Abstract
Liver disease is a major cause of mortality in individuals with HIV–HBV coinfection. The pathogenesis of liver disease in this setting is unknown, but is likely to involve drug toxicity, infection of hepatic cells with both HIV and HBV, and an altered immune response to HBV. The availability of therapeutic agents that target both HIV and HBV replication enable dual viral suppression, and assessment of chronic hepatitis B is important prior to commencement of antiretroviral therapy. Greater importance is now placed on HBV DNA levels and staging of liver fibrosis, either by liver biopsy or noninvasive measurement, such as transient elastography, since significant liver fibrosis may exist in the presence of normal liver function tests. Earlier treatment of both HIV and HBV is now generally advocated and treatment is usually lifelong.
Keywords: coinfection, HBV, HIV, therapy
There are approximately 36 million people living with HIV and 370 million with HBV. The prevalence of chronic HBV (CHB) infection in people with HIV varies between 5 and 20%, with the higher levels seen in areas in which CHB is endemic, such as countries in Africa and Asia [1]. Therefore, overall it is estimated that approximately 2–4 million people are coinfected with both HIV and HBV [2]. Liver disease has emerged as one of the most important causes of morbidity and mortality among HIV-positive individuals since the introduction of HAART, particularly in those coinfected with either HBV or HCV [3]. This review highlights recent advances in our understanding of the pathogenesis of liver disease in HIV–HBV coinfection. The assessment of HBV and staging of liver disease are discussed, as well as the timing and use of currently available therapies.
Natural history & pathogenesis of liver disease
HIV–HBV coinfection is characterized by high HBV DNA levels, infrequent HBV e antigen (HBeAg) seroconversion and frequent progression from acute hepatitis B to CHB [4–6]. Progression of fibrosis and development of cirrhosis is more common among people with HIV–HBV coinfection than in individuals infected with either HIV or HBV alone, and liver-related mortality is higher in coinfected individuals [6–8].
The pathogenesis of increased fibrosis in HIV–HBV coinfection remains unclear, and multiple factors may contribute. HIV infection of hepatocytes has been demonstrated both in vivo and in vitro [9], therefore, HIV may directly affect the HBV life-cycle, although evidence of this is currently lacking. HIV may also infect hepatic stellate cells, which deposit collagen and are the main effector cell leading to hepatic fibrosis [10]. Unique HBV mutations have been found in HIV–HBV coinfected individuals, which may potentially alter the pathogenicity of HBV [11] and high rates of HBV mutations that confer drug resistance have also been demonstrated [12]. The triple HBV polymerase mutant (rtL173V + rtL180M + rtM204V) was detected in 17% of 53 HIV–HBV coinfected patients who had received at least 6 months of lamivudine (LMV) therapy as part of HAART [12]. This mutant behaves as a vaccine-escape mutant in vitro. Another potential vaccine-escape mutant, rtV191I, has recently been described in a patient coinfected with HIV–HBV [13]. The rtV191I mutation creates a stop codon in the overlapping open-reading frame for the envelope protein leading to a loss of detectable HBV surface antigen (HBsAg) [13].
Immune dysfunction in HIV–HBV coinfection may also contribute to accelerated liver disease progression. For example, a reduced HBV-specific CD4 T-cell response is seen in HIV–HBV coinfection compared with HBV monoinfection, which may contribute to HBV chronicity [14]. Generalized immune activation in HIV may also contribute to liver damage [15]. HIV infection leads to severe depletion of gut mucosal immune cells, including CD4 T cells, which do not recover even after restoration of peripheral CD4 T cells [16]. The absence of gut mucosal CD4 T cells may increase microbial translocation, measured as increased lipopolysaccharide (derived from bacterial cell walls) in the blood of HIV-infected people [15]. These observations may provide an explanation for increased T-cell turnover and activation, and proinflammatory cytokines in HIV, which leads to disease progression [15,17]. Elevated concentrations of lipopolysaccharide have been described in chronic HCV infection and HIV–HCV coinfection but have not been studied in HBV monoinfection or HIV–HBV coinfection [18,19]. Together, intra-hepatic HIV infection, specific HBV mutations, reduced anti-HBV immunity and generalized immune activation are important areas for further research, which will ideally lead to novel therapies to limit the progression of liver disease in HIV–HBV coinfection.
Assessment of HBV in HIV-infected individuals
Occult HBV
Patients with HIV should undergo testing for other blood-borne viruses, specifically HBV and HCV, including HBsAg, hepatitis B surface antibody and hepatitis B core antibody tests. If HBsAg is positive, then further testing should be undertaken, including HBV DNA and HBeAg. If HBsAg is not detected, the role of testing HBV DNA remains unclear. Occult HBV (detectable HBV DNA in the absence of HBsAg) may reflect a phase of HBV infection between inactive disease and reactivation [20] or may be the result of immune depletion and lower CD4 T-cell counts in the case of HIV coinfection [21,22]. An insufficiently sensitive HBsAg assay may also lead to a diagnosis of occult HBV [23]. Occult HBV has been associated with an increased risk of hepatocellular carcinoma (HCC), particularly in HCV–HBV coinfection [24]. However, this association has not been demonstrated for occult HBV in the setting of HIV infection. Therefore, testing and treatment of occult HBV is not usually recommended [4,25,26].
Aminotransferase levels
Alanine aminotransferase (ALT) levels tend to be lower in HIV–HBV coinfection [26], owing to either an impaired HBV-specific immune response or reduced hepatic necro-inflammation, and yet, significant fibrosis may still be present. Therefore, the presence of normal ALT levels should be viewed with caution in HIV–HBV coinfection. Conversely, there are many potential causes for elevated ALT levels in HIV–HBV coinfection (summarized in Box 1), including HAART-related hepatic flare (HF) via a number of mechanisms [4]. Newer agents, such as the non-nucleoside reverse transcriptase inhibitor, etravirine, the integrase inhibitor raltegravir and CCR5 antagonist maraviroc, have not been associated with increased hepatotoxicity in HIV–hepatitis coinfected individuals to date [27–29].
Box 1. Possible causes for raised alanine aminotransferase in HIV–HBV coinfected individuals.
-
Direct drug toxicity
–Cumulative dose-related injury (e.g., nevirapine)
–Hypersensitivity (e.g., nevirapine, abacavir)
–Mitochondrial damage (e.g., didanosine, zidovudine, stavudine)
–Unknown mechanism (e.g., darunavir)
Immune restoration disease
HBsAg or HBeAg seroconversion or reversion
Selection of drug resistance
Superinfection with HAV, HCV, HDV or other infections
Other causes, including alcohol, other hepatotoxic drugs and fatty liver disease
HAV: Hepatitis A virus; HBeAg: Hepatitis B virus e antigen; HBsAg: Hepatitis B virus surface antigen; HDV: Hepatitis D virus.
During 72 weeks of HAART in 537 HIV-infected individuals in South Africa, of whom 106 (20%) were HBsAg positive, only 23 (4%) experienced HF [30]. Individuals with a high HBV DNA level (>2000 international units[IU]/ml) were more likely to experience HF than those with low HBV DNA levels or HBsAg-negative individuals [30].
HBeAg & hepatitis B e antibodies
HBV e antigen is the secreted form of the precore protein of HBV and differs from the capsid protein by the addition of 10 amino acids at the N-terminus and truncation at the C-terminus [31]. HBeAg is required for the establishment of persistent infection [32] and may lead to a reduced HBV-specific T-cell response [33]. HBeAg seroconversion (loss of HBeAg and/or detection of hepatitis B e antibodies) can occur spontaneously or following treatment with an anti-HBV agent. HBeAg seroconversion is usually associated with a significant decline in HBV DNA to undetectable levels, normalization of ALT and an improvement in disease outcome. Spontaneous HBeAg seroconversion is less common in HIV–HBV coinfection than HBV monoinfection [34]. However, following initiation of HBV-active HAART in a recent study of HIV–HBV coinfected patients in Thailand, HBeAg loss was relatively high (33% after 48 weeks) [35]. Quantitation of HBeAg and HBsAg is an emerging area of interest. Declining HBeAg or HBsAg levels may predict response to therapy and subsequent HBeAg or HBsAg seroconversion in HBV monoinfection [34,35]. However, the clinical significance of quantitative HBeAg or HBsAg measurement in HIV–HBV coinfection is currently unknown.
HBV DNA
HBV DNA level is a strong predictor of clinical disease (cirrhosis and HCC) in HBV mono-infection [36,37]. A baseline HBV DNA level greater than 20,000 IU/ml or 200,000 IU/ml increased the risk of developing cirrhosis by six and 10 times, respectively, compared with those with undetectable HBV DNA levels in a large cohort of Taiwanese HBV-monoinfected individuals.
Similar evidence for HBV DNA levels in HIV–HBV coinfected individuals is not available. In general, HBV DNA levels are significantly higher in HIV–HBV coinfected individuals compared with HBV-monoinfected individuals. Some recommendations for the treatment of HBV in HIV–HBV coinfection use similar thresholds as the treatment of HBV monoinfection [25,38]. More recent HIV–HBV coinfection guidelines have recommended a lower threshold of 2000 IU/ml for treating patients with CHB due to accelerated liver disease in HIV-infected individuals [4].
HBV polymerase sequencing
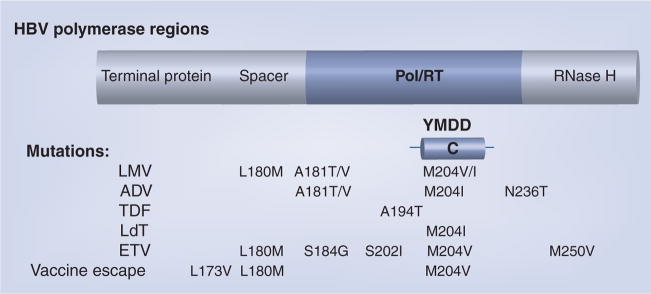
The polymerase protein encoded by the polymerase (Pol) open-reading frame is made up of four regions (Figure 1), including the DNA polymerase/reverse transcriptase (Pol/RT) [39]. HBV Pol/RT lacks a proof-reading capacity, so reverse transcription of the minus DNA strand from a RNA intermediate is error-prone. The YMDD motif (Tyr–Met–Asp–Asp) encodes amino acid positions rt203–206 in the Pol/RT region of the Pol protein [40]. Mutations within the YMDD motif at position rtM204 are typical of LMV and telbivudine (LdT) resistance [40]. Resistance to adefovir (ADV) occurs with rtN236T and/or rtA181T/V mutations [41]. Resistance to entecavir (ETV) requires the presence of at least three mutations: rtL180M, rtM204V and either rt184G/S, rtS202I or rtM250V [42].
Figure 1. HBV Polymerase & common drug-resistant mutations.
ADV: Adefovir; ETV: Entecavir; LdT: Telbivudine; LMV: Lamivudine; Pol/RT: Polymerase/reverse transcriptase region; TDF: Tenofovir; YMDD: Try–Met–Asp–Asp motif.
Adapted from [40].
Staging liver fibrosis
Liver biopsy has long been the ‘gold standard’ for assessing hepatic fibrosis, despite its many shortcomings, including cost, dependence on available expertise, interoperator variability, sampling error and risk of serious complications including death [43,44]. Noninvasive measures of liver stiffness, such as FibroScan® are now more widely available [45]. Other noninvasive assessments of fibrosis include algorithms based on a range of biochemical and haematological indices such as FibroTest®, Hepascore, or aspartate aminotransferase to platelet ratio index (APRI) [46]. More data are required before FibroScan and the algorithms can be recommended in HIV–HBV coinfection. However, as confidence in their utility grows, they are likely to become increasingly important owing to the inherent limitations of liver biopsy.
Transient elastography
Liver stiffness measurement by transient elastography (TE) or FibroScan is well validated for reliable detection of the presence of cirrhosis, regardless of the underlying liver condition, with an area under the receiver-operating characteristic curve of 0.94 (95% CI: 0.93–0.95) as found in a recent meta-analysis [45]. However, area under the receiver-operating characteristic curve was lower for the detection of significant fibrosis (0.84; 95% CI: 0.82–0.86) and varied depending on the underlying pathological condition. Most of our knowledge to date comes from the assessment of patients with HCV [45]. Few studies have focused on HIV–HBV coinfection [47], although TE in HIV–HCV coinfection has been reported [48,49] and a number of recent studies on patients with HBV monoinfection have been published [50–55]. In addition, a number of studies using TE in HIV monoinfected patients have described a small number of patients who have elevated liver stiffness and portal hypertension, with or without typical cirrhotic changes upon liver biopsy [56,57]. In some cases, this may be due to prolonged exposure to didanosine or nonalcoholic steatohepatitis [58]. TE requires further validation in HBV, especially in HIV–HBV coinfection.
Fibrosis staging following diagnosis of HIV–HBV coinfection is important to guide the timing of therapy, as well as prognosis, and for determining the role of intensive HCC screening or screening for esophageal varices.
Treatment
Therapeutic goals
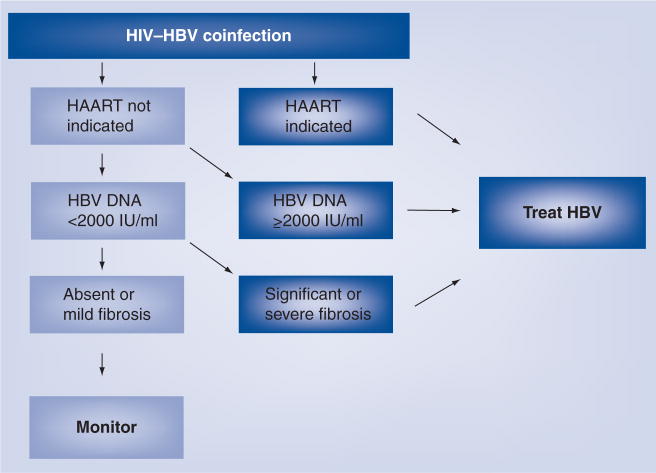
The long-term aim for treatment of HBV in both HBV monoinfection and HIV–HBV coinfection is to reduce the lifetime risk of progression to cirrhosis and HCC [59]. This is generally achieved by suppressing HBV DNA below detection using the most sensitive available assay [25]. In addition, minimizing HAART-associated hepatotoxicity is important. Cessation of HBV-active HAART, following HBeAg seroconversion, is not usually recommended, and treatment is therefore lifelong [20,25,38]. The need for HAART, primarily according to CD4 T-cell criteria (CD4 T cell <350 cells/μl), usually determines the timing and choice of treatment, although some recent guidelines recommend treatment at any CD4 T-cell count for those with HIV–HBV coinfection, on an individualized basis [60]. If HAART is required, the regimen usually includes two agents with complementary anti-HBV activity. If HAART is not required, agents with exclusive HBV and no HIV activity are used to avoid inducing HIV resistance (Figure 2).
Figure 2. Timing of HBV treatment in HIV–HBV coinfected individuals.
Adapted from [101].
Monitoring for drug resistance
Recent guidelines for the monitoring of on-treatment response to nucleoside/nucleotide analogs for HBV monoinfection, adhere to the ‘roadmap’ concept [61]. Primary nonresponse is defined as a failure to reduce the HBV DNA level by more than 1 log IU/ml at week 12. Week 24 response is defined as either complete (undetectable HBV DNA), partial (detectable but <2000 IU/ml) or inadequate (HBV DNA ≥2000 IU/ml). Treatment strategy is modified according to these results and polymerase sequencing for drug-resistant mutations. Such a concept has not yet been defined for the treatment of HIV–HBV coinfection, although little modification may be necessary [4]. The week 24 response may frequently be ‘inadequate’ even in the presence of combination therapy owing to high baseline HBV DNA levels in HIV–HBV coinfection and potentially a high rate of LMV resistance in therapy-naive patients [62].
Current treatment of HIV–HBV coinfection
Dual activity against HIV & HBV
Combination therapy with two HBV-active agents is preferable when HAART is required in HIV–HBV coinfected individuals, regardless of the HBV DNA level or fibrosis stage [4]. A NRTI backbone with HBV combination therapy can be delivered using Truvada®, a coformulation of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC). If HBV DNA is detectable and LMV has been used previously as part of HAART, then LMV-resistant HBV is invariably present [63] and an alternative combination is recommended. HBV treatment should therefore, be based on rescue therapy with TDF, ETV or ADV. ADV or ETV in combination with either FTC or LMV are alternatives to TDF. TDF alone or in combination with ETV is an alternative if FTC/LMV cannot be tolerated. However, there are very few controlled data to support the clinical use of these combinations. Such studies are urgently required.
Lamivudine & emtricitabine
Lamivudine, FTC and LdT are all L-nucleoside analogs and therefore share cross-resistance. LMV has been extensively studied for its efficacy against HBV (at 100 mg/day), and has good anti-HIV activity (at 300 mg/day). LMV is well tolerated and highly effective against HBV [64], but the barrier to resistance is low and therefore drug resistance common. Resistance rates of up to 20% per year are reported in HBV mono-infection [65], 50% after 2 years and 90% after 4 years in coinfected patients [12]. LMV-resistant mutations occur at a number of sites in the HBV polymerase, such as rtM204V/I, and have been extensively reported previously [66].
Emtricitabine and LMV should be considered interchangeable, and FTC offers no advantage in the setting of LMV resistance, due to structural similarities and identical resistance profiles [67].
Tenofovir
Tenofovir disoproxil fumarate is a nucleotide analog like ADV, but is more potent and less toxic. The anti-HBV effect of TDF was initially demonstrated in a subgroup analysis of both treatment-naive and LMV-resistant HBV-infected individuals in a study of HBV–HIV coinfection [68]. TDF caused greater suppression of HBV DNA than ADV in HBeAg-negative HBV-monoinfected individuals (91 vs 56% with undetectable HBV DNA after 48 weeks [p < 0.001]) [69]. Retrospective analyses and one small prospective study in HIV–HBV coinfected individuals have shown similar results [70].
It is unclear why complete virological suppression is not always achieved with TDF, even after 72 weeks, as no virological resistance has been described [71]. A novel HBV polymerase mutation (rtA194T) has been reported in individuals taking TDF, which conferred reduced susceptibility to TDF in vitro, alone or in combination with the LMV-resistance mutations, rtL180M and rtM204V [72]. This mutation has not yet been associated with clinical or virological treatment failure. Long-term prospective data for TDF in HIV–HBV coinfection are lacking. However, the dual antiviral activities of TDF, favorable side-effect profile and high genetic barrier to resistance, make it a popular choice for the treatment HIV–HBV coinfection.
Entecavir
Entecavir is a guanosine analog with high efficacy against HBV at 0.5 mg/day. ETV is contraindicated for the treatment of HBV in HIV–HBV coinfected individuals not receiving HAART, owing to its anti-HIV activity. ETV leads to a 1 log10 decline in HIV RNA and the emergence of the rtM184V mutation, associated with reduced susceptibility to LMV [73,74].
Genotypic HBV-resistance to ETV depends on at least three mutations: rtL180M and rtM204V and either rt184G/S, rtS202I or rtM250V, which can all occur on LMV therapy [42]. Therefore, ETV resistance is more common in LMV-experienced individuals, which necessitates a higher ETV dose of 1 mg daily in these patients. The main role for ETV in HIV–HBV coinfection is as a rescue therapy for LMV or TDF resistance or intolerance, in the presence of suppressed HIV under HAART. The safety and efficacy of this strategy was recently demonstrated in 65 HIV–HBV coinfected patients with LMV-resistant HBV [75]. Care should be taken when switching from TDF to ETV in individuals with a prior history of LMV-resistance and fully suppressed HBV DNA since viral rebound can occur in this setting [76].
Treatment of HBV alone
Anti-HBV agents that have no activity against HIV include the αIFNs (standard interferon [IFN] and pegylated IFN [pegIFN]), ADV and LdT.
Interferons
The use of IFNs to treat HBV in HIV–HBV coinfection has not been extensively studied. Small, open-label studies of IFN found poor response rates in HIV–HBV coinfection, probably due to immune deficiency [77,78]. A recent small study of pegIFN in combination with ADV in HIV–HBV coinfection (n = 18) showed no HBeAg seroconversion, only a modest decline in HBV DNA (median decrease of 3.6 log IU/ml after 48 weeks) and no significant sustained HBV DNA reduction [79]. PegIFN warrants further investigation in HIV–HBV coinfection, but the results from small studies are not encouraging. Treatment duration may also need to be extended in HIV–HBV coinfection, although this has not been studied.
PegIFN remains an option for the treatment of HBV where HIV does not yet require treatment owing to high CD4 T-cell counts. Finite duration of treatment (48 weeks) and the lack of potential for the emergence of drug-resistant HIV are advantages of pegIFN treatment. However, the lack of data in HIV–HBV coinfection, and the unfavorable side-effect profile are significant disadvantages.
Adefovir
Adefovir dipivoxil is not active against HIV at 10 mg/day, and HIV resistance following ADV monotherapy has not been demonstrated [80]. ADV is effective against both wild-type and LMV-resistant HBV, although ADV resistance mutations at rtN236T and rtA181V can be found in up to 29% of individuals after almost 5 years of treatment [41]. The incidence of resistance is markedly reduced when ADV is added to LMV rather than used as sequential monotherapy in LMV-resistant HBV [81]. Primary resistance to ADV is reported, particularly in the presence of polymorphisms such as rtI233V [4], and little effect on HBV DNA levels (reduction of less than 2 log over 48 weeks) is seen in up to 25% of HBV-monoinfected individuals taking ADV [82].
Telbivudine
Telbivudine is another L-nucleoside analog with anti-HBV activity. LdT (600 mg once daily) demonstrated a slightly greater anti-HBV effect than LMV (100 mg once daily) in a recent double-blind Phase III study of 1370 patients with CHB [83]. Unfortunately, LdT and LMV share cross-resistance, so that combination therapy with LdT and LMV is less effective than LdT monotherapy [84]. LdT should not be used in HIV–HBV coinfected individuals with previous or current LMV resistance.
Experience with LdT in HIV is limited. In vitro, LdT has no activity against HIV-1 [85]. LdT is currently recommended as the preferred treatment of HBV for HIV–HBV coinfected individuals when treatment of HIV is not required [4,86]. There are few data on the use of LdT in HIV–HBV coinfected individuals. A recent case report demonstrated a decline in HIV-1 RNA in a single HIV–HBV coinfected patient receiving combination therapy with LdT and ADV [87]. No rebound in HIV RNA or HIV resistance was reported. As this patient was receiving both ADV and LdT, it is currently unclear if LdT or ADV (at a dose of 10 mg/day) have a direct anti-HIV effect [87].
Combination therapy
Combination therapy with nucleoside/nucleotide analogs can result in the prevention of drug resistance in HBV monoinfection. Combination therapy using ADV and LMV resulted in less virological breakthroughs and fewer YMDD mutations than LMV monotherapy (2 vs 20%) over 52 weeks in a prospective, randomized study of 115 treatment-naive patients with CHB [88]. In the presence of LMV resistance, add-on therapy with ADV results in less ADV resistance and greater viral suppression than switching to ADV [81].
A recent prospective, randomized study in Thailand (TDF in coinfection [TICO]; n = 36) compared LMV/AZT with TDF/AZT and combination therapy using LMV/TDF together with efavirenz in treatment-naive HIV–HBV coinfected individuals [35]. HBV DNA suppression below 200 IU/ml was more common in TDF-based regimens (92% with TDF alone and 91% with LMV/TDF) compared with LMV monotherapy (46%, p = 0.013). However, there was no difference in the primary end point in median log10 decline in HBV DNA over the first 48 weeks. In addition, the kinetics of viral decline were not different with combination therapy [89]. Viral rebound and resistance only occurred in individuals receiving LMV alone (n = 2) [35]. Nonetheless, current recommendations favor combination HBV therapy, preferably with TDF and either LMV or FTC for HIV–HBV coinfection whenever possible.
It is unclear whether combination therapy is indicated when needing to treat HBV alone in the setting of HIV–HBV coinfection (i.e., when the CD4 count is >350 cells/μl and the patient is not receiving HAART). Guidelines from the European AIDS Clinical Society [90], and the HIV–Hepatitis B Virus International Panel [4] recommend combination therapy with LdT and ADV in this setting, although there are currently no data to support this.
Management of specific clinical situations
Immune restoration disease
Immune restoration disease (IRD) describes the worsening of an established infection following the initiation of HAART [91]. IRD may occur in approximately 10–30% of individuals following HAART, particularly those with low CD4 T-cell counts (<100 cells/μl). Hepatotoxicity (grade 3 [5–20× upper limit of normal] or Grade 4 [>20× upper limit of normal] transaminitis) after HAART occurs more frequently in HIV-infected individuals with either HBV or HCV coinfection [92]. Many factors may lead to abnormal ALT, or HF following the initiation of HAART, such as worsening of underlying liver disease, anti-retroviral hepatotoxicity, other medications and opportunistic infections, as well as IRD [93].
A newly regenerated immune response can result in significant hepatic inflammatory activity and marked reductions in HBV viremia, even in the absence of active HBV drugs [94]. In individuals with advanced HIV infection HF is common and is significantly associated with higher HBV DNA and ALT prior to the initiation of HAART [35]. Occasionally, HBV IRD is subsequently followed by HBeAg, and even HBsAg, seroconversion. In TICO, seroconversion rates were high; 33% lost HBeAg and 8% lost HBsAg [35]. HBeAg or HBsAg seroconversion following immune restoration predicts long-term HBV control, providing immunological restoration is maintained [94]. However, HBV IRD with HF can also be potentially serious, even fatal, in some circumstances [95]. Hepatic decompensation following HBV IRD is a recognized complication in patients with underlying cirrhosis and sometimes this has resulted in death. The relative risk of HBV IRD in individuals who initiate HAART with higher CD4 T-cell counts is currently unknown [93].
A potential strategy to reduce the incidence of HF or HBV-related IRD may be to initially treat HBV alone until there is a significant reduction in HBV DNA and then initiate HAART. This approach has been utilized to reduce the incidence of IRD secondary to other coinfections including Mycobacterium tuberculosis, Cytomegalovirus and Pneumocystis jerovicii pneumonia, although efficacy of this strategy varies with different pathogens [96]. If this strategy were to be used in HIV–HBV coinfection, the only currently available agents to use prior to intiating HAART are LdT or ADV alone or in combination.
Lamivudine resistance
Lamivudine-resistant HIV has reduced viral fitness in vitro and slower progression in vivo. Therefore, when LMV-resistant HBV is present, LMV should be continued to maintain selection pressure for LMV-resistant HIV and HBV. A second agent with a complementary resistance profile should be added. TDF has good activity against LMV-resistant HBV, but long-term experience with ADV suggests that continuing LMV in this setting may reduce the risk of TDF resistance [81,97]. ETV at 1 mg daily is effective when added to LMV [75], but is less preferred since LMV resistance predisposes to ETV resistance, as discussed earlier [40,42]. LdT is not effective in this setting.
Tenofovir intolerance or contraindication
Although TDF is very well tolerated, toxicities can occur and sometimes treatment needs to be stopped [98]. In the setting of renal toxicity, one can consider dose adjustment according to glomerular filtration rate [99]. However, if TDF cannot be continued and there is a history of LMV-resistance, the alternative agents to consider are ADV (in addition to LMV) or ETV at 1 mg daily. ADV should be used with caution as nephrotoxicity can occur but this is uncommon with the lower dose of 10 mg/day. When there is no history of LMV resistance, options include ETV, ADV or LdT in addition to HAART, although LdT is also associated with an increased risk of renal toxicity [100].
Conclusion & future perspective
HIV–HBV coinfection is common and the diagnosis and assessment of HBV should be considered in all HIV-infected individuals before commencing HAART. Effective combination therapy against both HIV and HBV is available using LMV/FTC and TDF. However, strategies for assessing and modifying therapy according to on-treatment response need to be developed. Further clinical trials are needed to address the optimal strategy to prevent or treat HBV–IRD, and the management of individuals with a high CD4 T-cell count. Earlier initiation of HBV-active HAART in all coinfected individuals may be the most effective approach. Insights into the pathogenesis of liver disease, defining the role of transient elastography and optimizing treatment strategies may help reduce the impact of HIV–HBV coinfection.
Executive summary
Liver disease in HIV–HBV coinfection
HIV–HBV coinfection leads to increased liver fibrosis and cirrhosis.
HIV–HBV coinfection is associated with high liver-related mortality compared with HIV or HBV monoinfection.
Pathogenesis may be related to unique HBV mutations or immune dysfunction (reduced HBV-specific CD4 and CD8 T-cell responses and/or immune activation).
Assessment of HBV in HIV–HBV coinfection
HBV surface antigen, hepatitis B surface antibody and hepatitis B core antibody should be tested and vaccination offered if no immunity is detected.
The relevance of occult HBV (detectable HBV DNA, but undetectable HBV surface antigen) is unclear.
Alanine aminotransferase levels may be normal even in the presence of significant fibrosis.
Hepatic flares may be due to drug toxicity, immune restoration, seroconversion, viral resistance or superinfection.
HBV DNA level is likely to be associated with clinical end points, such as cirrhosis or hepatocellular carcinoma in HIV–HBV coinfection, as demonstrated in HBV monoinfection.
Novel techniques in assessing HBV & liver disease
HBV polymerase sequencing is useful for detecting drug-resistance mutations and predicting cross-resistance.
Transient elastography (FibroScan®) reliably detects cirrhosis, but needs evaluation in HIV–HBV coinfection.
Therapy for HBV in HIV–HBV coinfection
Treatment of HBV is usually lifelong, aiming for suppression of HBV DNA to undetectable levels.
Failure to fully suppress HBV DNA should prompt a review of the treatment strategy.
Lamivudine/emtricitabine and tenofovir have dual activity against HIV and HBV, and is the preferred regimen when prescribing HBV-active HAART.
Adefovir and interferons do not appear to have significant anti-HIV activity.
Entecavir should only be used against HBV in conjunction with HAART owing to its anti-HIV effect.
The role of telbivudine for the treatment of HBV when HAART is not indicated requires further study.
Conclusion
Effective combination therapy with tenofovir and lamivudine/emtricitabine as part of HBV-active HAART reduces the emergence of drug resistance and leads to improved outcomes in HIV–HBV coinfection.
Treatment strategies need to be refined for patients with high CD4 T-cell counts not yet requiring HAART, and those with extremely low CD4 counts and advanced liver disease who are at greatest risk from HBV-immune restoration disease.
Footnotes
Financial & competing interests disclosure
David M Iser is a recipient of a postgraduate scholarship from the National Health and Medical Research Council (NHMRC). Sharon R Lewin has received sponorship and research funding from BMS and Gilead. Furthermore, SR Lewin is an NHMRC practitioner fellow, and receives funding from the NHMRC, Alfred Foundation and NIH (1 R01 AI060449). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Zhou J, Dore G, Zhang F, Lim P, Chen Y. Hepatitis B and C virus coinfection in the TREAT Asia HIV Observational Database. J Gastroenterol Hepatol. 2007;22:1510–1518. doi: 10.1111/j.1440-1746.2007.05062.x. [DOI] [PubMed] [Google Scholar]
- 2.Alter M. Epidemiology of viral hepatitis and HIV coinfection. J Hepatol. 2006;44:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4▪▪.Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV–Hepatitis B Virus International Panel. AIDS. 2008;22:1399–1410. doi: 10.1097/QAD.0b013e3282f8b46f. Excellent recent review of the major issues in treating HIV–HBV coinfection. [DOI] [PubMed] [Google Scholar]
- 5.Brook M, Gilson R, Wilkins E. British HIV Association guidelines on HIV and chronic hepatitis: coinfection with HIV and hepatitis B virus infection. HIV Med. 2005;6:84–95. doi: 10.1111/j.1468-1293.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 6▪.Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in coinfected patients. J Hepatol. 2006;44:S65–S70. doi: 10.1016/j.jhep.2005.11.015. Concise review of the natural history of HIV–HBV coinfection, including areas of controversy. [DOI] [PubMed] [Google Scholar]
- 7.Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [DOI] [PubMed] [Google Scholar]
- 8.Thio C, Seaberg E, Skolasky RJ, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicentre Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 9.Blackard J, Ma G, Nelson J, Sherman K. HIV entry into hepatocytes. Presented at: HIV and Liver Disease; Jackson Hole, WY, USA. 15–16 September 2006. [Google Scholar]
- 10.Tuyama A, Hong F, Schecter A, et al. HIV entry and replication in stellate cells promotes cellular activation and fibrogenesis: implications for hepatic fibrosis in HIV/HCV coinfectionPresented at: 15th Conference on Retroviruses and Opportunistic InfectionsBoston, MA, USA3–6 February 2008 [Google Scholar]
- 11.Revill P, Littlejohn M, Ayres A, et al. Identification of a novel hepatitis B virus precore/core deletion mutant in HIV/hepatitis B virus coinfected individuals. AIDS. 2007;21:1701–1710. doi: 10.1097/QAD.0b013e32826fb305. [DOI] [PubMed] [Google Scholar]
- 12.Matthews G, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV–HBV-infected individuals on extended lamivudine therapy. AIDS. 2006;20:863–870. doi: 10.1097/01.aids.0000218550.85081.59. [DOI] [PubMed] [Google Scholar]
- 13.Amini-Bavil-Olyaee S, Sheldon J, Lutz T, Trautwein C, Tacke F. Molecular analysis of an HBsAg-negative hepatitis B virus mutant selected in a tenofovir-treated HIV–hepatitis B virus coinfected patient. AIDS. 2009;23:268–272. doi: 10.1097/QAD.0b013e3283224316. [DOI] [PubMed] [Google Scholar]
- 14.Chang JJ, Wightman F, Bartholomeusz A, et al. Reduced hepatitis B virus (HBV)-specific CD4+ T-cell responses in human immunodeficiency virus type 1–HBV-coinfected individuals receiving HBV-active antiretroviral therapy. J Virol. 2005;79:3038–3051. doi: 10.1128/JVI.79.5.3038-3051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenchley J, Price D, Schacker T, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 16.Brenchley J, Schacker T, Ruff L, et al. CD4+ T-cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2005;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 18.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolganiuc A, Norkina O, Kodys K, et al. Viral and host factors induce macrophage activation and loss of Toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627–1636. doi: 10.1053/j.gastro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman C, Thio C. Clinical implications of HIV and hepatitis B coinfection in Asia and Africa. Lancet Infect Dis. 2007;7:402–409. doi: 10.1016/S1473-3099(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 21.Tsui J, French A, Seaberg E, et al. Prevalence and long-term effects of occult hepatitis B virus infection in HIV-infected women. Clin Infect Dis. 2007;45:736–740. doi: 10.1086/520989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen Stuart JW, Velema M, Schuurman R, Boucher CA, Hoepelman AI. Occult hepatitis B in persons infected with HIV is associated with low CD4 counts and resolves during antiretroviral therapy. J Med Virol. 2009;81:441–445. doi: 10.1002/jmv.21422. [DOI] [PubMed] [Google Scholar]
- 23.Raimondo G, Pollicano T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46:160–170. doi: 10.1016/j.jhep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Chemin I, Zoulim F. Hepatitis B virus induced hepatocellular carcinoma. Cancer Lett. doi: 10.1016/j.canlet.2008.12.003 (2009). Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Alberti A, Clumeck N, Collins S, et al. Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV coinfected patients. J Hepatol. 2005;42:615–624. doi: 10.1016/j.jhep.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 26.McGovern B. The epidemiology, natural history and prevention of hepatitis B. implications of HIV coinfection. Antivir Ther. 2007;12(Suppl 3):H3–H13. [PubMed] [Google Scholar]
- 27.Steigbigel R, Cooper D, Kumar P, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 28.Fatkenheuer G, Nelson M, Lazzarin A, et al. Subgroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med. 2008;359:1442–1455. doi: 10.1056/NEJMoa0803154. [DOI] [PubMed] [Google Scholar]
- 29.Campbell T, Mills A, Morlat P, et al. TMC125 safety and tolerability in treatment-experienced hepatitis B or C coinfected patients in DUET-1 and DUET-2. Presented at: HEP DART: Frontiers in Drug Development for Antiretroviral Therapies; Kohala Coast, HI, USA. 9–13 December 2007. [Google Scholar]
- 30.Hoffman C, Charalambous S, Martin D, et al. Hepatitis B virus infection and response to antiretroviral therapy (ART) in a South African ART program. Clin Infect Dis. 2008;47:1479–1485. doi: 10.1086/593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlier D, Jean-Jean O, Fouillot N, Will H, Rossignol J. Importance of the C terminus of the hepatitis B virus precore protein in secretion of HBe antigen. J Gen Virol. 1995;76:1041–1045. doi: 10.1099/0022-1317-76-4-1041. [DOI] [PubMed] [Google Scholar]
- 32.Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38:1075–1086. doi: 10.1053/jhep.2003.50453. [DOI] [PubMed] [Google Scholar]
- 33.Chen M, Sallberg M, Hughes J, et al. Immune tolerance split between hepatitis B virus precore and core proteins. J Virol. 2005;79:3016–3027. doi: 10.1128/JVI.79.5.3016-3027.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piroth L, Sene D, Pol S, et al. Epidemiology, diagnosis and treatment of chronic hepatitis B in HIV-infected patients (EPIB 2005 STUDY) AIDS. 2007;21:1323–1331. doi: 10.1097/QAD.0b013e32810c8bcf. [DOI] [PubMed] [Google Scholar]
- 35▪▪.Matthews GV, Avihingsanon A, Lewin SR, et al. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naive individuals in Thailand. Hepatology. 2008;48:1062–1069. doi: 10.1002/hep.22462. First randomized trial of mono versus combination anti-HBV therapy in HIV–HBV coinfection. [DOI] [PubMed] [Google Scholar]
- 36.Iloeje U, Yang H, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Yang H, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 38.Benhamou Y. Hepatitis B in the HIV-coinfected patient. J Acquir Immune Defic Syndr. 2007;45:S57–S65. doi: 10.1097/QAI.0b013e318068d1dd. [DOI] [PubMed] [Google Scholar]
- 39.Ganem D, Prince A. Hepatitis B virus infection – natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 40▪▪.Thio C, Locarnini S. Treatment of HIV/HBV coinfection: clinical and virological issues. AIDS Rev. 2007;9:40–53. Comprehensive review of available therapy, including a detailed discussion of drug resistance and mechanisms for cross-resistance. [PubMed] [Google Scholar]
- 41.Hadziyannis S, Tassopoulos N, Heathcote E, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Tenney D, Levine S, Rose R, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob Agents Chemother. 2004;48:3498–3507. doi: 10.1128/AAC.48.9.3498-3507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 44.Chan H. Revisiting the treatment recommendations for chronic hepatitis B. Hepatology. 2009;49:700. doi: 10.1002/hep.22714. [DOI] [PubMed] [Google Scholar]
- 45.Friedrich-Rust M, Ong M, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 46.Halfon P, Munteanu M, Poynard T. FibroTest-ActiTest as a non-invasive marker of liver fibrosis. Gastroenterol Clin Biol. 2008;32:22–39. doi: 10.1016/S0399-8320(08)73991-5. [DOI] [PubMed] [Google Scholar]
- 47.Maida I, Soriano V, Castellares C, et al. Liver fibrosis in HIV-infected patients with chronic hepatitis B extensively exposed to antiretroviral therapy with anti-HBV activity. HIV Clin Trials. 2006;7:246–250. doi: 10.1310/hct0705-246. [DOI] [PubMed] [Google Scholar]
- 48.Barreiro P, Labarga P, Martin-Carbonero L, et al. Sustained virological response following HCV therapy is associated with non-progression of liver fibrosis in HCV/HIV-coinfected patients. Antivir Ther. 2006;11:869–877. doi: 10.1177/135965350601100706. [DOI] [PubMed] [Google Scholar]
- 49.Macias J, Recio E, Vispo E, et al. Application of transient elastometry to differentiate mild from moderate to severe liver fibrosis in HIV/HCV coinfected patients. J Hepatol. 2008;49:916–922. doi: 10.1016/j.jhep.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 50.Chan H, Wong G, Choi P, et al. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (FibroScan®) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36–44. doi: 10.1111/j.1365-2893.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 51.Fung J, Lai C, But D, Wong D, Cheung T, Yuen M. Prevalence of fibrosis and cirrhosis in chronic hepatitis B: implications for treatment and management. Am J Gastroenterol. 2008;103:1421–1426. doi: 10.1111/j.1572-0241.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 52.Kim S, Ahn S, Park J, et al. Liver stiffness measurement in combination with noninvasive markers for the improved diagnosis of B-viral liver cirrhosis. J Clin Gastroenterol. 2008 doi: 10.1097/MCG.0b013e31816f212e. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 53.Marcellin P, Ziol M, Bedossa P, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242–247. doi: 10.1111/j.1478-3231.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 54.Wong G, Wong V, Choi P, et al. Clinical factors associated with liver stiffness in hepatitis B e antigen-positive chronic hepatitis B patients. Clin Gastroenterol Hepatol. 2009;7:227–233. doi: 10.1016/j.cgh.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 55.Wong G, Wong V, Choi P, et al. Evaluation of alanine transaminase and hepatitis B virus DNA to predict liver cirrhosis in hepatitis B e antigen-negative chronic hepatitis B using transient elastography. Am J Gastroenterol. 2008;103:3071–3081. doi: 10.1111/j.1572-0241.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 56.Maida I, Garcia-Gasco P, Sotgiu G, et al. Antiretroviral-associated portal hypertension: a new clinical condition? Prevalence, predictors and outcome. Antivir Ther. 2008;13:103–107. [PubMed] [Google Scholar]
- 57.Panos G, Holmes P, Valero S, et al. Cryptogenic pseudocirrhosis: a new clinical syndrome of non-cirrhotic portal hypertension (unassociated with advanced fibrosis) in patients with HIV that can be detected by transient elastography (TE) in differential diagnosis from HCV coinfection. Presented at: 4th International Workshop on HIV and Hepatitis Coinfection; Madrid, Spain. 19–21 June 2008. [Google Scholar]
- 58.Ingiliz P, Valantin M, Duvivier C, et al. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 monoinfected patients on antiretroviral therapy. Hepatology. 2009;49:436–442. doi: 10.1002/hep.22665. [DOI] [PubMed] [Google Scholar]
- 59.Sorrell M, Belongia E, Costa J, et al. National institutes of health consensus development conference statement: management of hepatitis B. Ann Intern Med. 2009;150:104–110. doi: 10.7326/0003-4819-150-2-200901200-00100. [DOI] [PubMed] [Google Scholar]
- 60.Hammer S, Eron J, Reiss P, et al. Antiretroviral treatment of adult HIV infection. 2008 recommendations of the International AIDS Society – USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 61.Keeffe E, Zeuzem S, Koff R, et al. Report of an international workshop: Roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007;5:890–897. doi: 10.1016/j.cgh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Selabe S, Lukhwareni A, Song E, Leeuw Y, Burnett R, Mphahlele M. Mutations associated with lamivudine-resistance in therapy-naive hepatitis B virus (HBV) infected patients with and without HIV coinfection: implications for antiretroviral therapy in HBV and HIV coinfected South African patients. J Med Virol. 2007;79:1650–1654. doi: 10.1002/jmv.20974. [DOI] [PubMed] [Google Scholar]
- 63.Matthews G, Dore G. Response to Schmutz et al. ‘Combination of tenofovir and lamivudine versus tenofovir after lamivudine failure for therapy of hepatitis B in HIV-coinfection’. AIDS. 2007;21:777–778. doi: 10.1097/QAD.0b013e3280b07774. [DOI] [PubMed] [Google Scholar]
- 64.Liaw Y. Results of lamivudine trials in Asia. J Hepatol. 2003;39:S111–S115. doi: 10.1016/s0168-8278(03)00155-7. [DOI] [PubMed] [Google Scholar]
- 65.Chang T, Lai C, Chien R, et al. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276–1282. doi: 10.1111/j.1440-1746.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 66.Westland C, Yang H, Delaney W, et al. Activity of adefovir dipivoxil against all major patterns of lamivudine-resistant HBV in patients. J Viral Hepat. 2005;12:67–73. doi: 10.1111/j.1365-2893.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 67.Yang H, Qi X, Sabogal A, Miller M, Xiong S, Delaney W. Cross-resistance testing of next-generation nucleoside and nucleotide analogs against lamivudine-resistant HBV. Antivir Ther. 2005;10:625–633. [PubMed] [Google Scholar]
- 68.Dore GJ, Cooper DA, Pozniak AL, et al. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J Infect Dis. 2004;189:1185–1192. doi: 10.1086/380398. [DOI] [PubMed] [Google Scholar]
- 69.Marcellin P, Buti M, Krastev Z, et al. A randomized, double-blind, comparison of tenofovir DF (TDF) versus adefovir dipivoxil (ADV) for the treatment of HBeAg-negative chronic hepatitis B (CHB): study GS-US-174–0102. Hepatology. 2007;46:80A. [Google Scholar]
- 70.Peters M, Andersen J, Lynch P, et al. Randomized controlled study of tenofovir and adefovir in chronic hepatitis B virus and HIV infection: ACTG A5127. Hepatology. 2006;44:1110–1116. doi: 10.1002/hep.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delaney W, Borroto-Esoda K. Therapy of chronic hepatitis B: trends and developments. Curr Opin Pharmacol. 2008;8:532–540. doi: 10.1016/j.coph.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Amini-Bavil-Olyaee S, Herbers U, Sheldon J, Luedde T, Trautwein C, Tacke F. The rtA194T polymerase mutation impacts viral replication and susceptibility to tenofovir in hepatitis B e antigen-positve and hepatitis B e antigen-negative hepatitis B virus strains. Hepatology. 2009;49(4):1158–1165. doi: 10.1002/hep.22790. [DOI] [PubMed] [Google Scholar]
- 73.Sasadeusz J, Audsley J, Mijch A, et al. The anti-HIV activity of entecavir: a multicentre evaluation of lamivudine-experienced and lamivudine-naive patients. AIDS. 2008;22:947–955. doi: 10.1097/QAD.0b013e3282ffde91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir – effects on HIV-1 replication and resistance. N Engl J Med. 2007;356:2614–2621. doi: 10.1056/NEJMoa067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pessôa M, Gazzard B, Huang A, et al. Efficacy and safety of entecavir for chronic HBV in HIV/HBV coinfected patients receiving lamivudine as part of antiretroviral therapy. AIDS. 2008;22:1779–1787. doi: 10.1097/QAD.0b013e32830b3ab5. [DOI] [PubMed] [Google Scholar]
- 76.Hull M, Toy J, Montessori V, et al. Rapid rebound in hepatitis B DNA in previously undetectable hepatitis B/HIV coinfected patients switching from tenofovir to entecavir therapy. Presented at: 9th International Congress on Drug Therapy in HIV Infection; Glasgow, UK. 9–13 November 2008. [Google Scholar]
- 77.Marcellin P, Boyer N, Colin J, et al. Recombinant α interferon for chronic hepatitis B in anti-HIV positive patients receiving zidovudine. Gut. 1993;34(2 Suppl):S106. doi: 10.1136/gut.34.2_suppl.s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Martino V, Thevenot T, Colin J, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology. 2002;123:1812–1822. doi: 10.1053/gast.2002.37061. [DOI] [PubMed] [Google Scholar]
- 79.Ingiliz P, Valantin M, Thibault V, et al. Efficacy and safety of adefovir dipivoxil plus pegylated interferon-α2a for the treatment of lamivudine-resistant hepatitis B virus infection in HIV-infected patients. Antivir Ther. 2008;13:895–900. [PubMed] [Google Scholar]
- 80.Delaugerre C, Marcelin A, Thibault V, et al. Human immunodeficiency virus (HIV) type 1 reverse transcriptase resistance mutations in hepatitis B virus (HBV)–HIV-coinfected patients treated for HBV chronic infection once daily with 10 milligrams of adefovir dipivoxil combined with lamivudine. Antimicrob Agents Chemother. 2002;46:1586–1588. doi: 10.1128/AAC.46.5.1586-1588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lampertico P, Vigano M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133:1445–1451. doi: 10.1053/j.gastro.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 82.Durantel S, Werle B, Durantel D, et al. Different profiles of response to adefovir dipivoxil and factors that may influence response in patients with chronic hepatitis B. Hepatology. 2004;40:654A. [Google Scholar]
- 83.Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 84.Lai C, Leung N, Teo E, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005;129:528–536. doi: 10.1016/j.gastro.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 85.Standring DN, Bridges EG, Placidi L, et al. Antiviral β-L-nucleosides specific for hepatitis B virus infection. Antivir Chem Chemother. 2001;12:119–129. [PubMed] [Google Scholar]
- 86.Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82–88. doi: 10.1111/j.1468-1293.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 87.Low E, Cox A, Atkins M, Nelson M. Telbivudine has activity against HIV-1. AIDS. 2009;23:546–547. doi: 10.1097/QAD.0b013e3283262f09. [DOI] [PubMed] [Google Scholar]
- 88.Sung J, Lai J, Zeuzem S, et al. A randomised double-blind Phase II study of lamivudine (LAM) compared with lamivudine plus adefovir dipivoxil (ADV) for treatment naive patients with chronic hepatitis B (CHB): week 52 analysis. J Hepatol. 2003;38(Suppl 2):25–26. [Google Scholar]
- 89.Lewin S, Ribeiro R, Avihingsanon A, et al. Viral dynamics of hepatitis B virus DNA in human immunodeficiency virus-1–hepatitis B virus coinfected individuals: similar effectiveness of lamivudine, tenofovir, or combination therapy. Hepatology. 2009;49(4):1113–1121. doi: 10.1002/hep.22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82–88. doi: 10.1111/j.1468-1293.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 91.French MA. Disorders of immune reconstitution in patients with HIV infection responding to antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:16–21. doi: 10.1007/s11904-007-0003-z. [DOI] [PubMed] [Google Scholar]
- 92.den Brinker M, Wit F, Wertheimvan Dillen P, et al. Hepatitis B and C virus coinfection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS. 2000;14:2895–2902. doi: 10.1097/00002030-200012220-00011. [DOI] [PubMed] [Google Scholar]
- 93.Crane M, Matthews G, Lewin S. Hepatitis virus immune restoration disease in the liver. Curr Opin HIV AIDS. 2008;3:446–452. doi: 10.1097/COH.0b013e3282fdc953. [DOI] [PubMed] [Google Scholar]
- 94.Miailhes P, Trabaud MA, Pradat P, et al. Impact of highly active antiretroviral therapy (HAART) on the natural history of hepatitis B virus (HBV) and HIV coinfection: relationship between prolonged efficacy of HAART and HBV surface and early antigen seroconversion. Clin Infect Dis. 2007;45:624–632. doi: 10.1086/520752. [DOI] [PubMed] [Google Scholar]
- 95.Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis. 2004;39:129–132. doi: 10.1086/421386. [DOI] [PubMed] [Google Scholar]
- 96.French MA. The immunopathogenesis of mycobacterial immune restoration disease. Lancet Infect Dis. 2006;6:461–462. doi: 10.1016/S1473-3099(06)70530-8. [DOI] [PubMed] [Google Scholar]
- 97.Delaney W. Progress in the treatment of chronic hepatitis B: long-term experience with adefovir dipivoxil. J Antimicrob Chemother. 2007;59:827–832. doi: 10.1093/jac/dkl551. [DOI] [PubMed] [Google Scholar]
- 98.Buchacz K, Brooks J, Tong T, et al. Evaluation of hypophosphataemia in tenofovir disoproxil fumarate (TDF)-exposed and TDF-unexposed HIV-infected out-patients receiving highly active antiretroviral therapy. HIV Med. 2006;7:451–456. doi: 10.1111/j.1468-1293.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 99.Tenofovir, package insert. Gilead Sciences, CA, USA.
- 100.Liaw Y, Gane E, Leung N, et al. 2-year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486–495. doi: 10.1053/j.gastro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 101.Iser D, Lewin S. Treatment of hepatitis B virus (HBV) in the setting of HIV–HBV co-infection. Asian Biomed. 2009;3:15–27. [Google Scholar]