Abstract
The embryonic mid-hindbrain organizer, which is composed of a transient cell population in the brainstem, controls the development of dopaminergic and serotonergic neurons. Different genes determining the position and activity of this embryonic structure have been implicated in dopamine and serotonin associated disorders. Mouse mutants with a caudally shifted mid-hindbrain organizer, are hyperactive, show increased numbers of dopaminergic neurons and a reduction in serotonergic cells.
In the present study we used these mutants to gain insights into the genetic and developmental mechanisms underlying motor activity and the response to psychostimulants. To this end we studied the motor activity of these animals after exposure to methylphenidate and amphetamine and characterized their dopaminergic and serotonergic innervation.
Saline-treated mutants showed increased locomotion, more stereotypic behaviour and a decrease in rearing compared to wild-type mice. This baseline level of activity was similar to behaviours observed in wild-type animals treated with high doses of psychostimulants. In mutants methylphenidate (5 or 30 mg/kg) or amphetamine (2 or 4 mg/kg) did not further increase activity or even caused a decrease of locomotor activity, in contrast to wild-type mice. Fluoxetine (5 mg or 10 mg/kg) reduced hyperactivity of mutants to levels observed in wild-types.
Transmitter measurements, dopamine and serotonin transporter binding assays and autoradiography, indicate a subtle increase in striatal dopaminergic innervation and a marked general decrease of serotonergic innervation in mutants.
Taken together, our data suggest that mice with an aberrantly positioned mid-hindbrain organizer show altered sensitivity to psychostimulants and that an increase of serotonergic neurotransmission reverses their hyperactivity. We conclude that the mid-hindbrain organizer, by orchestrating the formation of dopaminergic and serotonergic neurons, is an essential developmental parameter of locomotor activity and psychostimulant response.
Keywords: psychostimulants, isthmic organizer, hyperactivity, Otx2, dopamine, serotonin
INTRODUCTION
Dopaminergic (DA) neurons play a key role in regulating motor activity and the behavioural effects of psychostimulants such as methylphenidate (MP) and amphetamine (AMPH) (Scheel-Krüger, 1971). Both drugs increase locomotor activity by elevating extracellular DA. Although serotonin (5-HT) has also been implicated in motor activity control and psychostimulant response, its role is far less well understood (Breese et al., 1974; Jacobs and Fornal, 1997; Winstanley et al., 2003; Borycz et al., 2008). In particular, the role of 5-HT in the MP response has been controversial, since unlike AMPH there is no clear evidence that MP increases 5-HT (Kuczenski and Segal, 1997).
In contrast to the neurochemical and behavioural responses to psychostimulants, that have been extensively investigated, the genetic and developmental mechanisms determining these responses are poorly understood. In recent years substantial insight has been gained into the molecular mechanisms controlling the development of monoaminergic neurons, thereby providing the foundation for a better understanding of how genes and development affect the response to psychostimulants and motor activity.
During embryogenesis, the anterior neural tube gives rise to the forebrain, midbrain and hindbrain. Specialized cells forming a narrow band at the embryonic mid-hindbrain junction control, by secreting growth factors, induction, position and survival of neurons in the vertebrate brainstem. Hence these specialized cells are called the mid-hindbrain organizer (MHO) (Martinez et al., 1990; Wurst and Bally-Cuif, 2001). The position of MHO directs location and size of DA and 5-HT cell populations and is of functional relevance for adult locomotor activity (Brodski et al., 2003).
During neural tube closure, the transcription factors Otx2 and Gbx2 are, respectively, expressed in the prospective fore- and midbrain (Otx2) and prospective hindbrain (Gbx2). Mouse mutants, in which the Otx2/Gbx2 expression boundary had been shifted either anteriorly or posteriorly, indicate that this interaction border defines the position of the MHO (Broccoli et al. 1999; Millet et al., 1999).
In mutants with a caudally shifted Otx2/Gbx2 junction, one allele of the En1 coding sequence was replaced by the Otx2 coding sequence (En1+/Otx2). Thus, all cells that normally express the transcription factor, En1, ectopically express Otx2. Since En1 has a more posterior expression border than Otx2 in the neural tube, it shifts the Otx2/Gbx2 expression boundary slightly more posteriorly (Broccoli et al., 1999).
These mice are viable and fertile. At the dorsal aspect of the brainstem, they show an increased volume of the inferior colliculus and a decreased volume of the cerebellar vermis, accompanied by postural asymmetry and abnormal gait. Ventrally, they exhibit changes in the number of DA and 5-HT neurons (Broccoli et al., 1999; Brodski et al. 2003).
The major DA nuclei, ventral tegmental area and substantia nigra, develop in the midbrain, close to the MHO, whereas 5-HT raphe nuclei originate juxtaposed to the MHO in the hindbrain (Ye et al. 1998; Brodski et al., 2003).
In En1+/Otx2 mutants, the DA neuronal population expands to the posteriorly positioned MHO and is thus enlarged, resulting in a 15% increase in striatal DA. The ectopic DA neurons are predominantly a posterior extension of the substantia nigra and there is only a sparse expansion of the ventral tegmental area (Brodski et al., 2003). In contrast, the number of 5-HT neurons is reduced, resulting in a 25% decrease in striatal 5-HT (Brodski et al., 2003). Transmitter concentrations in other brain regions have not been reported. Interestingly, the changes in the monoaminergic transmitter populations are also reflected in behavioural changes, manifested by increased locomotor activity that is not correlated to atactic behaviour (Brodski et al., 2003).
In a complementary mouse mutant model, in which the caudal Otx2 expression border is shifted anteriorly, DA and 5-HT neuronal populations are changed in an opposite manner, compared to En1+/Otx2 mice. In these mutants the number of 5-HT neurons is increased while the number of DA neurons is reduced (Puelles et al., 2004; Borgkvist et al., 2006). The baseline level of motor activity of these animals is normal. However, their response to AMPH on horizontal motor activity is significantly higher than in wild-type (WT) controls (Borgkvist et al., 2006).
The aim of the present study was to further investigate the influence of the MHO on the DA and 5-HT systems and its influence on motor activity and response to psychostimulants. To this end, we studied the behavioural response of mouse mutants with a caudally repositioned MHO to drugs that stimulate monoaminergic neurotransmission. In addition, we characterized changes in the DA and 5-HT innervations in these animals.
EXPERIMENTAL PROCEDURES
Animals, treatments and behavioural tests
The generation of En1+/Otx2 mice has been described previously (Broccoli et al., 1999). Mutants were generated on a mixed C57Bl/6 and 129/Sv background. Mice were then backcrossed for at least 15 generations to a CD-1 background. CD-1 mice were purchased by Charles River, Israel. Male and female mice were separately housed in groups of 2–5 under standard laboratory conditions, with food and water ad libitum and a reversed 12 hour light–dark cycle. Behavioural tests were conducted between 10:00–16:00. The experiments were approved by the institutional Committee for Ethical Care and Use of Animals in Experiments, in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
WT and En1+/Otx2 littermates aged 4–6 month were placed in a transparent Perspex open field, measuring 40 × 40 × 20cm, and videotaped. The box was cleaned with a dilute ethanol solution between animals and carefully dried.
For the measurements of the distance traveled (cm), videotapes were analyzed off-line using the Noldus EthoVision® System, sampling at a rate of 5 pictures per second. The investigated time period was divided into 5-minute bins for analysis.
Locomotor activity in MP treated WT and En1+/Otx2 mice
Sixteen male WT and 16 male En1+/Otx2 mice were tested for locomotor activity during the dark phase of the light/dark cycle. The animals were weighed and placed in the open field for 10 minutes to habituate before being injected. Each animal was tested twice, once with 0.9% saline and once with either 5 mg/kg or 30 mg/kg body weight methylphenidate hydrochloride (Sigma-Aldrich Ltd.) in a volume of 10 ml/kg weight. The order of testing was counterbalanced, such that half the mice were tested first with saline and the other half were tested first with MP to control for the effects of novelty of the environment. At least 14 days separated the two test sessions. Since the order of treatment was counterbalanced, all the saline treated mice were analyzed as one group. Due to technical problems, data from one saline trial of each of the WT and En1+/Otx2 groups were discarded. Data were analyzed after a 10 minute habituation period for 45 min using the Noldus Ethovision ® system, as described above.
Locomotor activity in AMPH treated WT and En1+/Otx2 mice
Twenty five male WT and 24 male En1+/Otx2 mice were tested for locomotor activity during the dark phase of the light/dark cycle. The animals were weighed and injected i.p. with 0.9% saline, 2 or 4 mg/kg body weight D-amphetamine sulfate (Sigma-Aldrich Ltd.) in a volume of 10 ml/kg weight. Each animal was tested only once. Data were analyzed after a 10 minute habituation period for 60 min using the Noldus Ethovision® system, as described above.
Locomotor activity in fluoxetine treated WT and En1+/Otx2 mice
Eighteen male WT and 18 male En1+/Otx2 mice were tested for locomotor activity during the dark phase of the light/dark cycle. The animals were weighed and injected i.p. with saline, 5 mg/kg or 10 mg/kg body weight fluoxetine hydrochloride (FLU, Sigma-Aldrich Ltd.) in a volume of 10 ml/kg weight. Each animal was tested only once. Data were analyzed after a 10 minute habituation period for 60 min using the Noldus Ethovision ® system, as described above.
Stereotypies and rearing after exposure to MP, AMPH and FLU
Stereotypies and rearing were analyzed manually by an observer blind to the treatment by sampling for 60 seconds at the start of each 10 minute interval and measuring the duration of stereotyped behaviour. Stereotypical behaviours were defined as sniffing while immobile, grooming, licking the bottom or wall of the box, head nodding or repetitive lateral head movements. The total duration of stereotyped behaviour and rearing was compared between the mutant and WT mice using a three-way ANOVA for the effect of treatment (3 levels) and genotype (2 levels) and time as a repeated measure.
Center/Periphery ratio
In order to define the center/periphery exploration ratio, we defined the center area in the Noldus Ethovision system (20 × 20cm). Subsequently, the distances covered by the mice in the center and in the periphery were correlated with the total distance covered by the animals. Due to the fact that the number of mice in each experiment was too small to allow correlation analysis, the data from all saline-treated mice from the above three experiments were pooled.
Elevated plus maze
Thirteen En1+/Otx2 (6 females) and 19 WT (8 females) were tested on the elevated plus maze (EPM), which is a pharmacologically validated anxiety measure in rodents (File, 2001). The EPM was constructed of transparent Plexiglas and consisted of 2 opposing open arms and 2 opposing closed arms. The arms were 50 cm in length and 5 cm wide. The closed arms had walls that were 20 cm high, whereas the open arms had a ledge of 2 cm to prevent falling and the entire maze was elevated 50 cm from the floor. At the start of the trial, the mouse was placed in the center of the maze, facing an open arm and left to explore for 6 minutes. The trials were taped and duration spent in each of the arms was analyzed off line using the Noldus Ethovision System.
Autoradiography
Five WT and 5 mutant male mice were decapitated and brains quickly removed and frozen on dry ice. Five parallel series of consecutive coronal cryostat sections (10µm) were collected by thaw mounting onto coated glass slides.
Slides from each series were incubated with 160µl of 0.1nM [125I]-RTI-55 (from Perkin Elmer) (Fujita et al., 1994) in phosphate buffered saline at pH 7.4 for 1hr at room temperature. For the visualization of DAT, FLU (10µM) and desipramine (10µM) were added to RTI-55 in order to block RTI-55 binding to SERT or the noradrenaline transporter (NET). For the visualization of SERT, GBR-12909 (10µM) and desipramine (10µM) were added to RTI-55 in order to block RTI-55 binding to DAT and NET. Nonspecific binding was measured on a second series by incubating the radioactive ligand in the presence of GBR (10µM), FLU (10µM) and desipramine (10µM). Sections were washed, dried and exposed to film. For calibration, 1 cm2 filter paper were impregnated with RTI-55 dilutions of 1µM, 0.5µM, 0.25µM, 0.125µM and 0.1µM, and exposed to film.
Scanned autoradiograms were analyzed quantitatively with NIH ImageJ software. Anatomical regions were identified according to a mouse brain atlas (Paxinos and Franklin, 2003). Grey levels of selected regions were measured in triplicate and converted to nCi/mm2 via the calibrated standard curve. Specific binding values were obtained by subtracting non-specific binding from total binding. The mean specific binding values in brain regions of En1+/Otx2 mice were compared statistically to the mean value of WT mice.
Transporter binding assays
Mice were decapitated and brain cerebral cortex and caudate putamen (CPu) were quickly removed and frozen on dry ice.
[3H]Citalopram binding
Brain tissue was thawed and homogenized in 50 vol. of ice-cold buffer (50mM Tris.HCl buffer, pH 7.4 containing 120 mM NaCl and 5mM KCl) with a Brinkman polytron. The homogenate was centrifuged at 30000×g for 10 min (three times). The final pellet was resuspended with the buffer to yield a tissue concentration of approximately 30mg/ml (wet weight). A standard binding assay contained: 100µl homogenate, 300µl buffer and 100µl [3H]citalopram (specific activity 79 Ci/mmol; Perkin Elmer Life Sciences, Boston MA,USA) at concentrations of 0.25- 5 nM. Non-specific binding was measured in the presence of 1 µM fluvoxamine. The tubes were incubated at 25°C for 60 min. The homogenate was filtered under vacuum on GF/C filters. The filters were subsequently washed rapidly, and the radioactivity retained on the filters counted using a liquid scintillation beta counter (Packard 1600 TR). The ligand affinity (Kd) to the serotonin transporter and the transporter density (Bmax) were assessed separately for each brain area by Scatchard analysis of the binding data.
[3H]GBR12935 binding
Striatal tissue was thawed and homogenized in 10 volumes of ice cold 0.32 M sucrose in a glass Teflon homogenizer. The homogenate was centrifuged at 1000×g for 10 min. The supernatant was centrifuged for 15 min at 27000×g. The pellet was resuspended in 40 volumes (based on tissue wet weight) of buffer (120mM NaCl, 50mM Tris HCl pH 7.4) using Brinkman Polytron and 0.5 ml of the suspension was saved for protein determination. The rest of the homogenate was diluted 5 times (total dilution of 200) in the same buffer containing 0.01% bovine serum albumin. A standard binding assay contained: 100µl homogenate (5–10 µg protein), 350µl buffer and 50µl [3H]GBR 12935 (specific activity 38.5 Ci/mmol; Perkin Elmer Life Sciences, Boston MA, USA) at concentrations of 0.50–8 nM. Non-specific binding was measured in the presence of 50 µM mazindol. The tubes were incubated at 25°C for 45 min. The homogenate was filtered under vacuum on GF/C filters. The filters were subsequently washed rapidly, and the radioactivity retained on the filters counted using a liquid scintillation beta counter (Packard 1600 TR). The ligand affinity (Kd) to the serotonin transporter and the transporter density (Bmax) were assessed by Scatchard analysis of the binding data.
Transmitter measurements
Brains of naïve male mice (6 WT, 6 En1+/Otx2) were dissected, shock frozen on dry ice and cut on a cryostat to obtain 200-µm thick-thick sections. Cortex and nucleus accumbens were recovered by “punching” the region of interest. DA and 5-HT and their metabolites were measured by HPLC according to Sillaber et al., (1998) and referred to tissue weight to obtain neurotransmitter concentration.
Statistical analysis
Statistics: Statistical analyses were done using Statistica version 8.0 (Statsoft, 2007).
RESULTS
Motor behaviour of En1+/Otx2 mutants after MP exposure
First, we compared different parameters of motor activity (horizontal locomotor activity, rearing, and stereotypies) between saline and MP treated mutants and WT (Fig. 1).
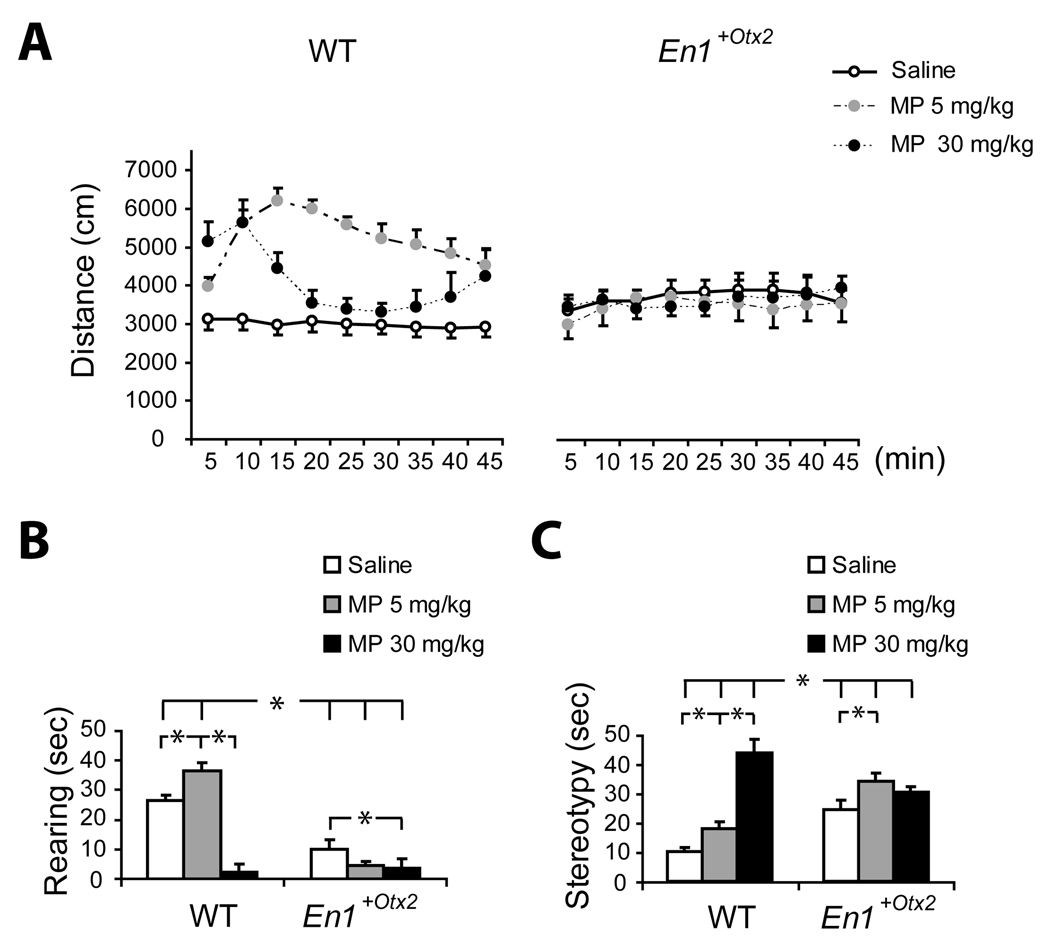
Fig. 1. Locomotor activity, rearing and stereotypies for animals treated with saline, 5 or 30 mg/kg MP.
A. Effect of MP on locomotor activity measured in distance traveled per 5 min time intervals (Mean ± SEM). WT mice showed increased activity following 5 mg/kg and 30 mg/kg MP. The hyperactivity elicited by the high dose declined rapidly, as stereotypy increased. Locomotor activity of En1+/Otx2 mutants after MP exposure was not significantly different from saline treated mutants.
B. Time (sec) spent engaged in rearing behaviour (Mean ± SEM) following MP treatment. The first minute of each 10 minute interval was sampled. Mutant mice have less rearing behaviour compared to WT after saline and 5 mg/kg MP, but following 30 mg/kg MP there was no difference between mutant and WT mice. In the mutant mice, 30 mg/kg MP significantly reduced rearing, and 5 mg/kg had no effect. In WT mice, 5 mg/kg MP increased rearing and 30 mg/kg decreased rearing.
C. Time (sec) spent engaged in stereotypic behaviour (Mean ± SEM) following MP treatment. The first minute of each 10 minute interval was sampled. WT animals treated with 5 or 30mg/kg MP show a significant increase in stereotypies. Mutant mice showed more stereotypy than WT mice following saline or the low dose of MP and less stereotypy than WT following the high dose of MP.
Both saline groups n=14, all other groups, n=8
Horizontal Locomotor Activity
There was a significant interaction between genotype, drug treatment and time, F (16, 448)=3.51, p<.00001 (adjusted after Greenhouse Geisser correction, p<.001), as well as significant interactions between time and treatment, genotype and treatment and main effect for treatment. The three way interaction was further analyzed by examining the difference between En1+/Otx2 and wild-type littermates for each drug treatment.
The WT mice showed significantly more activity than En1+/Otx2 mice following treatment with 5 mg/kg MP, F (1, 56) =27.89, p<.000005 as well as 30 mg/kg MP, F (1,56) =9.35, p<.005 (Fig. 1A). However, as suggested by the significant interaction between treatment, genotype and time, the pattern of locomotor activity in the WT mice following 30 mg/kg MP was biphasic. Twenty minutes from the start of the observation period, the locomotor activity was attenuated below the level observed with 5mg/kg MP. This reduction in locomotion was due to the increased stereotyped behaviour which precluded locomotor activity (Fig. 1C).
In contrast to the marked MP-induced hyperactivity in WT mice, there was no change in the level of locomotion between En1+/Otx2 mice treated with saline or with MP, 5 mg/kg F(1, 56) =.08, p=.77 or 30 mg/kg, F (1,56)=.003, p=.95. Confirming the previous studies (Brodski et al., 2003), the saline treated En1+/Otx2 mice were more active than the saline-treated WT mice, F (1,56)=4.50, p<.05 (Fig. 1).
Since in this experiment mice were tested twice in a counterbalanced order, we investigated whether the same pattern of results would be obtained if only the first session were analyzed. There was a significant three way interaction between dose, genotype and time F(16,208)=4.06, p<.001, indicating that even with a small number of mice, the WT mice showed a significant increase in activity after 5 mg/kg or 30 mg/kg MPH, p<.001, whereas the En1+/Otx2 did not show a change in activity.
Rearing
A preliminary analysis of the effects of genotype and treatment over time (repeated measure) revealed no main effect or interaction with time. The data for rearing and stereotypy are presented, therefore, as the total scores for the recording period. There were significant effects of genotype, F (1, 51)=79.4, p<.000001 and MP treatment, F (2, 51)= 35.82, p<.000001 on rearing behaviour, but these main effects were modulated by an interaction between genotype and MP, F (2,51)=25.6, p<.000001.
Planned comparisons of the rearing behaviour of the WT and En1+/Otx2 mice at each treatment showed that WT mice showed more rearing than mutant mice after injections of saline or 5 mg/kg MP, but not after 30 mg/kg MP. The high dose of MP led to a significant decrease in rearing in both WT and mutant mice, compared to the saline-treated mice (Fig. 1B).
Stereotypies
Similarly, there were significant effects of genotype, F (1, 51) = 7.07, p<.05, and MP treatment, F (2, 51) = 31.12, p<.000001 on stereotypy, but these main effects were modulated by an interaction between genotype and MP, F (2,51) = 19.3, p<.00001.
Contrast analysis of the interaction revealed that in the WT mice, both the low (t=2.21, p<.05) and high dose (t=9.87, p<.000001) of MP increased the level of stereotypy. In contrast, in the En1+/Otx2 mice, only the low dose of MP significantly increased stereotypy compared to saline treatment (t=2.66, p<.05). Comparing the two genotypes revealed that the En1+/Otx2 mice showed more stereotypy than the WT mice after treatment with saline or 5 mg/kg MP, but at the 30 mg/kg dose, the stereotypy of the WT mice exceeded that of the mutant mice (Fig. 1C).
Motor behaviour of En1+/Otx2 mice after AMPH exposure
In order to investigate whether En1+/Otx2 mutants are less responsive to the locomotor enhancing effects of another psychostimulant, AMPH, we tested the effect of two doses of this substance. Although, AMPH has similar behavioural and neurochemical effects as MP, there are significant differences between these substances, such as the ability of AMPH to increase 5-HT synaptic levels (Kuczenski and Segal, 1997).
Horizontal locomotor activity
Figure 2 shows a significant genotype x treatment interaction F (2, 43) = 5.74, p<.01. In addition, there was a significant interaction between time and genotype, F (11, 473) = 1.96, p<.05, however the adjusted p after the Greenhouse Geisser correction (p=.13) was not significant.
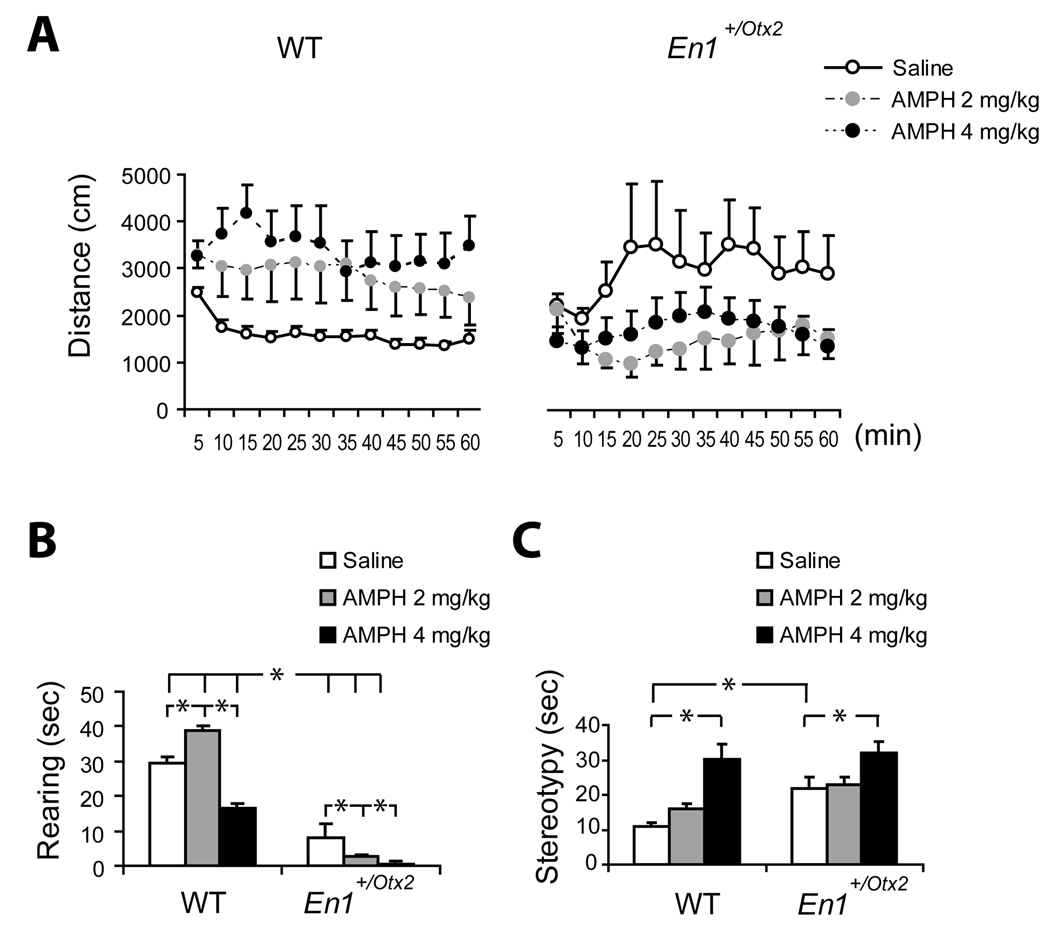
Fig. 2. Locomotor activity, rearing and stereotypies for animals treated with saline, 2 or 4mg/kg AMPH.
A. Effect of AMPH on locomotor activity measured in distance traveled per 5 min time interval (Mean ± SEM). In WT mice 2 as well as 4 mg/kg AMPH led to an increase in locomotor activity. En1+/Otx2 showed reduced activity after AMPH exposure and were more active than WT mice after saline treatment.
B. Time (sec) spent engaged in rearing behaviour (Mean ± SEM) following AMPH treatment. The first minute of each 10 minute interval was sampled. In the mutant mice, both doses of AMPH led to a decrease in rearing, whereas in WT mice decreased rearing was observed after the higher dose.
C. Time (sec) spent engaged in stereotypic behaviour (Mean ± SEM) following AMPH treatment. The first minute of each 10 minute interval was sampled. Stereotypies were significantly increased for wild-type as well as for mutants with the higher dose.
Both saline groups, n=11, WT 2 mg/kg and 4 mg/kg AMPH and En1+/Otx2 2 mg/kg, n=7, and En1+/Otx2 4 mg/kg, n=6.
The genotype x treatment interaction was analyzed using planned comparisons between the WT and En1+/Otx2 for each treatment. En1+/Otx2 mice were more active than the WT mice when treated with saline, t= 2.07, p<.05, confirming the results of the MP experiment and our previous studies (Brodski et al, 2003). Following 4 mg/kg AMPH, the mutant mice showed significantly less activity than WT mice, t=2.09, p<.05 and following 2 mg/kg AMPH there was no significant difference between the WT and mutant mice, t =1.81, p=.08.
Since the En1+/Otx2 mice appeared to show decreased activity following AMPH treatment, the activity of the mice treated with each of the two doses of AMPH was compared to the activity of the mice treated with saline. This analysis revealed a significant reduction in activity following 2 mg/kg AMPH, t =2.05, p<.05 but not following 4 mg/kg AMPH t =1.64, p=.11 (Fig. 2A).
Rearing
Significant effects of genotype, F (1,43)=240.6, p<.000001 and of treatment F (2,43)=20.7, p<.000005 on rearing were found, modified by a genotype x treatment interaction, F (2,43)=12.96, p<.00005. In the WT mice, rearing was significantly increased by 2 mg/kg AMPH (t=3.61, p<.001), and significantly decreased by 4 mg/kg AMPH (t=5.03, p<.00001). However, in the mutant mice, both doses of AMPH significantly decreased rearing (t=2.15, p<.05 for 2 mg/kg and t=2.90, p<.01 for 4 mg/kg) (Fig. 2B).
Stereotypies
There were more stereotypies in the mutant mice compared to the WT mice F (1, 43) = 8.72, p<.01. Mice treated with a high dose of AMPH showed more stereotypies compared to mice treated with saline or the low dose F (2, 43) = 16.6 p<.000005 (Fig. 2C), in both groups.
Motor behaviour of En1+/Otx2 mice after FLU exposure
Next, we investigated whether the increase of 5-HT induced by AMPH, but not by MP, could contribute to the different behavioural responses of mutants to these two substances. To this end we increased 5-HT levels in mutants by FLU.
Horizontal locomotor activity
The three way ANOVA for the effects of genotype, treatment (saline, 5 mg/kg or 10 mg/kg FLU) and time interval (repeated measure), after Greenhouse-Geisser adjustment for repeated measures, revealed a significant interaction between genotype, treatment and time, F (18, 270) = 2.53, unadjusted p<.001, (adjusted p<.05) as well as significant interactions between genotype and treatment F (2, 30) = 22.47, p<.000005, time and treatment F (18, 270) = 2.66, p<.0005 (adjusted p<.05).
In order to analyze the genotype by treatment interaction, planned comparisons were made between the En1+/Otx2 and WT mice at each dose level over the entire time period. This analysis replicated the finding that at baseline En1+/Otx2 mice showed more locomotor activity than the WT mice, F (1, 30) = 51.03, p<.000001. This difference was eliminated, however in mice treated with 5 mg/kg FLU, F (1, 30) = .003, p = .96 or 10 mg/kg FLU F (1, 30) = 3.42, p = .07. Further analysis revealed that in the first 40 minutes of the session, the mutant mice treated with 10 mg/kg FLU showed significantly lower locomotor activity than the WT mice with the same dose F (1, 30)=4.37, p< .05 (Fig. 3A).
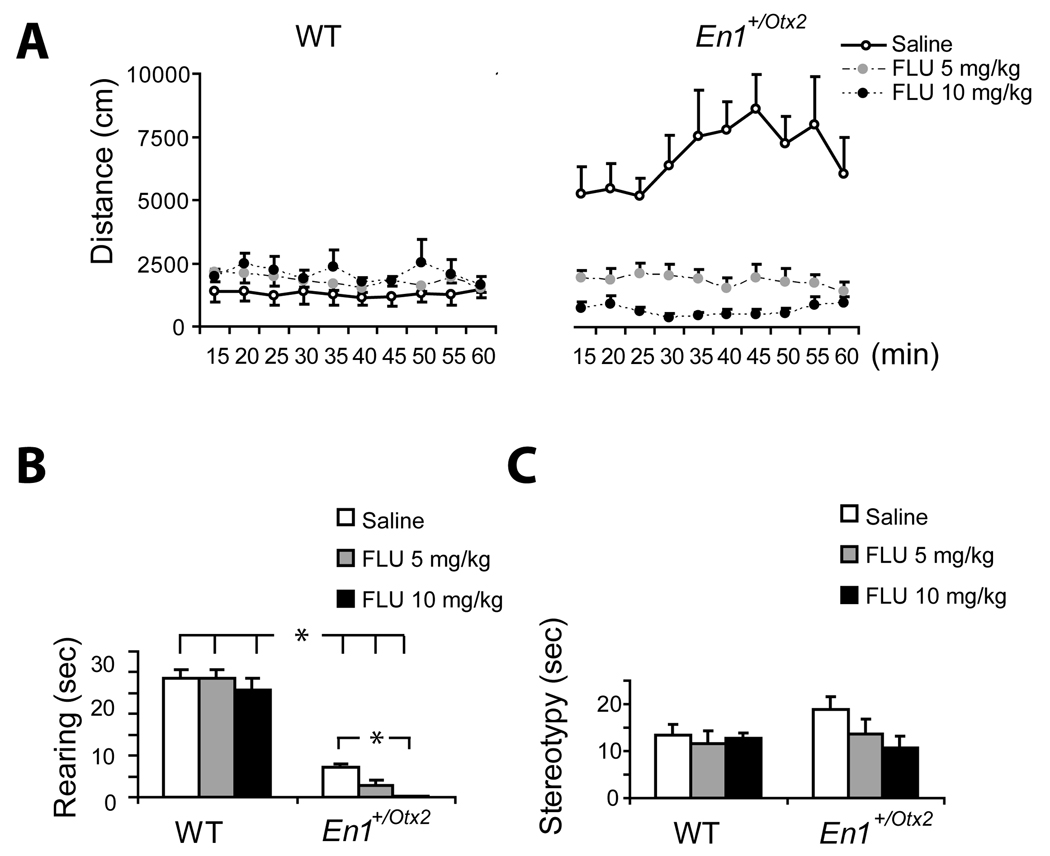
Fig. 3. Locomotor activity, rearing and stereotypies for animals treated with saline, 5 or 10 mg/kg FLU.
A. Effect of FLU on locomotor activity measured in distance traveled per 5 min time interval (Mean ± SEM). Saline treated En1+/Otx2 were more active than saline-treated WT mice. FLU treated mutants showed a dose dependant decrease in locomotor activity.
B. Time (sec) spent engaged in rearing behaviour (Mean ± SEM) following FLU treatment. The first minute of each 10 minute interval was sampled. FLU did not affect rearing in WT but the higher dose of FLU reduced rearing in mutants.
C. Time (sec) spent engaged in stereotypic behaviour (Mean ± SEM) following FLU treatment. The first minute of each 10 minute interval was sampled. There were 6 mice in each group.
Rearing
As shown in the previous experiments mutant mice had a significant lower rate of rearing F (1,30) = 274.9 p<.000001 which was further decreased by FLU treatment F (2,30) = 3.66, p<.05. Planned comparisons revealed that mutant but not WT mice treated with 10 mg/kg dose of FLU had significantly less rearing than the mice in the saline group, (t = 2.76, p<.01) (Figure 3B).
Stereotypies
The two way ANOVA for the effects of genotype, F (1,30)=.77, p=.38, and FLU treatment F (2,30)=1.08, p=.35, did not reveal any group differences for stereotypy (Figure 3C).
Center/Periphery ratio
To further explore the behaviour of En1+/Otx2 mice in the open field, we tested whether there is a correlation of the center/periphery ratio with the total level of activity. A significant correlation was found between the center/periphery ratio and the total level of activity (r=.68, <.01) in the En1+/Otx2 mutant mice, but not the WT mice (r=.002, p=.99) in saline-treated mice. Thus, the hyperactivity in the mutant mice could be a consequence of reduced anxiety which facilitates a broader range of exploration of an open field.
Elevated plus maze
In order to test the possibility that the mutants show less anxiety than WT, a separate cohort of mice was tested in the elevated plus maze (EPM) which is a standard test of anxiety that is sensitive to anxiolytic drugs (File 2001).
The ratio of time spent in the open arms to the total time in the maze was analyzed by ANOVA for the effect of genotype and sex. No significant effects of genotype, F (1,28) =.07, p= .79, sex F (1,28) =.15, p= .71 and no interaction between genotype and sex F (1,28) =.64, p= .43 were found. The En1+/Otx2 mice spent 30.5% (SD 37.1) of the total time in the open arms, whereas the WT mice spent 26.7% (SD 19.0) of the total time in the open arms. These data suggest that hyperactivity of En1+/Otx2 mutants is not linked to altered anxiety levels of these animals.
SERT and DAT Autoradiography in En1+/Otx2 mutants
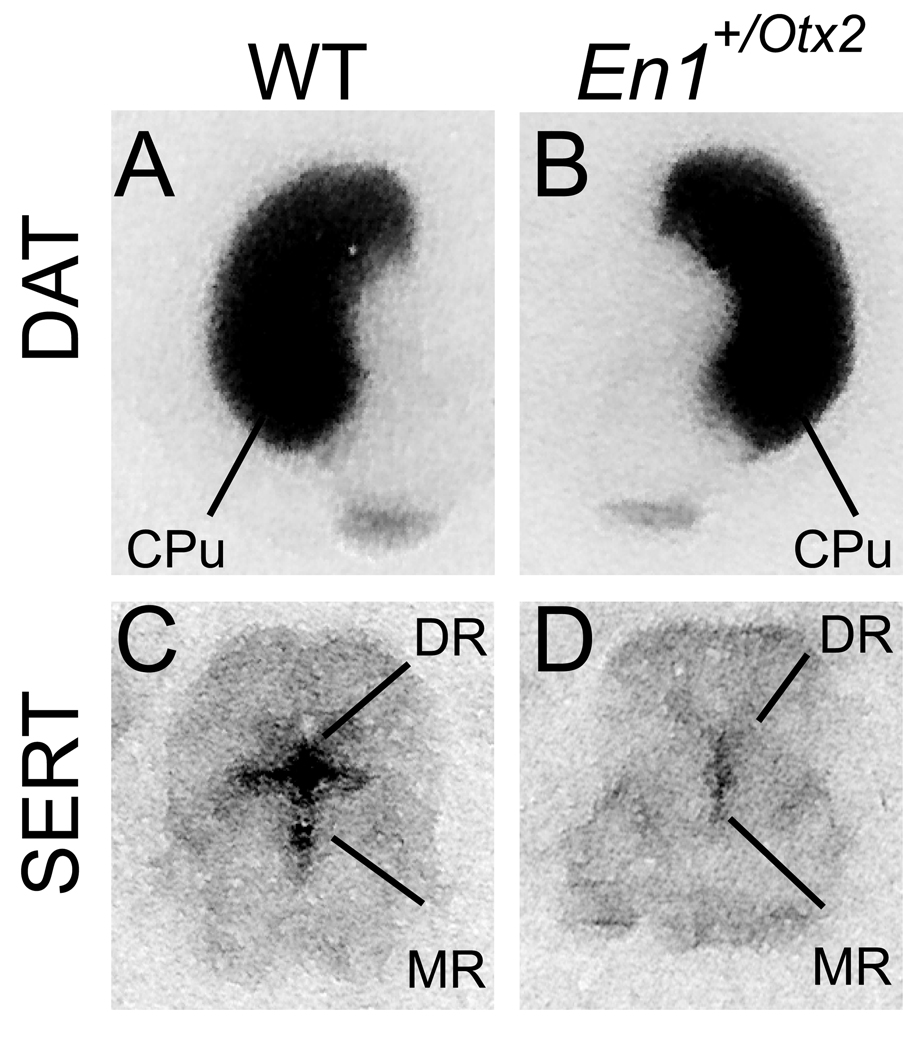
In order to characterize in mutants changes in the monoaminergic system associated with the altered response to psychostimulants we investigated DA and 5-HT innervation using [125I]-RTI-55 autoradiography in these animals (Fujita et al., 1994).
T tests comparing the mutant and WT mice indicated lower SERT binding in each of the regions tested: CPu, nucleus accumbens, substantia nigra, raphe nucleus, frontal cortex and prefrontal cortex.
In contrast, the comparison for DAT binding revealed no significant differences between WT and mutant mice in the CPu, nucleus accumbens, ventral tegmental area, substantia nigra, frontal cortex, prefrontal cortex. (Table 1, Fig. 4)
Table 1. Quantitative autoradiographic analysis of specific [125I]RTI-55 binding to 5-HT and DA uptake sites in WT and En1+/Otx2brains.
Values represent the mean binding density measured in triplicates and converted to nCi/mm2 via the calibrated standard curve. Asterisks indicate significant difference in t testing (p<0.05)
| WT | En1+/Otx2 | |||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | t-value | p-value | |
| SERT | ||||
| Caudate putamen | 0.083 (.02) | 0.048 (.007) | 4.26 | 0.003* |
| Nucleus accumbens | 0.075 (.02) | 0.038 (.004) | 3.86 | 0.005* |
| Frontal cortex | 0.041 (.006) | 0.020 (.002) | 7.09 | 0.0001* |
| Prefrontal cortex | 0.043 (.007) | 0.024 (.001) | 6.03 | 0.003* |
| Substantia nigra | 0.13 (.02) | 0.037 (.006) | 9.47 | 0.00001* |
| Raphe nuclei | 0.15 (.03) | 0.063 (.02) | 6.28 | 0.0002* |
| DAT | ||||
| Caudate putamen | 0.36 (.71) | 0.46 (.14) | 1.57 | 0.16 |
| Nucleus accumbens | 0.17 (.06) | 0.20 (.03) | 0.829 | 0.44 |
| Frontal cortex | 0.016 (.001) | 0.014 (.002) | 1.13 | 0.29 |
| Prefrontal cortex | 0.022 (.004) | 0.019 (.005) | 0.99 | 0.35 |
| Substantia nigra | 0.13 (.05) | 0.17 (.06) | 1.22 | 0.26 |
| Ventral tegmental area | 0.19 (.05) | 0.21 (.05) | 0.73 | 0.49 |
Fig. 4. Representative autoradiograms of [125I]RTI-55 binding to coronal sections of WT and mutant brains.
A, B visualization of DAT. C, D visualization of SERT. SERT binding was significantly reduced in the dorsal as well as in the median raphe. Caudate Putamen (CPu), dorsal raphe nucleus (DR), median raphe nucleus (MR).
DAT and SERT binding assays with En1+/Otx2 mutants
In mutants a small (15%) increase of DA cells, associated with the substantia nigra and an increase in striatal DA has been reported (Brodski et al., 2003). In order to detect subtle changes in DA innervation, we used a highly sensitive saturation binding assay, labeling DAT with [3H]GBR12935.
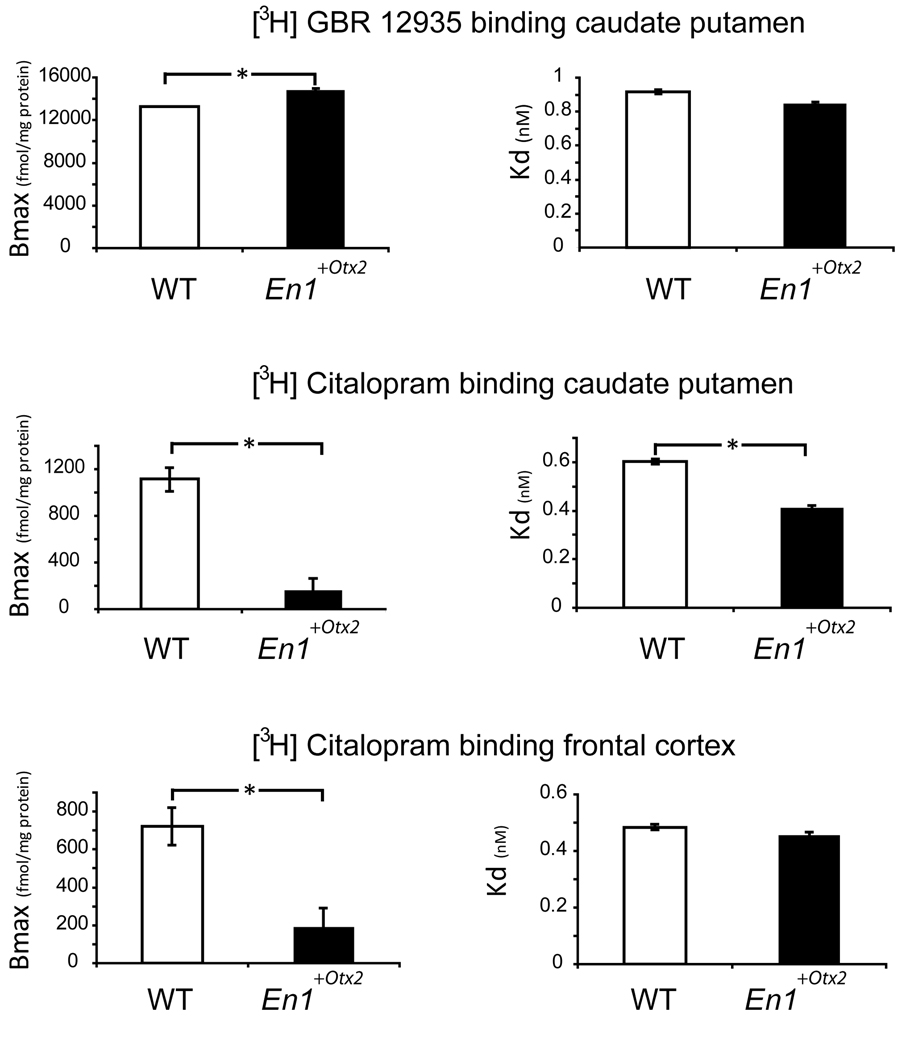
The maximal binding capacity (Bmax) of [3H]GBR12935 binding was higher in the CPu (n=10) of the En1+/Otx2 mutants as compared to the WT (14836±1346 vs. 13263±1809 fmol/mg protein, p< 0.05, df=18, t=−2.2). The affinity (Kd) of [3H]GBR12935 to the DA transporter was indistinguishable between the two groups (0.85±0.13 vs. 0.92±0.17 nM)
In addition, we used [3H]citalopram to quantify changes in SERT in mutant animals. The maximal binding capacity (Bmax) of [3H]citalopram was significantly lower in the CPu (n=5) as well as in the cerebral cortex (n=6) of the En1+/Otx2 mutants as compared to the WT (CPu:162 ± 19 vs. 1115 ± 202 fmol/mg protein, p<0.0001, df=8, t= 10.5; cortex: 145 ± 22 vs. 794 ± 154 fmol/mg protein, p<0.0001, df=10, t=10.2). The affinity (Kd) of [3H]citalopram to the serotonin transporter was significantly lower in the CPu (n=5) of the mutants as compared to the WT and tended to be lower in the cerebral cortex (n=6) of the mutants compared to the WT (CPu: 0.41 ± 0.05 vs. 0.60 ± 0.05 nM, p<0.0001, df=8, t=5.9; cortex: 0.45 ± 0.07 vs. 0.53 ± 0.06 nM, p=0.053, df=10, t=2.2) (Fig. 5).
Fig. 5. Maximal binding capacity (Bmax) and affinity (Kd) of [3H]GBR12935 and [3H]Citalopram in WT and En1+/Otx2 mutants.
DAT measured by [3H]GBR12935 binding was significantly increased in the caudate putamen of mutants. SERT measured by [3H]Citalopram binding was significantly reduced in the caudate putamen and in the frontal cortex of mutants. Values are expressed as mean ± SD.
Asterisks indicate significant difference in t testing (p<0.05)
DA and 5-HT tissue concentrations in the nucleus accumbens and frontal cortex of En1+/Otx2 mice
Earlier experiments have shown that En1+/Otx2 mice show an increase in DA concentration in the striatum and a decrease in serotonin. In order to further characterize the neurochemical changes in these mutants, we measured the concentration of these two neurotransmitters in the cortex and nucleus accumbens of WT and mutants, by high performance liquid chromatography (HPLC). In addition, we assessed the metabolites of these neurotransmitters, HVA, DOPAC and 5-HIAA (Table 2).
Table 2.
Tissue concentrations of dopamine (DA), homovanillic acid (HVA), dihydroxyphenylacetic acid (DOPAC), serotonin (5-HT), 5-hydroxyindoleacetic acid (5-HIAA) of WT and En1+/Otx2 mutants. Values are reported as ng/mg tissue weight. Asterisks indicate significant difference in t testing (p<0.05).
| Nucleus Accumbens |
Frontal Cortex |
|||
|---|---|---|---|---|
| Substance | WT | En1+/Otx2 | WT | En1+/Otx2 |
| DA | 23.08 (10.19) | 21.35 (7.78) | 0.35 (0.19) | 0.56 (0.31) |
| HVA | 4.66 (1) | 4.18 (1.64) | 0.54 (0.15) | 0.74 (0.21) |
| DOPAC | 12.31 (4.03) | 9.68 (6.28) | 0.32 (0.08) | 0.56 (0.18)* |
| 5-HT | 0.9 (0.40) | 0.5 (0.18)* | 0.79 (0.22) | 0.26 (0.11)* |
| 5-HIAA | 1.32 (0.47) | 0.62 (0.20)* | 0.62 (0.31) | 0.23 (0.07)* |
DA concentrations in the cortex of WT mice did not differ significantly (t=1.22, p=0.29) from En1+/Otx2 mice. Also the DA metabolite HVA did not show a significant difference between WT and En1+/Otx2 mice (t= 1.89 p=0.09). In contrast, the concentration of the DA metabolite DOPAC was significantly higher (t=2.95, p<0.015) in mutants compared to WT mice.
In the nucleus accumbens, the DA (t=0.34, p=0.75), HVA (t=0.61, p=0.55) and DOPAC (t=0.86, p=0.41) concentrations of En1+/Otx2 mice were not significantly different from those of WT mice.
The 5-HT and 5-HIAA concentration in the cortex of En1+/Otx2 mice were significantly decreased compared to WT animals (t=9.92, p<.0005 and t=3.10, p<0.01, respectively). A similar decrease in serotonin and 5-HIAA concentrations was found in the nucleus accumbens of En1+/Otx2 mice compared with WT (t=2.78, p<.05 and t=3.31, p=0.01, respectively).
DISCUSSION
Here we report that En1+/Otx2 mice do not show increased locomotor activity after MP exposure and even show a reduction after treatment with AMPH. Increasing 5-HT activity in these animals with FLU leads to a robust reduction of locomotor activity. Transmitter measurements, transporter binding assays and autoradiographic studies indicate that these mutants show a subtle increase in the nigrostriatal innervation and a general dramatic reduction of 5-HT innervation.
Alterations of the DA and 5-HT system in En1+/Otx2 mice
The measurement of tissue concentrations of 5-HT and SERT binding studies in En1+/Otx2 mice demonstrated a dramatic reduction in serotonergic innervation in all measured brain areas. These results are in accordance with the earlier reported reduction of the number of 5-HT neurons in the raphe nuclei (Brodski et al., 2003) which project to a wide area of brain regions (Tork, 1990).
Our binding assays revealed a significant increase of DAT expression in the CPu of En1+/Otx2 mice. We did not assess DAT expression in the cerebral cortex by binding studies as the high concentration of the piperazine acceptor sites in this brain area interferes with the binding of [3H]GBR12935 to the DA transporter (Gordon et al. 1995).
In contrast to the binding assay, the autoradiography showed a trend for an increase of DAT in En1+/Otx2 mice in the CPu, which did not reach statistical significance. We attribute this discrepancy, to the relatively subtle increase of the DA neurons (15%) in mutants and to the use of a single point analysis that does not enable the calculation of Bmax. This small increase could be detected by the more sensitive saturation binding assay. A specific increase in striatal innervation is in accordance with previous findings indicating that mutants show a significant increase in the DA substantia nigra cells, which project to the striatum, but not in the ventral tegmental area cells, which project to the accumbens and cortex (Brodski et al., 2003). In the present study, the decrease in the 5-HT projections was more pronounced than the subtle increase in DA projections, in accordance with the previous difference observed in transmitter measurements in these mice (Brodski et al., 2003).
Effects of AMPH and MP on the motor activity of En1+/Otx2 mutants
Consistent with previous findings En1+/Otx2 mice showed an increase in locomotor activity, compared to WT animals in all three behavioural experiments (Brodski et al., 2003). However, WT as well as of En1+/Otx2 mutants showed some variability in their baseline locomotor activity, between different experiments. We attribute these fluctuations to small changes in the experimental environment and to the outbred CD-1 background of the mice, which can increase variability. In order to avoid having the genetic background confound the analysis of mutants, standards have been suggested for the choice of WT control animals. Implementing these precautions allow the reliable characterization of mutant animals of outbred strains within an experiment (Wolfer et al., 2002; Crusio et al., 2009). To this end, we used in each experiment WT control animals that were littermates of En1+/Otx2 mice. In addition, equal numbers of WT and mutant littermates were taken from different litters. Finally, WT and mutants were derived from at least 5 different breeding pairs for each experiment. Therefore, we do not expect that differences observed between WT and En1+/Otx2 are significantly confounded by the genetic background.
Rodents show a typical dose-dependent behavioural response to AMPH and MP. Vertical activity is increased first, followed by a prolonged period of enhanced horizontal locomotion, which is then displaced by stereotypic behaviour at higher doses (Fog et al., 1969; Segal and Kuczenski, 1987; Yates et al., 2007). The activating effects of lower and medium doses of psychostimulants are initiated by increased DA transmission in the nucleus accumbens. In contrast, stereotypies induced by high doses have been associated with augmented DA activity in the striatum (Kelly et al., 1975; Kelly et al., 1976; Sellings et al., 2003).
In our experiments, WT mice showed, as expected, a strong increase in activity after the lower dose of MP (5 mg/kg) and AMPH (2 mg/kg). At a higher dose of MP (30 mg/kg), the initial rise in horizontal and vertical activity decreased rapidly, due to the increase in stereotypies. In contrast, stereotypies induced by a higher dose of AMPH (4 mg/kg) led only to a decrease in rearing and but did not affect the prolonged rise in horizontal activity. The differences between MP and AMPH are in accordance with previous observations indicating that a dose of 30 mg/kg MP is more potent in inducing stereotypies than a dose of 4 mg/kg AMPH (Pechnick et al., 1979).
En1+/Otx2 mice showed a very different behaviour pattern compared to WT animals. The lack of increased locomotor activity after psychostimulants, suggest that En1+/Otx2 mice show a decreased sensitivity to these substances.
Although we did not detect any alterations of DA concentrations in the frontal cortex of En1+/Otx2 mice, the augmented DOPAC level in this region suggests increased DA turnover in these animals. Previous findings indicate that DA activation in the frontal cortex blocks the locomotor-activating effects of psychostimulants mediated by the nucleus accumbens (Vezina et al., 1991). Therefore, increased turnover of DA in the frontal cortex in En1+/Otx2 mutants could contribute to the lack of psychostimulant- induced hyperactivity in mutants.
Interestingly, a complementary mouse model in which the MHO was shifted rostrally leads to an increased sensitivity to amphetamine (Borgkvist et al., 2006). Taken together, we conclude that the position of the MHO plays a central role in psychostimulant response
AMPH and MP are chemically related substances having partially overlapping neurochemical and behavioural effects. One important difference however, is the ability of AMPH to increase 5-HT synaptic levels (Kuczenski et al., 1989; Kuczenski et al., 1997). It is, therefore, conceivable that the observed increase in 5-HT might account for the behavioural differences. Indeed, the pronounced decrease in activity following FLU suggests that the reduction of locomotor activity in En1+/Otx2 mutants observed after exposure to AMPH, but not to MP, is mediated by an AMPH-induced increase in 5-HT. The inhibitory effects of 5-HT induced by AMPH and FLU might be particularly pronounced due to a possible compensatory upregulation of 5-HT receptors in these mutants.
Effects of FLU on En1+/Otx2 mice
Since there was a dissociation between rearing and stereotyping, these behaviours were analyzed separately. Rearing in mutants was decreased by both psychostimulants and FLU. In contrast, stereotypies were not increased by FLU, as they were following treatment with the psychostimulants. There was even a tendency for FLU to reduce stereotypic behaviour in mutants, which did not, however, reach statistical significance. These results are in accordance with previous findings suggesting that FLU does not increase stereotypies, but does rather decrease this behaviour under different experimental conditions and species (Wennemer and Kornetsky 1999; Hugo et al., 2003). Importantly, these results demonstrate that the decrease of locomotor activity in En1+/Otx2 mutants is not due to an increase in stereotypies.
The robust decrease of hyperactivity in mutants by FLU further supports the importance of 5-HT in controlling activity levels in interaction with DA. Hyperdopaminergic and hyperactive Dat knockout mice show normal levels of 5-HT. Interestingly, increasing 5-HT levels in these animals, led to a reduction of locomotor activity (Gainetdinov et al., 1999). Taken together, these findings suggest that activation of the 5-HT system can very effectively block DA induced hyperactivity.
A wide range of observations has implicated 5-HT in motor activity control. The functional interaction between the 5-HT and DA system are particularly well documented and relevant for the effects of 5-HT on locomotor activity (Di Giovanni et al., 2008; Di Matteo et al., 2008; Rothman et al., 2006). 5-HT neurons project to the ventral tegmental area, the substantia nigra, as well as to their terminal fields, striatum, nucleus accumbens and cortex. Interestingly, the substantia nigra receives the densest 5-HT innervation of the brain (Di Giovanni et al., 2008).
5-HT has complex effects on the activity of midbrain DA neurons, depending on the activation of the various 5-HT receptor subtypes and the state of activation of DA neurons (Di Giovanni et al., 2008; Di Matteo et a., 2008; Rothman et al., 2006). However, the main control seems to be inhibitory (Di Giovanni et al., 2008; Rothman et al., 2006). Electrical stimulation of the raphe nuclei was shown to inhibit the activity of DA neurons (Gervais and Ruillard, 2000). Consistently, the selective lesions of 5-HT neurons enhanced the firing activity of DA neurons (Guiard et al., 2008). Conversely, FLU resulted in a dose-dependent inhibition of the firing rate of the DA neurons (Prisco and Espositio, 1995). Finally, drugs releasing 5-HT, antagonize the stimulant properties of DA-releasing drugs (Rothman et al., 2006).
Alterations in En1+/Otx2 not connected to monoaminergic neurons
Can all the behavioural alterations in En1+/Otx2 mice described here be explained by a change in monoaminergic neurons? Among ventrally developing nuclei only monoaminergic neurons are known to be affected in En1+/Otx2 mutants. Other neuronal populations, forming immediately rostral or caudal to the MHO like the nucleus oculomotorius, trochlearis, trigeminalis and the pedunculopontine nucleus are not affected (Brodski et al., 2003). However, among dorsally-derived structures, the shift leads to an enlargement of the inferior colliculus and to a decrease of the cerebellar vermis (Broccoli et al., 1999). So far there is no evidence that the inferior colliculus is directly involved in the behavioural phenotype of En1+/Otx2 mice.
The function of the cerebellum for motor coordination is very well established. Hence, the most likely explanation for the postural asymmetry and ataxia observed in En1+/Otx2 mutants is a reduction in the cerebellar vermis (Brocolli et al., 1999). The cerebellar vermis has also been associated with coordinated locomotion (Mori et al., 1999). However, hyperactivity of En1+/Otx2 mutants is not correlated to their atactic behaviour and can be reversed by FLU. It therefore seems more likely that hyperactivity of these animals results from an increase in striatal DA and a decrease in 5-HT, rather than to a cerebellar defect (Brodski et al., 2003).
There are some reports on psychostimulant effects on the vermis. These include evidence that repeated metamphetamine exposure regulates gene expression in the rodent vermis (Hamamura et al., 1999). In humans, the effect of MP on the regional cerebral blood flow of the vermis predicted the therapeutic response in adults with Attention Deficit Hyperactive Disorder (ADHD) (Anderson et al., 2002; Schweitzer et al., 2003). However, the primary molecular and cellular targets for psychostimulants are neurotransmitter transporters located on monoaminergic neurons (Elliott and Beveridge 2005). We therefore favor the view that the altered response in psychostimulants in En1+/Otx2 mutants is caused primarily by changes in the development of monoaminergic neurons in these animals.
MHO genes and human disorders
Genes controlling the positioning and activity of the MHO have been associated with a variety of psychiatric disorders. The secreted molecule WNT1 is a central component of the MHO and has been implicated, together with its downstream signalling pathways, in schizophrenia (Kozlovsky et al., 2000; Emamian et al., 2004; Miyaoka et al., 1999). The transcription factor, En2, a close homologue of En1 and also expressed at the MHO, has been associated in different genetic studies with autism (Petit et al., 1995; Kuemerle et al, 2007). Finally Otx2 has recently been identified as a possible susceptibility gene for bipolar disorder (Sabunciyan et al., 2007). Alterations in monoaminergic neurotransmission, changed sensitivity to psychostimulant response and stereotypies reported here in En1+/Otx2 mutants are important aspects of the above-mentioned disorders. Our results, therefore, indicate that En1+/Otx2 mice are a useful tool to model gene-behaviour interactions related to these disorders.
Acknowledgements
This work was supported by research grants to C.B. from the National Institute for Psychobiology in Israel – Founded by the Charles E. Smith Family (7-2004-5, 212-2007-08), the Israel Science Foundation grant No. 864/05 and by the Israeli Ministry of Health, Chief Scientist's Office (3-00000-3546).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson CM, Polcari A, Lowen SB, Renshaw PF, Teicher MH. Effects of methylphenidate on functional magnetic resonance relaxometry of the cerebellar vermis in boys with ADHD. Am J Psychiatry. 2002;159:1322–1328. doi: 10.1176/appi.ajp.159.8.1322. [DOI] [PubMed] [Google Scholar]
- Björklund A, Lindvall O. Handbook of Chemical Neuroanatomy, Vol. 2, Classical Transmitter in the CNS, Part I. Amsterdam: Elsevier; 1984. dopamine containing systems in the CNS. [Google Scholar]
- Borgkvist A, Puelles E, Carta M, Acampora D, Ang SL, Wurst W, Goiny M, Fisone G, Simeone A, Usiello A. Altered dopaminergic innervation and amphetamine response in adult Otx2 conditional mutant mice. Mol Cell Neurosci. 2006;31:293–302. doi: 10.1016/j.mcn.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Breese GR, Cooper BR, Mueller RA. Evidence for involvement of 5-hydroxytryptamine in the actions of amphetamine. Br J Pharmacol. 1974;52(2):307–314. doi: 10.1111/j.1476-5381.1974.tb09714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccoli V, Boncinelli E, Wurst W. The caudal limit of Otx2 expression positions the isthmic organizer. Nature. 1999;401:164–168. doi: 10.1038/43670. [DOI] [PubMed] [Google Scholar]
- Borycz J, Zapata A, Quiroz C, Volkow ND, Ferré S. 5-HT 1B receptor-mediated serotoninergic modulation of methylphenidate-induced locomotor activation in rats. Neuropsychopharmacology. 2008 Feb;33(3):619–626. doi: 10.1038/sj.npp.1301445. [DOI] [PubMed] [Google Scholar]
- Brodski C, Weisenhorn DM, Signore M, Sillaber I, Oesterheld M, Broccoli V, Acampora D, Simeone A, Wurst W. Location and size of dopaminergic and serotonergic cell populations are controlled by the position of the midbrain-hindbrain organizer. J Neurosci. 2003;23:4199–4207. doi: 10.1523/JNEUROSCI.23-10-04199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8(1):1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Pierucci M, Esposito E. Serotonin-dopamine interaction: electrophysiological evidence. Prog Brain Res. 2008;172:45–71. doi: 10.1016/S0079-6123(08)00903-5. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Pierucci M, Esposito E. Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Prog Brain Res. 2008;172:7–44. doi: 10.1016/S0079-6123(08)00902-3. [DOI] [PubMed] [Google Scholar]
- Elliott JM, Beveridge TJ. Psychostimulants and monoamine transporters: upsetting the balance. Curr Opin Pharmacol. 2005;5(1):94–100. doi: 10.1016/j.coph.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36(2):131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125(1–2):151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- Fog R. Stereotyped and non-stereotyped behaviour in rats induced by various stimulantdrugs. Psychopharmacologia. 1969;14(4):299–304. doi: 10.1007/BF02190114. [DOI] [PubMed] [Google Scholar]
- Fujita M, Shimada S, Fukuchi K, Tohyama M, Nishimura T. Distribution of cocaine recognition sites in rat brain: in vitro and ex vivo autoradiography with [125I]RTI-55. J Chem Neuroanat. 1994;7(1–2):13–23. doi: 10.1016/0891-0618(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Gervais J, Rouillard C. Dorsal raphe stimulation differentially modulates dopaminergic neurons in the ventral tegmental area and substantia nigra. Synapse. 2000;35(4):281–291. doi: 10.1002/(SICI)1098-2396(20000315)35:4<281::AID-SYN6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Gordon I, Weizman R, Rehavi M. [3H]GBR 12935 labels mainly the piperazine acceptor site in the rat prefrontal cortex. Brain Res. 1995;674(2):205–210. doi: 10.1016/0006-8993(95)00007-d. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol. 2008;11(5):625–639. doi: 10.1017/S1461145707008383. [DOI] [PubMed] [Google Scholar]
- Hamamura M, Ozawa H, Kimuro Y, Okouchi J, Higasa K, Iwaki A, Fukumaki Y. Differential decreases in c-fos and aldolase C mRNA expression in the rat cerebellum after repeated administration of methamphetamine. Brain Res Mol Brain Res. 1999;64:119–131. doi: 10.1016/s0169-328x(98)00306-4. [DOI] [PubMed] [Google Scholar]
- Hugo C, Seier J, Mdhluli C, Daniels W, Harvey BH, Du Toit D, Wolfe-Coote S, Nel D, Stein DJ. Fluoxetine decreases stereotypic behavior in primates. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):639–643. doi: 10.1016/S0278-5846(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;(6):820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94(3):507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40(1):45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. Low GSK-3beta immunoreactivity in postmortem frontal cortex of schizophrenic patients. Am J Psychiatry. 2000;157(5):831–833. doi: 10.1176/appi.ajp.157.5.831. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioural and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;(6):2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997 May;68(5):2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Kuemerle B, Gulden F, Cherosky N, Williams E, Herrup K. The mouse Engrailed genes: a window into autism. Behav Brain Res. 2007;176(1):121–132. doi: 10.1016/j.bbr.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez S, Alvarado-Mallart RM. Expression of the homeobox Chicken gene in chick/quail chimeras with inverted mes-metencephalic grafts. Dev Biol. 1990;139:432–436. doi: 10.1016/0012-1606(90)90312-7. [DOI] [PubMed] [Google Scholar]
- Millet S, Campbell K, Epstein DJ, Losos K, Harris E, Joyner AL. A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature. 1999;401:161–164. doi: 10.1038/43664. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K. Stimulation of a restricted region in the midline cerebellar white matter evokes coordinated quadrupedal locomotion in the decerebrate cat. J Neurophysiol. 1999;82:290–300. doi: 10.1152/jn.1999.82.1.290. [DOI] [PubMed] [Google Scholar]
- Miyaoka T, Seno H, Ishino H. Increased expression of Wnt-1 in schizophrenic brains. Schizophr Res. 1999;38(1):1–6. doi: 10.1016/s0920-9964(98)00179-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Second Edition. Academic Press; 2003. [Google Scholar]
- Pechnick R, Janowsky DS, Judd L. Differential effects of methylphenidate and d-amphetamine on stereotyped behavior in the rat. Psychopharmacology (Berl) 1979;65(3):311–315. doi: 10.1007/BF00492220. [DOI] [PubMed] [Google Scholar]
- Petit E, Hérault J, Martineau J, Perrot A, Barthélémy C, Hameury L, Sauvage D, Lelord G, Müh JP. Association study with two markers of a human homeogene in infantile autism. J Med Genet. 1995;32(4):269–274. doi: 10.1136/jmg.32.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisco S, Esposito E. Differential effects of acute and chronic FLU administration on the spontaneous activity of dopaminergic neurones in the ventral tegmental area. Br J Pharmacol. 1995;116(2):1923–1931. doi: 10.1111/j.1476-5381.1995.tb16684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles E, Annino A, Tuorto F, Usiello A, Acampora D, Czerny T, Brodski C, Ang SL, Wurst W, Simeone A. Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development. 2004;131:2037–2048. doi: 10.1242/dev.01107. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Balance between dopamine and serotonin release modulates behavioural effects of amphetamine-type drugs. Ann N Y Acad Sci. 1074:245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- Sabunciyan S, Yolken R, Ragan CM, Potash JB, Nimgaonkar VL, Dickerson F, Llenos IC, Weis S. Polymorphisms in the homeobox gene OTX2 may be a risk factor for bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1083–1086. doi: 10.1002/ajmg.b.30523. [DOI] [PubMed] [Google Scholar]
- Scheel-Krüger J. Comparative studies of various amphetamine analogues demonstrating different interactions with the metabolism of the catecholamines in the brain. Eur J Pharmacol. 1971;14(1):47–59. doi: 10.1016/0014-2999(71)90121-x. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Lee DO, Hanford RB, Tagamets MA, Hoffman JM, Grafton ST, Kilts CD. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28:967–973. doi: 10.1038/sj.npp.1300110. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioural characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242(3):917–926. [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23(15):6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillaber I, Montkowski A, Landgraf R, Barden N, Holsboer F, Spanagel R. Enhanced morphine-induced behavioural effects and dopamine release in the nucleus accumbens in a transgenic mouse model of impaired glucocorticoid (type II) receptor function: influence of long-term treatment with the antidepressant moclobemide. Neuroscience. 1998;85:415–425. doi: 10.1016/s0306-4522(97)00607-6. [DOI] [PubMed] [Google Scholar]
- StatSoft, Inc. STATISTICA (data analysis software system), version 8.0. 2007 www.statsoft.com.
- Tork I. Anatomy of the serotonergic system. Ann. N. Y. Acad. Sci. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. [DOI] [PubMed] [Google Scholar]
- Vezina P, Blanc G, Glowinski J, Tassin JP. Opposed Behavioural Outputs of Increased Dopamine Transmission in Prefrontocortical and Subcortical Areas: A Role for the Cortical D-1 Dopamine Receptor. Eur J Neurosci. 1991;3(10):1001–1007. doi: 10.1111/j.1460-9568.1991.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Wennemer HK, Kornetsky C. Fluoxetine blocks expression but not development of sensitization to morphine-induced oral stereotypy in rats. Psychopharmacology (Berl) 1999;146(1):19–23. doi: 10.1007/s002130051083. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170(3):320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Crusio WE, Lipp HP. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25(7):336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- Yates JW, Meij JT, Sullivan JR, Richtand NM, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neurosci Lett. 2007;427(1):66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]