Abstract
Although the positive clinical benefits of levodopa have fostered the concept of an abnormality in the dopaminergic system in Restless Legs Syndrome (RLS), research into the nigro–striatal (PET/SPECT studies) or tubero-infundibular (i.e., prolactin secretion) dopaminergic pathways has shown limited positive results. Some research groups have focused on the A11 dopaminergic system in the hypothalamus as this is the primary source of descending dopaminergic input into the spinal cord, an area of the nervous system believed by some investigators to be involved in RLS symptom development. Some investigators have now proposed lesioning or toxin-inhibiting the A11 system as a model of RLS, even though there has been no clear clinical or autopsy data to suggest that RLS is a neurodegenerative disorder. In this study, the A11 cell bodies were identified in 6 RLS and 6 aged-matched control autopsy cases. Cells were stained for tyrosine hydroxylase (TH), and stereological measure of the individual TH (+) cell volume was made. Regional assessment of gliosis as assessed by immunostaining for glial fibrillary acidic protein (GFAP) was made in the surrounding tissue. General histological staining was also performed on the tissue. This study found no significant difference between RLS or control cases on any measure used: TH (+) cell volume, fractional GFAP staining, or general histological examination. Nor was there histological indication of any significant inflammation or concurrent ongoing pathology in these RLS cases. The findings do not support the concept of dramatic cell loss or of a neurodegenerative process in the A11 hypothalamic region of patients with RLS. However, that does not exclude the possibility that the A11 system is involved in RLS symptoms. Changes at the cellular level in dopaminergic metabolism or at the distal synapse with changes in receptors or transporters were not evaluated in this study.
Keywords: Restless Legs Syndrome, A11 dopaminergic system, Hypothalamus, Tyrosine hydroxylase, Gliosis, Hypothalamus, Human autopsy, Brain
1. Introduction
Several pathologies have been advanced as the most fundamental for the common sensory-motor disorder, Restless Legs Syndrome (RLS). The effectiveness of dopaminergic agents for treating RLS symptoms has been seen as indicating involvement of a primary dopaminergic pathology [1]. But any such pathology has not been clearly demonstrated. PET/SPECT studies of striatal dopamine-2 receptor have not shown consistent findings: 3 studies report decrease, 2 studies found no change and 1 study showed an increase [1,3]. There have been two SPECT studies of striatal DAT which found no change [1]. One hypothesis, which has evolved from RLS dopamine research, is that a non-nigro–striatal pathway may be involved. Pathology in the A11 dopaminergic system whose cell bodies are located in the hypothalamus and whose axons, in part, project to the spinal cord, has been proposed as the basis for RLS symptoms [11]. It is argued that leg movements seen in sleep in patients with RLS are driven at the level of the spinal cord [4]. If that is true and if the dopaminergic system is involved, then the A11, as the only significant spinal dopamine system, is likely to play a critical role. Animal models involving the A11 dopaminergic system involved mostly lesions of the A11 system in rodents [9,10]. But careful evaluation of RLS autopsy brains has failed to find any indication of either neurodegeneration or gliosis on general neuropathic assessments of the nigro–striatal dopamine system or of any loss of TH-staining neurons [5,6]. This does not, however, exclude the possibility of a neurodegenerative process or decrease in dopaminergic neurons selectively involving the A11 system.
The current study was undertaken to ask two basic questions: (1) Is the volume of tyrosine hydroxylase (TH) immunoreactive neurons in the A11 region altered in RLS compared to aged-matched controls? (2) Is there a reactive astrogliosis of the A11 in RLS?
2. Methods
Tissues from patients with primary RLS were obtained from the McLean/Harvard Brain Bank as described previously [5]. The cases of RLS had been screened prior to their death as part of the brain donation project supported by the RLS Foundation in concert with the Harvard Brain Bank. All RLS cases met the 4 basic criteria for RLS [2] and all had symptom onset before 45 years of age. Six cases with no known neurological condition nor any significant pathological findings were used as control cases [5]. Two of the 6 control cases had prior screening for RLS that indicated they did not have RLS (currently or in the past); the other 4 controls had no information about RLS. We received formalin-fixed tissue samples and paraffin-embedded tissue blocks of the posterior hypothalamus. Fixed tissues were processed and embedded in paraffin. The A11 cells make up a well-defined nuclear group in rodents, but in humans, the TH (+) neurons of the A11 system do not form well demarcated nuclei. Therefore, specific anatomical markings had to be standardized to locate these cells. The A11 region (see Fig. 1) was identified in the posterior hypothalamus dorsal to the mammillary bodies, immediately lateral to the third ventricle, and medial to the mammillotegmental tract [7,8]. Also the inhomogeneity in the rostal-caudal distribution of TH (+) cells in the tissue meant millimeter differences in a tissue slice could dramatically change the number of TH (+) cell present in that slice. Therefore, actual counting of total number of TH (+) cells present in the A11 area would not be possible. However, the region of interest (i.e., A11) could be identified and all of the TH (+) cells in the region evaluated for volume, and markers of gliosis measured within that area. Following sectioning and examination of scout tissue sections, we determined that the A11 region was present and could be examined in the tissue samples from 6 RLS and 6 control cases.
Fig. 1.
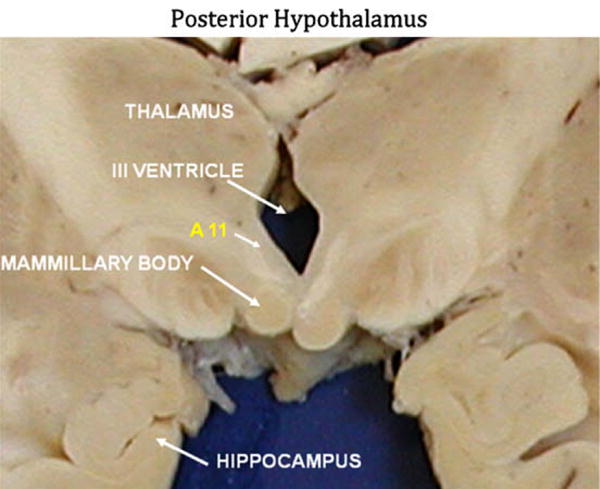
Representative coronal section of the brain at the level of the posterior hypothalamus. The anatomical landmarks for defining the A11 region are indicated along with the area which was subsequently evaluated as A11 in this study.
2.1. Immunostaining
TH immunoreactivity was assessed using a polyclonal antibody (1:250; Pel-Freez). Astrocytes were evaluated using a polyclonal antibody against glial fibrillary acidic protein (GFAP) (1:400; DAKO). Immunoreactivity was detected with the ABC kit (Vector Laboratories).
2.2. Volume of TH (+) neurons
Stereological measurements were made with a Zeiss light microscope equipped and interfaced with a StereoInvestigator system (MBF Bioscience, Williston, Vermont). The images were captured with a microFire video camera (Optronics, Goleta, California). We used an isotropic nucleator, an unbiased stereological probe, to measure the volume of all TH (+) in a 10-μm section (Fig. 1) with a 40× objective.
2.3. Measurement of the fractional area of glial fibrillary acidic protein (GFAP) immunostaining
We examined the exact same region in which we measured the volumes of TH (+) neurons. This was achieved by copying the contour of the TH-stained slide onto the GFAP-stained section. Fractional areas were measured with the “area fraction fractionator” software using a 100× oil objective.
2.4. General microscopic examination
In addition to the stereological studies, all sections were screened for microscopic changes on H&E and TH, GFAP, and α-synuclein immunostains.
3. Results
The RLS and control cases were well matched for age but the control cases were a majority of male (4/2) cases, while the RLS cases were all female (see Table 1). There is a suggestion of a possible gender effect only for the number of cells counted, with the females showing greater variance on this issue, but completely overlapping male cases. More importantly, the number of cells counted per case in the RLS and control groups are nearly identical. The gender differences between the samples would, therefore, be unlikely to obscure differences based on diagnosis.
Table 1.
The individual gender and age of RLS and Control cases along with the number of TH (+) cells counted, mean cell volume of these TH (+) cells and the fractional area of tissue associated with GFAP (+) staining.
| Gender | Age (years) | No. of cells counteda | Mean cell volumec,b (μm3) | GFAP Area fractionc (%) | |
|---|---|---|---|---|---|
| Control | M | 66 | 32 | 3766.4 | 5.8 |
| F | 55 | 53 | 4130.1 | 7.7 | |
| F | 92 | 43 | 2428.1 | 5.7 | |
| M | 65 | 16 | 1512.2 | 9.8 | |
| M | 64 | 31 | 1943.6 | 8.0 | |
| M | 94 | 23 | 4305.3 | 7.8 | |
| Mean ± SD | 73 ± 16 | 33 ± 13 | 3014 ± 1202 | 7.5 ± 1.5 | |
| RLS | F | 83 | 22 | 2455.4 | 7.3 |
| F | 53 | 32 | 2757.2 | 5.1 | |
| F | 76 | 35 | 2021.2 | 6.6 | |
| F | 77 | 8 | 1693.0 | 6.1 | |
| F | 86 | 58 | 4373.8 | 6.4 | |
| F | 106 | 46 | 3744.0 | 4.9 | |
| Mean ± SD | 80 ± 17 | 34 ± 18 | 2841 ± 1031 | 6.1 ± 0.9 | |
| p-Value, Mann–Whitney | U = 14, p = 0.52 | U = 16.5, p = 0.81 | U = 18, p = 1.0 | U = 8, p = 0.11 |
The number of cells that were identified in the A11 region of the hypothalamus that were positively stained for tyrosine hydroxylase (TH).
The calculated mean cell volume of all TH (+) cells identified in the A11 region.
Fractional area of GFAP immunoreactivity in the A11 region.
The TH antibody yielded good staining of neuronal cell bodies and axons (Fig. 2). For each RLS case, we measured from 8 to 58 neurons; for controls between 16 and 53 neurons. The mean volume in cases of RLS was 2841 μm3 (SEM = 420.7), and in controls it was 3014 μm3 (490.7), a non-significant difference (p = 0.79) (see Table 1).
Fig. 2.
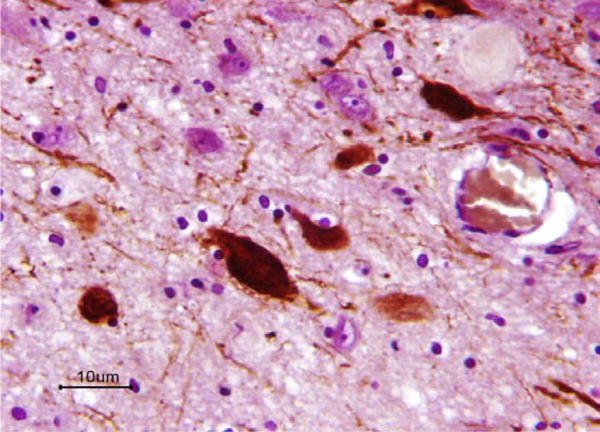
Neurons, dendrites, and axons immunopositive (dark maroon-colored) for TH in the A11 region. Note also the presence of unstained neurons. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
The mean fractional area of GFAP–immunoreactivity was 6.1% in RLS and 7.5% in controls. These differences were not significant (p = 0.132, Mann–Whitney).
On general microscopic examination, the RLS tissues did not reveal obvious loss of neurons or neurites, axonal swellings, spongiform changes or rarefaction of the neuropil, neuronal inclusions, vascular abnormalities, or inflammation.
4. Conclusion
The A11 region in the posterior hypothalamus shows no evidence of changes in the volume of TH (+) neurons, neither atrophy nor hypertrophy, for RLS compared with age-matched control cases. Moreover, there was no gliosis, a nonspecific marker of pathologic change. Thus, the region examined does not reveal the type of changes that characterize neurodegenerative, metabolic, vascular or infectious disorders. It could be argued that the number of cases used in this study were too small to appreciate real changes in the neurons. However, one of the purposes of this study was to ascertain whether the dopaminergic cells in the A11 region showed the same degree of cell loss typically seen in Parkinson’s disease and thus justify the lesioning of the A11 as a model for RLS as proposed by some investigators [9,10]. If taken in the context of other known neurodegenerative conditions like Parkinson’s or Alzheimer’s Diseases, half the number of cases would have been adequate to appreciate the severe pathology underlying those conditions. Therefore, these findings do not support the concept of cell loss or neurodegeneration in the A11 region of patients with RLS, at least not to the degree seen in other neurodegenerative diseases. Animal models of RLS, which utilize lesioning of the A11 cell bodies, may not be representative of the neurological status of the RLS patient.
Although our observations do not support an overt pathological change in the A11 region, the possibility that the A11 system plays a role in RLS should not be excluded. The clinical manifestations of RLS may be attributed to a small subset of TH neurons or neurons not expressing TH. Our measurements could miss changes in these hypothetical neuronal populations. Also the manifestations of RLS may be secondary to cellular changes in dopamine metabolism or changes in the distal A11 synapses, i.e., up- or down-regulation of receptors or transporters, which would not be as detectable as structural changes in the cell body.
Acknowledgments
We thank Ms. Gay Rudow for excellent technical assistance. Financial support for this study came from NIH Grant, PO1-AG21190. We thank the RLS Foundation, the Harvard Brain Tissue Resource Center (R24 MH/NS068855) and the NIH/NIA Baltimore Longitudinal Study on Ageing (BLSA) for contributing the brains.
References
- 1.Allen RP, Earley CJ. Dopamine and iron in restless legs syndrome. In: Chokroverty S, Hening W, Walters AS, editors. Sleep and movement disorders. Philadelphia: Butterworth Heinemann; 2003. pp. 333–40. [Google Scholar]
- 2.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 3.Cervenka S, Palhagen SE, Comley RA, Panagiotidis G, Cselenyi Z, Matthews JC, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain. 2006;129:2017–28. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- 4.Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–30. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- 5.Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Earley CJ. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–9. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 6.Connor JR, Wang XS, Patton SM, Menzies SL, Troncoso JC, Earley CJ, et al. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology. 2004;62:1563–7. doi: 10.1212/01.wnl.0000123251.60485.ac. [DOI] [PubMed] [Google Scholar]
- 7.Kitahama K, Ikemoto K, Jouvet A, Nagatsu I, Sakamoto N, Pearson J. Aromatic L-amino acid decarboxylase- and tyrosine hydroxylase-immunohistochemistry in the adult human hypothalamus. J Chem Neuroanat. 1998;16:43–55. doi: 10.1016/s0891-0618(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 8.Ondo WG, Zhao HR, Le WD. Animal models of restless legs syndrome. Sleep Med. 2007;8:344–8. doi: 10.1016/j.sleep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Qu S, Le W, Zhang X, Xie W, Zhang A, Ondo WG. Locomotion is increased in A11-lesioned mice with iron deprivation: a possible animal model for restless legs syndrome. J Neuropathol Exp Neurol. 2007;66:383–8. doi: 10.1097/nen.0b013e3180517b5f. [DOI] [PubMed] [Google Scholar]
- 10.Mai JK, Assheuer J, Paxinos G. Atlas of Human Brain. Academic Press; San Diego: 1997. [Google Scholar]
- 11.Qu S, Ondo WG, Zhang X, Xie WJ, Pan TH, Le WD. Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp Brain Res. 2006;168:152–6. doi: 10.1007/s00221-005-0075-1. [DOI] [PubMed] [Google Scholar]


