Summary
For migrating cells, budding yeast, and many other cells, it is critical that polarization occur towards one, and only one, site (the singularity rule). Polarity establishment involves amplification of Cdc42 foci via positive feedback, but the basis for singularity was unclear. To assess whether or not singularity is linked to Cdc42 amplification, we disabled the yeast cell’s endogenous amplification mechanism and synthetically re-wired the cells to employ a different positive feedback loop to generate Cdc42 foci. Re-wired cells violated the singularity rule, occasionally making two buds. Mathematical modeling indicated that, given sufficient time, competition between foci would promote singularity. In re-wired cells, slower competition sometimes resulted in a failure to develop a single “winning” focus before budding. Manipulations predicted to slow competition in normal cells also allowed occasional formation of two buds, suggesting that singularity is enforced by rapid competition between Cdc42 foci.
Keywords: CDC42, actin, polarity, yeast, positive feedback, symmetry breaking
Introduction
A polarized cell usually has a single directional axis: a “front” and a “back”. One of the central questions in polarity establishment concerns how cells polarize to one and only one "front" (here referred to as singularity). Singularity does not depend on pre-oriented polarization cues, because in many cases cells deprived of such cues polarize spontaneously towards a randomly oriented, but nevertheless unique, "front" (Wedlich-Soldner and Li, 2003). Although much has been learned about the molecular components responsible for polarity establishment, the basis for the singularity of polarization remains unclear.
Polarity establishment in animals and fungi involves the highly conserved Rho-family GTPase, Cdc42p (Etienne-Manneville, 2004). Polarization signals act through Cdc42p-directed guanine nucleotide exchange factors (GEFs) and/or GTPase activating proteins (GAPs) to trigger the localized accumulation of membrane-bound GTP-Cdc42p at the site destined to become the "front" of the cell. This localized GTP-Cdc42p then organizes cytoskeletal elements through a variety of effectors to yield the polarized morphology appropriate to the cell type (Etienne-Manneville, 2004).
The budding yeast Saccharomyces cerevisiae has served as a tractable model system to investigate the mechanism of polarity establishment (Park and Bi, 2007). In this system, when a cell commits to begin a new cell cycle, activation of G1 cyclin/cyclin-dependent kinase (CDK) complexes triggers rapid actin polarization leading to bud formation (Lew and Reed, 1993). Polarization is normally directed to predictable sites by the "bud site selection" machinery, which employs fixed landmarks that communicate with the Cdc42p system via the Ras-related GTPase Rsr1p. However, mutational inactivation of that machinery (e.g., in rsr1Δ mutants) does not block polarization: it simply randomizes the budding location (Park and Bi, 2007). Importantly, such "symmetry breaking" polarization occurs with a timing, efficiency, and singularity similar to that in wild-type cells (Bender and Pringle, 1989; Chant and Herskowitz, 1991).
Theoretical analyses of how cells with initially homogeneous distributions of polarity factors might develop asymmetric patterns date back to a landmark paper by Alan Turing (Turing, 1952). Turing pointed out that small clusters of "morphogens" (in our context, polarity factors) would arise at random sites due to stochastic fluctuations, and that if an autocatalytic amplification mechanism were present, then a stochastic cluster could grow by positive feedback to generate a dominating asymmetry.
We recently proposed a molecular mechanism for the positive feedback that amplifies a stochastic cluster of GTP-Cdc42p at a random cortical site during symmetry breaking in yeast (Goryachev and Pokhilko, 2008; Kozubowski et al., 2008). A key player is the scaffold protein, Bem1p, which links the Cdc42p-directed GEF to a Cdc42p effector kinase (p21-activated kinase, or PAK). GTP-Cdc42p at the cortex binds and thereby recruits the PAK, together with the associated GEF, which induces neighboring Cdc42p molecules to exchange their GDP for GTP. The Bem1p-GEF-PAK complex thereby "grows" a cluster of GTP-Cdc42p at the cortex (Fig. 1A). This mechanism requires Bem1p-GEF-PAK complexes to diffuse rapidly in the cytoplasm, so that they can be recruited to GTP-Cdc42p molecules already resident at the cortex: for that reason we refer to this as the “diffusion-mediated” amplification mechanism in what follows.
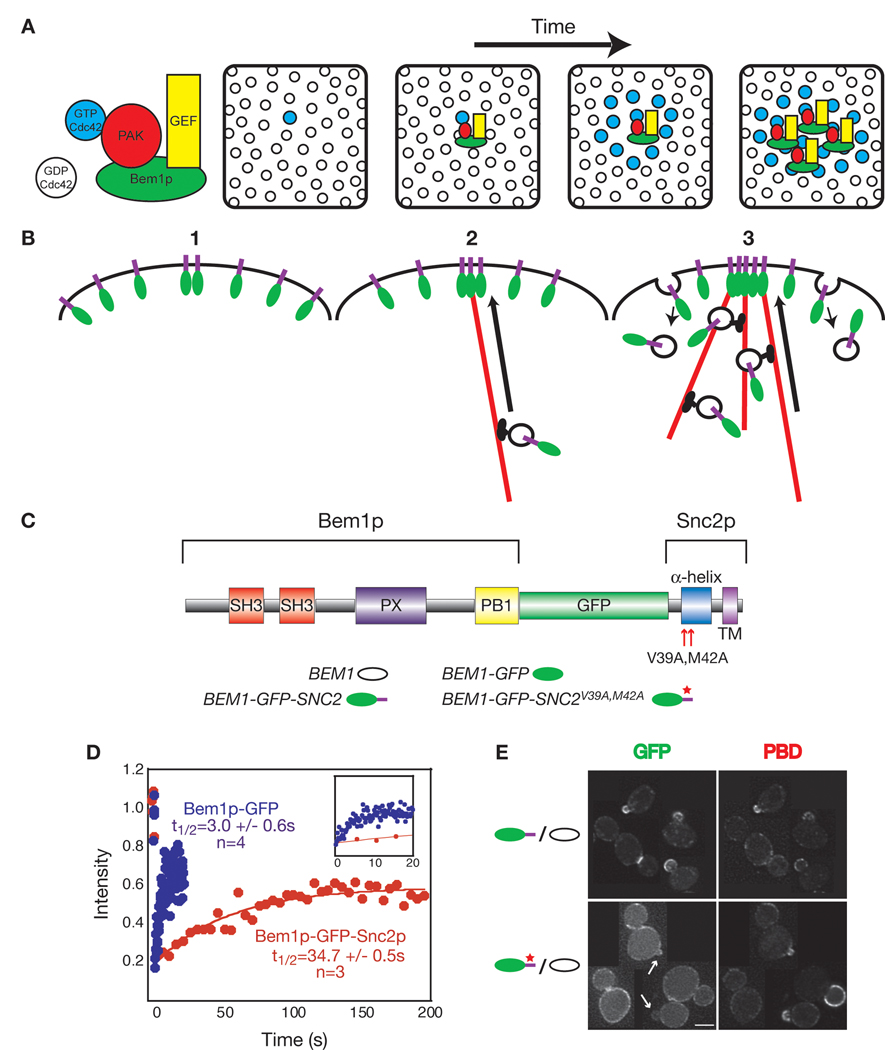
Fig. 1. Re-wiring the yeast polarization feedback loop.
(A) Diffusion-mediated amplification (Kozubowski et al., 2008). The panels represent sequential snapshots of the plasma membrane as seen from the cell interior. GTP-Cdc42p arising stochastically at random sites may recruit a GEF-PAK complex from the cytoplasm through direct binding of the PAK (a Cdc42p effector). The associated GEF then promotes GTP-loading of Cdc42p in the immediate vicinity, causing growth of a GTP-Cdc42p cluster. (B) Actin-mediated amplification (Wedlich-Soldner et al., 2003). (1) A membrane protein able to promote actin cable attachment may (2) capture (or nucleate) an actin cable, which then delivers vesicles containing more of the protein towards that site on the plasma membrane, growing a cluster. (3) Slow diffusion and balanced endocytosis maintains the polarized state (Marco et al., 2007). (C) Synthetic Bem1p-GFP-Snc2p protein used to re-wire amplification. Key: oval = Bem1p, green oval = Bem1p-GFP, green oval with a purple tail = Bem1p-GFP-Snc2p. Red star = mutations in Snc2p endocytosis motif. (D) FRAP analysis of diploids homozygous for BEM1-GFP-SNC2 or BEM1-GFP. Average intensities plotted relative to pre-bleach signal. Recovery half-times indicated (mean ± sd). Inset: same data, expanded timescale. (E) Bem1p-GFP-Snc2p, but not the endocytosis-deficient Bem1p-GFP-Snc2pV39A,M42A, concentrates at polarization sites (arrows). Bar = 5 µm.
Amplification mechanisms can explain how a random site, benefiting from a stochastic initial advantage, can develop a concentrated cluster of polarity factors. However, amplification could in principle allow several sites to develop such clusters: why does only a single site become the "front"? Theoretical analyses indicate that singularity could arise via competition between “fronts” for factors needed to establish a successful polarization site (Goryachev and Pokhilko, 2008), or by dissemination of anti-polarity factors that squelch polarity establishment at other sites (Gierer and Meinhardt, 1972; Onsum and Rao, 2009). A wide variety of other mechanisms to enforce singularity, distinct from the amplification process itself, can also be envisaged. In the fast block to polyspermy during sea urchin fertilization (another process in which singularity is important), ion fluxes induced by the first sperm to fuse with the egg rapidly make the entire cortex unwelcoming for new sperm (Jaffe, 1976). One could imagine that similarly rapid processes (which could involve ion fluxes, or changes in cell wall tension induced by local cell wall remodeling (Klis et al., 2006), or other factors) would favor a single cluster of Cdc42p and block others from forming.
To address the singularity question, yeast geneticists sought to identify mutants that cause cells to form more than one bud. Most "multibudded" mutants were uninformative, involving defects in cell abscission such that buds from sequential cell cycles remained attached to the mother. However, a pioneering study identified a point mutant, cdc42-22, in which multiple buds grew simultaneously (Caviston et al., 2002). In that mutant, polarization was no longer dependent on the Cdc42p-directed GEF, suggesting that it must polarize without using the diffusion-mediated mechanism discussed above. Precisely why cdc42-22 cells make >1 bud is unclear, but one possibility is that the loss of singularity reflects the use of a distinct amplification mechanism to polarize Cdc42p.
To ask whether specifically altering the Cdc42p amplification mechanism would impact singularity, we created a novel fusion protein designed to "re-wire" the endogenous yeast polarization pathway to use an engineered feedback loop to break symmetry. We show that such re-wired cells can polarize and successfully proliferate, but often polarize to two sites simultaneously and sometimes make two buds. Combined experimental and theoretical analysis of both wild-type and re-wired cells suggests that when more than one Cdc42p cluster forms, the amplification mechanisms engender competition between the clusters, eventually producing a single winner. However, if competition is slow (as in re-wired cells) and fails to be completed within the time allotted prior to bud emergence, then singularity is violated and two buds are formed. We conclude that singularity is enforced by an intrinsic competitive property of the Cdc42p positive feedback mechanism that underlies polarity establishment.
Results
Re-wiring the yeast polarization feedback loop
Previous work on an artificial system involving overexpression of Cdc42pQ61L (a "constitutively active" GTP-locked mutant that no longer uses a GEF to become GTP-loaded) suggested that an alternative amplification pathway, quite distinct from the diffusion-mediated pathway, could be used to grow clusters of GTP-Cdc42p (Wedlich-Soldner et al., 2003). Because overexpression of Cdc42pQ61L is lethal to yeast, we did not use that system, but we did follow the conceptual model emerging from it, which is illustrated in Fig. 1B. Here, a membrane-bound polarity factor can influence membrane attachment of actin cables. Actin-mediated delivery of vesicles containing the polarity factor then increases the local concentration of the factor (assuming that it is highly concentrated on vesicles), leading to further actin cable attachment in a positive feedback loop. Eventually, of course, the membrane protein will diffuse away, but a stable focused polarization site can persist if endocytosis removes the polarity factor from the membrane before it diffuses too far (Marco et al., 2007)(Fig. 1B).
Actin-mediated amplification (Fig. 1B) relies on a protein with the following characteristics: it must (i) traffic at high concentration on secretory vesicles, (ii) diffuse slowly in the plasma membrane, (iii) enhance the local attachment of actin cables, and (iv) undergo endocytosis before it diffuses too far from its site of delivery. The yeast exocytic v-SNAREs (Snc1p and Snc2p) fulfill three (i, ii, and iv) of the four requirements (Valdez-Taubas and Pelham, 2003), but cannot influence actin cables. To create a protein that fulfills all four requirements, we fused the scaffold protein Bem1p (which can influence local actin cable attachment via Cdc42p) to the v-SNARE Snc2p (Fig. 1C). Our goal was to drive actin-mediated amplification without the toxic side-effects of Cdc42pQ61L overexpression.
Using fluorescence recovery after photobleaching (FRAP), we found that whereas Bem1p-GFP was highly dynamic (recovery t1/2~3 s), recovery of Bem1p-GFP-Snc2p was much slower (t1/2~35 s) (Fig. 1D), consistent with previously reported dynamics for Bem1p (Wedlich-Soldner et al., 2004) and v-SNAREs (Valdez-Taubas and Pelham, 2003), respectively. Thus, the dynamics of Bem1p-GFP-Snc2p are dominated by the Snc2p moiety.
When expressed in wild-type cells, Bem1p-GFP-Snc2p was concentrated together with GTP-Cdc42p at pre-bud sites and bud tips early in the cell cycle, and at the mother-bud neck late in the cell cycle (Fig. 1E). GTP-Cdc42p was detected using the PBD-RFP reporter linking the GTP-Cdc42p-binding domain from the effector Gic2p (PBD) to td Tomato (Tong et al., 2007). In principle, polarization could occur either by delivery and endocytosis (Fig. 1B) or by lateral diffusion within the membrane and concentration at the polarization site through binding interactions. However, the slow diffusion of integral plasma membrane proteins in yeast (D=0.0025 µm2/s: Valdez-Taubas and Pelham, 2003) impairs the latter mechanism, and Bem1p-GFP-Snc2V39A,M42Ap, carrying point mutations that inactivate the Snc2p endocytosis signal (Fig. 1C) (Grote et al., 2000; Lewis et al., 2000) was no longer polarized to pre-bud sites or bud tips (Fig. 1E), indicating that its polarization is dependent on recycling.
Because diffusion-mediated amplification (Fig. 1A) requires cycling of Bem1p through the cytoplasm, where diffusion is much faster (for GFP, D=11 m2/s: Slaughter et al., 2007), tethering Bem1p to the membrane would disable this mechanism. At the same time, as a synthetic protein with all four of the requisite properties listed above, Bem1p-GFP-Snc2p should enable actin-mediated amplification (Fig. 1B).
Bem1p-GFP-Snc2p promotes proliferation of rsr1Δ cells, but biases polarization towards the previous division site
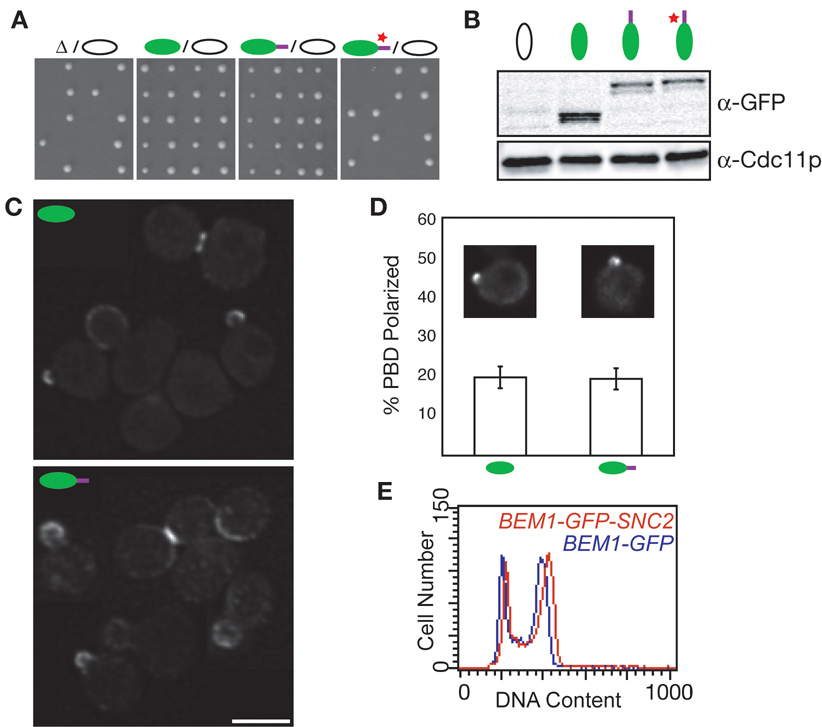
To ask whether actin-mediated amplification could replace diffusion-mediated amplification, we replaced one copy of BEM1 in a diploid with BEM1-GFP-SNC2. Upon tetrad dissection, all BEM1-GFP-SNC2 haploids were viable, even if they lacked spatial cues for bud emergence (Fig. 2A). Bem1p-GFP-Snc2p was expressed at similar levels to Bem1p-GFP (Fig. 2B), and localized to polarization sites as well as to the mother-bud neck (Fig. 2C). GTP-Cdc42p was also polarized at quantitatively similar levels in re-wired and wild-type cells (Fig. 2D). BEM1-GFP-SNC2 rsr1Δ cells proliferated with a normal cell cycle profile (Fig. 2E) and a doubling time only slightly longer than that of controls (102 min versus 90 min). BEM1-SNC2 lacking the GFP moiety also promoted robust growth (not shown). As yeast proliferation occurs by budding and absolutely requires polarization, these findings indicate that Bem1p-GFP-Snc2p can establish polarity.
Fig. 2. Bem1p-GFP-Snc2p promotes polarization and proliferation of rsr1Δ cells.
(A) Tetrads from sporulation of rsr1Δ/rsr1Δ strains heterozygous for bem1, BEM1-GFP, BEM1-GFP-SNC2, or BEM1-GFP-SNC2V39A,M42A as indicated. (B) Bem1p-GFP, Bem1p-GFP-Snc2p and Bem1p-GFP-Snc2pV39A,M42A are expressed at similar levels. Blot probed with anti-GFP and anti-Cdc11p (loading control). (C) BEM1-GFP-SNC2 rsr1Δ cells display polarized Bem1p-GFP-Snc2p. Bar = 5 µm. (D) Wild-type and re-wired cells polarize comparable amounts of GTP-Cdc42p, assessed using PBD-RFP probe. Inset: examples of live cells. (E) Wild-type and re-wired cells displayed a similar cell-cycle profile (all are rsr1Δ).
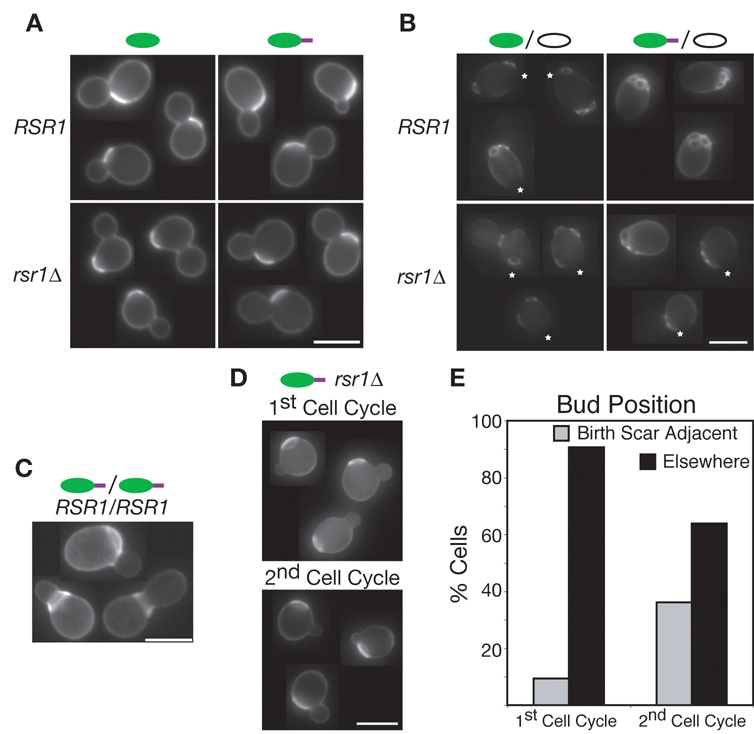
We expected that BEM1-GFP-SNC2 rsr1Δ cells would break symmetry and pick random bud sites like rsr1Δ cells. However, bud scar and birth scar staining indicated that new buds often formed adjacent to previous division sites (Fig. 3A). We speculate that because of its concentration at the neck during cytokinesis and its slow diffusion, Bem1p-GFP-Snc2p remains near the division site, seeding polarization towards that site in the next cell cycle. Consistent with this view, when Bem1p-GFP-Snc2p was expressed in wild-type diploid cells (i.e. cells with functional bud site selection and Bem1p, in which daughters almost always bud towards the distal site marked by the landmark Bud8p (Chant and Pringle, 1995; Zahner et al., 1996)), it skewed the budding pattern towards the division site instead (Fig. 3B). Some cells even budded directly within the previous division site (Fig. 3C), a behavior that is normally prohibited by a Cdc42p GAP (Tong et al., 2007).
Fig. 3. Bem1p-GFP-Snc2p biases polarization towards the previous division site, but can also break symmetry.
(A) Budding of BEM1-GFP-SNC2 rsr1Δ haploids is biased towards the previous division site. First-time mothers stained to label the cell wall and birth scar (bright patch, which marks previous division site). (B) Budding of RSR1/RSR1 BEM1/BEM1-GFP-SNC2 diploids is biased towards the previous division site. Multiple-time mothers stained to label bud scars (location of previous division sites). * indicates birth scars, when not obscured by bud scars. (C) Budding of BEM1-GFP-SNC2/BEM1-GFP-SNC2 diploids can occur directly into the birth scar. (D) Following centrifugal elutriation (which lengthens G1 in the first cycle), BEM1-GFP-SNC2 rsr1Δ cells break symmetry. In the second cell cycle budding was again biased. Cells fixed and stained at 80 min (first cycle) and 180 min (second cycle) after elutriation. Bar = 5 µm in all panels. (E) Bud site position for first- and second-time mothers from D. n>100.
Re-wired cells break symmetry by actin-mediated positive feedback
If slow diffusion of Bem1p-GFP-Snc2p from the division site biases polarization towards that site, then lengthening the early G1 interval (before polarization) should provide time for dissipation of the Bem1p-GFP-Snc2p gradient to a homogeneous distribution, forcing the cells to break symmetry. We used centrifugal elutriation (a size-selection procedure) to isolate small early-G1 daughter cells that have a longer G1 interval, and shifted the cells to 37°C to depolarize actin following elutriation (Lillie and Brown, 1994). BEM1-GFP-SNC2 rsr1Δ cells budded at random sites in the first cycle after elutriation, although the preference for the division site returned in the second cycle (Fig. 3D,E). Thus, Bem1p-GFP-Snc2p can promote symmetry breaking.
If Bem1p-GFP-Snc2p polarizes via actin-mediated amplification instead of diffusion-mediated amplification, then polarity in this strain (unlike in wild-type or rsr1Δ cells) should be abolished upon actin depolymerization. Indeed, whereas Latrunculin A-treated control cells polarized Bem1p, GTP-Cdc42p, Spa2p (a polarisome component (Sheu et al., 1998)), and Cdc3p (a septin (Gladfelter et al., 2001)), none of these markers became polarized in Latrunculin A-treated Bem1p-GFP-Snc2p cells (Fig. 4A,B). At the dose employed (200 µM), Latrunculin A depolymerizes all detectable F-actin (both the cables that mediate vesicle delivery and the cortical patches that mediate endocytosis). Moreover, the endocytosis-deficient Bem1p-GFP-Snc2V39A,M42Ap was unable to rescue proliferation of bem1Δ rsr1Δ cells (Fig. 2A), presumably because endocytosis of the construct is key to polarization. These results indicate that Bem1p-GFP-Snc2p cannot engage an actin-independent polarization mechanism. Thus, Bem1p-GFP-Snc2p can break symmetry, but it does so by actin-mediated positive feedback (Fig. 1B).
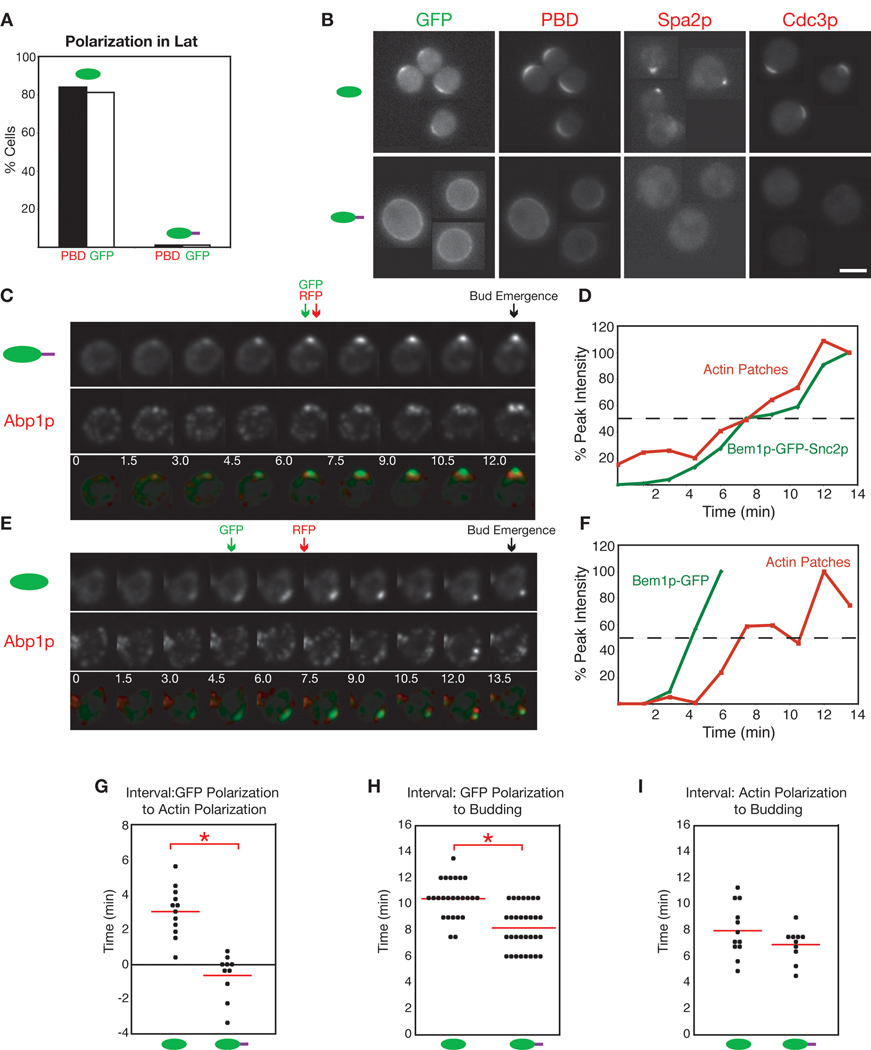
Fig. 4. Comparison of actin-mediated and diffusion-mediated polarization.
(A) Polarization in re-wired cells is actin-dependent. Polarization of PBD-RFP and either Bem1p-GFP or Bem1p-GFP-Snc2p was scored following complete actin depolymerization (200 µM Lat-A, 2 h). n>90. (B) Lat-A treated re-wired cells do not polarize PBD-RFP, Spa2p-RFP, or Cdc3p-RFP. Montages of live-cell images. Bar = 5 µm. (C) Dynamics of Bem1p-GFP-Snc2p and actin patch polarization (frames from movie 1). Actin patches visualized with Abp1pmCherry. Arrows: times of 50% of peak Bem1p-GFP-Snc2p polarization (green), actin patch polarization (red), and bud emergence (black: scored from DIC images). Time is in min. Bar = 2 µm. (D) Quantitation of polarization in C. Integrated GFP or RFP intensity in the focus is plotted as % of peak intensity for that cell. (E) Dynamics of Bem1p-GFP and actin patch polarization (frames from movie 2). (F) Quantitation of polarization in E. (G) Interval between polarization of Bem1p-GFP or Bem1p-GFP-Snc2p and actin patches, scored from times when integrated GFP and RFP intensities reached 50% of peak. Line indicates average. * The difference is statistically significant (p<0.001, Student’s t-test). (H) Interval from first detection of polarized GFP signal to bud emergence. Because polarization of Bem1p-GFP is more abrupt than that of Bem1p-GFP-Snc2p, use of the “50% of peak” criterion for GFP polarization would lead to a bigger difference than the “first detection” criterion used here. * The difference is statistically significant (p<0.001, Student’s t-test). (I) Interval from actin patch polarization (50% of peak) to bud emergence. The difference is not statistically significant (p = 0.14). All cells are rsr1Δ.
The demonstration that a synthetic re-wiring of the yeast polarization pathway to employ the actin-based mechanism can work to break symmetry provides an important validation of the actin-mediated positive feedback concept (Wedlich-Soldner et al., 2003). Moreover, it indicates that such polarization can occur in a sufficiently rapid timeframe to be useful to yeast, which was unclear from the previous work as polarization of Cdc42pQ61L takes much longer (Gulli et al., 2000; Wedlich-Soldner et al., 2003). We then directly compared the kinetics of polarization in wild-type and re-wired cells.
Dynamics of polarization in wild-type and re-wired cells
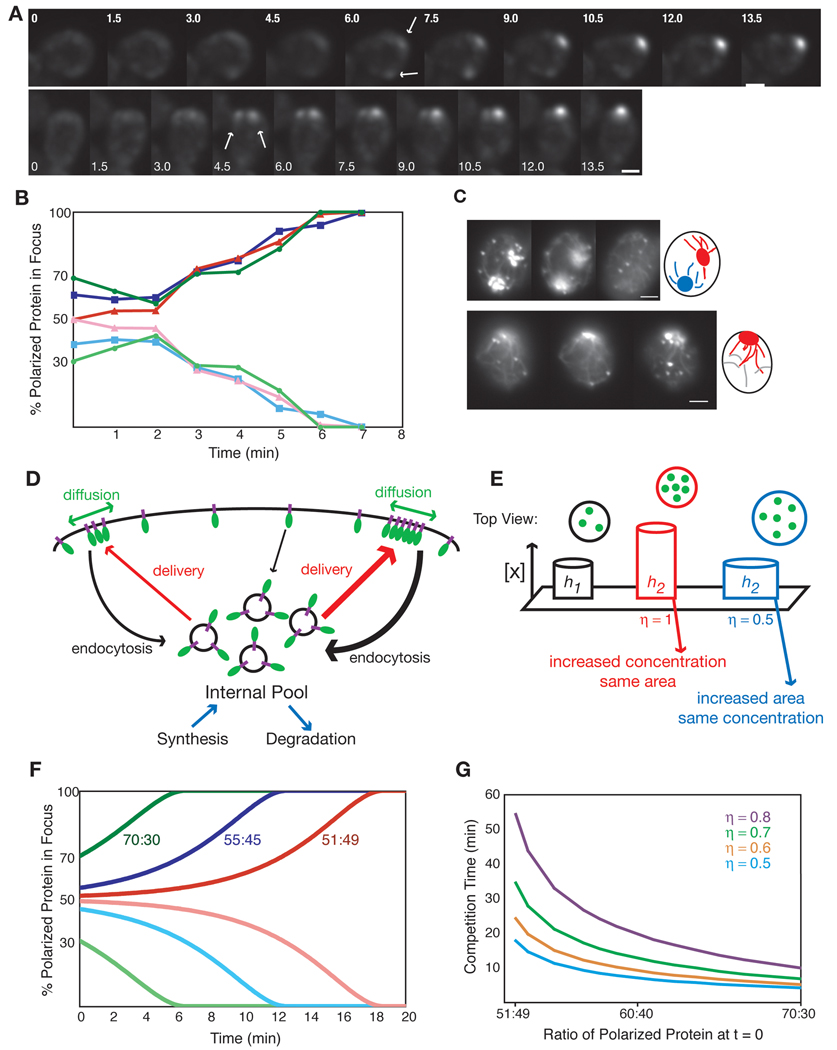
Side-by-side comparisons indicated that although superficially similar, polarization in wild-type and re-wired cells displayed three notable differences. First, in re-wired cells Bem1p-GFP-Snc2p accumulated at a small focus gradually (Fig. 4C,D and supplemental movie 1), whereas in wild-type cells Bem1p-GFP accumulated more abruptly and to a wider zone (~1.9 µm diameter) that subsequently condensed to a small (<1 µm diameter) focus (Fig. 4E,F and supplemental movie 2). Second, whereas Bem1p-GFP-Snc2p polarization occurred approximately coincident with that of the actin patch marker Abp1p-mCherry, polarization of Bem1p-GFP occurred 3.0 ± 0.41 min (mean ± SEM, n=12) before that of Abp1p-mCherry (Fig 4C–G). Third, bud emergence occurred 10.4 ± 0.29 min (mean ± SEM, n = 25) after Bem1p-GFP polarization was first detected, but only 8.2 ± 0.3 min (mean ± SEM, n = 31) after Bem1p-GFP-Snc2p polarization was first detected (Fig. 4H). To avoid potential differences stemming from variations in temperature or slide composition, the data in Fig. 4H were collected from mixed-cell experiments where BEM1-GFP rsr1Δ and BEM1-GFP-SNC2 rsr1Δ cells were imaged simultaneously (the cells were distinguished by the presence of an mCherry marker in the BEM1-GFP-SNC2 rsr1Δ strain). We also measured the interval between Abp1p polarization and bud emergence, which was similar wild-type and re-wired cells (Fig. 4I).
We conclude that in wild-type cells, polarization of Bem1p is rapid and precedes actin polarization by about 3 min, whereas in re-wired cells, polarization of Bem1p-GFP-Snc2p is gradual and coincident with actin polarization. In both cases, bud emergence occurs about 8 min after actin polarization. These findings are entirely consistent with (and strongly support) the premise of the re-wiring approach: in wild-type cells, diffusion-mediated amplification generates a focus of Bem1p (and GTP-Cdc42p) that subsequently recruits actin, whereas in the re-wired cells actin-mediated amplification leads to simultaneous polarization of Bem1p-GFP-Snc2p and actin.
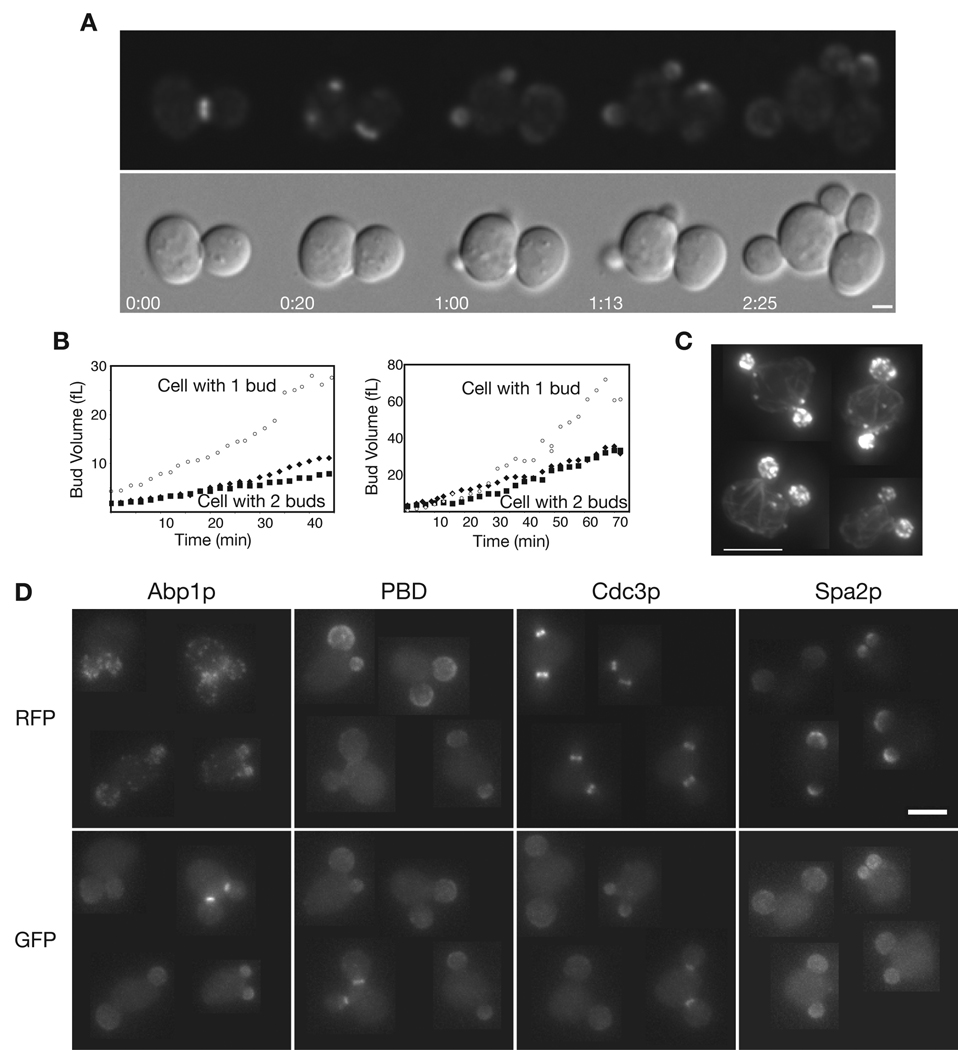
Re-wired cells sometimes make two buds simultaneously
In diploid BEM1-GFP-SNC2 rsr1Δ cells growing on minimal media at 24°C, 4.9% of budded mothers had two buds (n > 1000). This number was reduced to ~1–2% under optimal growth conditions (rich media at 30°C), in which the cells budded predominantly towards the division site (see above). Time-lapse analysis revealed that the two buds emerged and grew simultaneously, though at a reduced rate compared to neighboring single-budded cells (Fig 5A,B and supplemental movie 3 & supplemental movie 4). The two buds in such cells were both “true buds” in the sense that they displayed polarized actin cables and patches, septin hourglass structures at the neck, and polarized localization of a GTP-Cdc42p reporter as well as Spa2p (Fig. 5C,D). The location of the two buds relative to each other varied widely: some cells had buds right next to each other while other cells had buds at opposite ends (Fig. 5C,D). Thus, switching from diffusion-mediated amplification (Fig. 1A) to actin-mediated amplification (Fig. 1B) caused the occasional breakdown of singularity, suggesting that the normal prohibition restricting cells to form only one bud is conferred by the nature of the amplification mechanism itself.
Fig. 5. Re-wired cells violate singularity.
(A) Re-wired cells can grow two buds simultaneously. BEM1-GFP-SNC2 cells (frames from movie 3). Time in h:min. Bar = 2 µm. (B) Comparison of bud growth in side by side one- vs two-bud cells, measured from DIC images (e.g. movie 4). Open symbols: one-bud cell. Closed symbols: two-bud cell. Left, haploid cells. Right, diploid cells. (C) Actin cables and patches are polarized towards both buds. Cells were fixed and stained with rhodamine-phalloidin. Bar = 5 µm. (D) Montage of two-bud cells containing BEM1-GFP-SNC2 (lower panels) and either ABP1-mCherry, CDC3-mCherry, PBD-RFP, or SPA2-mCherry (upper panels). Bar = 5 µm.
We tested whether the two-budded phenotype could be suppressed either by reinstating diffusion-mediated positive feedback loop (through addition of BEM1-GFP) or by restoring bud site selection (through addition of RSR1). BEM1-GFP reduced the frequency of two-budded cells (2.5% vs 4.9%, n > 1000), but RSR1 had little effect (5.6% vs 4.9%, n > 1000).
Competition between polarization foci
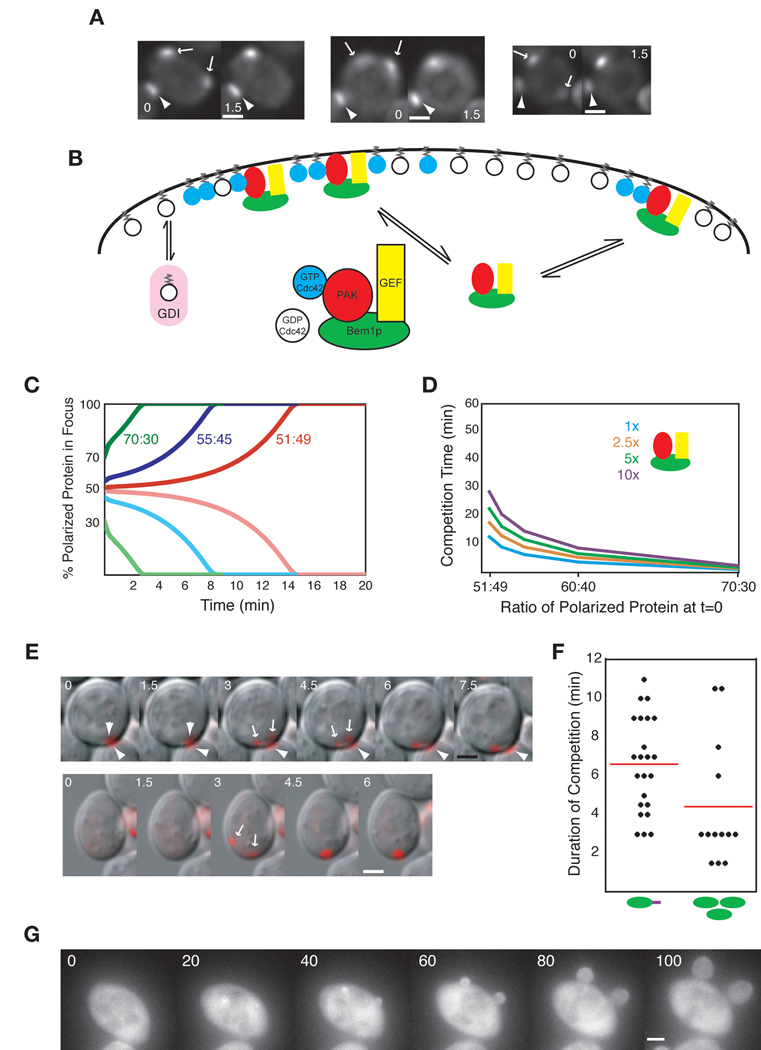
Time-lapse microscopy of polarization in 144 BEM1-GFP-SNC2 rsr1Δ cells revealed that 27 (19%) initiated polarization at two foci, yet only 4 (2.8%) made two buds. Thus, in a majority of the cells that generated two Bem1p-GFP-Snc2p foci, one of the foci subsequently disappeared, and the cells formed a single bud (Fig. 6A,B and supplementary movie 5 & supplementary movie 6). Two foci could coexist for up to 10 min before one focus disappeared leaving a single bud site (see Fig. 7F below).
Fig. 6. Competition between foci in re-wired cells.
(A) Re-wired BEM1-GFP-SNC2 rsr1Δ cells formed two foci but then one focus grew while the other disappeared prior to budding: selected frames from movie 5 & movie 6. Time in min. Bar = 2 µm. (B) Integrated GFP intensity in each focus for three illustrative BEM1-GFP-SNC2 rsr1Δ cells. Foci from the same cell are colored in dark vs light red, blue, or green. (C) Actin cables are directed towards both foci. Selected z-planes of representative 1-focus and 2-focus BEM1-GFP-SNC2 rsr1Δ cells fixed and imaged as in Fig. 5C. Tracing shows cables that could be unambiguously assigned to a (color-coded) focus. Grey: no clear attachment. (D) Model for Bem1p-GFP-Snc2p dynamics in cells with two foci. Red arrows: vesicular trafficking along actin cables. Black arrows: endocytosis. Green arrows: diffusion in the plane of the membrane. (E) Effect of focus geometry on diffusion-mediated escape of Bem1p-GFP-Snc2p. Circles represent a top-down view of the cylinders illustrating distribution of Bem1p-GFP-Snc2p (green dots). (F) Simulation of competition between foci with η = 0.5. Bem1p-GFP-Snc2p content of foci from the same cell are colored in dark vs light red, blue, or green. Simulations started with the indicated ratios of protein. (G) Dependence of the competition time on the initial ratio and η.
Fig. 7. Competition between foci in wild-type cells and cells overexpressing Bem1p.
(A) Two-focus (arrows) intermediates in wild-type cells resolve within 1.5 min. Deconvolved images of Bem1p-GFP. Arrowhead: neck. Time in min. Bar = 2 µm. (B) Model for competition between foci in wild-type cells. A small limiting pool of Bem1p-GEF-PAK complex diffuses rapidly in the cytoplasm and can bind GTP-Cdc42p in either focus. GTP-Cdc42p is concentrated in each focus, and can bind the complex or hydrolyze GTP. GDP-Cdc42p can bind to GDI and be extracted from the membrane or can be re-loaded with GTP by neighboring GEFs. (C) Simulation of competition between foci. Bem1p content of foci from the same cell are colored in dark vs light red, blue, or green. Simulations started with the indicated ratios of protein. (D) Dependence of the competition time on the initial ratio and the abundance of the Bem1p-GEF-PAK complex. (E) Cells overexpressing Bem1p display competition between foci. SPA2-mCherry cells containing a high-copy 2 µm-BEM1-GFP plasmid were imaged, Spa2p-mCherry (polarity marker)/DIC overlays shown (selected frames from movie 7). Time in min. Bar = 2 µm. (F) Quantitation of competition times (interval between first detection of two foci and first detection of a single winning focus). Comparison of re-wired (BEM1-GFP-SNC2 rsr1Δ) cells and Bem1p overexpressors. (G) Bem1p overexpressors can violate singularity and make two buds: selected frames from movie 8. Bar = 2 µm.
In contrast to cells with two foci, we never saw the focus disappear in the 117 cells that only made a single focus. This suggests that the “disappearance” of one focus was due to the presence of the second focus, implicating some form of competition between foci as the basis for the disappearance of one focus.
Cells that established two foci of Bem1p-GFP-Snc2p always did so within < 3 min of each other, and in most cases any potential differences in the focus initiation times were not resolved by our 1.5 min image acquisition intervals. Thus, it appears that once a dominant focus becomes established, new foci do not arise. This was not due to progression of the cell cycle (which eventually terminates polarization) because new foci did not arise even if the cell cycle was arrested at a polarizing stage (Fig. S1). A competitive mechanism that favors the stronger focus would account for this observation, as newly-growing foci would be unable to compete with a well-established focus.
What is the basis for the observed competition between foci? In cells with two foci, secretory vesicles carrying Bem1p-GFP-Snc2p would encounter actin cables oriented towards either focus (Fig. 6C). If we assume (as seems likely) that stronger foci (those containing more Bem1p-GFP-Snc2p) sustain more actin cables, then delivery will be biased towards the stronger focus, forming a potential basis for competition (red arrows in Fig. 6D). To assess whether this would yield the observed behavior, we turned to mathematical modeling.
Mathematical model for competition between Bem1p-GFP-Snc2p foci
A model incorporating delivery of Bem1p-GFP-Snc2p from internal pools to the polarization site by actin cables, diffusion in the plasma membrane, and retrieval by endocytosis (Fig. 6D) is presented in the supplement. We assume that in the relevant timeframe for polarization (a few minutes), the system is at a global steady state in which synthesis and degradation of Bem1p-GFP-Snc2p are balanced, and can be ignored.
The simplest scenario is that delivery of Bem1p-GFP-Snc2p is biased in a manner proportional to the amount of Bem1p-GFP-Snc2p that is already present in each focus. However, because endocytic retrieval of Bem1p-GFP-Snc2p (black arrows in Fig. 6D) is also expected to be proportional to the amount already present, this would not necessarily lead to a net change in the relative amounts of protein in the two different foci. The outcome in that scenario would depend on diffusion in the plasma membrane (green arrows in Fig. 6D).
The rate of diffusion-mediated "escape" of Bem1p-GFP-Snc2p from a focus will depend on the precise geometry of the focus (i.e. the concentration profile in two dimensions). An unrealistic but instructive geometry is illustrated in Fig. 6E, where a reference focus (black) is depicted as a circular region containing evenly-distributed Bem1p-GFP-Snc2p. Here, diffusion-mediated escape of Bem1p-GFP-Snc2p from the focus is proportional both to the length of the circle's perimeter and to the concentration of Bem1p-GFP-Snc2p within the focus.
We now consider two extreme scenarios for the distribution of Bem1p-GFP-Snc2p in a stronger focus (depicted in red or blue in Fig. 6E). At one extreme (red), the size (and hence perimeter) of the circle remains unchanged, and the Bem1p-GFP-Snc2p concentration in the circle is higher. In that case, diffusion-mediated escape from each focus will simply be proportional to the total protein content in the focus, just as we assumed for delivery and endocytosis. It can then be demonstrated that in cells with two foci containing amounts of Bem1p-GFP-Snc2p designated as h1 and h2, the ratio h1/h2 will tend to remain the same regardless of the fraction of Bem1p-GFP-Snc2p in each focus (supplement: Analysis of the proportional model). Thus, in this scenario there is no net competition between foci.
In the second scenario (Fig. 6E, blue), the concentration of Bem1p-GFP-Snc2p in the stronger focus remains the same as in the reference focus, but the area of the circle is increased. Because protein content is proportional to area, and diffusion-mediated escape is proportional to the length of the circle’s perimeter, in this scenario diffusion-mediated escape would be proportional to [total protein in focus]0.5. These extremes (red, blue) allow us to infer the general form of the escape term for a realistic focus geometry in between these extremes: diffusion-mediated escape will be proportional to [total protein in focus]η, where 0.5 < η < 1. It can then be shown that the ratio, h1/h2, of protein content in one focus to protein content in the other, will change according to
where k is a positive quantity dependent on diffusion rate and focus geometry (supplement: Analysis of the non-proportional model).
This means that if h1/h2 > 1 (i.e. focus 1 is stronger than focus 2), d(h1/h2)/dt will be positive and focus 1 will grow at the expense of focus 2. Conversely, if h1/h2 < 1 (i.e. focus 2 is stronger than focus 1), d(h1/h2)/dt will be negative and focus 2 will grow at the expense of focus 1. Unless the foci have precisely equal content (h1/h2 = 1, in which case d(h1/h2)/dt = 0), the stronger focus will out-compete the weaker focus. Thus, inclusion of a realistic diffusion scenario (0.5 < η < 1) in a simple model of Bem1p-GFP-Snc2p behavior is sufficient to promote competition and, given sufficient time, singularity.
To assess the timeframe in which this competitive mechanism would operate, we constrained model parameters based on published observations and experimental data (Fig. 1D, 2B, and S2; see supplement: Parameter estimation). Examples of model behavior using these parameters and setting η = 0.5 are shown in Fig. 6F. When two foci of Bem1p-GFP-Snc2p were initiated at different ratios, the stronger focus grew while the weaker one disappeared. The bigger the initial asymmetry, the faster the resolution of two foci to one (Fig. 6F,G). With increasing η, competition took progressively longer (Fig. 6G). Thus, with realistic parameter values, competition for Bem1p-GFP-Snc2p can lead to growth of one focus at the expense of the other within a biologically relevant (several minute) timeframe, as long as stronger foci are also larger.
When two foci start out with almost equivalent amounts of Bem1p-GFP-Snc2p, competition takes longer (Fig. 6F,G), and one would expect two buds to emerge. The fact that we detected 2-budded cells shows that competition is not always completed within the allotted interval between polarization and bud emergence. We observed a total of 38 Bem1p-GFP-Snc2p cells in which two buds emerged (including both RSR1 and rsr1Δ strains). In two cases, a tiny bud was then "abandoned", but in the other 36 instances both buds grew for prolonged periods (Fig. 5 and movie 3 & movie 4), suggesting that competition is terminated soon after bud emergence. Because recycling endosomes and golgi quickly enter small buds (Preuss et al., 1992), the recycling pools of Bem1p-GFP-Snc2p in each bud may become "insulated" from each other soon after bud emergence, terminating competition.
Modeling competition between Bem1p-GFP foci in wild-type cells
We then asked whether competition between foci also occurs in wild-type cells as well as in re-wired cells. At our time-lapse rates (one z-stack every 1.5 min, which was the fastest rate that did not induce phototoxic damage upon prolonged filming), we observed 5 apparent instances of two-foci intermediates out of 79 rsr1Δ cells containing Bem1p-GFP (6%: Fig. 7A). These were fainter than in re-wired cells and required deconvolution to detect. By the next timepoint, these intermediates had resolved to a single focus (Fig. 7A), suggesting that competition occurs more rapidly in wild-type than in re-wired cells, and resolves within 1.5 min.
A mathematical model of diffusion-mediated amplification (Goryachev and Pokhilko, 2008) suggested that competition between GTP-Cdc42p clusters for a limiting cytoplasmic pool of Bem1p-GEF complexes should lead to the eventual growth of the biggest cluster at the expense of the others (Fig. 7B). However, with the parameter values estimated by Goryachev and Pokhilko, the model predicts that competition occurs on a timescale even slower than that of our re-wired cell model (supplement: Modeling competition in the diffusion-mediated amplification system; Fig. S3).
Kozubowski et al. (2008) recently showed that an unstable Bem1p-GEF-PAK complex mediates positive feedback, while Goryachev and Pokhilko modeled a stable Bem1p-GEF complex. We altered the model accordingly (supplement: Adapting the model to account for the Bem1p-GEF-PAK complex). We also re-estimated the GEF and GAP rate constants based on biochemical assays using yeast cell lysates (supplement: Estimation of GEF and GAP activities; Fig. S4). With these modifications, the model predicted faster competition between clusters than the fastest model of the re-wired cells (η = 0.5: compare Fig. 7C with Fig. 6F), and further tuning of model parameters could make competition even faster (supplement: Factors affecting competition timescale in the diffusion-mediated model). Thus, competitive features of the diffusion-mediated mechanism might underlie singularity.
Competition between foci in cells overexpressing Bem1p
Mathematical modeling indicated that competition between foci in the diffusion-mediated mechanism would be slower if the abundance of the Bem1p-GEF-PAK complex were increased (Fig. 7D). To test this prediction, we increased Bem1p-GFP expression using a high-copy plasmid. Overexpression of Bem1p did not noticeably slow cell proliferation. Most of the overexpressed protein was cytoplasmic, presumably because much of the Bem1p was either monomeric or in distinct complexes that did not polarize. Because of the elevated cytoplasmic background, polarization (though visible) was more difficult to detect, so we added a separate polarity marker, Spa2p-mCherry, to monitor focus formation in these strains. Strikingly, 18 out of 127 (14%) of cells overexpressing Bem1p initially developed two polarization foci, but then (with one exception: see below) one focus grew and the other disappeared prior to budding (Fig. 7E and Supplemental movie 7). These findings strongly suggest that competition between foci is a feature of the normal polarization process, and that competition is slowed by additional Bem1p as predicted by the model.
Competition between foci was somewhat faster in the Bem1p overexpressors than in the re-wired cells (Fig. 7F). In addition, in a small number of the Bem1p overexpressors we noted three behaviors that we had not seen in the re-wired cells. First, in 2 out of 18 two-foci cells, an initially dimmer focus became brighter and successfully competed against an initially brighter focus (supplement Fig. S5). Second, in 4 out of 109 one-focus cells we observed apparent disappearance of the focus, immediately followed by re-appearance of a focus at a distinct site (supplement Fig. S6). Third, in 5 cells (4 of which were initially scored as having only one focus), close examination revealed that at the beginning of focus growth, the cells had two foci close to each other, which appeared to merge forming a single focus at an intermediate position (supplement Fig. S7). Interestingly, this was predicted to occur by the mathematical model (Goryachev and Pokhilko, 2008).
One of the two-focus cells discussed above went on to form two buds, violating the singularity rule. In other experiments (with cells that lacked the Spa2p marker) we also observed rare (<1%) cells budding simultaneously at two sites (Fig. 7G and Supplemental movie 8). Thus, as in the re-wired cells, when competition between foci is too slow, then both foci give rise to buds, violating singularity.
Discussion
Singularity in polarization is guaranteed by competition between foci
A key result from this work is that cells synthetically re-wired to use a different positive feedback loop to polarize Cdc42p sometimes violated the singularity rule and made two buds simultaneously. This finding suggests that singularity is an intrinsic property of the Cdc42p-amplifying positive feedback system, and that there is no separate singularity-guaranteeing process.
What aspect of the Cdc42p amplification pathway confers singularity? Our findings on both re-wired cells polarizing via actin-mediated feedback and Bem1p overexpressors polarizing via diffusion-mediated feedback indicate that at least in some cells, polarization occurs through an intermediate “multiple foci” stage. Thus, the stochastic processes that initiate amplification can occur at more than one site. In most instances where two foci appeared, one focus subsequently grew while the other disappeared, and a single bud emerged from the site of the winning focus. This finding provides strong evidence that foci interact with each other in a competitive manner that leads to the growth of one focus at the expense of the other. Cells that did establish two foci did so almost simultaneously, and new foci no longer appeared once a strong focus had formed, even if the cell cycle was arrested at a polarization-competent stage. This observation is also consistent with a competitive process, as the first focus would effectively prevent the growth of subsequently-initiated foci. Thus, singularity can result from a competitive mechanism built into the Cdc42p amplification feedback system.
In rsr1Δ cells filmed at 1.5 min time-lapse intervals, the intermediate “multiple foci” stage did not persist beyond a single timepoint. The transitory nature of this stage suggests that competition occurs very rapidly, making the intermediate difficult to detect. In addition, polarization occurred more abruptly in wild-type than in re-wired cells, and this feature would be expected to reduce the incidence of two-spot intermediates. If the first site to begin amplification grows a focus very quickly, then this “first focus” may grow to a dominant size before other sites begin their amplification, precluding growth of subsequent foci.
With current parameter estimates, foci in our mathematical model appear to compete somewhat more slowly than foci in the wild-type cell: according to the model, foci that had starting ratios more equal than 60:40 should have taken longer than 3 min to compete. It is certainly possible to alter parameters so as to speed competition (supplement: Factors affecting competition timescale in the diffusion-mediated model), and further work may yield more accurate parameter estimates that can explain the rapid competition of wild-type cells. Alternatively, a full accounting of the speed of competition may require that additional aspects of the polarization process become incorporated into the model. One feature absent from the model is noise. In cells, both molecular and vesicular noise would be expected to introduce a random element into the competition. Perhaps noise is responsible for the rare instances (which we cannot currently explain) of cells in which an initially weaker “underdog” focus went on to win the competition.
Given the above considerations, we suggest that the singularity rule boils down to having a rapid competitive mechanism built into the polarization process. Our re-wired cells, with a competition mechanism operating in the timeframe of several minutes, disobey singularity in <5% of cells. Bem1p overexpressors, with a faster competition mechanism, disobey singularity in <1% of cells. And wild-type cells, with a competition mechanism that resolves all competitions within 1.5 min, can effectively guarantee singularity.
Basis for competition between polarization foci
In the re-wired cells, the simplest scenario is that foci compete via their attached actin cables for the vesicles that deliver additional Bem1p-GFP-Snc2p to the foci. Because foci are constantly losing Bem1p-GFP-Snc2p by diffusion and by endocytosis, delivery of new vesicles is required to maintain a focus. We present a simple mathematical model for such competition, and show that if delivery and loss are both proportional to the Bem1p-GFP-Snc2p content of a focus, then the situation is balanced and different foci can co-exist indefinitely. However, if stronger foci are also larger in extent (even if only slightly so) than weaker foci, then diffusion-mediated loss will no longer be proportional to protein content, and the stronger focus will grow at the expense of the weaker focus. This simple and realistic assumption about focus geometry suffices to make the 2-foci situation competitive rather than balanced. When model parameters were estimated based on experimental findings, the mathematical model was able to promote competition in a relevant timeframe, suggesting that this mechanism is powerful enough to account for competition in the re-wired cells. Of course, it remains entirely possible that there are other factors that could enhance competition.
In wild-type cells, mathematical modeling predicted that foci would compete for a limiting pool of cytoplasmic Bem1p-GEF-PAK complex, and that increasing the concentration of the Bem1p-GEF-PAK complex would slow competition. Our finding that cells overexpressing Bem1p displayed readily detectable competition and occasionally violated singularity confirms this prediction, and supports the validity of the model for diffusion-mediated amplification.
Comparison with cdc42-22 and other mutants
Prior to this work, the major experimental study to address singularity in polarization was focused on cdc42-22 mutants capable of growing two or even more buds simultaneously (Caviston et al., 2002). In those mutants, polarization was uncoupled from cell cycle control and new buds continued to emerge and grow throughout the cell cycle. Strikingly, the presence of an established bud did not prevent the initiation of a subsequent bud in the same cell, after which the buds both grew, yielding a remarkable 45% of the population with >1 bud (Caviston et al., 2002). This observation suggests that unlike the cells described in our study, cdc42-22 mutants do not exhibit significant competition between polarization foci. Because cdc42-22 mutants no longer need the GEF in order to polarize, they clearly do not use the diffusion-mediated Bem1p-GEF-PAK complex amplification mechanism (Fig. 1A). It would be very interesting to determine what amplification mechanism functions in that mutant, and why it is not subject to competition.
Heterozygous diploids containing one copy of cdc42-22 and one of wild-type CDC42 obey the singularity rule, leading Caviston et al. (2002) to suggest that wild-type Cdc42p polarizes much more efficiently than Cdc42p-22, so that the polarization site established by the wild-type would (by polarizing associated proteins and downstream factors) deprive the weaker Cdc42p-22 of the werewithal to establish secondary polarization sites. Interestingly, heterozygous diploids containing one copy of BEM1 and one of BEM1-SNC2 were still able to make two-budded cells, albeit at reduced frequency. Thus, the re-wired polarization mechanism would appear to operate more efficiently than that of cdc42-22, allowing establishment of a second polarization site even when the wild-type mechanism is active.
Occasional multi-budded cells have also been reported in other strains that are very sick and slow-growing, including bem1Δ (Wedlich-Soldner et al., 2004) and bem2Δ (Knaus et al., 2007) mutants. In bem1 mutants (which require Rsr1p to polarize), we also detected very rare 2-budded cells in which the buds grew simultaneously, but we noticed that those cells were also multinucleate. Proliferation in the presence of an almost-lethal mutation like bem1 leads the cells to accumulate a historical legacy of defects (and perhaps also adaptations), including large cell size, abandoned buds, and multiple nuclei. It is therefore difficult to discern why the rare two-budded cells occur in such strains, or what the link to multinuclearity might be. In contrast, the re-wired cells and Bem1p-overexpressors discussed above proliferate almost as well as wild-type cells, allowing much cleaner interpretation.
Tuning competition to obey or flout singularity
Not all polarized cells obey the singularity rule. Filamentous fungi can sustain many growing tips in the same (multinucleate) cell (Harris, 2008), and neurons initially form several neurite extensions from the same cell body (da Silva and Dotti, 2002). Yet, it appears that many of the same polarity regulators that obey singularity in other cell types are similarly employed in these multipolar cells. It may be that evolution has fine-tuned the speed and effectiveness of competition to allow the same molecular elements to promote or disregard singularity in different cell types.
Supplementary Material
Acknowledgements
We thank David Amberg, Erfei Bi, Tony Bretscher, John Cooper, David Drubin, and Bruce Goode for advice and reagents, and Steve Haase, Joe Heitman, Dan Kiehart, Sally Kornbluth, John York, and members of the Lew lab for comments on the manuscript. A.S.H. was supported by an NSF predoctoral fellowship. Work by N.S.S. and A.B.G. was supported by UK BBSRC grant G001855. Work by H.F.N. and M.C.R. was supported by NSF grant DMS-061670. This work was supported by NIH grant GM62300 to D.J.L.
Abbreviations
- GEF
guanine nucleotide exchange factor
- GAP
GTPase activating protein
- PAK
p21-activated kinase
- CDK
cyclin-dependent kinase
- v-SNARE
vesicle-associated soluble NSF attachment protein receptor
- PBD
p21-binding domain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bender A, Pringle JR. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc. Natl. Acad. Sci. U.S.A. 1989;86:9976–9980. doi: 10.1073/pnas.86.24.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Tcheperegine SE, Bi E. Singularity in budding: a role for the evolutionarily conserved small GTPase Cdc42p. Proc Natl Acad Sci U S A. 2002;99:12185–12190. doi: 10.1073/pnas.182370299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J, Herskowitz I. Genetic control of bud site selection in yeast by a set of gene products that constitute a morphogenetic pathway. Cell. 1991;65:1203–1212. doi: 10.1016/0092-8674(91)90015-q. [DOI] [PubMed] [Google Scholar]
- Chant J, Pringle JR. Patterns of bud-site selection in the yeast Saccharomyces cerevisiae. J. Cell Biol. 1995;129:751–765. doi: 10.1083/jcb.129.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3:694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Cdc42 - the centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- Gierer A, Meinhardt H. A theory of biological pattern formation. Kybernetik. 1972;12:30–39. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 2001;4:681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Goryachev AB, Pokhilko AV. Dynamics of Cdc42 network embodies a Turing-type mechanism of yeast cell polarity. FEBS Lett. 2008;582:1437–1443. doi: 10.1016/j.febslet.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Grote E, Vlacich G, Pypaert M, Novick PJ. A snc1 endocytosis mutant: phenotypic analysis and suppression by overproduction of dihydrosphingosine phosphate lyase. Mol Biol Cell. 2000;11:4051–4065. doi: 10.1091/mbc.11.12.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulli MP, Jaquenoud M, Shimada Y, Niederhauser G, Wiget P, Peter M. Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol. Cell. 2000;6:1155–1167. doi: 10.1016/s1097-2765(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Harris SD. Branching of fungal hyphae: regulation, mechanisms and comparison with other branching systems. Mycologia. 2008;100:823–832. doi: 10.3852/08-177. [DOI] [PubMed] [Google Scholar]
- Jaffe LA. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature. 1976;261:68–71. doi: 10.1038/261068a0. [DOI] [PubMed] [Google Scholar]
- Klis FM, Boorsma A, De Groot PW. Cell wall construction in Saccharomyces cerevisiae. Yeast. 2006;23:185–202. doi: 10.1002/yea.1349. [DOI] [PubMed] [Google Scholar]
- Knaus M, Pelli-Gulli MP, van Drogen F, Springer S, Jaquenoud M, Peter M. Phosphorylation of Bem2p and Bem3p may contribute to local activation of Cdc42p at bud emergence. EMBO J. 2007;26:4501–4513. doi: 10.1038/sj.emboj.7601873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L, Saito K, Johnson JM, Howell AS, Zyla TR, Lew DJ. Symmetry-Breaking Polarization Driven by a Cdc42p GEF-PAK Complex. Curr Biol. 2008;18:1719–1726. doi: 10.1016/j.cub.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ, Reed SI. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J. Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Nichols BJ, Prescianotto-Baschong C, Riezman H, Pelham HR. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol Biol Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie SH, Brown SS. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J. Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onsum MD, Rao CV. Calling heads from tails: the role of mathematical modeling in understanding cell polarization. Curr Opin Cell Biol. 2009;21:74–81. doi: 10.1016/j.ceb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HO, Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev. 2007;71:48–96. doi: 10.1128/MMBR.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Franzusoff A, Segev N, Botstein D. Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol Biol Cell. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu YJ, Santos B, Fortin N, Costigan C, Snyder M. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol Cell Biol. 1998;18:4053–4069. doi: 10.1128/mcb.18.7.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter BD, Schwartz JW, Li R. Mapping dynamic protein interactions in MAP kinase signaling using live-cell fluorescence fluctuation spectroscopy and imaging. Proc Natl Acad Sci U S A. 2007;104:20320–20325. doi: 10.1073/pnas.0710336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Gao XD, Howell AS, Bose I, Lew DJ, Bi E. Adjacent positioning of cellular structures enabled by a Cdc42 GTPase-activating protein-mediated zone of inhibition. J Cell Biol. 2007;179:1375–1384. doi: 10.1083/jcb.200705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing A. The Chemical Basis of Morphogenesis. Philos Trans R Soc Lond B Biol Sci. 1952;237:37–72. doi: 10.1098/rstb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Taubas J, Pelham HR. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr Biol. 2003;13:1636–1640. doi: 10.1016/j.cub.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Li R. Spontaneous cell polarization: undermining determinism. Nat Cell Biol. 2003;5:267–270. doi: 10.1038/ncb0403-267. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Wai SC, Schmidt T, Li R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J Cell Biol. 2004;166:889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner JE, Harkins HA, Pringle JR. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:1857–1870. doi: 10.1128/mcb.16.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.