.1136/sti.2005.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDavid K, Li J, Lee LM. Racial and ethnic disparities in HIV diagnoses for women in the United States. J Acquir Immune Defic Syndr. 2006;42:101–7. doi: 10.1097/01.qai.0000199353.11479.08. [DOI] [PubMed] [Google Scholar]
- 46.Harper S, Lynch J, Burris S, Davey Smith G. Trends in the black-white life expectancy gap in the United States, 1983-2003. JAMA. 2007;297:1224–32. doi: 10.1001/jama.297.11.1224. [DOI] [PubMed] [Google Scholar]
- 47.Asch SM, Kerr EA, Keesey J, et al. Who is at greatest risk for receiving poor-quality health care? N Engl J Med. 2006;354:1147–56. doi: 10.1056/NEJMsa044464. [DOI] [PubMed] [Google Scholar]
- 48.Women & HIV/AIDS: Programs and Events. [June 15 2009];2008 Available at: http://www.4woman.gov/hiv/programs/
- 49.DOH Launches Six-Month HIV Screening Campaign. [June 14 2006]; Media Advisory June 19, 2006. Available at: http://app.doh.dc.gov/news_room_dsf/release.asp?id=332&mon=200606.
- 50.New York State Department of Health 2005 Guidance for HIV Counseling and Testing and New Laboratory Reporting Requirements, Rev. 2006. [June 14 2009];2006 Available at: http://www.nyhealth.gov/diseases/aids/regulations/2005_guidance/docs/guidance.pdf.
- 51.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE1-4. [PubMed] [Google Scholar]
- 52.Torian L, Wiewel E, Liu K, Sackoff J, Frieden T. Risk factors for delayed initiation of medical care after diagnosis of human immunodeficiency virus. Arch Intern Med. 2008;168:1181–7. doi: 10.1001/archinte.168.11.1181. [DOI] [PubMed] [Google Scholar]
- 53.Mocroft A, Phillips A, Soriano V, et al. Seventh International Congress on Drug Therapy in HIV Infection. Glasglow, UK: 2004. Why do patients stop antiretrovirals used as part of an initial HAART regimen? Results from the EuroSIDA study group. [Abstract PL 14.1] [Google Scholar]
- 54.Yuan Y, L'Italien G, Mukherjee J, Iloeje UH. Determinants of discontinuation of initial highly active antiretroviral therapy regimens in a US HIV-infected patient cohort. HIV Med. 2006;7:156–62. doi: 10.1111/j.1468-1293.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 55.Cunningham WE, Markson LE, Andersen RM, et al. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the United States. HCSUS Consortium. HIV Cost and Services Utilization. J Acquir Immune Defic Syndr. 2000;25:115–23. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- 56.O'Brien ME, Clark RA, Besch CL, Myers L, Kissinger P. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J Acquir Immune Defic Syndr. 2003;34:407–14. doi: 10.1097/00126334-200312010-00008. [DOI] [PubMed] [Google Scholar]
- 57.Cheever LW. Engaging HIV-infected patients in care: their lives depend on it. Clin Infect Dis. 2007;44:1500–2. doi: 10.1086/517534. [DOI] [PubMed] [Google Scholar]
- 58.Giordano TP, Gifford AL, White AC, Jr, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 59.Webb M, Vanable P, Carey M, Blair D. Cigarette smoking among HIV+ men and women: examining health, substance use, and psychosocial correlates across the smoking spectrum. J Behav Med. 2007;30:371–83. doi: 10.1007/s10865-007-9112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood E, Hogg RS, Lima V, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–4. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 61.Wolfe D. Paradoxes in antiretroviral treatment for injecting drug users: access, adherence and structural barriers in Asia and the former Soviet Union. Int J Drug Policy. 2007;18:246–54. doi: 10.1016/j.drugpo.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 62.Nijhawan A, Kim S, Rich J. Management of HIV infection in patients with substance use problems. Curr Infect Dis Rep. 2008;10:432–8. doi: 10.1007/s11908-008-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staszewski S, Morales-Ramirez J, Tashima KT, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999;341:1865–73. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 64.Gallant JE, DeJesus E, Arribas J, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 65.Grinsztejn B, Nguyen B, Katlana, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistance virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–9. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 66.Lalezari J, Goodrich J, DeJesus E, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3. [abstract 104bLB]. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]