Abstract
Phosphatidylcholine transfer protein (PC-TP, a.k.a. StarD2) is a highly specific intracellular lipid binding protein that catalyzes the transfer of phosphatidylcholines between membranes in vitro. Recent studies have suggested that PC-TP in vivo functions to regulate fatty acid and glucose metabolism, possibly via interactions with selected other proteins. To begin to address the relationship between activity in vitro and biological function, we undertook a high throughput screen to identify small molecule inhibitors of the phosphatidylcholine transfer activity of PC-TP. After adapting a fluorescence quench assay to measure phosphatidylcholine transfer activity, we screened 114,752 compounds of a small molecule library. The high throughput screen identified 14 potential PC-TP inhibitors. Of these, 6 compounds exhibited characteristics consistent with specific inhibition of PC-TP activity, with IC50 values that ranged from 4.1 – 95.0 µM under conditions of the in vitro assay. These compounds should serve as valuable reagents to elucidate the biological function of PC-TP. Because mice with homozygous disruption of the PC-TP gene (Pctp) are sensitized to insulin action and relatively resistant to the development of atherosclerosis, these inhibitors may also prove to be of value in the management of diabetes and atherosclerotic cardiovascular diseases.
Keywords: phospholipid, lipid binding protein, START domain
Introduction
Phosphatidylcholine transfer protein (PC-TP, a.k.a. StarD2) is a soluble lipid binding protein and a member of the steroidogenic acute regulatory protein–related transfer (START) domain superfamily [1; 2]. Expression of PC-TP is accentuated in highly oxidative tissues in the mouse, including liver, brown fat, heart and muscle [3]. Among the phenotypes of mice with homozygous disruption of the Pctp gene (Pctp−/−) are increased hepatic insulin sensitivity and redistribution of body fat, which appear to be attributable to preferential utilization of fatty acids over glucose as energy substrates [4]. Mice that lack expression of both PC-TP and ApoE are relatively resistant to atherosclerosis when compared with ApoE-deficient mice [5]. Moreover, an unbiased genetic screen of a well characterized human population has revealed that a coding region polymorphism in the Pctp gene is associated with larger, less atherogenic LDL particles [6].
PC-TP was identified and has been characterized extensively based upon its in vitro activity [7], which is to bind and catalyze the intermembrane exchange of phosphatidylcholines, but no other lipid. Recently, yeast two-hybrid screening [8] has led to the suggestion that the function(s) of PC-TP in vivo may be dictated at least in part by interacting proteins. One such protein is thioesterase superfamily member 2 (Them2), which is mitochondrial-associated [9] and exhibits acyl-CoA thioesterase activity [8]. This suggests the possibility that PC-TP may participate in mitochondrial fatty acid metabolism [3; 10]. The observation that it also interacts with developmentally expressed transcription factor Pax3 raises the possibility PC-TP could also regulate transcription in certain cell types [8].
It is unclear at present whether the phosphatidylcholine transfer activity of PC-TP is related to its biological function(s). We reasoned that this issue could ultimately be addressed using a small molecule inhibitor of PC-TP activity. Because Pctp−/− mice are sensitized to insulin action and protected against atherosclerosis, the development of small molecule inhibitors might also eventually hold implications as a novel approach to the control of diabetes and reduction of cardiovascular risk.
Methods
Materials
Egg yolk trans-phosphatidylethanolamine, egg yolk phosphatidylcholine, egg sphingomyelin, egg phosphatidic acid and bovine heart cardiolipin were from Avanti Polar Lipids (Alabaster, AL) and were of the highest available purities (>99%). 2-(12-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)dodecanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine (NBD-PC) and Lissamine™ rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (rhodamine DHPE) were from Invitrogen (Carlsbad, CA). 1-palmitoyl-2-[1-14C]linoleoyl L-3-phosphatidylcholine (53 mCi/mmol) and [1α,2α (n)-3H] cholesteryl oleate (45 Ci/mmol) were from GE Healtchare (Piscataway, NJ). Unless otherwise specified, all other chemical reagents were from Sigma-Aldrich (St. Louis, MO).
Expression and purification of recombinant PC-TP
For bacterial expression of His-tag PC-TP, we utilized a previously described synthetic gene encoding human PC-TP [11]. This cDNA was codon-optimized to achieve maximal recombinant protein expression in E. coli. The synthetic gene was subcloned into pET19b (Novagen, EMD Biosciences, Madison, WI), which introduced an in-frame N-terminal His-tag (i.e. ten sequential His residues) followed by an enterokinase recognition site that facilitated its removal following affinity purification. In preliminary experiments, we found that the His-tag was not removed efficiently by enterokinase digestion when sufficiently large quantities of PC-TP were purified for inhibitor screening (i.e. > 100 mg). Therefore, in order to minimize the potential influence of added amino acid residues on PC-TP activity, we modified the pET19b-PCTP plasmid so that the nucleotide sequence encoding the enzyme cleavage site was removed and the His-tag was shortened from 10 to 6 His residues. This reduced number of amino acid residues fused to the N-terminus of PC-TP from 23 to 8. This plasmid was transformed into E. Coli BL21(DE3), and His-tag PC-TP was induced by the addition of 1 mM IPTG to LB followed by 14 h of shaking (250 rpm) at room temperature [11]. Bacteria were harvested by centrifugation and lysed with BugBuster (Novagen) supplemented with EDTA-free protease inhibitor. The bacterial lysate was clarified by centrifugation at 16,000 x g for 20 min at 4 °C, followed by centrifugation at 100,000 x g for 60 min at 4 °C.
For protein purification, bacterial lysates were filtered using a 0.22 µm filter and then adsorbed to a 5 ml His-trap HP column (GE Healthcare). To remove proteins that were bound non-specifically, the column was first washed with 5 ml of PBS containing 150 mM imidazole. His-tag PC-TP was then eluted using 5 ml of PBS containing 500 mM imidazole. Finally, the protein was dialyzed into 150 mM NaCl, 10 mM HEPES at pH 7.4 (buffer) plus 0.1 mM DTT, 3 mM NaN3, 0.2 mM EDTA and 0.1 mM PMSF. Purified His-tag PC-TP yielded a single band by SDS-PAGE followed by Coomassie brilliant blue staining and was shown to be immunoreactive by Western blot analysis [12]. These analyses indicated that shortening of the His-tag from 10 to 6 had no appreciable influence on the purification process. PC-TP concentrations were determined according to its molar extinction coefficient at 280 nm, which was calculated based the amino acid sequence (www.expasy.org). This procedure produced a yield of approximately 15 mg of pure His-tag PC-TP per L of bacterial culture.
Fluorescence quench assay of PC-TP activity
Phosphatidylcholine transfer activity of PC-TP was measured by modification of an assay in which the fluorescence of NBD-PC is utilized to detect movement of phosphatidylcholine molecules from donor to acceptor small unilamellar vesicles [8; 13]. Small unilamellar vesicles were prepared by sonication of phospholipids suspended in buffer [8]. Donor small unilamellar vesicles were composed of egg yolk trans-phosphatidylethanolamine, egg yolk phosphatidylcholine, egg sphingomyelin, egg phosphatidic acid, NBD-PC plus rhodamine DHPE in a molar ratio 55:14:15:10:1:5. Acceptor small unilamellar vesicles contained egg trans-phosphatidylethanolamine, egg phosphatidylcholine, egg sphingomyelin, egg phosphatidic acid in a molar ratio of 60:15:15:10. Small unilamellar vesicles were stored under an atmosphere of N2 at 4 °C until use.
Upon addition of acceptor vesicles, time dependent increases in fluorescence intensity (F(t)) reflected transfer of NBD-PC from donor small unilamellar vesicles containing rhodamine DHPE to acceptor small unilamellar vesicles. The fluorescence of NBD-PC in donor small unilamellar vesicles was quenched by the rhodamine DHPE. In preliminary experiments, optimal excitation and emissions wavelengths were determined to be 475 and 530, respectively. Due to its higher water solubility compared with native phosphatidylcholines, there was appreciable spontaneous transfer of NBC-PC, but with a much slower time course in this assay [13]. In preliminary experiments, we determined that the shortened His-tag did not influence the activity of PC-TP in this assay compared with protein in which the His-tag was removed enzymatically.
Adaptation of PC-TP activity assay to 384-well microplate format
In order to conduct the high throughput inhibitor screen, the PC-TP activity assay was adapted to a microplate format. The final volume of each well was 48.4 µL. First, a 38 µL mixture that contained acceptor small unilamellar vesicles and purified recombinant His-tag PC-TP in buffer was added to Nunc 384-well plates (Fisher Scientific, Pittsburgh, PA) containing 0.4 µl dimethyl sulfoxide (DMSO). DMSO was included because the compounds in the small molecule library were dissolved in this solvent. Following the addition of 10 µL donor vesicles, the plate was immediately loaded into a Spectramax M5 fluorimeter (Molecular Devices, MDS, Sunnyvale, CA). Following 2 sec of shaking, 10 readings of F(t) were averaged for each well every 15 sec for 6 min. Final conditions for the assay were 82 nM PC-TP, 188 µM acceptor phospholipid, 52 µM donor phospholipid in buffer (150 mM NaCl, 10 mM HEPES at pH 7.4) containing 1 vol% DMSO at room temperature. Transfer rates of NBD-PC were determined by fitting ΔF(t) = ΔFmax(1-e−kt) [8; 14] using Prism 4 (GraphPad Software, San Diego, CA), where k is the apparent first-order rate constant and ΔFmax is a constant that reflects the total number of NBD-PC molecules that are transferred from donor to acceptor small unilamellar vesicles in the assay. In preliminary experiments, it was demonstrated that this DMSO concentration did not influence the assay. For purposes of the high throughput screen, the negative control (c−) for inhibition was defined as all components plus 0.4 µL DMSO. The positive control (c+) substituted an equal volume of buffer for PC-TP. The Z’ factor [15], a measure of the suitability of an assay for high throughput screening, was calculated as: Z’(t) = 1 − 3(SDc− + SDc+)/(F(t)c− − F(t)c+).
Small molecule compound library
The compound library belonging to the Laboratory for Drug Discovery in Neurodegeneration (LDDN) at the Partners Center for Drug Discovery (Brigham and Women’s Hospital, Boston, MA) [16] consisted of approximately 120,000 small molecules, including compounds approved by the Food and Drug Administration (FDA), a purified natural products library, compounds purchased from Peakdale (High Peak, UK), Maybridge Plc. (Cornwall, UK), Cerep (Paris, France), Bionet Research Ltd. (Cornwall, UK), Prestwick (Ilkirch, France), Specs and Biospecs (CP Rijswijk, the Netherlands), ENAMINE (Kiev, Ukraine), I.F. Lab LTD (Burlington, Canada), and Chemical Diversity Labs (San Diego, CA), as well as small molecules from different academic institutions including our own LDDN chemists. Compounds were selected from the different vendors by applying a series of filters. All small molecules generally adhere to Lipinski’s rules and contain a low proportion of known toxicophores and unwanted functionalities, and have been optimized for maximization of molecular diversity.
High throughput screen
The PC-TP activity assay in the 384-well plate format was used to screen the small molecule library. Briefly, 0.4 µL of each compound (1.67 mM) dissolved in DMSO was first added in an automated fashion to the 384-well assay plates (Corning Inc., Corning, NY) [16] to achieve a final concentration of 14 µM for each compound in the high throughput screen. A µFill Microplate Dispenser (BioTek/Labtech International LTD, Ringmer, East Sussex, UK) was utilized to add the 38 µL mixture of small unilamellar vesicles and purified recombinant His-tag PC-TP in buffer to the 384-well plates. The plates were then loaded into a PheraStar plate reader (BMG Labtech, Offenberg, Germany) fitted with optical filters for excitation wavelength of 480 nm and emission wavelength of 520 nm. In preliminary experiments, these wavelengths were found to be sufficiently close to the optimal values that there was no appreciable impact on the PC-TP activity assay. Plates were shaken for 2 sec and then 10 baseline values of fluorescence intensity were averaged for each well to calculate the baseline (bl) fluorescence Fbl. The % Fbl was calculated from the fluorescence intensity of the inhibitor plus small unilamellar acceptor vesicles plus PC-TP in buffer divided by the value of fluorescence of the small unilamellar acceptor vesicles plus PC-TP in buffer. % Fbl provided a measure of whether a compound fluoresced (i.e. % Fbl > 100) or absorbed emitted light (i.e. % Fbl < 100) in the presence of the protein and small unilamellar acceptor vesicles. Wells with compounds exhibiting % Fbl < 60 were excluded as having the potential to generate false positive results due to absorbance of emitted light.
To initiate phosphatidylcholine transfer, 10 µL of donor vesicles were added to each well using the µFill Microplate Dispenser, and plates were put on a stacker. After 120 sec, plates were again loaded into the plate reader, shaken for 2 sec, and 10 readings were averaged for each well to obtain F(120 sec). The fluorescence intensity reflected net transfer of NBD-PC by PC-TP from quenched donor vesicles containing rhodamine DHPE to unquenched acceptor vesicles. For each plate, controls included 16 each of c− and c+ wells. A potential inhibitor (inh) was selected for confirmation if it demonstrated >75% inhibition of PC-TP transfer activity as measured by: ((Fc− (120 sec) − Finh(120 sec))/(Fc− (120 sec) − Fc+(120 sec))) × 100, where Fc− (120 sec) and Fc+(120 sec) represent average fluorescence intensities of the c− and c+ wells, respectively. F(120 sec) values from c− and c+ well also allowed for the calculation of a Z’ factor for each plate
Confirmatory screening
Potential small molecule inhibitors were confirmed manually in a 384-well plate. Values of F(t) were measured at 60 sec intervals for 10 min as described above for each compound in triplicate. As was the case for the high throughput screen, the plate included 16 each of c− and c+ wells. Inhibition of PC-TP activity was measured at the single time point of 60 sec. In preliminary experiments, it was observed that fluorescence intensity increased curvilinearly during the first several minutes following the mixing of PC-TP with donor and acceptor small unilamellar vesicles. Therefore, inhibition by compounds was further confirmed according to the change in fluorescence (ΔF) during the initial 180 sec (ΔF = (F(180 sec) − F(0)), where F(0) is the fluorescence intensity that was measured immediately the addition of donor vesicles. This was used to calculate % inhibition = ((ΔFc− − ΔFinh)/(ΔFc− − ΔFc+)) × 100, where ΔFc− and ΔFc+ represent the average changes in intensities over 180 sec for the c− and c+ wells, respectively.
IC50 determinations
For the most promising compounds, IC50 values were determined using a 96-well format to measure PC-TP activity. Compounds were initially dissolved in DMSO at 10 mM concentrations and then diluted to final concentrations in buffer. The final volume of each well was 100 µL. First, purified His-tag PC-TP (final concentration of 225 nM per well) and 10 µL inhibitor were added to each well of a 96-well Nunc plate (Fisher Scientific). In the c− and c+ wells, PC-TP or inhibitor was substituted with buffer. This was followed by the addition of acceptor small unilamellar vesicles to yield a final acceptor phospholipid concentration of 225 µM. At time zero, 20 µL of donor small unilamellar vesicles were added to yield a final donor phospholipid concentration of 50 µM. The plate was immediately loaded into the Spectramax M5 fluorimeter. Following 2 sec of shaking, 10 readings fluorescence intensity were averaged for each well every 30 sec for 45 min.
Transfer rates of NBD-PC were determined by fitting ΔF(t) = ΔFmax(1−e−kt). Each compound was assayed in triplicate for each inhibitor concentration. Duplicate c− and c+ wells were also measured for each inhibitor concentration. An additional well contained donor and acceptor small unilamellar vesicles plus inhibitor, but no PC-TP. This well insured that the inhibitor itself did not induce transfer of NBD-PC or increase ins fluorescence by disrupting vesicle integrity. For these experiments, % inhibition = (kc− − kinh)/(kc− − kc+) × 100, where kc− and kc+ represent the average k values for the c− and c+ wells, respectively. IC50 values were determined by fitting measurements of % inhibition to a sigmoidal dose-response curve using Prism 4.
Confirmatory assay of PC-TP inhibition
Inhibition of PC-TP transfer activity was verified using an independent assay that measures intermembrane transfer of radiolabeled phospholipids [17; 18]. Donor small unilamellar vesicles were prepared by sonication of 1 mg of phosphatidylcholine, 0.1 × 106 dpm [14C]-phosphatidylcholine, 1 × 106 dpm [3H]-cholesteryloleate and the antioxidant butylated hydroxytoluene (2 µg) dispersed in 1 ml of 5 mM EDTA, 50 mM Tris at pH 7.2. Multilamellar vesicles were prepared by rotary shaking (250 rev/min) of 104.6 mg phosphatidylcholine plus 10.4 mg cardiolipin (95:5 mol%) and 10 µg butylated hydroxytoluene dispersed in 10 ml of the same buffer. In order to isolate a population of multilamellar vesicles that could be separately physically from small unilamellar vesicles by centrifugation, rotary shaken suspensions were centrifuged at 55,000 x g for 30 min. The supernatant was then discarded, and the pellet was gently resuspended in 6 ml of Tris-EDTA buffer. Solutions containing PC-TP (final concentration of 280 nM) plus inhibitor or an equal volume of DMSO were incubated together in a 37°C shak ing water bath with 50 µl donor small unilamellar vesicles and 100 µl acceptor multilamellar vesicles adjusted to a final volume of 500 µl with Tris-EDTA buffer. Control samples contained no added protein. Following 30 min of incubation with shaking at 37°C, multilamellar accepto r vesicles were pelleted by centrifugation (55,000x g) at 4°C for 30 min. The [ 14C]-phosphatidylcholine and [3H]-cholesteryl oleate contents in 350 µl of small unilamellar vesicles that remained suspended were determined by liquid scintillation counting (Liquid Scintillation System LS 6500TD, Beckman, Fullerton, CA). Because cholesteryl oleate is not transferred by PC-TP [19], [3H]-cholesteryl oleate concentrations were employed to correct for small losses of SUV during centrifugation. In this manner, phosphatidylcholine transferred from small unilamellar donor to multilamellar acceptor vesicles was calculated from decreases observed in the [14C]/[3H] ratios of the donor vesicles.
Results
Miniaturization of fluorescence assay for PC-TP activity
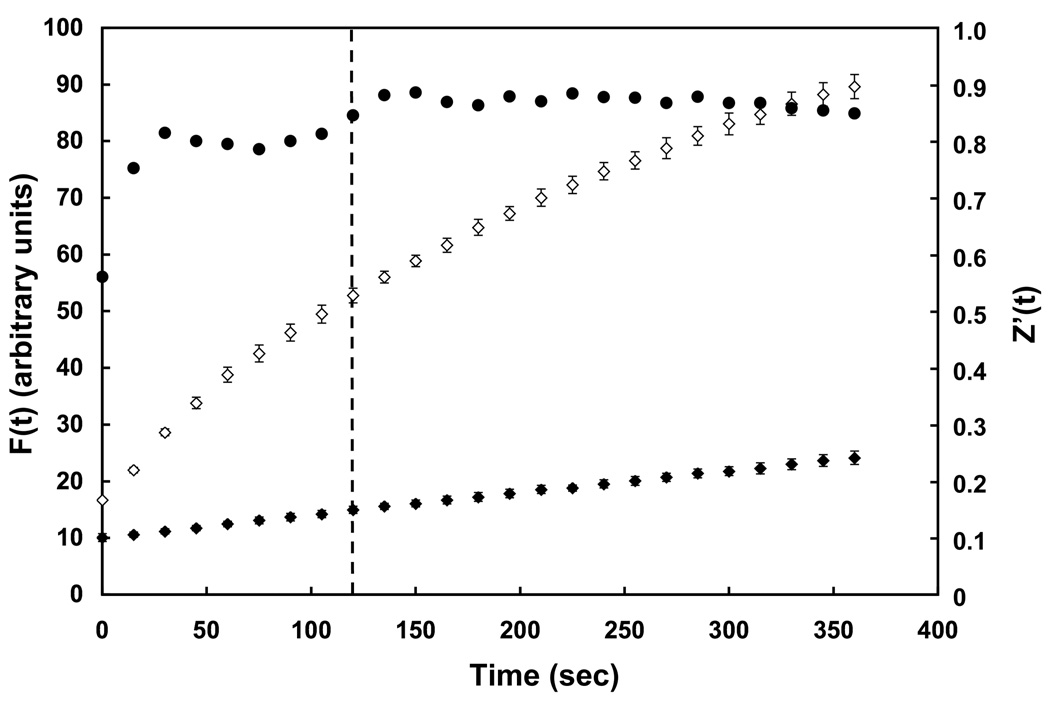
Figure 1 shows the PC-TP activity assay adapted to a 384-well plate. Compared with the spontaneous transfer of NBD-PC, PC-TP-mediated transfer was much more rapid. During the 360 sec time course of the experiment, values of F(t) increased sharply for PC-TP-mediated NBD-PC transfer and began to level off at the latest time points in the experiment. The time course of fluorescence intensity was well fit (R2 > 0.99) to the equation ΔF(t) = ΔFmax(1−e−kt). The fitted parameters (mean ± SD, n = 6) were k = 0.0040 ± 0.0003 sec−1 and ΔFmax = 172 ± 11 in arbitrary units. Using the mean values, 50% of Fmax was reached at 172 sec and 80% was achieved at 360 sec (the total time of the experiment). As measures of leveling off, calculated times to reach values for F(t) of 90% and 95% of Fmax were 570 sec and 741 sec, respectively. By contrast, there was a much more linear-appearing, slow spontaneous transfer. Figure 1 also plots Z’(t) during the course of the assay. Throughout the assay, the Z’ factor readily exceeded 0.5, which is generally considered to be the threshold for a robust high throughput screen [15]. At a time point of 120 sec (indicated by the dashed line), values of F(t) were increasing rapidly and Z’(120 sec) was 0.84. To minimize the effect of the slow spontaneous transfer, 120 sec was chosen as the time to make a suitable endpoint measurement of F(120 sec) for the high throughput screen. At this time point, the F(t) was 40% of Fmax. This time was about the shortest time point that was also feasible for automated processing of the 384-well plates.
Figure 1.
Fluorescence assay of PC-TP activity assay scaled to a 384-well plate. Fluorescence intensities were measured in arbitrary fluorescence units (mean ± 1SD) as functions of time following mixing of donor unilamellar vesicles with acceptor unilamellar vesicles in the presence (◇, n = 6) or absence (◆, n = 6) of PC-TP. Values for Z’(t) (●) were calculated at each time point as described in Methods. The dashed line indicates the time point (120 sec) that was chosen for a single time-point measurement of PC-TP inhibition during the high throughput screen.
High throughput inhibitor screen
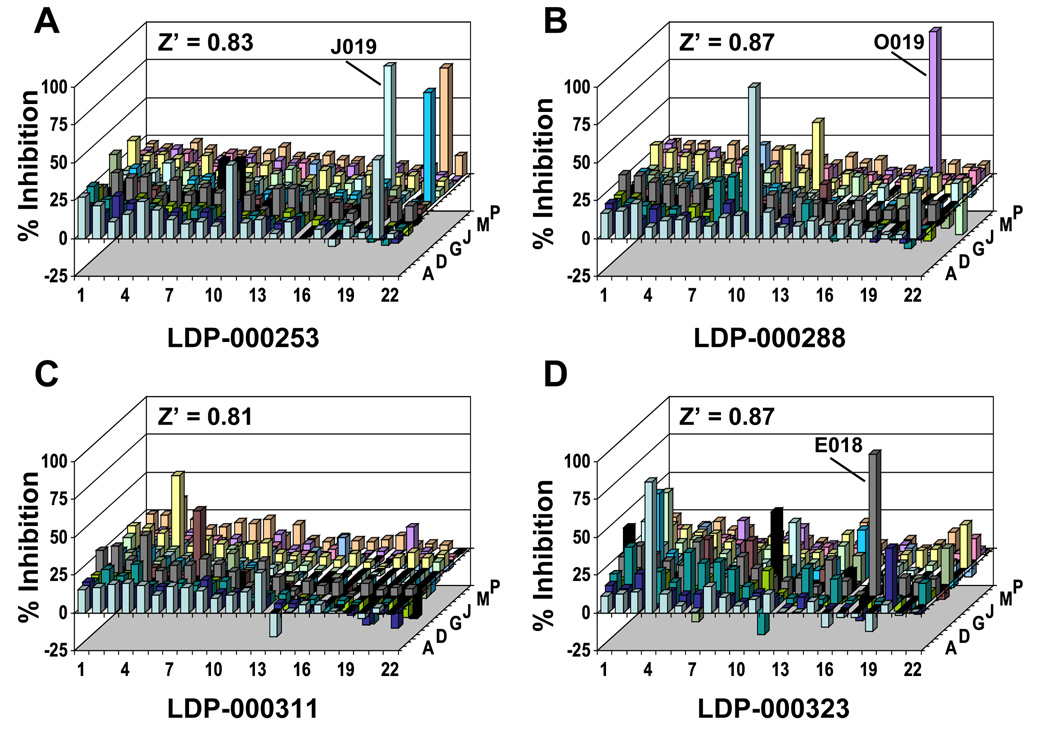
The miniaturized PC-TP activity assay was used to screen the LDDN small molecule library. A total of 326 plates were assayed with each plate containing 352 compounds, so that a total of 114,752 compounds were screened. Figure 2 shows the Z’ factor for each plate. In all cases, the Z’ factor exceeded the threshold of 0.5 for an “excellent assay” [15]. Mean ± SD values of Z’(120 sec) were 0.88 ± 0.06 (range: 0.53 to 0.92), with a median value of 0.86. Figure 3 displays experimental values of % inhibition and Z’ factors for the four representative plates denoted by arrows labeled A–D in Figure 2. These data demonstrate that the assay yielded few if any compounds per plate that met the criterion of 75% inhibition and that most compounds did not exceed 25% inhibition. The well positions of the three individual compounds contained on the four plates that initially met both criteria set for inhibition (i.e. > 75% inhibition and % Fbl > 60) are indicated. Overall, 139 compounds fulfilled these criteria.
Figure 2.
Quality control for high throughput screening of the LDDN small molecule library for inhibitors of PC-TP activity. Compounds numbering 114,752 were distributed among 326 384-well plates at 352 compounds/plate. Each plate contained 16 wells that served as positive controls and 16 wells of negative controls. These wells were used to calculate values of Z’(120 sec) for each plate as described in the text and plotted sequentially. Arrows labeled A–D denote representative plates, which are fully illustrated in Figure 3.
Figure 3.
Values of % inhibition for individual compounds on representative plates. Inhibition of PC-TP activity for individual wells of plates indicated in Figure 2 were calculated from values of F(120 sec), as described in the text. The Z’ factor of each plate (Z’(120 sec)) is indicated. The well positions are indicated for three compounds (one each in panels A, B and D), which fulfilled the initial screening criteria. Negative values for % inhibition are attributable to the absorbance of a minority of compounds.
Confirmatory screening
In order to confirm and refine the initial screen, each of the 139 compounds was re-screened in triplicate based on a single time point of 60 sec, at which F(60 sec) was 20% of Fmax (Figure 1). We also assessed the influence of compounds on the increase in fluorescence, ΔF, during the first 180 sec, which was the time required for F(t) to increase to 50% of Fmax. Based on these data, the 50 compounds that were most effective potential inhibitors were chosen for further scrutiny. An analysis of the chemical structures of each these compounds suggested 14 potential inhibitors that represented unique structural classes and are listed in Table 1.
Table 1.
Candidate Small Molecule Inhibitors of PC-TP Activitya
| Primary Screen |
Secondary Screenb |
||||||
|---|---|---|---|---|---|---|---|
| Plate | Well | Compound | % Inhibition F(120 sec) |
% Fbl | % Inhibition F(60 sec) |
% Inhibition ΔF |
% Fbl |
| LDP-000042 | O012 | LDN-0008837 | 84.2 | 753.3 | 78.8 ± 0.6 | 104.5 ± 2.9 | 684.8 ± 17.0 |
| LDP-000072 | G002 | LDN-0024298 | 114.6 | 92.4 | 106.6 ± 1.0 | 109.3 ± 2.9 | 81.4 ± 1.4 |
| LDP-000083 | I010 | LDN-0028202 | 95.2 | 110.8 | 97.6 ± 0.3 | 86.9 ± 3.8 | 121.0 ± 2.4 |
| LDP-000095 | O009 | LDN-0032514 | 83.0 | 66.3 | 58.0 ± 0.4 | 45.3 ± 3.4 | 78.1 ± 3.1 |
| LDP-000122 | F017 | LDN-0042264 | 78.4 | 341.6 | 80.7 ± 2.2 | 87.4 ± 4.1 | 326.9 ± 3.3 |
| LDP-000179 | F013 | LDN-0060192 | 83.5 | 560.5 | 80.9 ± 1.7 | 101.9 ± 2.0 | 530.8 ± 30.9 |
| LDP-000187 | E003 | LDN-0062847 | 107.5 | 109.8 | 51.4 ± 7.4 | 20.7 ± 9.4 | 160.0 ± 3.9 |
| LDP-000204 | J011 | LDN-0068964 | 100.9 | 83.7 | 81.6 ± 3.5 | 85.4 ± 7.7 | 80.3 ± 1.7 |
| LDP-000216 | C005 | LDN-0073085 | 112.4 | 194.1 | 80.1 ± 2.9 | 57.4 ± 4.6 | 149.2 ± 2.1 |
| LDP-000227 | G015 | LDN-0077121 | 82.4 | 87.4 | 74.5 ± 7.4 | 43.9 ± 15.0 | 102.1 ± 1.6 |
| LDP-000227 | I015 | LDN-0077123 | 82.3 | 96.9 | 65.4 ± 7.2 | 34.9 ± 12.9 | 107.5 ± 6.2 |
| LDP-000253 | J019 | LDN-0085978 | 89.7 | 104.1 | 81.0 ± 1.8 | 65.5 ± 3.7 | 106.1 ± 0.6 |
| LDP-000288 | O019 | LDN-0097251 | 99.3 | 115.2 | 72.7 ± 10.3 | 54.9 ± 14.9 | 117.6 ± 5.9 |
| LDP-000290 | C019 | LDN-0097715 | 114.8 | 110.7 | 98.8 ± 1.2 | 96.0 ± 1.9 | 107.3 ± 1.5 |
Calculations for % inhibition and % Fbl are as described in the text.
Data are mean ± SD.
Determination of IC50 values
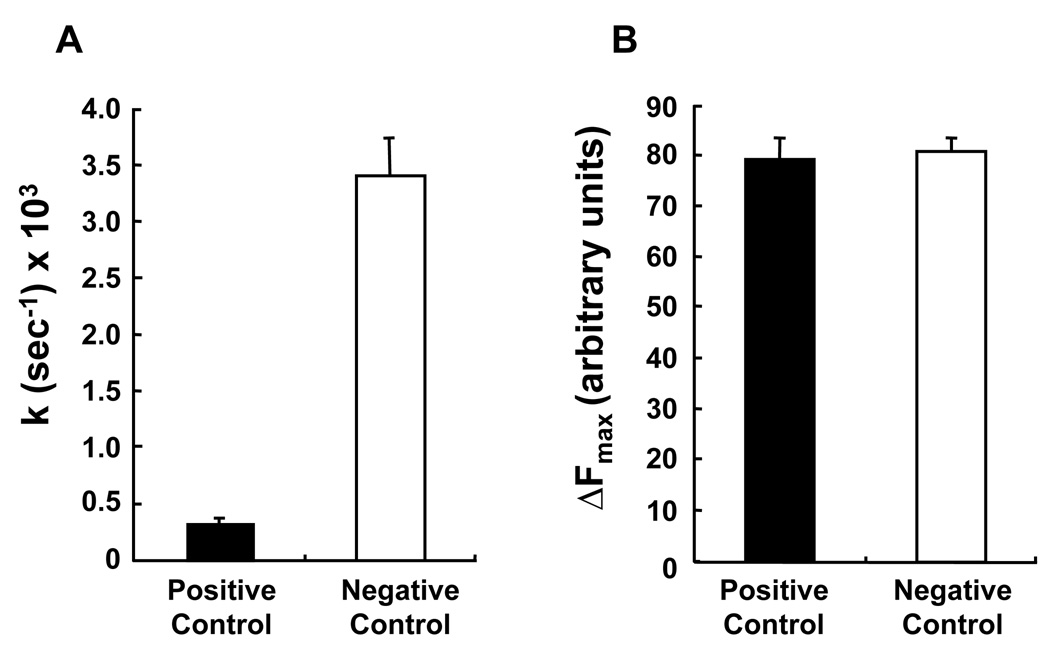
A dose-response curve was generated for each of the 14 compounds Table 1. For this purpose, the PC-TP activity assay was adapted to 96-well format, with optimization for formal kinetic analysis of PC-TP activity [14]. Figure 4 demonstrates the reproducibility and dynamic range of this assay. For each experiment, the 91 values of F(t) obtained during the course of the fluorescence transfer assay were fit (R2 ≥ 0.99) to the function ΔF(t) = ΔFmax(1−e−kt). Figure 4A shows a 10-fold difference between the values of k obtained for the positive and negative control samples. Figure 4B shows that ΔFmax did not differ between positive and negative control samples. This was expected because PC-TP catalyzes phosphatidylcholine transfer, so that its presence in the sample would be expected to increase only the rate and not the total number of NBD-PC molecules transferred, which is quantified as ΔFmax.
Figure 4.
Characteristics of the PC-TP activity assay when scaled to a 96-well plate. Values of (A) k and (B) ΔFmax were determined as described in Methods for positive control (open bars; n = 16) and positive control (closed bars; n = 16) conditions. Error bars represent 1SD.
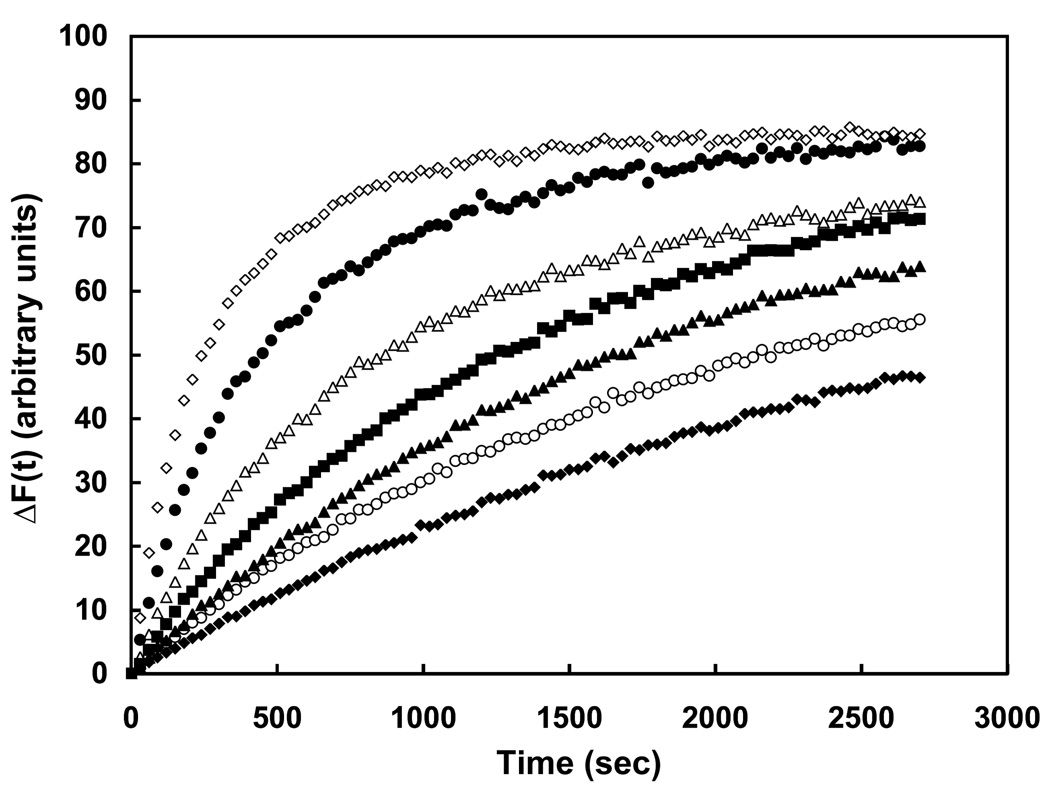
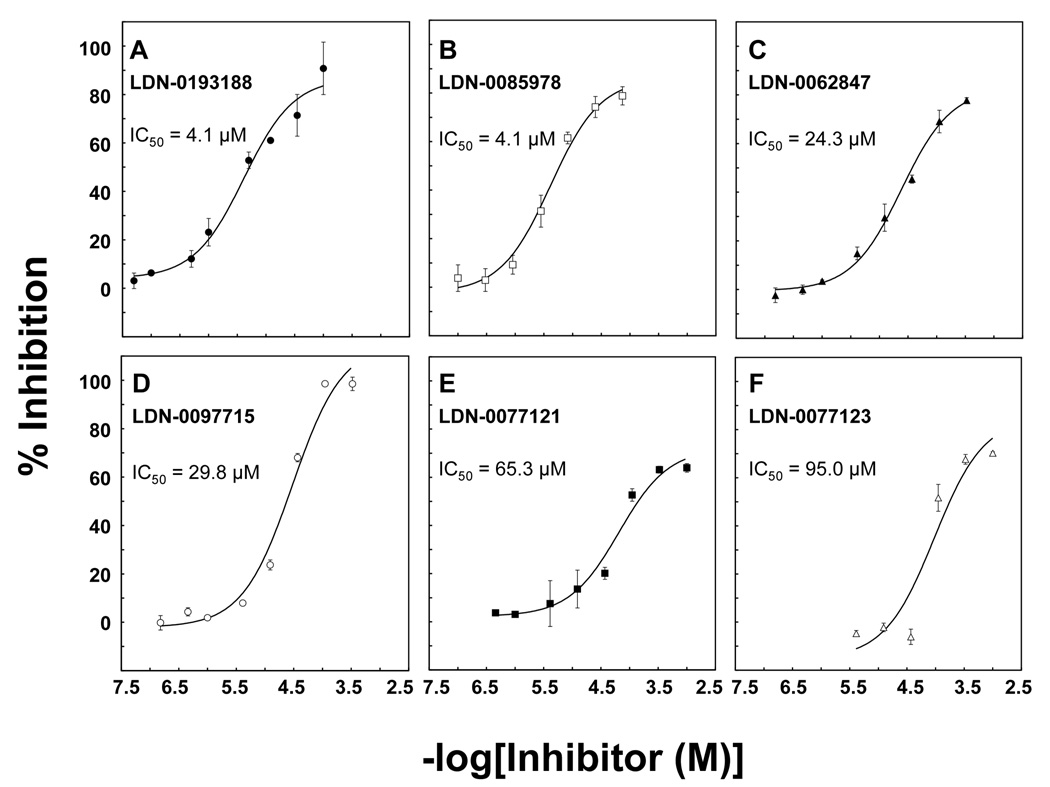
Of the 14 compounds tested using a range of concentrations, 6 altered the kinetics of NBD-PC transfer in a manner consistent with inhibition of PC-TP activity: These compounds decreased the value of k, without influencing ΔFmax. One of these (LDN-0028202), was not sufficiently soluble to characterize reproducibly, but a close analogue (LDN-0193188) proved to be both soluble and effective as a PC-TP inhibitor, as demonstrated by Figure 5. As the inhibitor concentration was increased from 1 – 100 µM, there was a progressive decrease in the rate of NBD-PC transfer. Figure 6 presents the dose response curve for this compound (Figure 6A) and the 5 other PC-TP inhibitors. The other 8 compounds generally yielded rapid initial increases in fluorescence suggesting disruption of the small unilamellar vesicles rather than protein-mediated fluorescence transfer (data not shown).
Figure 5.
Small molecule inhibition of PC-TP activity. Time dependent increases were measured in the absence (◆, positive control) or presence (◇, negative control) of PC-TP in a 96-well plate. Progressive inhibition of PC-TP activity was observed following the addition of LDN-0193188 at concentrations of 1 µM (●), 5 µM (△), 12 µM (■), 35 µM (▲) and 100 µM (○).
Figure 6.
Potencies of small molecule inhibitors of PC-TP activity. As described in the text, IC50 values were determined for the most promising inhibitors identified by high throughput screening: A) LDN-0193188, B) LDN-0085978, C) LDN-0062847, D) LDN-0097715, E) LDN-0077121 and F) LDN-0077123. Data points are mean ± SD of three determinations.
To further confirm inhibition, we tested two compounds using a semiquantitative assay in which PC-TP activity is determined according to the transfer of [14C]-phosphatidylcholine molecules from small unilamellar donor to multilamellar acceptor vesicles. Because it was performed at a single time-point and required the mechanical separation of donor and acceptor vesicles, this radiolabeled assay provided much more limited information than the fluorescence assay. The lack of detailed kinetic data did not permit us to establish detailed dose-response curves, but did allow the estimation of percentages of inhibition at threshold concentrations of compounds. These were in qualitative agreement with the results from the fluorescence assays: the more potent compound LDN-0085978 (Figure 6B) inhibited PC-TP transfer by 67 ± 6% at 1 µM, and this was sustained at concentrations ranging up to 20 µM. The less potent compound LDN-0097715 (Figure 6C) inhibited PC-TP transfer by 35 ± 11% at 10 µM, which was sustained at concentrations ranging up to 100 µM.
Discussion
After miniaturizing a fluorescent phosphatidylcholine transfer assay, we conducted a high throughput small molecule screen that has led to the identification of several inhibitors of the phosphatidylcholine transfer activity of PC-TP. To accomplish this aim, we took advantage of certain biochemical characteristics of PC-TP [3; 7].
Previous studies by other investigators [7; 20; 21; 22] and our group [3; 14] have demonstrated that PC-TP-mediated phosphatidylcholine exchange between membranes is not representative of a classical enzymatic reaction. The activity of PC-TP in vitro is influenced by the concentration and composition of both donor and acceptor small unilamellar vesicles [17; 23], as well as the concentration of PC-TP [14]. Mechanistic models [14; 20; 22] suggest that these parameters control the fraction of PC-TP that is free in solution to transfer phosphatidylcholines versus a fraction of PC-TP that becomes membrane-bound and unavailable to participate. In preliminary experiments, these parameters were carefully optimized in order to yield appropriate phosphatidylcholine transfer rates so that the small molecule screen could be executed efficiently. For the initial screen, a single time point at 120 sec represented an interval following addition of all reagents to the 384-well plate, which yielded a value of F(120 sec) that fell on the early portion of the curve for PC-TP-mediated NBD-PC transfer (i.e. < 50% of Fmax). As a result, the single reading approximated the rate of NBD-PC transfer. The criteria set for confirmatory screening was also based on this notion. As is evident from Figure 1, the earlier single time point of 60 sec was in a region of more rapid change in F(t), while maintaining a high Z’ factor. Whereas such a rapid reading was not feasible in the high throughput screen due to the technical constraints of the assay, it could be achieved in the confirmatory screen when a limited number of compounds were tested. By also using ΔF to set criteria for the confirmatory screen, we sought to account for the small fraction of NBD-PC molecules that were transferred prior to the first reading that could be achieved after loading of the plate into the fluorimeter.
The characteristics of the fluorescence transfer assay did not allow us to distinguish inhibition of PC-TP activity from the possibility that a compound absorbed light emitted by unquenched NBD-PC molecules. Therefore, a cutoff of 60 for % Fbl was set in the initial screen. Because we also included ΔF to set criteria for the confirmatory screen, this cutoff value for % Fbl could be reduced (Table 1). This is because the intrinsic absorbance of a compound from the library would be expected to remain constant over time. The initial prioritization of compounds involved strict criteria, but compounds that were eliminated can always be subsequently re-examined in more detail or in an alternative assay to separate truly active compounds from compounds that interfere with the fluorescent signal.
The choice of final concentration for compounds in the high throughput screen was based on several key considerations. Because intact membranes in the form of small unilamellar vesicles were a key component of the assay, this limited the volume % of DMSO that could be added. The use of 1% DMSO in the assay allowed us to achieve a compound concentration of 14 µM, which represented a 170-fold molar excess compared with PC-TP. The large excess was considered to be important because it was assumed that some compounds would partition into the membrane bilayers of the small unilamellar vesicles. At the same time, the final phospholipid concentration of donor plus acceptor small unilamellar vesicles was 17-fold higher than the concentration of compound. This reduced the likelihood that amphipathic or hydrophobic compounds would disrupt vesicle integrity [24] and lead to an increase fluorescence of NBD-PC molecules by physically separating them from rhodamine DHPE. Nevertheless, the elimination of 8 of the 14 compounds in Table 1 was based on unusual kinetic properties during the determination of IC50 values, which may have been due to alterations in vesicle structures and not inhibition of PC-TP. This suggests that the use of a membrane-based assay imposed important constraints in our experiments.
In adapting the PC-TP activity assay to 96-well format, we optimized the conditions of NBD-PC transfer for formal kinetic analyses. Although this was associated with a modest (15%) increase in total phospholipid concentration in the assay, there was a 2.7–fold increase in PC-TP concentration. In this setting, we observed a higher range of IC50 values for the 6 compounds in Figure 6 relative to the 75% percent inhibition at 14 µM that was required for their selection during the initial screening process. In standard situations in which the concentration of inhibitor is much greater than an enzyme concentration, IC50 of a reversible inhibitor is independent of enzyme concentration. However, when the range of inhibitor concentrations is similar or less that the enzyme concentration, IC50 will vary with the concentration of enzyme. This may have explained the variations in IC50 with PC-TP concentration, appreciating that the presence of small unilamellar vesicles most likely altered the free concentrations of both the protein and the compounds. In support of this possibility, we have found that reduced PC-TP concentrations in the 96-well assay format were associated with reduced IC50 values (data not shown).
The observation that increased PC-TP concentrations required higher concentrations of inhibitor at similar phospholipid concentrations suggested an inhibition mechanism that involves binding of inhibitor to the protein, leading to competitive or non-competitive inhibition. However, alternative explanations are possible. The mechanism by which PC-TP transfers phosphatidylcholines between membranes requires several steps [2; 3]. PC-TP must first associate with a donor small unilamellar vesicle and then exchange a phosphatidylcholine. This is followed by dissociation from the vesicle, association with an acceptor small unilamellar vesicle, exchange of a phosphatidylcholine and finally dissociation. Inhibition of the transfer of NBD-PC could theoretically occur at any of these steps and further study will be required to distinguish from among these possibilities. It is also important to note that the assays utilized in this study do not discriminate between exchange and net transfer of phosphatidylcholines, although others [7] have demonstrated that PC-TP preferentially promotes exchange of phosphatidylcholines under similar conditions.
For this inhibitor screen, we adapted a previously published and validated NBD-PC-based assay [13] in which the PC-TP-mediated transfer rates of this fluorescent phosphatidylcholine were the same as a radiolabeled phosphatidylcholine. When taken together with the previously described binding properties of PC-TP for phosphatidylcholine (reviewed in [7]), Nichols and Pagano concluded that PC-TP binds the NBD-PC (which is modified by the addition the more hydrophilic NBD group to the end of sn-2 acyl chain) in a similar fashion as natural phosphatidylcholines. However, the possibility remained that the mechanism(s) of inhibition of PC-TP-mediated NBD-PC transfer may not apply to the transfer of natural phosphatidylcholines. Accordingly, we tested two of the compounds identified in the high throughput screen for inhibition of PC-TP-mediated transfer of a radiolabeled phosphatidylcholine [17]. This assay offers the advantage that the phosphatidylcholine molecule that is bound and transferred by PC-TP was not chemically modified. However, the assay is limited by the requirements for physical separation of donor and acceptor vesicles, as well as higher total concentrations of phospholipids and PC-TP. Notwithstanding these differences, qualitatively similar inhibition was observed for two different compounds.
In summary, we have utilized high throughput screening to identify small molecule inhibitors of PC-TP activity in vitro. Currently, there are not validated methods for PC-TP-mediated intermembrane phosphatidylcholine transfer intracellularly, the development of which is needed allow additional testing. If effective, these inhibitors should prove useful in identifying the relationship between the phosphatidylcholine transfer activity of PC-TP in vitro and the function of PC-TP in vivo, and in rationalizing the phenotypes of the Pctp−/− mouse [4; 5]. It is also possible that the compounds may be of therapeutic value in the management of diabetes and atherosclerosis.
Acknowledgments
This work was supported by National Institutes of Health (grants DK56626 and DK48873) and a pilot and feasibility grant from Partners Center for Drug Discovery (D.E.C). During the course of these studies, Neil Wagle was a Howard Hughes Medical Institute Medical Research Training Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ponting CP, Aravind L. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem. Sci. 1999;24:130–132. doi: 10.1016/s0968-0004(99)01362-6. [DOI] [PubMed] [Google Scholar]
- 2.Roderick SL, Chan WW, Agate DS, Olsen LR, Vetting MW, Rajashankar KR, Cohen DE. Structure of human phosphatidylcholine transfer protein in complex with its ligand. Nature Struct. Biol. 2002;9:507–511. doi: 10.1038/nsb812. [DOI] [PubMed] [Google Scholar]
- 3.Kanno K, Wu MK, Scapa EF, Roderick SL, Cohen DE. Structure and function of phosphatidylcholine transfer protein (PC-TP)/StarD2. Biochim. Biophys. Acta. 2007;1771:654–662. doi: 10.1016/j.bbalip.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scapa EF, Pocai A, Wu MK, Gutierrez-Juarez R, Glenz L, Kanno K, Li H, Biddinger S, Jelicks LA, Rossetti L, Cohen DE. Regulation of energy substrate utilization and hepatic insulin sensitivity by phosphatidylcholine transfer protein/StarD2. Faseb J. 2008;22:2579–2590. doi: 10.1096/fj.07-105395. [DOI] [PubMed] [Google Scholar]
- 5.Wang WJ, Baez JM, Maurer R, Dansky HM, Cohen DE. Homozygous disruption of Pctp modulates atherosclerosis in apolipoprotein E deficient mice. J. Lipid Res. 2006;47:2400–2407. doi: 10.1194/jlr.M600277-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Dolley G, Berthier MT, Lamarche B, Despres JP, Bouchard C, Perusse L, Vohl MC. Influences of the phosphatidylcholine transfer protein gene variants on the LDL peak particle size. Atherosclerosis. 2007;195:297–302. doi: 10.1016/j.atherosclerosis.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Wirtz KW. Phospholipid Transfer Proteins. Annu. Rev. Biochem. 1991;60:73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]
- 8.Kanno K, Wu MK, Agate DA, Fanelli BK, Wagle N, Scapa EF, Ukomadu C, Cohen DE. Interacting proteins dictate function of the minimal START domain phosphatidylcholine transfer protein/StarD2. J. Biol. Chem. 2007;282:30728–30736. doi: 10.1074/jbc.M703745200. [DOI] [PubMed] [Google Scholar]
- 9.Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell. 2003;115:629–640. doi: 10.1016/s0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- 10.de Brouwer AP, Westerman J, Kleinnijenhuis A, Bevers LE, Roelofsen B, Wirtz KWA. Clofibrate-induced relocation of phosphatidylcholine transfer protein to mitochondria in endothelial cells. Exp. Cell Res. 2002;274:100–111. doi: 10.1006/excr.2001.5460. [DOI] [PubMed] [Google Scholar]
- 11.Feng L, Chan WW, Roderick SL, Cohen DE. High-level expression and mutagenesis of recombinant human phosphatidylcholine transfer protein using a synthetic gene: evidence for a C-terminal membrane binding domain. Biochemistry. 2000;39:15399–15409. doi: 10.1021/bi001076a. [DOI] [PubMed] [Google Scholar]
- 12.Shoda J, Oda K, Suzuki H, Sugiyama Y, Ito K, Cohen DE, Feng L, Kamiya J, Nimura Y, Miyazaki H, Kano M, Matsuzaki Y, Tanaka N. Etiologic significance of defects in cholesterol, phospholipid, and bile acid metabolism in the liver of patients with intrahepatic calculi. Hepatology. 2001;33:1194–1205. doi: 10.1053/jhep.2001.23936. [DOI] [PubMed] [Google Scholar]
- 13.Nichols JW, Pagano RE. Resonance energy transfer assay of protein-mediated lipid transfer between vesicles. J. Biol. Chem. 1983;258:5368–5371. [PubMed] [Google Scholar]
- 14.Cohen DE, Leonard MR, Carey MC. In vitro evidence that phospholipids secretion into bile may be coordinated intracellularly by the combined actions of bile salts and the specific phosphatidylcholine transfer protein of liver. Biochemistry. 1994;33:9975–9980. doi: 10.1021/bi00199a021. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 16.Bandyopadhyay S, Ni J, Ruggiero A, Walshe K, Rogers MS, Chattopadhyay N, Glicksman MA, Rogers JT. A high-throughput drug screen targeted to the 5'untranslated region of Alzheimer amyloid precursor protein mRNA. J. Biomol. Screen. 2006;11:469–480. doi: 10.1177/1087057106287271. [DOI] [PubMed] [Google Scholar]
- 17.DiCorleto PE, Zilversmit DB. Protein-catalyzed exchange of phosphatidylcholine between sonicated liposomes and multilamellar vesicles. Biochemistry. 1977;16:2145–2150. doi: 10.1021/bi00629a016. [DOI] [PubMed] [Google Scholar]
- 18.Feng L, Cohen DE. Baculovirus-mediated expression of recombinant rat phosphatidylcholine transfer protein. J. Lipid Res. 1998;39:1862–1869. [PubMed] [Google Scholar]
- 19.Johnson LW, Hughes ME, Zilversmit DB. Use of phosphatidylcholine exchange protein to measure inside-outside transposition in phosphatidylcholine liposomes. Biochim. Biophys. Acta. 1975;375:176–185. doi: 10.1016/0005-2736(75)90187-x. [DOI] [PubMed] [Google Scholar]
- 20.Berkhout TA, van den Bergh C, Mos H, de Kruijff B, Wirtz KWA. Regulation of the activity of phosphatidylcholine transfer protein by vesicle phosphatidic acid and membrane curvature: A fluorescence study using 2-parinaroylphosphatidylcholine. Biochemistry. 1984;23:6894–6900. [Google Scholar]
- 21.Helmkamp GM., Jr Concerning the mechanism of action of bovine liver phospholipids exchange protein: Exchange or net transfer. Biochem. Biophys. Res. Comm. 1980;97:1091–1096. doi: 10.1016/0006-291x(80)91487-4. [DOI] [PubMed] [Google Scholar]
- 22.van den Besselaar AMHP, Helmkamp GM, Wirtz KWA. Kinetic model of the protein-mediated phosphatidylcholine exchange between single bilayer liposomes. Biochemistry. 1975;14:1852–1858. doi: 10.1021/bi00680a008. [DOI] [PubMed] [Google Scholar]
- 23.Somerharju PJ, van Paridon PA, Wirtz KWA. Application of fluorescent phospholipids analogues to studies on phospholipid transfer proteins. Subcellular Biochemistry. 1990;16:21–43. doi: 10.1007/978-1-4899-1621-1_2. [DOI] [PubMed] [Google Scholar]
- 24.Cohen DE, Thurston GM, Chamberlin RA, Benedek GB, Carey MC. Laser light scattering evidence for a common wormlike growth structure of mixed micelles in bile salt- and straight-chain detergent-phosphatidylcholine aqueous systems: relevance to the micellar structure of bile. Biochemistry. 1998;37:14798–14814. doi: 10.1021/bi980182y. [DOI] [PubMed] [Google Scholar]