Abstract
Mesenchymal stem cells have generated much interest because of their potential use in regenerative medicine. The major draw back in the application of these cells is that there is no single marker or markers that have been established to identify and aid in isolating the cells from a variety of other cell types. The commonly expressed mesenchymal stem cell surface antigens include CD44, CD73, CD90.2, CD105, and CD146. In the present study we examined the stability of these surface antigens in culture and their potential application in identifying and isolating murine derived adipose derived stem cells. The data showed that the expression of these markers increased with culturing and appeared to stabilize by passage 8; the cells were sorted positively for the surface markers at this passage. Each subset was maintained in culture and evaluated for differentiation toward osteogenic lineage in vitro and in vivo. The CD73 and CD105 positive cell subsets demonstrated robust differentiation toward osteogenic lineage in vitro; the CD90.2+ cell subset exhibited the least differentiation toward osteogenic lineage. Assessment of the cell subpopulations for in vivo differentiation demonstrated that all the cell subsets exhibited potential to differentiate into osteoblasts. Taken together, these data suggest that this panel of markers although useful in identifying cells with potential to differentiate toward osteogenic lineage, cannot prospectively be used for enriching for ADSC from a variety of other cell types.
INTRODUCTION
Mesenchymal stem cells (MSCs) have generated much interest because of the potential they possess in regenerative medicine. However, the biology of these cells is still poorly understood primarily because there are no specific markers available to identify them. The application of these cells in regenerative medicine will require isolation and identification of the cells from a mixture of other cell types. Numerous surface antigens have been shown to be expressed on MSCs isolated from various sources. These surface antigens include CD105, CD90.2, CD73, CD146, and many others. Although these surface markers are regarded as putative MSCs markers, they are also expressed on a variety of other cell types [1–8]. The use of these markers for the isolation of the putative stem cells from a variety of other cell types is therefore limited. Several other putative MSCs markers have been postulated. STRO-1, an antibody raised against human marrow derived cells expressing low levels of CD34 was shown to recognize stromal cells that express low levels of CD34 [9]. Cells isolated based on Stro-1 antibody were shown to have high potential to differentiate into several cell lineages, including osteogenic, adipogenic, chondrogenic, and myogenic cell lineages [10–12]. The cells, however, sorted based on this antibody still represent a mixed population. SSEA-1 and SSEA-4 antigens are stage specific antigens expressed by murine and human embryonic stem cells respectively [13, 14]. These antigens have been identified on marrow preparations and are thought to be expressed by primitive MSCs. The usefulness of these markers in the isolation of the putative MSCs remains undefined. In the present study we focused on the commonly recognized markers CD44, CD73, CD90.2, CD105, and CD146 expressed by MSCs isolated from adipose tissue (ADSCs) to determine their stability in culture and their potential effectiveness in isolating MSCs from a variety of other cell types. We compared cell subsets sorted for these antigens for their efficiency in osteoblast differentiation in vitro and in vivo. Here we report the stability and differentiation of each subset toward osteogenic lineage in vitro and in vivo.
The rationale behind the present studies is to understand how the surface antigens can be utilized to isolate the cells with high osteogenic potential from a variety of other cells. It is well established that there are no specific markers available for the identification of the adult stem cells; analysis of the established surface antigens expressed by ADSCs with culturing offers means of assessing markers that can be applied to prospectively isolate a population of cells that exhibit high osteogenic potential as well as differentiate into other cell lineages.
MATERIALS AND METHODS
Isolation of the Adipose Derived Mesenchymal Stem Cells (ADSC)
Murine adipose derived stem cells (ADSCs) were isolated as described previously [15, 16]. Briefly, adipose tissue obtained from the murine inguinal fat pad was minced, washed extensively, and digested with 0.075% Type II collagenase at 37°C for 30 minutes with gentle agitation. The digested fat was centrifuged at 2,000 rpm for 10 minutes. The pellet was washed then passed through 70 μm mesh filter. The filtrate was centrifuged as above and plated in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin v/v (P/S) and incubated at 37 °C in 5% CO2. At passage 3, the cells were transduced with a retrovirus containing GFP-Zeocin to allow for visualization and cell selection.
Transduction of the Cells with a Retrovirus Carrying eGFP and Zeocin Resistant Genes
Cell transduction was performed as described previously [17, 15]. Briefly, the cells were plated in six-well plates in DMEM and maintained in culture to 60% confluence. The cells were treated with 1 ml of a high-titer DFG based retro virus [18] in DMEM supplemented with 10% FBS and 8 μg/ml polybrene and incubated for 24 h. After 24 h, the medium was replaced with new medium supplemented with 10% FBS and treated with retroviral vector and incubated for further 24 h. After two more rounds of medium changes with addition of viral vector, new medium without the viral vector was added and the cells were incubated further for 24 h. To select a population of cells expressing GFP, cells were maintained in DMEM supplemented with 10% FBS, 25 μg/ml of Zeocin and 50 μg/ml of ascorbic acid. The GFP+ ADSCs were maintained in culture in presence of Zeocin until use.
Differentiation Assays
Alkaline Phosphatase Activity (ALP) Assay
Cells were treated with 100 ng/ml of BMP-2 for three days followed by analysis of ALP activity by densitometry using the Revelation Program (Dynex Tech, Virginia). Cells were grown to 80% confluence before addition of BMP-2 containing media to allow for equivalent cell quantity for each subset. Cells were also stained for ALP activity at passage 4. For cytochemical staining of ALP activity, the cells were fixed in methanol/acetone (1:1), and stained for 30 minutes at room temperature with Naphthol Phosphate (0.05mg/ml) and Fast Red (1mg/ml). Wells were examined under the light microscope and photographed. Each sample was run in triplicate, three independent times.
Osteogenic Differentiation
Osteogenic differentiation in vitro was assessed by staining cells with Alizarin Red S at 21 days after culturing in osteogenic medium. Cells were grown to 70% confluence before the introduction of osteogenic media to allow for equivalent cell quantity for each subset. Osteogenesis induction medium contained: α-minimum essential medium (α-MEM), 5% FBS, 1% P/S, 10 mM Na-β-glycerophosphate, 0.2mM Ascorbic Acid Phosphate (ASA), and 10−8 M Dexamethasone (Sigma Aldrich, St Louis MO). Each sample was run in triplicate at three independent times.
Adipogenic Differentiation
Adipogenic differentiation in vitro was assessed by staining cells with Oil Red O at 14 days after plating in adipogenic medium. Adipogenesis induction medium contained: DMEM, 5%FBS, 1% P/S, 0.5mM 3-isobugyl-1-methylxanthine (IBMX), 1μM Indomethacin, 1μM Insulin, and 10−7M Dexamethasone.
Flow Cytometry/Cell Sorting
Isolated cells were treated with PE-labeled antibodies against: CD44 (eBioscience, San Diego, CA; Cat. No. 12-0441-81), CD73 (BD Pharmingen, San Jose; Cat. No. 550741), CD90.2 (Thy-1.2) (BD Pharmingen; Cat. No. 553005), CD105 (Endoglin) (R&D, Minneapolis, MN; Cat. No. MAB1320), and CD146 (MelCAM) (Santa Cruz, Santa Cruz, CA; Cat. No. sc-18837PE). Cells were treated with each antibody at a concentration of 20 μg/mL. The expression level of each antigen was analyzed by flow cytometry. Each trial was measured in triplicate and each experiment was performed 3 times. The cells were sorted positively based on this panel of markers using the MoFlo High Performance Cell Sorter (Dako Cytomation, Denmark, located in the core facility, Penn State College of Medicine). The sorted cells were expanded to obtain adequate cells for in vivo assessment of osteoblast differentiation.
Injection of the Cell Subpopulations into Mice Femur Cavities
Subsets of cells sorted positively for the CD73, CD90.2, CD105, and also the unsorted cells were injected at a density of 1×106cell/ml into sublethally irradiated wild type mice femurs. All the animal experiments were performed under approved protocol by Penn State University Animal use committee. For cell injection, mice were anesthetized with 1mL/Kg mouse weight Ketamine and Xylazine (100mg/ml in 2:1 Ketamine/Xylazine) prior to cell injection. The cells for injection were trpysinized from plates and washed in sterile PBS. The washed cells were suspended in sterile PBS at a concentration of 1×106cell/ml and injected into the femurs of the irradiated mice through the femoral chondyle. The recipient mice were sacrificed at 1, 2, 4 weeks after infusion. For donor cells retrieval from bone marrow, the marrow was flushed from the recipient femurs, centrifuged, and plated in Petri dishes. For donor cells retrieval from bone, after the marrow flush the femurs were cut into small pieces and placed in Petri dishes. The cells that grew out of the bone pieces were expanded in culture in presence of Zeocin. All the retrieved cells were cultured in DMEM/10% FBS, supplemented with Zeocin (25 μg/ml) to select for the GFP+ cells. The GFP+ cells were then assessed for gene expression of osteoblast associated differentiation genes, Runx2, Osterix (OSX), Osteoponin, and Osteocalcin (OCN).
Gene Expression Analysis
Gene expression analysis was performed on cells retrieved from two mice per experimentation group (i.e. unsorted, CD105+, CD73+, CD90.2+); gene expression analysis was performed in triplicates and each experiment was performed three times. Gene expression analysis was performed as described previously [15] and the primers used are shown in Table I. Briefly, total RNA was extracted from 1×106 of the donor unsorted and the sorted cell subsets, using Trizol (Invitrogen, CA) per manufacturer’s instructions. The mRNA was reverse transcribed to cDNA using SuperScript™ First-Strand Synthesis System for RT-PCR (Invitrogen, CA) according to the manufacturer’s instructions. cDNA was amplified using an Eppendorf PCR System (Eppendorf AG, Hamburg, Germany) at 94°C for 30 sec, 65°C for 30 sec, and 72°C for 1 min for 30 or 35 cycles, after initial denaturation at 94°C for 5 min. All primer sequences that were used were determined using established GenBank sequences and are indicated in Table 1. Triplicate PCR reactions were amplified using primers designed for β-actin as a control for assessing PCR efficiency. The PCR fragments were analyzed by agarose gel electrophoresis.
Table 1.
Primer Sequences used to Assess Expression of the Mouse Osteoblast Associated Differentiation Genes
| Runx2 | F5′-CCA ACT TCC TGT GCT CCG TG-3′ R5′-TCT TGC CTC GTC CGC TCC-3′ |
| Osterix | F5′-ACC AGG TCC AGG CAA CAC-3′ R5′-GGG CAG TCG CAG GTA GAA-3′ |
| Osteopontin | F5′-CTG GTG CCT GAC CCA TCT C-3′ R5′-TGC CCT TTC CGT TGT TGT C-3′ |
| Osteocalcin | F5′-CAG GAG GGC AAT AAG GTA GT-3′ R5′-GAG GAC AGG GAG GAT CAA G-3′ |
| Beta-actin | F5′-AGA GGG AAA TCG TGC GTG AC-3′ R5′-CAA GAA GGA AGG CTG GAA AA-3′ |
RESULTS
Isolation of ADSC
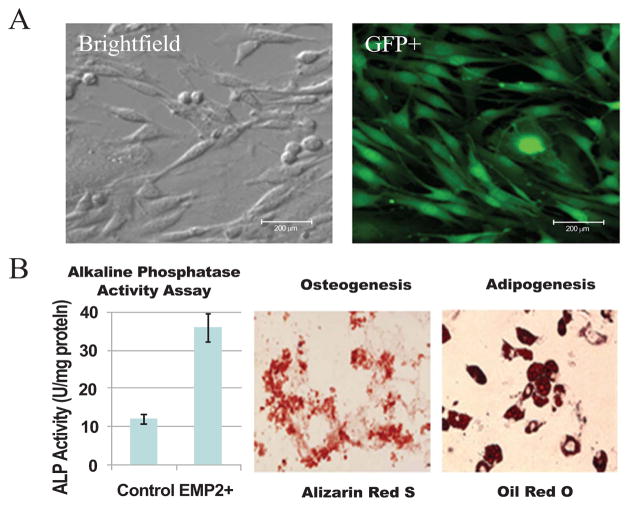
The isolated ADSCs expanded rapidly in culture and at passage 3 they were transduced with the retrovirus carrying eGFP and the Zeocin resistant genes. Fig. (1) shows the morphological appearance of the cells in bright field and under fluorescence for GFP Fig. (1A). Transduction of the cells with the retrovirus carrying eGFP and Zeocin resistant genes did not affect their growth or differentiation toward osteogenic and adipogenic cell lineages. Unsorted non-transduced ADSC as well as unsorted eGFP/Zeocin-transduced cells were analyzed for surface antigen expression, growth, differentiation and morphological appearance; there were no differences noted between the cell types in terms of the above characteristics. Alkaline phosphatase activity was assessed after BMP-2 treatment, mineral deposition was assessed by Alizarin Red S staining, and lipid vacuole formation was assessed by Oil Red O staining. The results showed that the cells have potential differentiate at least toward osteogenic and adipogenic cell lineages Fig. (1B).
Fig. 1. Morphological appearance of the unsorted ADSCs isolated from the murine inguinal fat pad.
(A) The cells are shown under bright field and under fluorescence imaging for GFP (scale bars are 200μm). (B) Differentiation of the Unsorted ADSCs; ALP activity after BMP-2 treatment, Alizarin Red S staining of the cells after 21 days in osteogenic medium, Oil Red O staining of the cells maintained in culture for 14 days in adipogenic medium. BMP-2 treatment induced increase in ALP activity. The cells show differentiation toward osteogenic and adipogenic cell lineages in vitro.
ADSC Cell Surface Antigen Expression
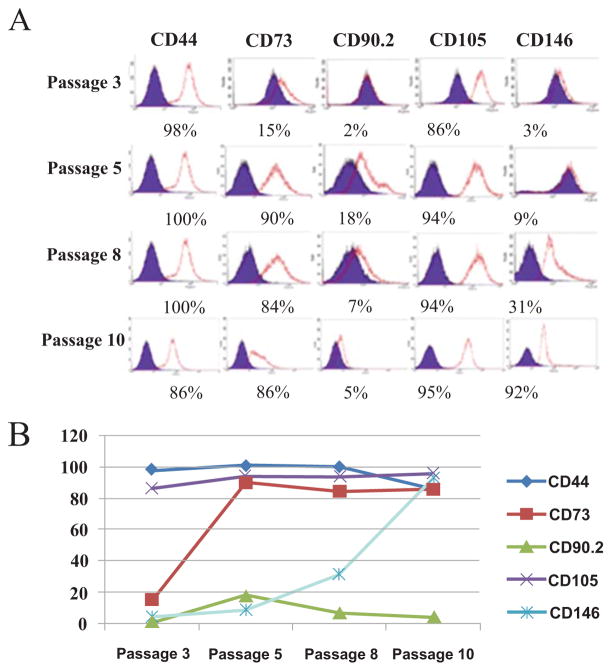
A cell surface marker profile was generated on the isolated cells over a culturing period from passage 3 to passage 10. The data showed that the expression levels of CD73, and CD146 was initially low. However, the levels increased significantly in culture with passaging and were maximal by passage 10 Fig. (2A). The expression levels of CD44 and CD105 were consistently very high and remained at these levels throughout the culture period. The expression of CD90.2 remained consistently low. These findings were similar in two different ADSCs preparations. The changes of the surface antigens upon culturing and passing are presented in the graphical form Fig. (2B). These data indicate that the cells expressing CD73 and CD146 increase the expression of these antigens in culture or with culturing the cells that express these antigens become predominant during culturing.
Fig. 2. Expression of selected surface antigens by the sorted ADSCs during expansion in culture.
(A) The panel of putative murine MSC markers analyzed by flow cytometry during culturing from Passage 3 to Passage 10 are shown. The percentage of cells in the total population expressing each marker is noted below each histogram. Note the changes in marker expression during passaging. (B) Changes of the antigen expression during cell expansion analyzed by flow cytometry in the graphical form. CD73 and CD146 expression increased with passaging; CD44, CD105 stayed constant during culturing; CD 90.2 remained low throughout the expansion period with a slight decrease during expansion in culture.
Sorting of the ADSCs for Specific Subpopulations
At passage 8, when marker expression appeared stable, the ADSCs were sorted into positive subsets according to the proposed panel of markers: CD73, CD90.2, and CD105 markers. CD44 and CD146 were used to characterize the marker expression of ADSCs, however they were not chosen as markers that would specifically sort out murine ADSCs from a mixture of other cell types. CD44 was found to be highly expressed by the murine ADSC population, with 100% of the cells expressing CD44 at all passages. CD44 is expressed by most mesenchymal cells and other cell types, including adipocytes, fibroblasts, macrophages, and adipose derived stem cells. Because this antigen is expressed by several other cell types, it is not considered a marker of MSCs and can not be used to isolate MSCs from other cell mixtures. This antigen was not further analyzed because of these properties. CD146 is used most commonly as an epithelial marker we did not pursue it further.
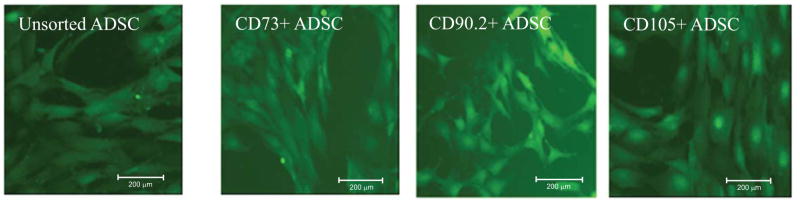
The morphological appearance of the sorted cell subpopulations are shown in Fig. (3). The CD105+ and CD73+ sorted cells appeared to exhibit similar morphologies; both cell types were long, thin, and similar in size Fig. (3). These two cell subsets greatly resembled the unsorted cell population because cells that express these antigens are predominant in the preparation. This resemblance in morphology is likely due to the fact that the unsorted cell population contains cells that highly expresses CD105 and CD73, and expresses a low level of CD90.2. The CD90.2+ cells exhibited a slightly different morphology; these cells were smaller when compared to the unsorted or CD73+, and CD105+ cells. The sorted ADSCs retained their designated marker expression throughout experimentation.
Fig. 3. Morphological appearance and stability of the sorted cell subsets.
The unsorted, CD73+ and CD105+ cells appear to exhibit similar morphologies because cells expressing these antigens are predominant in the preparation. The CD90.2+ subsets display a different morphology because they represented a smaller population of the cells in the preparation. The cells expressing CD73 also express CD105. Because these cells are predominant in the preparation they exhibit similar morphologies with unsorted cells. (scale bars are 200μm). CD90.2+ cells appear to comprise cells that are smaller with a round morphological appearance whereas the other cell subsets appear spindle shaped.
ADSC Substances Differentiate towards the Osteogenic Linkage
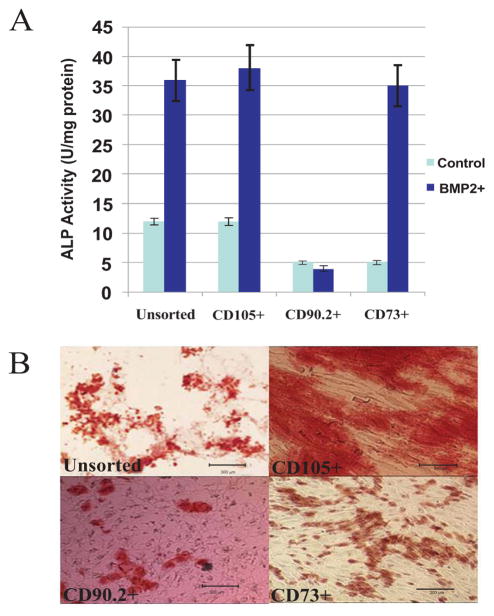
The differentiation potential of each cell subset toward osteogenic lineage was assessed in vitro. The Alkaline Phosphatase (ALP) activity of each cell subset was determined by treating the cells with BMP-2 for 3 days followed by analysis of ALP activity by densitometry. Alkaline phosphatase plays a role in mineralization; it is an early biochemical marker of osteoblast differentiation. Alkaline phosphatase (ALP) activity data showed that unsorted ADSCs and the cells sorted for CD105+, and CD73+ exhibited higher ALP activity; while the CD90.2+ ADSCs expressed low ALP activity Fig. (4A). ALP activity assessment following BMP-2 treatment provides a faster means of assessing osteogenic potential of MSCs preparation and does not require addition of other osteogenic supplements. To evaluate potential of mineral deposition by differentiating MSCs requires addition of osteogenic supplements and in this case BMP-2 is not necessary. The cell subsets were further evaluated for osteogenic potential by analyzing the ability of each subset to deposit calcium in vitro based on Alizarin red staining. Unsorted cells and the cells sorted for CD105+ and CD73+ADSCs stained heavily for calcium deposition as indicated by Alizarin Red S staining when cultured in osteogenic medium Fig. (4B). The CD90.2+ ADSCs again demonstrated minimal staining for calcium deposition (Fig. 4B). These data suggest that CD 90.2+ cells have less potential to differentiate toward osteogenic lineage at least in vitro. The CD146+ cell subset was not evaluated.
Fig. 4. Osteogenic differentiation of the sorted cell subsets in vitro.
(A) ALP activity of each cell subset. The Unsorted, CD105+ and CD73+ showed similar level of ALP activity. The CD90.2+ cells showed reduced ALP activity expression. (B) Alizarin Red S staining of the same cell subsets for calcium deposition (scale bars are 300μm). The Unsorted, CD105+ and CD73+ cells show extensive staining for calcium deposition. The CD90.2+ cells show reduced staining for calcium confirming the ALP activity data. The basal ALP activity of CD73 is lower than the other cell subsets suggesting that the other cell subsets might contain cells that already express higher levels of ALP activity.
In vivo Differentiation of the ADSC Subsets
In order to further evaluate the different cell subsets for differentiation toward osteogenic lineage, the cell subsets were injected into the mice femurs and were then retrieved at 1, 2, and 4 weeks following injection. The retrieved cells were selected for GFP expression by culturing them in a medium supplemented with Zeocin for a period of 10–21 days. The length of time the cells were cultured in Zeocin containing medium depended on the number of ADSC retrieved from the bone or bone marrow. Also at least 10 days was necessary for the complete elimination of endogenous cells. Retrieved ADSC were assessed osteoblast differentiation genes.
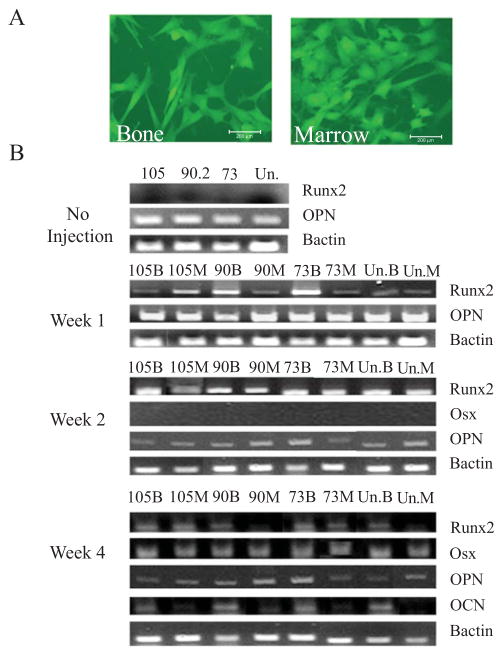
The donor cells retrieved from the bone and marrow appeared to exhibit distinct cell morphologies Fig. (5A). The cells retrieved from bone marrow exhibited a round morphology whereas the cells retrieved from bone exhibited spindle shaped morphology. Analysis of genes associated with osteoblast differentiation by the retrieved cells at different time points showed differential expression of some genes Fig. (5B). Prior to injection, all the cell subsets constitutively expressed osteopontin. The donor cells of all subsets that were retrieved from the bone and marrow of the recipient femurs 1 week after cell infusion demonstrated expression of Runx2, an early marker of osteoblast differentiation. Donor cell subsets retrieved at week 2 after cell infusion from the bone and marrow also showed expression of Runx2; analysis for Osterix (OSX) and Osteocalcin (OCN) did not reveal any expression at this time point. At week 4, Runx2 was still expressed by the entire donor cell subsets retrieved from bone and marrow; however OSX was also expressed at this time point Fig. (5B). Interestingly, at week 4 osteocalcin was also expressed by all the donor cell subsets retrieved from bone but not the marrow. The data indicate that the donor cells that become associated with bone differentiate into osteoblasts in vivo while the donor cells that remain associated with marrow are progenitors. These data clearly demonstrate that ADSCs undergo gradual differentiation toward osteoblast lineage in vivo. The data also indicate that each of the donor cell subset had potential to differentiate into osteoblasts in vivo. These data differ from in vitro data; because CD90.2+ cells were less efficient in differentiating toward osteogenic lineage in vitro but they differentiated efficiently into osteoblasts and equally well as the CD 73+ and CD105+ cell subsets in vivo. The data suggest that in vitro differentiation data may not correlate with the in vivo differentiation data. Nevertheless because we did not perform quantitative gene expression for each subset we cannot rule out subtle differences in the expression of these genes by each of the cell subsets in vivo. Each of the cell subset retrieved from bone expressed osteocalcin suggesting that they all had potential to differentiate into osteoblast in vivo. These data indicate that the surface markers may not be useful in discriminating cells with higher osteogenic potential at least in vivo, the data however suggest that cells enriched in CD105 or CD73 exhibit higher levels of osteogenic potential at least in vitro.
Fig. 5. Expression of the osteoblast differentiation genes at different time points by each of the donor cell subset retrieved from the femurs infused with the cells.
(A) Representative morphological appearance of ADSCs retrieved from the bone and bone marrow. (B) Before infusion of the cell subsets into femurs, all the cell subsets analyzed expressed osteopontin constitutively. At 1 week, all the donor cell subsets retrieved from bone and marrow expressed Runx2. At 2 weeks, the donor cell subsets retrieved from bone and marrow continued to show expression of Runx2. At 4 weeks, expression of osterix is seen as well as osteoclacin. Osteocalcin is expressed by cells retrieved from bone, not marrow. The donor cells were retrieved from the recipient femurs at the indicated time points, cultured in presence of Zeocin and assessed for expression of the indicated genes. B= bone, M= marrow, Un= unsorted cells.
DISCUSSION
The majority of mesenchymal stem cell studies focus on bone marrow derived stem cells. However, adipose tissue has become an appealing source for MSCs due to its abundance and ease of isolation [19]. Adipose derived stem cells are highly proliferative and multipotent [20]. Because of these properties adipose derived stem cells hold great promise in regenerative medicine.
Although MSCs have generated a lot of interest, there is no single marker available to identify these cells. Numerous markers have been proposed, however their usefulness in isolating cells from a myriad of other cells has not been established. The pioneering work of Friedstein showed that within adult marrow a rare 1 in 104–105 bone marrow mononuclear cells are clonogenic precursors associated with the marrow stroma [21–23]. These cells were shown to be capable of forming bone and reconstituting the microenvironment [24]. Since then, numerous markers have been proposed that are capable of identifying these cell populations, however none has been established as an exclusive marker for these cells. CD105 or endoglin has been shown to be expressed on all putative MSCs. This antigen is however also expressed by numerous other cells including endothelial cells and the breast cancer cell line MDA-231 [1, 4]. The antibody recognizing this antigen was originally raised against MSCs harvested from bone marrow and called SH2. Later studies identified endoglin or CD105 as the antigen recognized by the SH2 antibody [25]. Endoglin is a dimeric glycoprotein present on human vascular endothelium; it is a component of the TGF-β signaling system that acts as a type III receptor [25]. The ADSCs express very high levels of this antigen and remained relatively stable during culturing. The usefulness of this antigen to isolate ADSCs from other cell types is limited because it is expressed by almost all the cells isolated from the adipose tissue; the expression of CD105 is 94% from Passage 4 through Passage 10. This suggests that CD105 is expressed by a large heterogeneous population of cells in the isolate.
CD73 is a GPI anchored ecto-5′-nucleotidase which functions to catalyze the conversion of certain mononucleosides to nucleosides. This antigen was expressed at high levels by the ADSCs; CD73 was expressed by 84–90% of the ADSC population from Passage 5-Passage 10. The finding that ADSCs express high levels of this antigen and its expression is increased during culturing makes it an ineffective marker for the isolation of the putative ADSCs from a variety of other cell types. The CD73 antigen is however useful for characterizing culture expanded human MSCs. The antigen has previously been used to isolate cells with the potential to differentiate toward myoblasts from a human embryonic stem cell line [26], thus suggesting that the majority of MSCs express this antigen.
The human CD90.2 antigen is also known as Thy-1; is a member of the immunoglobulin gene superfamily. The CD90.2 molecule is expressed in nervous tissue, connective tissue, various stromal cell lines, and by a rare subset of human fetal bone marrow cells that contains multipotent hematopoietic progenitor activity. The antigen is also expressed on a subset of CD34 positive cells from human bone marrow, cord blood or fetal liver [27]. In mouse ADSC preparation, the cells expressing CD90 represented a small population. In human ADSCs, the CD90 antigen is expressed at high levels, the difference in expression level of this antigen by human and mouse ADSC is not clear here. The level of cells expressing this antigen remained low through passaging and expansion. This is contrary to human MSCs which express high levels of this antigen [28]. Interestingly cells sorted for this antigen exhibited reduced capacity to differentiate toward osteogenic lineage in vitro but were able to differentiate into osteoblasts in vivo. These data suggest that the cells require some other factors to induce their differentiation, however these factors may be absent during the in vitro conditions used to assess osteogenic differentiation.
The in vivo assessment for osteogenic differentiation demonstrated that the ADSCs underwent sequential differentiation in vivo. These findings are in agreement with what is known regarding the expression of Runx2 and OSX during osteoblasts differentiation. Osteopontin appeared to be constitutively expressed by all the cell subsets. Runx2 was expressed by all the cells subsets in vivo at all the time points assessed. Osterix was expressed equally at Week 4 by all the cells subsets retrieved from both the marrow and bone. Interestingly osteocalcin was expressed by all the donor cell subsets retrieved from bone while the cells retrieved from bone marrow expressed very low levels. These findings demonstrated that the cells which engraft in bone differentiate into osteoblasts while those that remain in the marrow are progenitors. Osteoblasts differentiate from mesenchymal cells, and their differentiation is regulated by specific factors that are expressed at different time points during differentiation. Runx2 and OSX are transcription factors that are expressed selectively at high levels in osteoblasts. Null mutations of either factor leads to complete absence of bone in mice. Runx2 is required early during osteoblast differentiation, whereas OSX is required later by committed osteoprogenitors to mature osteoblasts [29]. Our findings on ADSCs differentiation in vivo appeared to follow this sequence. Runx2 was expressed early by the injected cells. OSX expression was seen four weeks after cell injection in cells retrieved from both the bone and marrow. However only the cells retrieved from the bone express Osteocalcin, a gene expressed by mature functioning osteoblasts, by week 4. These results suggest that the differentiation of ADSCs in vivo is slow and for their application they may require factors to accelerate differentiation into osteoblasts in vivo.
CONCLUSION
Taken together, the present findings imply that the putative markers assessed here which are attributed to MSCs may not be useful in isolating MSCs from a variety of other cell types because they undergo changes in culture and some are expressed by most cells in the preparation. The antigens are useful for characterizing culture expanded MSCs. The antigens may also be useful as guides to indicate that certain cell preparations contain cells that exhibit MSCs characteristics. The cells were cultured in standard conditions used for maintenance and expansion of MSCs. Because the cells are removed from their natural niche, the analysis of cell differentiation in vitro and in vivo may differ from the behavior of the cells when they are in their natural niche. In addition the cells used here were transduced with a retrovirus carrying GFP/Zeocin resistant genes, therefore the possibility that transduction could affect the behavior of the cells in vitro and in vivo can not be ruled out. Nevertheless, the present findings suggest that further investigations are necessary to discover markers that characterize ADSC and their utility in isolating MSCs with high potential for osteogenic differentiation.
Acknowledgments
This work was supported in part by NIH grant AR049688.
We would like to thank Drs. Paul Robbins and Zhibao Mi for the generous gift of EGFP-Zeocin retrovirus used to transduce ADSCs.
References
- 1.Arthur HM, Ure J, Smith AJ, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217(1):42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 2.Elshal MF, Khan SS, Raghavachari N, et al. A unique population of effector memory lymphocytes identified by CD146 having a distinct immunephenotypic and genomic profile. BMC Immunol. 2007;13:8–29. doi: 10.1186/1471-2172-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krupinski J, Turu MM, Luque A, Badimon L, Slevin M. Increased PrPC expression correlates with endoglin (CD105) positive microvessels in advanced carotid lesions. Acta Neuropathol. 2008;116(5):537–45. doi: 10.1007/s00401-008-0427-6. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Gómez E, Eleno N, López-Novoa JM, et al. Characterization of murine S-endoglin isoform and its effects on tumor development. Oncogene. 2005;24(27):4450–61. doi: 10.1038/sj.onc.1208644. [DOI] [PubMed] [Google Scholar]
- 5.Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189(1):4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita M, Kajiyama H, Terauchi M, et al. Involvement of aminopeptidase N in enhanced chemosensitivity to paclitaxel in ovarian carcinoma in vitro and in vivo. Int J Cancer. 2007;120(10):2243–50. doi: 10.1002/ijc.22528. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita Y, Hooker SW, Jiang H, et al. CD73 expression and fyn-dependent signaling on murine lymphocytes. Eur J Immunol. 1998;28(10):2981–90. doi: 10.1002/(SICI)1521-4141(199810)28:10<2981::AID-IMMU2981>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, Altala A. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180(5):2226–33. doi: 10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78(1):55–62. [PubMed] [Google Scholar]
- 10.Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotent. Cells Tissues Organs. 2002;170(2–3):73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- 11.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–73. [PubMed] [Google Scholar]
- 12.Gronthos S, Zannettino AC. A method to isolate and purify human bone marrow stromal stem cells. Methods Mol Biol. 2008;449:45–57. doi: 10.1007/978-1-60327-169-1_3. [DOI] [PubMed] [Google Scholar]
- 13.Berrill A, Tan HL, Wuang SC, Fong WJ, Choo AB, Oh SK. Assessment of stem cell markers during long-term culture of mouse embryonic stem cells. Cytotechnology. 2004;44(1–2):77–91. doi: 10.1023/B:CYTO.0000043414.90681.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109(4):1743–51. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 15.Liao X, Li F, Wang X, Yanoso J, Niyibizi C. Distribution of murine adipose-derived mesenchymal stem cells in vivo following transplantation in developing mice. Stem Cells Dev. 2008;17(2):303–14. doi: 10.1089/scd.2007.0086. [DOI] [PubMed] [Google Scholar]
- 16.Zuk P, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Wang X, Niyibizi C. Distribution of single-cell expanded marrow derived progenitors in a developing mouse model of osteogenesis imperfecta following systemic transplantation. Stem Cells. 2007;25(12):3183–93. doi: 10.1634/stemcells.2007-0466. [DOI] [PubMed] [Google Scholar]
- 18.Gambotto A, Dworacki G, Cicinnati V, et al. Immunogenicity of enhanced green fluorescent prote in (EGFP) in BALB/c mice: identification of an H2-Kd-restricted CTL epitope. Gene Ther. 2000;7:2036–40. doi: 10.1038/sj.gt.3301335. [DOI] [PubMed] [Google Scholar]
- 19.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion, and differentiation. Methods. 2008;45(2):115–20. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mischen BT, Follmar KE, Moyer KE, et al. Metabolic and functional characterization of human adipose-derived stem cells in tissue engineering. Plast Reconstr Surg. 2008;122(3):725–38. doi: 10.1097/PRS.0b013e318180ec9f. [DOI] [PubMed] [Google Scholar]
- 21.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–90. [PubMed] [Google Scholar]
- 22.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 23.Friedenstein AJ. Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo. Haematol Blood Transfus. 1980;25:19–29. doi: 10.1007/978-3-642-67319-1_3. [DOI] [PubMed] [Google Scholar]
- 24.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20(3):263–72. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 25.Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem Biophys Res Commun. 1999;265(1):134–9. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- 26.Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–8. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 27.Haeryfar SM, Hoskin DW. Thy-1 more than a mouse pan-T cell marker. J Immunol. 2004;173(6):3581–8. doi: 10.4049/jimmunol.173.6.3581. [DOI] [PubMed] [Google Scholar]
- 28.Hoogduijn MJ, Crop MJ, Peeters AM, et al. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 2007;16(4):597–604. doi: 10.1089/scd.2006.0110. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Bone. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]