Abstract
Vaccination with tumor antigens has not been an effective treatment for solid tumors. The goal of the current study was to determine whether a combination of vaccination and hematopoetic cell transplantation (HCT) can effectively treat primary, disseminated or metastatic CT26 and MC38 murine colon tumors. Vaccination of tumor bearing mice with irradiated tumor cells and CpG adjuvant failed to alter progressive tumor growth. However, mice bearing primary, disseminated lung or metastatic liver tumors were uniformly cured after administration total body irradiation (TBI) followed by the transplantation of hematopoetic progenitor cells and T cells from syngeneic but not allogeneic vaccinated donors. Requirements for effective treatment of tumors included irradiation of hosts, vaccination of donors with both tumor cells and CpG, transfer of both CD4+ and CD8+ T cells along with progenitor cells, and ability of donor cells to produce IFN-γ. Irradiation markedly increased the infiltration of donor T cells into the tumors, and the combined irradiation and HCT altered the balance of tumor infiltrating cells to favor CD8+ effector memory T cells as compared to CD4+CD25+FoxP3+ Treg cells. The combination of vaccination and autologous hematopoetic cell transplantation was also effective in treating tumors. In conclusion, these findings show that otherwise ineffective vaccination to solid non-hematologic tumors can be dramatically enhanced by HCT.
Introduction
Despite the potency and specificity of the immune system, vaccination with tumor antigens generally fails to eradicate cancer in mice and humans (1,2). Currently, the most successful form of immunotherapy is adoptive cell therapy, which includes ex-vivo activation of tumor-infiltrating lymphocytes (TILs) and re-infusion of these cells along with high doses of cytokines. This approach is limited by cytokine toxicity and by the limited range of tumors from which sufficient TILs can be obtained (melanoma) (3). We hypothesized that the adoptive transfer of T cells from mice vaccinated against tumor antigens into tumor bearing mice given total body irradiation would result in marked expansion of the T cells, and their subsequent infiltration and eradication of tumors.
The results of the current study show that mice that have developed disseminated tumors or bulky primary tumors established for 2 weeks following inoculation with the CT26 colon carcinoma cells can be cured, when treated with a combination of tumor vaccination and hematopoetic cell transplantation (HCT), without ex-vivo T cell activation or use of TILs. Prior attempts at effective treatment by vaccination of mice with unmodified CT26 cells have failed due presumably to the low immunogenicity of this tumor (4). Several strategies have been used to overcome this problem including vaccination with GM-CSF transfected CT26 cells as well as with altered ligands with heteroclitic activity (4–7). Surprisingly, by combining vaccination with HCT, we reproducibly induced eradication of unmodified CT26 tumor cells that was dependent on the transfer of CD4+ and CD8+ T cells, and the ability of the transferred cells to produce IFN-γ.
Materials and methods
Animals
Wild-type male BALB/c (H-2d) mice, male BALB/c Rag2−/− mice, wild-type male DBA2/J (H-2 d) mice, and wild-type female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were 5–8 weeks old. The Stanford University Committee on Animal Welfare (Administration Panel of Laboratory Animal Care) approved all mouse protocols used in this study.
Cell lines
The CT26 cell line (an N-nitro-N-methylurethane-induced BALB/c murine colon carcinoma) was purchased from ATCC (Manassas, VA). The MC38 cell line (dimethyl-hydrazine induced C57BL/6 colon adenocarcinoma) was kindly provided by Dr. David Bartlett of the University of Pittsburgh (8). Cell lines were maintained in RPMI-1640 complete medium supplemented with 10% fetal calf serum, L-glutamine, 2 mercaptoethanol, streptomycin and penicillin. For intrasplenic injection, animals were anesthetized with ketamine/xylazine. Laparotomy was performed, and 5×105 CT26 cells were injected in the spleen. Abdominal wall was closed with surgical sutures.
Vaccination
Five-week-old male BALB/c mice were immunized by subcutaneous injection of 1×106 irradiated (10,000cGy) CT26 cells and 30 μg of CpG. Five-week-old female C57BL/6 mice were immunized by subcutaneous injection of 1×106 irradiated (10,000cGy) MC38 cells and 30 μg of CpG. AH-1 peptide (300μg per vaccination) used in this study was obtained from Sigma-Genosys. The peptide was >95% pure as indicated by analytical HPLC. Lyophilized peptide was diluted in DMSO and stored at −20°C until use. Oligonucleotide containing unmethylated CG motifs (CpG) (TCCATGACGTTCCTGACGTT) was synthesized and phosphorothioate-stabilized by Oligos, Etc. The oligonucleotide was reconstituted in sterile pyrogen-freewater and then diluted in PBS for in vivo injections. 30μg of ultrapure LPS (Invivogen) was used in some experiments instead of CpG.
Irradiation
The irradiation was performed with a Philips X-ray unit (200 kV, 10 mA; Philips Electronic Instruments Inc., Rahway, NJ, USA) at a rate of 84 cGy/min with a 0.5 mm Cu filter.
Donor cell preparation
Single cell suspensions of bone marrow and spleen prepared according to previously described procedures (9). Some samples were enriched either for CD4+ cells, CD8+ T cells or Thy1.2+ cells with anti-CD4, anti-CD8 magnetic microbeads (Miltenyi Biotech) or anti-Thy1.2-biotin monoclonal antibodies (mAb) (5a-8; Caltag, Burlingame, CA) and streptavidin-magnetic beads (Miltenyi Biotech) respectively using the MidiMACS system (Miltenyi Biotech, Auburn, CA). Enriched cells were stained with anti-TCR-allophycocyanin (APC) and anti-CD4 or anti-CD8-fluorescein isothiocyanate (FITC) mAbs to check for purity, and preparations were uniformly at least95% pure.
Purified HSCs were obtained by modification of the methods described by Spangrude et all (10). Thy-1lolin−/loSca-1+ c-Kit+ cells were sorted on a dual laser FACS (Becton Dickinson, Mountain View, CA) made available throughthe FACS shared-user group at Stanford University using FlowJo software (TreeStar, Ashland, OR) for data analysis. After sorting cells were checked by FACS reanalysis and determined to be >99%pure.
Histopathology
Animals were killed when moribund as per Stanford Animal Welfare protocol guidelines, or at 100 days after transplantation if they survived without morbidity. Histopathological specimens were obtained from lungs and livers of hosts. Tissues were fixed in 10% formalin, stained with hematoxylin and eosin and images were obtained using an Eclipse E1000M microscope (Nikon, Melville, NY, USA) as described before (11).
Analysis of donor cell accumulation in host spleens and tumor nodules
Single-cell suspensions were prepared from spleens and tumor nodules of BALB/c recipients. The following reagents were used for flow cytometric analysis: unconjugated anti-CD16/32 (2.4G2 BD Biosciences), anti-CD4-FITC (RM4-5 BD Biosciences), anti-TCR-APC (H57-597 BD Biosciences), anti-CD8-APC-Cy7, (53-6.7 BD Biosciences), anti-Thy1.1 PE-Cy7 (HIS51, eBioscience), anti-Thy1.2-biotin (5a-8; Caltag) mAbs, and streptavidin-PE (SAv-phycoerythin, BecktonDickenson). All stainings were performed in PBS/1% calf serum in the presence of purified anti-CD16/32mAbs.
Statistical analysis
Kaplan–Meier survival curves were generated using Prism software (SAS Institute Inc., Cary, NC, USA), and statistical differences were analyzed using the log-rank (Mantel–Cox) test. Statistical significance in differences between mean percentage of donor cells in host spleens and tumors was analyzed using the two-tailed Student’s t-test of means. For all tests, P<0.05 was considered significant.
Results
Syngeneic HCT from vaccinated BALB/c donor mice can cure established CT26 colon tumors
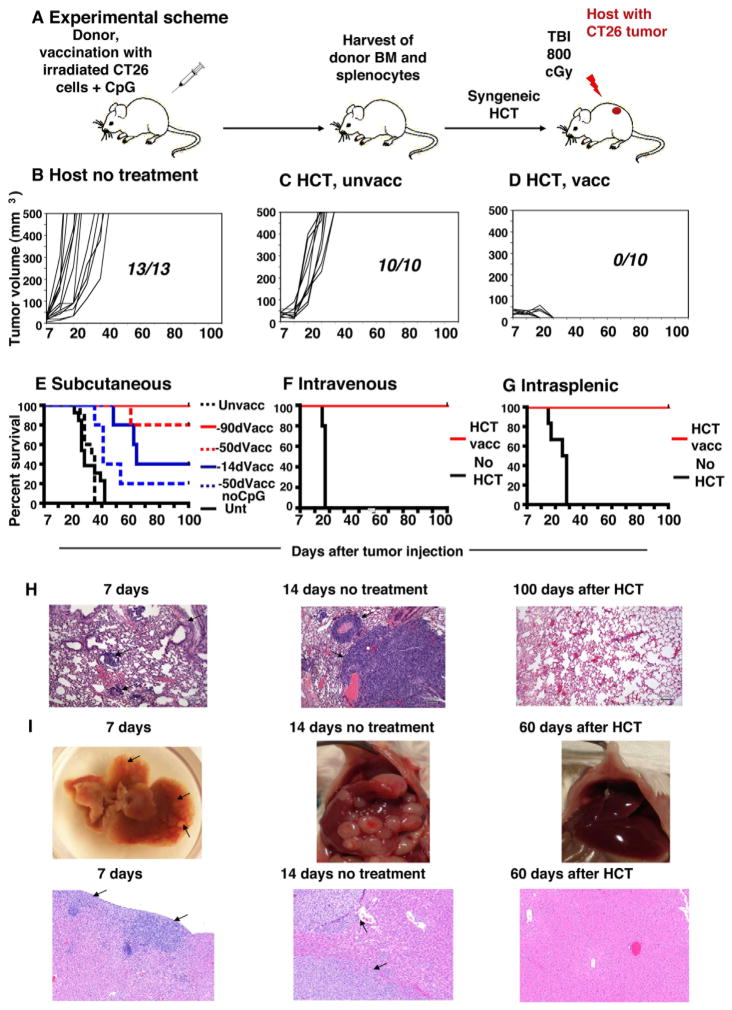
Figure 1A shows our experimental scheme, which uses HCT from tumor-vaccinated donors to treat CT26 colon tumors in syngeneic mice. In all instances, normal BALB/c donor mice were vaccinated subcutaneously (s.c.) with 106 irradiated CT26 tumor cells mixed with 30 μg CpG, an adjuvant that stimulates antigen presenting cell via TLR-9 (12,13). After 90 days, spleen and bone marrow cells were harvested, and transplanted intravenously (i.v.) into tumor-bearing BALB/c host mice following a single dose of total body irradiation (TBI). Seven days prior to TBI, hosts had been given live tumor cells via s.c. (2.5×104), i.v. (2×105) or intrasplenic (5×105) routes. Figure 1B shows the progressive growth of s.c. tumors in all untreated mice. Similarly, tumor bearing recipients of 50 ×106 bone marrow cells and 60 × 106 spleen cells from unvaccinated donors had uniformly progressive tumors (Figure 1C). In contrast, after HCT from vaccinated donors, tumor bearing mice displayed a steady regression of tumor volume over a 100 day observation period (Figure 1D), which remained stable until the end of study (day 180; data not shown). Shortening the time interval between immunization of the donor and harvesting the graft from 90 to 14 days, but not to 50 days, resulted in lower anti-tumor effect (p=0.005 and p=0.3, respectively; log rank test; Figure 1E). Omission of CpG from the donor vaccine resulted in a further loss of efficacy (p=0.01), and only 20% of hosts survived 100 days.
Figure 1. Syngeneic HCT from vaccinated BALB/c donor mice can cure established CT26 colon tumors.
A, Experimental scheme. B–D, Fraction of mice with progressive subcutaneous tumor growth and kinetics are shown: B, untreated mice (n=13), C, mice that received bone marrow (BM) and splenocyte transplants on day 7 from unvaccinated (unvacc) donors (n=10), or (D) vaccinated (vacc) donors (n=10). E, Survival of hosts that received transplants from donors vaccinated 90 (n=10), 50 (n=5) and 14 days (n=5) earlier, as well as from donors vaccinated with tumor cells without CpG (n=5). p=0.005 90 days vs 14 days F–G, Survival of hosts given tumor cells by intravenous (F) or intrasplenic (G) injection that received transplants on day 7 from vaccinated donors or were left untreated (unt). p<0.01 for intravenous tumor untreated vs treated, p=0.001 for intrasplenic tumor untreated vs treated. H, Histological sections (100x magnification) of host lungs after i.v. tumor cell injection. I, Gross specimens and histological sections of host livers after intrasplenic injection of tumor (I). Arrows show tumor nodules.
The same HCT strategy was also successful in recipients given tumor cells by i.v. administration. By day 7, tumor cells had disseminated into the lungs and formed multiple tumor clusters (Figure 1H). By day 20 all untreated control mice succumbed to progressive disease with large, nearly confluent tumor nodules (Figure 1F and 1H). In contrast, recipients of HCT from vaccinated donors all survived at least 100 days, with no histologic evidence of residual tumor (Figure 1H and 1H). Accordingly, improvement of survival was significant as compared to untreated mice (p <0.01) (Figure 1F). When tumor cells were injected into the spleen, by day 7 tumor nodules became established in the parenchyma of the liver (Figure 1I), and by day 14 there was evidence of blood vessel invasion (arrows, Figure 1I). All untreated animals died by day 30 (Figure 1G) with multiple visible, as well as microscopic, tumors. Treated mice survived beyond day 100 (Figure 1G), easily exceeding the survival of untreated mice (p=0.001). The liver of treated mice displayed no abnormalities and also no histologic evidence of residual tumor at day 60 (Figure 1I). HCT from vaccinated donors also cured peritoneal carcinomatosis, which had been created by intraperitoneal injection of 5×106 tumor cells and which displayed multiple peritoneal nodules and ascites by the time of transplant (data not shown). All untreated mice died by day 20, and all transplanted mice survived at least 100 days without any peritoneal tumor growth.
Vaccination and HCT induces long-term anti-tumor immunity
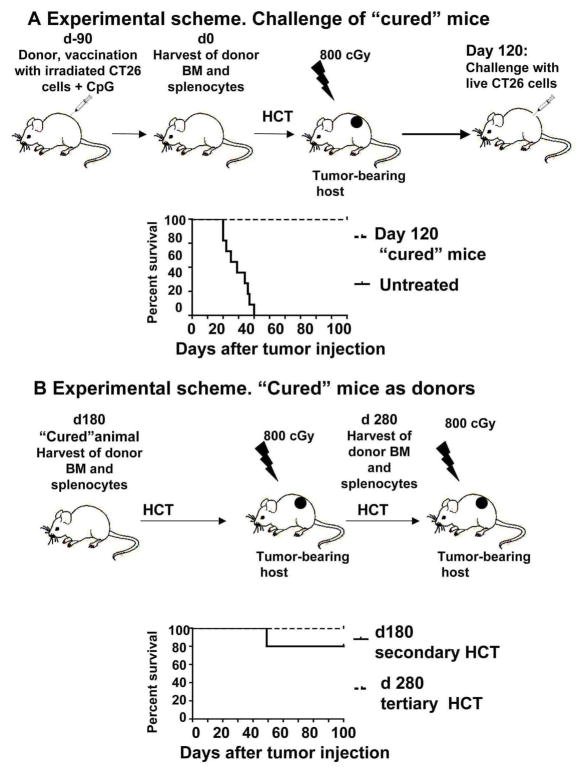
To assess the duration of effect of vaccination combined with HCT, we challenged “cured” animals from the experiment in Figure 1D with 2.5× 104 live tumor cells at day 120 as shown in the experimental scheme in Figure 2A. The results show that these animals were completely protected and survived for at least 100 days (Figure 2A). Moreover, harvesting of spleen and bone marrow cells from “cured” recipients at day 180 after HCT, and secondary transfer resulted in 80 % of the new recipients surviving for more than 100 days (Figure 2B). At least 100 days later those secondary recipients were used as donors for another HCT into irradiated tumor-bearing tertiary hosts which was also effective since all tertiary hosts survived more than 100 days (Figure 2B). Thus, anti-tumor immunity generated by a single vaccination could eradicate tumors 370 days later (Figure 2B).
Figure 2. “Cured” animals are protected from tumor challenge and can serve as donors of immune cells for HCT into syngeneic tumor-bearing hosts.
A, Animals with a single subcutaneous nodules cured by HCT from vaccinated animals were challenged at day 120 with live tumor cells subcutaneously. p<0.001 for “cured” animals (n=5) vs untreated controls (n=9).
B, Bone marrow and splenocytes from “cured” animals were harvested at day 180 and transferred into lethally irradiated tumor-bearing hosts. 100 days after bone marrow and splenocytes were harvested from secondary hosts and transferred into lethally irradiated tumor-bearing hosts. p<0.001 for transplanted animals (n=5) vs untreated (n=9) controls.
Tumor eradication requires lethal irradiation of hosts, as well as transfer of CD4+ and CD8+ T cells from vaccinated donors
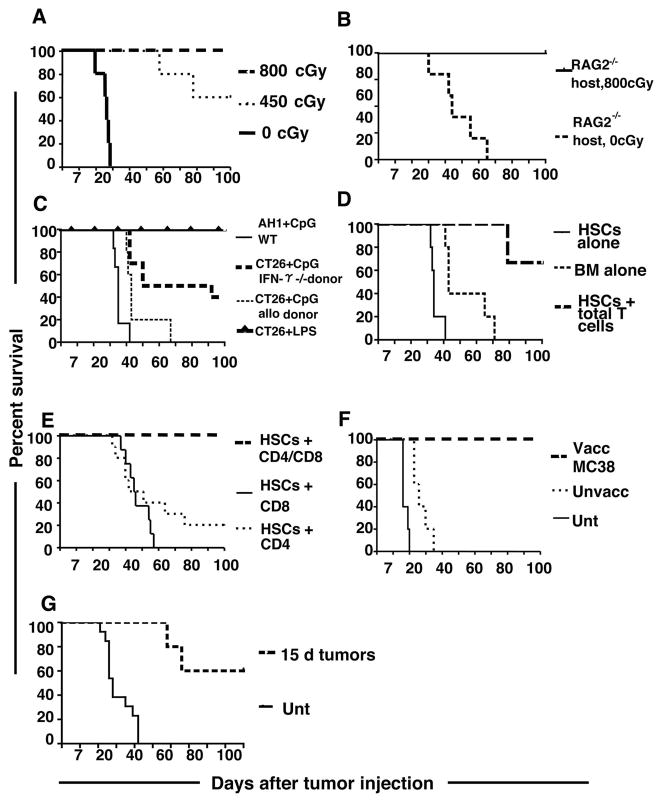
Figure 3A illustrates the significant role of the host conditioning regimen in our vaccine strategy. All tumor bearing recipients of bone marrow and splenocytes from vaccinated donors were cured when conditioned with myeloablative TBI (800cGy). In contrast, only 60% of hosts survived 100 days with a non-myeloablative radiation dose (450 cGy) (p <0.05), while none survived more than 40 days without irradiation (p<0.0001). Radiation causes lymphodepletion which might deplete host regulatory T cells that suppress anti-tumor immune responses. In order to test whether radiation mediates tumor eradication through such a mechanism, we studied unirradiated tumor-bearing RAG2−/− recipients that lack T cells. Rag 2−/− BALB/c hosts were given myeloablative radiation or no radiation immediately before transplantation of cells from vaccinated donors. Figure 3B shows that although there was a significant delay in mortality in non-irradiated Rag 2−/− mice as compared to non-irradiated wild type mice (p=0.001), all mice died by day 65. Conditioning of the Rag 2−/− mice with 800cGy resulted in significant improvement in survival as compared to the non-irradiated mice (p=0.001), and all hosts survived at least 100 days (Figure 3B).
Figure 3. Requirements for host irradiation and donor T cells in transplants.
A, Survival of BALB/c hosts that received either 800 cGy TBI (n=5), 450 cGy TBI (n=5) or no conditioning (n=5) before transplantation. p<0.05 for 800cGy vs 450cGy and p<0.0001 for 800 cGy vs 0cGy.
B, Rag 2−/− BALB/c hosts received transplants after either 800 cGy TBI (n=5) or no irradiation (n=5). p=0.001 for irradiated RAG2−/− vs unirradiated. C, BALB/c hosts received transplants from BALB/c IFN-γ−/− donors (n=10)(p=0.004), DBA/2J donors (n=5)(p<0.001) or BALB/c donors vaccinated with either AH-1 peptide and CpG (n=6)(p<0.0001) or irradiated tumor cells and lipopolysaccharide (LPS) instead of CpG (n=5) (p=1.0). (Survival compared with 90 day CpG group from Figure 1E). D, Hosts were given either donor whole bone marrow cells (n=5), or c-kit+Sca-1+ HSCs alone (n=5), or HSCs and purified splenic T cells (n=6) (p=0.008). E, Hosts received HSCs with either CD4+ (n=10) or CD8+ (n=8) T cells, or transplants containing a mixture of CD4+ and CD8+ T cells (n=5) (p<0.008). F, Survival of C57BL/6 mice with MC38 subcutaneous colon tumor nodules given TBI and transplants from C57BL/6 donors that had been vaccinated with irradiated MC38 tumor cells and CpG (n=10) as compared with untreated control mice (n=5). (p<0.008). G, Survival of hosts (n=5) with large subcutaneous tumors established for 15 days after HCT from vaccinated donors compared with survival of untreated animals (p=0.0005).
When IFN-γ−/− BALB/c mice were vaccinated and used as bone marrow and splenocyte donors, the survival of hosts was decreased as compared to wild type syngeneic donors (p=0.004)(Figure 2C). Likewise, survival was reduced in all five mice given grafts from vaccinated MHC-matched, minor antigen-mismatched DBA/2J donors (H-2d) (p<0.001) (Figure 3C). While 4/5 animals had progressive tumor growth, one mouse displayed tumor regression, but succumbed due to GVHD. Substitution of whole tumor cells with the tumor-associated immunodominant AH-1 peptide (4) for vaccination of donors resulted in decreased survival (p<0.0001) (Figure 3C). Use of lipopolysaccharide (30μg) as an adjuvant for vaccination was just as effective as CpG, based on survival of hosts post-HCT (p=1.0) (Figure 3C). Grafts from vaccinated donors consisting of bone marrow or FACS-purified c-kit+Sca1highlin− hematopoietic stem cells (HSC) failed to prevent tumor progression (Figure 3D). Addition of 5×106 splenic T cells to the HSCs increased survival (p=0.008) such that the majority of hosts were alive at 100 days without detectable tumors (Figure 3D). When HSC grafts were supplemented with both CD4+ (3.5×106) plus CD8+ T cells (1.8×106), survival was improved (p<0.008) as compared to supplementation with CD4+ or CD8+ T cells only (Figure 3E).
The utility of HCT from vaccinated donors was further validated in studies of another colon cancer, MC38, which grows only in C57BL/6 (H-2b) mice (8). For these experiments, donor mice were vaccinated with 1×106 MC38 tumor cells (Figure 3F). Again, while syngeneic recipients of grafts from vaccinated donors were cured, recipients of transplants from unvaccinated donors did not survive beyond 35 days (p<0.0001). HCT from tumor-vaccinated donors could also significantly improve survival of animals with large (>10 mm) tumors established for 15 days (p=0.0005) (Figure 3G). After 100 days, 60% of treated animals were completely tumor free
Analysis of donor T cells in host tumors and spleens after transplantation: irradiation promotes T cell accumulation in tumors
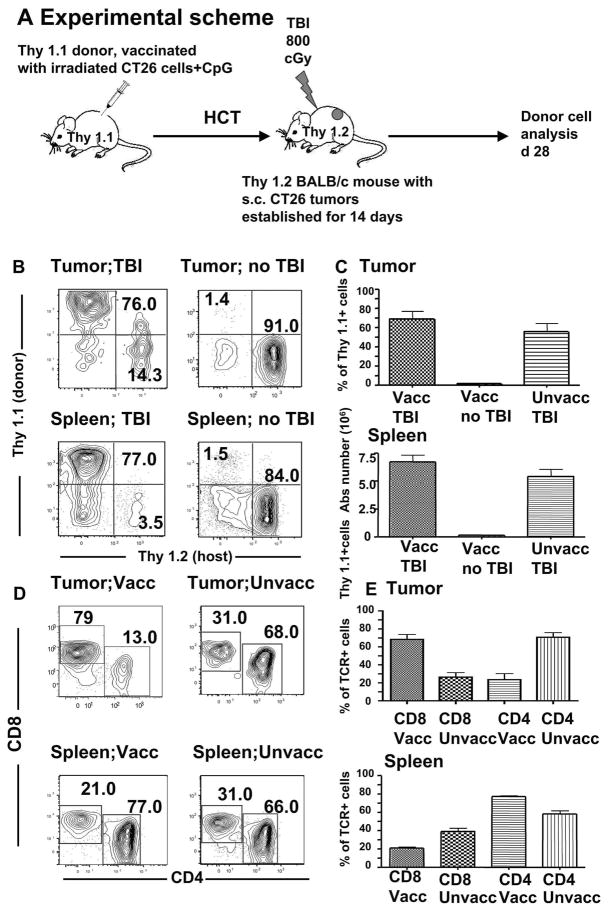
To delineate the donor-derived cell populations involved in the anti-tumor response, we transplanted bone marrow and spleen cells from BALB/c Thy1.1 donors into tumor bearing BALB/c Thy 1.2 hosts, as depicted in Figure 4A. To assure that there would be sufficient donor cells for analysis at day 28, HCT was performed in animals bearing tumors that had been established for 14 instead of 7 days. In irradiated recipients of vaccinated donor grafts approximately 70% of T cells infiltrating the tumors were of donor origin (Figure 4B and C), while donor T cells accounted for <2% of tumoral T cells when hosts were not irradiated (p<0.001). A similar facilitation of donor cell accumulation in the spleen was observed in irradiated versus non-irradiated hosts (p<0.01) (Figure 4B and C). Differences in accumulation of total T cells from vaccinated and unvaccinated donors were not significant (p>0.05). However, the majority of tumor T cells from vaccinated donors were CD8+, whereas most of the tumoral T cells from unvaccinated donors were CD4+ (p=0.01) (Figure 4D and E). CD4+ T cells were in the majority in the spleens with both vaccinated and unvaccinated donors (p<0.001).
Figure 4. Donor T cells accumulate in host tumors after transplantation.
A, Experimental scheme. B, Thy 1.1 and Thy 2.2 analysis of single cell suspensions of the tumors and spleens obtained on day 28 from tumor-bearing hosts given unvaccinated or vaccinated donor transplants with or without TBI. C, Mean percentages and SE of tumor-infiltrating Thy1.1+ cells at day 28 in top panel (n=5 in each group). Mean absolute numbers of Thy1.1+ cells in the host spleen in bottom panel (n=5). (p<0.001 for differences between TBI and no TBI, and p>0.05 for unvaccinated donors vs vaccinated after TBI). D–E, Analysis of CD4+ and CD8+ T cells among Thy1.1 + cells. Vaccinated donors are compared with unvaccinated donors (n=5 in each group) (p<0.001 for tumors and p<0.001 for spleens).
HCT alters the balance between regulatory and effector cells at the tumor site
Previous studies have shown that CD4+CD25+FoxP3+ Treg cells can suppress tumor immunity (14). Moreover, this suppression was mediated at the tumor site and was lost after intra-tumoral depletion of Tregs (14). We showed above (Figure 3B) that conditioning and HCT was required to cure tumors in RAG2−/− mice lacking T regulatory cells. Thus, the requirement for irradiation is not based on host Treg depletion.
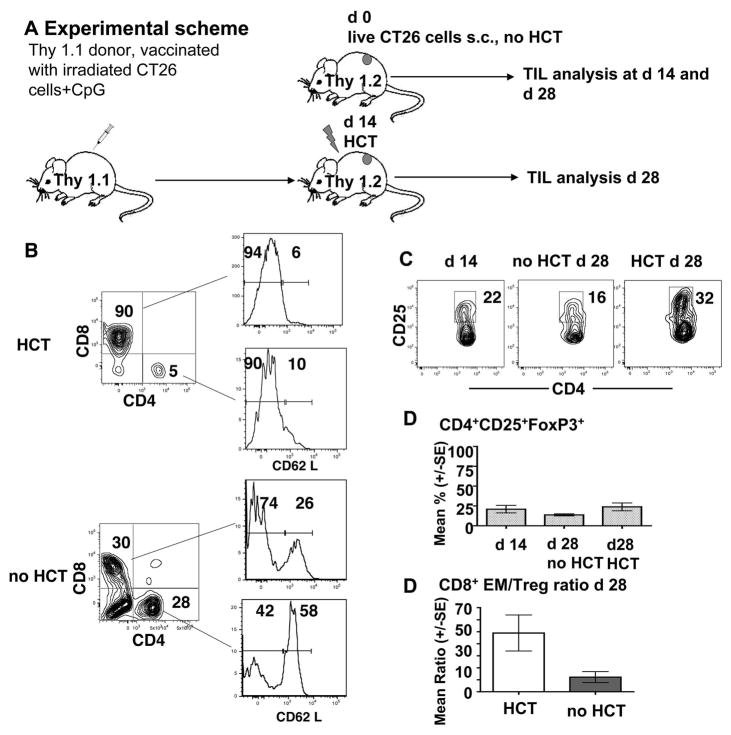
However, regulatory T cells of donor or host origin may be capable of infiltrating tumors when wild-type hosts are used. We examined host and donor T cell subsets infiltrating CT26 subcutaneous tumor nodules in wild-type BALB/c mice before and after HCT, and in controls without HCT as shown in the experimental scheme in Figure 5A. Control Thy1.2 mice given CT26 cells subcutaneously were euthanized 14 or 28 days later, and single cell suspensions from tumors were analyzed for tumor infiltrating T lymphocytes (TIL) subsets.
Figure 5. Effector/regulatory T cell ratios changes in favor of CD8 effector memory cells as a result of vaccination and HCT.
A, Experimental scheme. Syngeneic Thy 1.1 donors were vaccinated with 106 irradiated CT26 cells and CpG. Bone marrow and splenocytes were transplanted into lethally irradiated Thy 1.2 hosts with s.c. tumors established for 14 days. Tumor-infiltrating cells and host spleens were analyzed d 14 after HCT (28 days after tumors were induced). Control tumor-bearing animals did not receive irradiation and HCT. B, Analysis of CD62L expression on CD8+ and CD4+ tumor-infiltrating T cells form irradiated hosts that received HCT (gated Thy1.1+ cells) or untreated control animals (no HCT)(gated Thy1.2+cells) at day 28. The data are representative for the group of animals (n=5).
C–D, CD25 expression of tumor-infiltration CD4+ T cells and analysis of CD4+CD25+Foxp3+ T cells in tumors obtained from untreated animals on day 14, untreated animals on day 28 as well as tumor-bearing animals that received HCT (day 28). Mean percentages and SE are shown (n=5 in each group). E, CD8+ effector memory/Treg ratio in tumors in animals receiving HCT versus untreated animals on day 28. Mean percentages and SE are shown (n=5 in each group) (p<0.05).
Experimental mice were lethally irradiated and given HCT from vaccinated Thy 1.1 donors after 14 days of tumor growth, and tumor cell suspension were analyzed 14 days after HCT. Figure 5B shows the representative staining patterns for CD4+ and CD8+ T cells in cell suspensions using gated Thy1.2+ T cells from control mice and gated Thy1.1+ from mice given HCT at 28 days after the subcutaneous injection of tumor cells (14 days after HCT).
Whereas CD8+ and CD4+ cells accounted for about 90% and 5% respectively of Thy1.1+ cells in mice given HCT, the CD8+ and CD4+ cells accounted for 30% and 28% respectively of Thy1.2+ cells in mice without HCT. Almost all of the CD8+ and CD4+ T cells in mice given HCT were CD62Llo (Figure 5B). The staining pattern indicates that few naive or central memory cells were found in these tumors, almost all were effector memory cells, since the CD8+ and CD4+ cells were almost all CD44hi (data not shown).
In contrast, the gated CD8+ and CD4+ cells from tumors in control mice contained discrete subsets of both CD62 Llow and CD62 Lhi cells. The CD62 Lhi cells accounted for 26% of CD8+ cells and 58% of CD4+ cells (Figure 5B). Staining of gated CD4+ tumor cells from control mice and those given HCT for CD4 versus CD25 showed that about 16% of CD4+ cells were CD25+ in controls, and 32% were CD25+ in those given HCT at the day 28 time point (Figure 5C). At day 14, 22% of CD4+ cells were CD25+. The results of additional staining for intracellular FoxP3+ showed that the mean percentage of CD4+CD25+ FoxP3+ Treg cells among gated CD4+ cells in the tumors of all 3 groups of mice varied from about 15% to 25% (Figure 5D). The differences in the means were not statistically significant (p>0.05) as judged by the Student t test.
Despite the similar percentages of Treg cells among total CD4+ T cells in the tumor cell infiltrate, there was a marked difference in the balance of CD8+CD62 LloCD44 hi effector memory T cells versus Treg cells. Whereas, day 28 tumors from control mice showed a mean ratio of about 5:1 CD8+ effector memory to Treg cells in the infiltrate, the day 28 tumors from mice given HCT showed mean ratio of about 50:1. The differences in ratios were statistically significant (p<0.05). Thus, while HCT did not deplete Tregs at the tumor site, the balance of tumor infiltrating cells was altered to favor CD8+ effector memory T cells as compared to Treg cells.
Tumor vaccination becomes effective when combined with HCT and vaccine induced anti-tumor immunity is not prevented by the presence of growing tumors
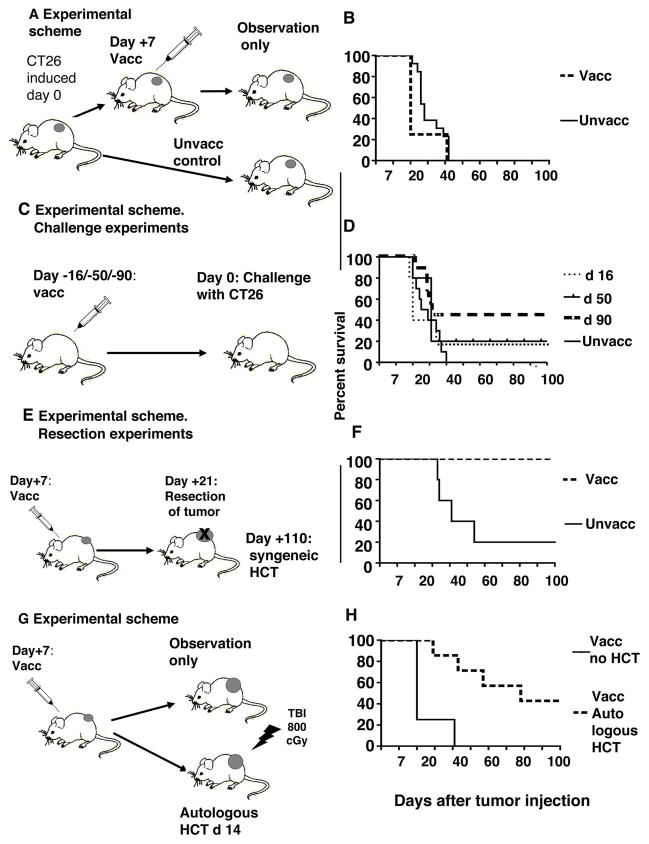
Figure 6A shows the experimental scheme used to determine the effect of vaccination alone on survival of tumor-bearing mice. Figure 6B shows that the survival of vaccinated, but not HCT treated, animals with 7-day tumors did not improve as compared to unvaccinated tumor-bearing animals and all animals died by day 40. Moreover, when vaccinated non tumor-bearing animals were challenged with as few as 2.5×104 CT26 cells 16 and 50 days after vaccination (Figure 6C), only 20% of mice survived 100 days (Figure 6D). Some degree of protection developed in animals vaccinated given the tumor challenge 90 days after vaccination, as indicated by the observation that 50% of animals remained tumor free (p=0.03)(Figure 6D). Next, to assess the potential effect of larger tumors on the response to vaccination, we vaccinated mice with tumors growing for 7 days and then waited 14 additional days before resecting the growing tumors at day 21 (Figure 6E). Bone marrow and splenocytes were harvested from donors on day 110 and transferred into lethally irradiated tumor-bearing hosts. All hosts survived with complete tumor regression for at least 100 days (Figure 6F). Only 20% of hosts given transplants from unvaccinated donors survived 100 days, and the difference using vaccinated versus unvaccinated donors was significant (p<0.05) (Figure 6F). Thus, HCT from vaccinated animals into syngeneic tumor bearing hosts resulted in cure of tumors, indicating that growing tumors in donors do not prevent the development of potent anti-tumor effector cells in adoptive hosts.
Figure 6. Tumor vaccination without HCT is not effective, but vaccination combined with HCT is highly effective, even in the presence of growing tumor.
A, Experimental scheme. Tumors were induced subcutaneously at day 0. Animals were unvaccinated or vaccinated at day 7 with irradiated tumor cells and CpG. B, Survival of vaccinated versus unvaccinated mice (n=8). C, Non tumor-bearing animals were vaccinated with irradiated tumor cells and CpG, and challenged with live tumor cells 16 (n=5), 50 (n=5) or 90(n=10) days after vaccination. p=0.03 for 90 days vaccination vs control. D, Survival of vaccinated mice after tumor challenge. E, Experimental scheme. Mice with subcutaneous tumors were vaccinated at day 7 after tumor was induced. Tumors were resected at day 21. At day 110 after tumor induction, bone marrow and splenocytes were harvested and transferred into lethally irradiated tumor-bearing hosts, F, Survival of hosts given bone marrow and spleen cell transplants from vaccinated or unvaccinated donors (n=5 each group) (p<0.05). G, Donors were injected with tumor cells on day 0, vaccinated on day 7 and splenectomized on day 14. Splenectomized donors had their abdominal incisions closed with surgical sutures before receiving a single dose of 800 cGy TBI. Within 6 hours of TBI, the donors were given an autotransplant of all harvested spleen cells injected intravenously. H, Survival of hosts with (n=7) and without (n=8) autologous HCT (p<0.001).
Autologous HCT enhances tumor immunity after vaccination
A model of autolous HCT was studied as shown in Figure 6G. In this scheme a group of donors was vaccinated 7 days after live tumor cell injection, splenectomized 7 days later, conditioned with TBI immediately after recovery from surgery, and spleen cells were injected intravenously within 6 hours after TBI. Bone marrow cells were not required for rescue of these myeloablated hosts, since mouse spleen cells contain both immune cells and HSCs. Donors that received the autologous transplants had significantly improved survival as compared to those without transplants, and about 40% survived at least 100 days with complete tumor regression (p<0.001) (Figure 6H). Thus, large 14 day tumors were either cured or their growth significantly delayed after autologous HCT.
Discussion
These data show for the first time that it is possible to eradicate primary, metastatic, or disseminated solid tumors by treating tumor bearing hosts with HCT containing sensitized T cells from vaccinated donors. While there is growing evidence that hematologic cancers can be effectively treated with a combination of tumor vaccination and HCT (15,16), the effect of such treatment on solid tumors has not been tested. Our outcome measure for tumor immunity in the current study was eradication of the CT26 or MC38 colon tumors. Investigating the specific tumor antigen epitopes recognized by sensitized T cells after whole tumor cell vaccination is the subject of our continuing studies.
Allogeneic HCT from tumor antigen vaccinated donors was not effective in our model, although this approach has been reported to induce complete remissions of primary melanoma tumors or metastatic breast tumors in mice (17,18). An important limitation of allogeneic HCT is the development of graft versus host disease (GVHD), which occurs in a severe form in about 30–50% of humans who receive this therapy (19–21). GVHD is likely to be aggravated by vaccinating donors with tumors expressing host alloantigens. By combining tumor vaccination of the donor with syngeneic HCT for the treatment of primary and metastatic colon cancer in mice, we not only avoided GVHD but also achieved a potent and durable anti-tumor response. The results demonstrate a powerful synergy between tumor vaccination and HCT in two genetically distinct mouse strains with two different colon tumors. A similar synergy has been reported in mice with primary melanoma treated with ex vivo expanded, T cell antigen receptor transgenic CD8+ T cells specific to a tumor antigen in combination with syngeneic HCT (22,23).
The latter approach required infusion of IL-2 to facilitate the expansion and persistence of the T cells, and was not used for metastatic tumors (22,23). In our model, a robust anti-tumor immune response could be transferred to tumor bearing mice without ex-vivo T cell expansion or treatment of the mice with cytokines. The finding that CD4+ and CD8+ T cells needed to be included in the transplant to achieve cures indicates that effective vaccination requires epitopes recognized by both types of T cells. Such epitopes were lacking in a vaccine consisting of the immunodominant AH-1 peptide and CpG, which may explain why this vaccine was ineffective, in contrast to vaccines containing whole tumor cells, which are a source of multiple CD4 and CD8 epitopes. CD4+ T cells provide help to memory CD8+ T cells by enhancing their immune potency, expansion, and persistence after exposure to antigen (24). The efficacy of the transferred donor cells in the current study required that they produce IFN-γ, since cells obtained from IFN-γ−/− donors lost potency.
Irradiation of tumor bearing hosts was also required for tumor cures, and markedly augmented the expansion of transplanted T cells in the spleen and their infiltration into tumors. Since lethal irradiation was considerably more effective than sublethal irradiation, hematopoetic stem cells had to be included in the transplants to rescue hosts from marrow aplasia in the current study. Previous studies indicate that the hematopoetic stem cells injected into irradiated mice not only prevented marrow aplasia, but also facilitated the expansion of CD8+ T cells directed to melanoma tumor antigens by enhancing IL-7 and IL-15 production (22).
Irradiation and HCT have been shown previously to promote expansion and efficacy of transplanted anti-tumor T cells by inducing secretion of IL-7 and IL-15, and activating antigen-presenting cells through TLR-4 and CD14 receptors (22,23).
In the current study, we found that irradiation and HCT altered the balance of T cell subsets infiltrating the tumors rather than simply depleting T regulatory cells at the tumor site. Since CD4+CD25+FoxP3+ Treg cells can suppress tumor immunity (14), and CD8+ effector memory T cells can mediate tumor cell killing, the balance of the subsets was determined in tumor bearing mice with or without HCT. Mice given irradiation and HCT had a 10 fold higher ratio of CD8+ effector memeory T cells:Treg cells in the tumors as compared to control mice without HCT. Thus, the HCT procedure not only increases the absolute number of T cells that infiltrated the tumors, but also favors the T cell subsets that kill tumor cells versus the subset that suppresses tumor immunity.
Tumor vaccination without HCT was not effective against established tumors. However, vaccination of tumor-bearing animals provided long-term, transferable immunity, which could be enhanced by HCT. These data suggest that patients whose primary tumors are resected but remain at high risk for relapse, might benefit from early vaccination combined with HCT in the event of relapse.
Acknowledgments
We thank G. Fisher for advice, and G. Letsinger for help with manuscript preparation. We acknowledge Dr. J. Slansky form the University of Colorado for kindly providing Ld-AH-1 tetramers.
The work was supported by a grant from the National Cancer Institute (P01 CA-49605), and the Nishimura Fund for Cancer Research.
References
- 1.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC. Cancer immunotherapy: moving beyond current vaccines. Nature Medicine. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, Rardoll DM, Jaffee EM. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci U S A. 1996;93:9730–9735. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slansky JE, Rattis FM, Boyd LF, Fahmy T, Jaffee EM, Schneck JP, Margulies DH, Pardoll DM. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13:529–38. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 6.McMahan RH, McWilliams JA, Jordan KR, Dow SW, Wilson DB, Slansky JE. Relating TCR-peptide-MHC affinity to immunogenicity for the design of tumor vaccines. J Clin Invest. 2006;116:2543–51. doi: 10.1172/JCI26936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McWilliams JA, Sullivan RT, Jordan KR, McMahan RH, Kemmler CB, McDuffie M, Slansky JE. Age-dependent tolerance to an endogenous tumor-associated antigen. Vaccine. 2008;26:1863–73. doi: 10.1016/j.vaccine.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett TH, Griswold DP, Roberts BJ. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35:2434–2439. [PubMed] [Google Scholar]
- 9.Dutt S, Ermann J, Tseng D, Liu YP, George TI, Fathman CG, Strober S. L-selectin and beta7 integrin on donor CD4 T cells are required for the early migration to host mesenteric lymph nodes and acute colitis of graft-versus-host disease. Blood. 2005;106:4009–4015. doi: 10.1182/blood-2005-06-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1998;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 11.Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol. 2007;15(178):6242–51. doi: 10.4049/jimmunol.178.10.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 13.Okano F, Merad M, Furumoto K, Engleman EG. In vivo manipulation of dendritic cells overcomes tolerance to unmodified tumor-associated self antigens and induces potent antitumor immunity. J Immunol. 2005;174:2645–2652. doi: 10.4049/jimmunol.174.5.2645. [DOI] [PubMed] [Google Scholar]
- 14.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX. Intra-tumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borrello I, Sotomayor EM, Rattis F, Cooke SK, Gu L, Levitsky HI. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood. 2000;95:3011–3019. [PubMed] [Google Scholar]
- 16.Mirmonsef P, Tan G, Zhou G, Morino T, Noonan K, Borrello I, Levitsky H. Escape from suppression: tumor-specific effector cells outcompete regulatory T cells following stem-cell transplantation. Blood. 2008;111:2112–2121. doi: 10.1182/blood-2007-06-096586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meunier MC, Delisle JC, Bergeron J, Rineau V, Baron C, Perreault C. T cells targeted against a single minor histocompatibility antigen can cure solid tumors. Nature Medicine. 2005;11:1222–1229. doi: 10.1038/nm1311. [DOI] [PubMed] [Google Scholar]
- 18.Luznik L, Slansky JE, Jalla S, Borrello I, Levitsky HI, Pardoll DM, Fuchs FJ. Successful therapy of metastatic cancer using tumor vaccines in mixed allogeneic bone marrow chimeras. Blood. 2003;101:1645–1652. doi: 10.1182/blood-2002-07-2233. [DOI] [PubMed] [Google Scholar]
- 19.Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Lietman S, Read EJ, Tisdale J, Dunbar C, Linehan WM, Young NS, Barrett AK. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 20.Massenkeil GM, Nagy S, Neuburger S, Tamm I, Lutz CP, le Coutre O, Rosen O, Wernecke K-D, Dorken B, Arnold R. Survival after reduced-intensity conditioning is not inferior to standard high-dose conditioning before allogeneic haematopoietic cell transplantation in acute leukaemias. Bone Marrow Transplantation. 2005;36:683–689. doi: 10.1038/sj.bmt.1705123. [DOI] [PubMed] [Google Scholar]
- 21.Carnevale-Schianca F, Cignetti A, Capaldi A, Vitaggio K, Vallario A, Ricchiardi A, Sperti E, Ferraris R, Gatti M, Grignan G, Rota-Scalabrini D, Geuna M, Fizzotti, Sangiolo D, Sottile A, De Rosa G, Bucci A, Lambertenghi-Deliliers G, Benedetti E, Nash R, Aglietta M. Allogeneic nonmyeloablative hematopoietic cell transplantation in metastatic colon cancer: tumor-specific T cells directed to a tumor-associated antigen are generated in vivo during GVHD. Blood. 2006;107:3795–3803. doi: 10.1182/blood-2005-10-3945. [DOI] [PubMed] [Google Scholar]
- 22.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrzesinski C, Paulos CM, Gattinoni L, Palmer DC, Kaiser A, Yu Z, Rosenberg SA, Restifo NP. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]