Abstract
Njoku-Obi, Augustine N. (School of Veterinary Medicine, Tuskegee Institute, Ala.), Edward M. Jenkins, Jessie C. Njoku-Obi, Joanne Adams, and Verdell Covington. Production and nature of Listeria monocytogenes hemolysins. J. Bacteriol. 86:1–8. 1963.—Hemolysin produced by various strains of Listeria monocytogenes varied in quality and quantity, depending on medium, incubation temperature and time, and biological variations in the organisms. The hemolysin was inactivated by filtration (through Seitz, Selas, or sintered-glass filters), heat, oxygen, and formalin. Sodium thiosulfate reactivated hemolysin inactivated by filtration and oxygen. The hemolysin was protein in nature, migrating electrophoretically as a gamma-globulin, and highly antigenic in the rabbit. Although no toxicity was observed in intact mice injected with hemolysin, a possible leukocytolysis was noted with isolated mice peritoneal exudate cells. Due to the high antihemolytic activity of normal sera from various species, the possible use of an antilisteriolysin test in serological diagnosis is questioned.
Full text
PDF
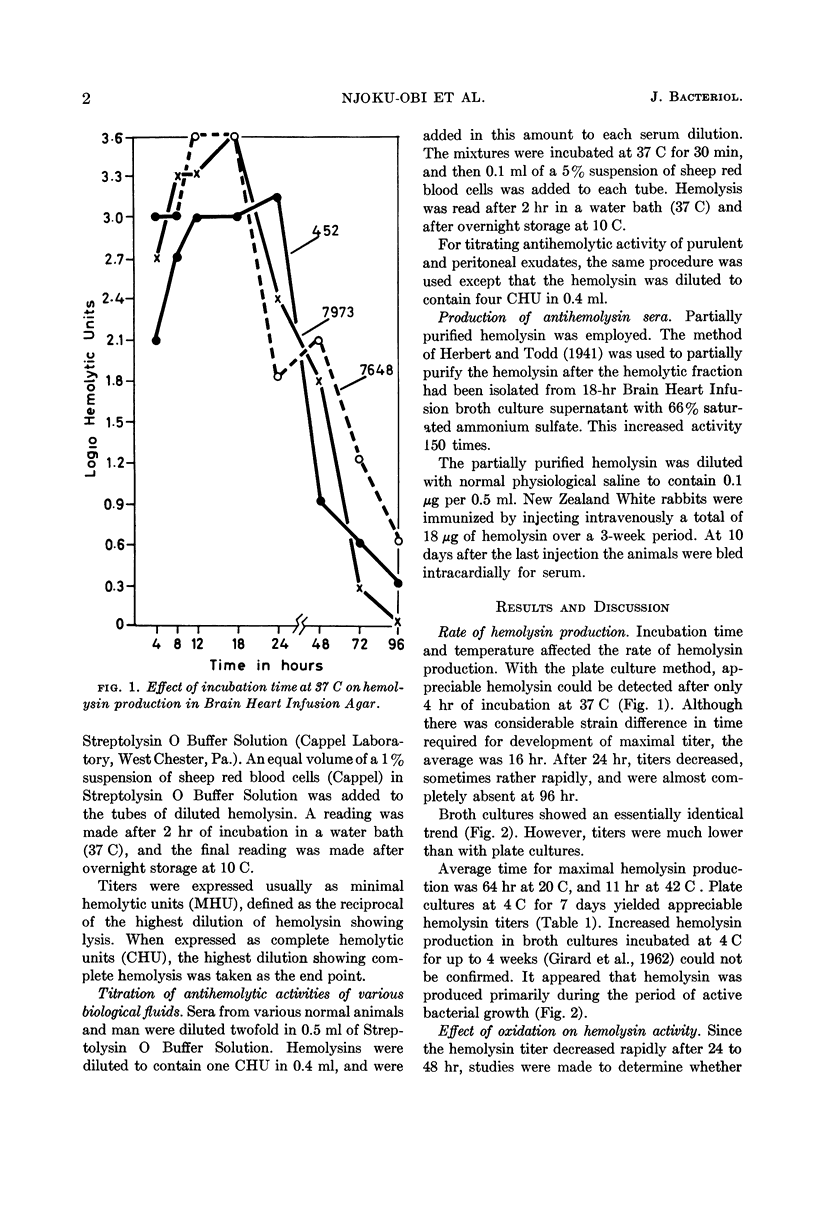



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Herbert D., Todd E. W. Purification and properties of a haemolysin produced by group A haemolytic streptococci (streptolysin O). Biochem J. 1941 Nov;35(10-11):1124–1139. doi: 10.1042/bj0351124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON J., THATCHER F. S., GAGNON J. Studies with staphylococcal toxins. IV. The purification and metallic requirements of specific hemolysins. Can J Microbiol. 1958 Aug;4(4):345–361. doi: 10.1139/m58-037. [DOI] [PubMed] [Google Scholar]



