Abstract
OBJECTIVE
To estimate the probabilities and identify risk factors for entering the menopausal transition and moving into each subsequent transition stage.
METHODS
Estimations of probabilities of entry into each menopause transition stage and predictors associated with each transition stage were conducted in a population-based cohort of midlife women.
RESULTS
The likelihood of entering the menopausal transition and moving into each subsequent stage was increased for each unit increase in FSH (P<0.001) and with each unit decrease in inhibin b (P<0.001) in the adjusted multivariable model. The largest observed change in average FSH levels was comparing women in the late transition (stage 4), with an average of 24.78 miU/mL, to the early transition (stage 3), with 10.38 miU/mL. Women experiencing this amount of change in FSH had an odds of transitioning from stage 3 to stage 4 of 1.90 (95% CI: 1.86-1.95). Decreases in inhibin b resulted in odds ratios similar to the magnitude of changes in FSH. Current smoking increased the odds of transition into each stage by about 30% (OR=1.30, 95% CI 1.28, 1.32). Average estradiol levels did not change dramatically between stages. However, higher estradiol significantly increased the odds of entering the transition (P=0.013). Age and race predicted transitions into some but not all stages. Body mass index, alcohol use and age at menarche did not predict entrance into any stage of the menopausal transition after adjusting for other study variables.
CONCLUSIONS
These results show that increased FSH, decreased inhibin b and smoking strongly predict entry into the earliest stages of the menopausal transition as defined by changes in bleeding patterns. African Americans entered the transition before white women but race did not predict entry into late transition stages. Higher estradiol levels predict entry into the earliest transition stage but not subsequent stages.
Keywords: Menopausal stages, menopause, perimenopause, estradiol, FSH, Inhibin b, smoking
INTRODUCTION
Menopause marks the end of women's reproductive capacity, but it signals more than end of fertility. Health issues such as low bone mineral density, sexual problems, mood disorders and disturbed sleep increase around menopause, although the extent to which these problems are associated with diminished ovarian reserve is not well understood.1
Associations between hormone changes, menopausal symptoms and other health issues associated with menopause have been aided by staging the menopausal transition. We adapted the stages as proposed by the Stages of Reproductive Aging Workshop (STRAW),2 which continue to undergo validation, and identified differential associations between menopausal stages and reproductive hormones and between the stages and common menopausal symptoms that occurred well before the point of menopause.3-5 However, the probabilities of an individual women moving from one menopausal stage to the next, particularly in the earliest transition stages, have not been quantified and the effects of risk factors on the probabilities have not been determined. Such information is essential to individualize the treatment of health issues associated with menopause.
The objectives of this study were to estimate the probability of entry into each sequential stage of the menopausal transition and identify risk factors associated with these transitions. We hypothesized that reproductive hormone levels, age, race, smoking, body mass index (BMI), alcohol use and age at menarche are predictors of transition from one menopausal stage to the next. We suggest that if the probabilities of progressing to the next menopausal stage can be calculated, then individual treatment of menopausal symptoms may become more feasible. For example, women may be more willing to tolerate symptoms for a short time period, but seek treatment when symptoms persist. The risk/benefit of treatments is also affected by the duration of treatment. A woman may accept an effective treatment with troublesome side effects for a short time period, but choose an alternative with fewer side effect when the expected duration is long.
METHODS
Cohort Participants
The Penn Ovarian Aging Studies are based on a population-based cohort of 436 women. The cohort was identified by random-digit dialing to households in Philadelphia County, Pennsylvania as described in previous reports.5 Sampling was stratified to obtain equal numbers of African American and Caucasian women (N=218 in each group). The Institutional Review Board of the University of Pennsylvania approved the study, and written informed consent was obtained from all participants.
At enrollment in the cohort, the participants were ages 35 to 47 years with regular menstrual cycles in normal range (22-35 days) for the previous three cycles, had an intact uterus and at least one ovary. Exclusion criteria included current use of psychotropic or hormonal medications including hormonal contraception and hormone therapies; pregnancy or breast feeding; serious health problems known to compromise ovarian function (e.g., diabetes mellitus, liver disease, and breast or endometrial cancer); and alcohol or drug abuse within the past year.
During the 9-year interval, 125 cohort members discontinued. Baseline comparisons of all variables in this report between the discontinuers and the participants who continued throughout the study identified only one variable that differed significantly between the two groups (Table 1). The discontinuers had slightly higher FSH levels on average, although this did not appear to be clinically meaningful and both groups were clearly at premenopausal levels. We previously reported a study of the demographic and hormonal characteristics of the sample and found that in the first 5 years of the cohort with 58% (73/125) of the discontinuers, there were no substantial differences compared between the active and inactive participants, indicating that discontinuation was spread equally across study characteristics.6
Table 1.
Comparison of Study Variables at Baseline Between Continuers and Dropouts
| Continuers N=309 | Dropouts N=127 | P Value | |
|---|---|---|---|
| Age (years) | 41.5 (3.55) | 41.2 (2.66) | 0.343 |
| Cycle length (days) | 27.2 (3.21) | 27.4 (7.17) | 0.689 |
| FSH (mIu/mL) | 7.14 (3.16, 16.13) | 8.10 (3.07, 21.35) | 0.010 |
| Inhibin b (ng/mL) | 65.5 (18.6, 230.7) | 66.1 (17.4, 251.6) | 0.898 |
| Estradiol (pg/mL) | 36.3 (10.36, 127.1) | 33.2 (8.35, 131.8) | 0.216 |
| BMI (kg/m2) | 29.2 (8.29) | 29.4 (6.84) | 0.833 |
| Smoking (yes) | 113 (36.7) | 53 (41.7) | 0.325 |
| Alcohol (>1/wk) | 30 (9.7) | 12 (9.5) | 0.933 |
| Race | 0.272 | ||
| African American | 150 (49) | 69 (54) | |
| Caucasian | 159 (51) | 58 (46) |
Data are given as mean with standard deviation (SD), geometric mean with 95% confidence interval, or number with percentage of participants as appropriate for the variable.
Study Design
The cohort was followed for 9 years with 10 assessment periods. The first 5 periods were at approximately 8-9 month intervals; the later assessments were at one year intervals. Blood samples for the hormone assessments were obtained in each assessment period during the first 6 days of two consecutive menstrual cycles or one month apart in non-cycling women, yielding a possible maximum of 20 hormone samples per participant.
Trained research interviewers obtained the blood samples, anthropometric measures and all other study data in individual in-person interviews at the participants' homes. The study was explained to the participants as a general women's health study. At each assessment period, a structured interview questionnaire focused on overall health, and subjects completed a set of validated self-report measures to assess health and other variables of the study as previously described.3, 5
Study Variables
Menopausal Stage
We defined 5 stages of menopausal transition based on menstrual bleeding patterns and adapted from the Stages of Reproductive Aging Workshop (STRAW)2 in order to capture the early changes in the menopausal transition. We previously compared these stages to other staging definitions and demonstrated significant associations of the stages with reproductive hormone changes.5, 7
The following 5 categories were defined in this study: 1) premenopausal: regular menstrual cycles in the 22-35 day range; 2) late premenopausal: a change in cycle length of >=7 days either direction from the participant's personal baseline at enrollment in the cohort and observed for at least once cycle in the study; 3) early transition: changes in cycle length of >=7 days either direction from the participant's personal baseline at enrollment in the cohort and observed for at least two consecutive cycles in the study or 60 days amenorrhea; 4) late transition: 90 days to 11 months amenorrhea; 5) postmenopausal: >=12 months amenorrhea excluding hysterectomy.
Menopausal stage was identified at each assessment period using the menstrual dates at each study visit (visits were conducted within 6 days of bleeding) and the two previous menstrual dates obtained at each visit. Additional confirmatory data were obtained from the daily symptom diaries that participants recorded for one menstrual cycle at each assessment period, (the diary date was used in cases of disagreement), the reported number of menstrual periods between assessments, cycle length and number of bleeding days.
Hormones
Follicular phase blood samples were collected during days 1-6 of the menstrual cycle. Assays were conducted in the Clinical and Translational Research Center of the University of Pennsylvania. All assays were performed in duplicate, with the means of the duplicates used in analysis. Estradiol and follicle stimulating hormone (FSH) were measured by radioimmunoassay using Coat-A-Count commercial kits (Diagnostic Products, Los Angeles, CA). The inter and intra assay coefficients of variation were less than 5%. Dimeric inhibin b was measured in serum by Patrick Sluss, PhD, Massachusetts General Hospital, Boston. Inhibin b assays were performed with a solid-phase sandwich ELISA (Diagnostic Systems Laboratories, Inc., Beckman Coulter, Houston, TX) based on the use of plates coated with a monoclonal antibody specific for the alpha-subunit for detection.8, 9 The limit of measurement for the assay was 15 pg/mL (CV=20%). Values below the sensitivity threshold (15 pg/mL) were given the threshold value. The assay was controlled in triplicate using samples with mean concentrations of 155.3, 316.3, and 919.3 pg/mL, with interassay CVs of 11.6, 7.6 and 9.7%, respectively. The reference ranges for women are as follows: normally cycling women, follicular phase: 64 to 146; normally cycling women, mid-cycle: 47 to 169; normally cycling women, luteal phase: <15 to 72; postmenopausal women not on hormone therapy:<15 pg/mL.
Other covariates
Covariates of age, race (African American or Caucasian), current smoking (yes, no), BMI (kg/m2), alcohol use (yes, no) and age at menarche were obtained in the study interviews and selected for this report based on their significance in previous reports and the goals of this study.
Statistical Analysis
A Markov transition model was used to characterize the 5-stage transition to menopause. The Markov assumption is that the past menstrual history affects the future stages only through the information in the current stage, and risk factors influence the progress to menopause by influencing the transition probabilities. Unlike logistic regression and survival analysis, which use only the information from the first and last visit and only describe the event of postmenopause, the Markov model utilizes the information from all study visits to characterize transitions among the five stages and facilitate the prediction of the future stage based purely on the information in the current stage. These procedures are highly analogous to clinical practice.
For this particular application, an ordinal Markov transition model was utilized, where a subject can get to stage k+1 only by first reaching stage k. However, the periodic/annual evaluations of study participants imposed challenges for the ordinal Markov transition model, inasmuch as the exact transition time was rarely observed and the intervals between adjacent visits were not equally spaced. Although these challenges could be handled by modeling the transition intensities in the continuous time setting instead of the transition probabilities,10 the approach is computationally expensive and the parameters are difficult to interpret clinically. To address this problem, we exploited the fact that menstrual cycles are approximately monthly. We discretized the continuous time with the minimal step as one month and approximated the transition intensity matrix in the continuous time setting by a one-step transition probability matrix in a discrete time scale. We investigated the effects of covariates on the transition probabilities through a proportional odds model (Appendix 1). This formulation led to an efficient parametrization, a computationally efficient estimation procedure, and interpretable parameters in terms of the progression to menopause.
We first screened each covariate to determine that it had sufficient prevalence for valid inferences and a differential distribution across the menopausal stages in order to be considered a predictor of menopause. We then fit the ordinal discrete time Markov transition model11 for each selected covariate individually. We fit these univariate models with distinct slopes, which means the covariate had different effects depending on the current stage, and with common slopes, which means the covariate had a similar association for every transition. A likelihood ratio test was performed to select between the distinct slopes and the common slopes for further analyses. The set of significant covariates was then evaluated jointly in a multivariate model, and backward model selection was performed based on the likelihood ratio test to achieve model parsimony.
In these models, odds ratios greater than 1 indicate an increased odds of reaching the next menopausal stage for each unit increase in the covariate (for continuous variables such as age and hormone levels). For categorical covariates (smoking, race), the odds ratio represents the comparison between the 2 groups defined by the variable. Odds ratios less than 1 indicate that the odds of reaching the next menopausal stage increase as the measured levels decrease.
The full cohort was analyzed (N=436), with 10 subjects (2.3%) omitted in the Markov model due to missing variables. The two hormone values obtained in each study period were averaged for each subject. In cases where two hormone values were not obtained in an assessment period, the single value was used. The hormone values were transformed to the natural log in all analyses. Mean hormone values are expressed as the geometric mean with 95% confidence intervals. Analyses were performed using a FORTRAN program ORDMKV,12 which uses the Quasi-Newton method to maximize the likelihood and simultaneously gives the parameter estimates and the large sample variances. Statistical tests were 2-sided with P<0.05 considered significant.
RESULTS
Table 2 shows the summary statistics for the study variables at each menopausal stage. The mean age for all observations in Stage 1 (premenopausal) was 42.5 (SD 3.5) years and 50.2 (SD 3.2) years in Stage 5 (postmenopausal). All participants were premenopausal at the study baseline; 65 subjects were postmenopausal at endpoint. Age, cycle length, FSH levels and BMI increased with menopausal stages. Inhibin b levels decreased with menopausal stages, while estradiol levels initially increased and then decreased in Stages 4 and 5. The proportion of African Americans and Caucasians varied at each stage, with more African American than Caucasian women entered in the earliest transition stages (Stages 2 and 3) but more Caucasians in Stages 4 and 5. Reported smoking and alcohol use slightly increased between Stage 1 and Stage 5. The mean age at menarche was 12.66 (SD 1.76) years.
Table 2.
Level of Study Covariates at Each Menopausal Stage
| Age | Cycle Length | FSH mIU/mL | Inhibin B pg/mL | Estradiol pg/mL | BMI kg/m2 | Smoking | Alcohol | Race | ||
|---|---|---|---|---|---|---|---|---|---|---|
| African American | Caucasian | |||||||||
| Stage1 Premenopausal N=1395 (426 subjects) | 42.54(3.51) | 28.03(3.66) | 7.69 3.38-17.52 | 69.41 18.67-258.06 | 38.09 12.22-118.72 | 28.69(7.45) | 33.36% | 8.82% | 43.16% | 56.84% |
| Stage 2 Late Premenopausal N=580 (252 subjects) | 43.54(3.43) | 32.44(7.57) | 8.166 3.06-21.76 | 61.56 14.43- 262.54 | 39.25 12.59- 122.34 | 29.22(7.73) | 37.07% | 11.55% | 50.38% | 49.62% |
| Stage 3 Early transition N=957 (271 subjects) | 45.68(3.63) | 40.94(15.24) | 10.38 2.63-40.94 | 40.04 8.35- 192.10 | 41.68 8.52- 203.89 | 30.57(8.26) | 36.57% | 14.42% | 53.47% | 46.53% |
| Stage 4 Late transition N=310 (136 subjects) | 48.27(3.81) | 141.69(81.07) | 24.78 4.08-150.39 | 20.29 7.18-57.33 | 33.45 5.20-215.29 | 31.09(8.31) | 31.93% | 14.84% | 43.66% | 56.34% |
| Stage 5 Postmenopausal N=169 (65 subjects) | 50.22(3.20) | 829.26(527.86) | 44.26 9.98-196.29 | 16.78 6.42-43.83 | 20.70 2.30, 185.90 | 30.11(8.85) | 38.46% | 20.71% | 39.51% | 60.49% |
Data are for the observation N at each stage and are given as mean with standard deviation (SD), geometric mean with 95% confidence interval, or percentage of participants as appropriate for the variable. The mean age at menarche was 12.66 (SD 1.76) years.
The covariates most strongly associated with entry into menopausal stages in univariate Markov models were increasing FSH levels (P<0.001), decreasing inhibin b levels (P<0.001), and age (P<0.001). These variables had highly significant associations with each transition stage, including the earliest stages, and significant common slopes, indicating that the effects of these variables were similar across all stages. Decreasing estradiol (P=0.033), current alcohol use (P=0.026) and current smoking (P=0.057) also had a similar effect across all stages in the univariate models (Table 3).
Table 3.
Univariate Association of Each Covariate with Transition to Menopausal Stages
| Variable | Distinct Slopes | Common Slope | P Value* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage Transition | Odds Ratio | 95% CI | P Value | Overall P Value | Odds Ratio | 95% CI | P Value | ||
| Age | Late Premenopausal | 1.05 | 1.02-1.09 | <0.001 | <0.001 | 1.11 | 1.09-1.13 | <0.001 | 0.001 |
| Early Transition | 1.12 | 1.08-1.16 | <0.001 | ||||||
| Late Transition | 1.19 | 1.14-1.25 | <0.001 | ||||||
| Post Menopausal | 1.14 | 1.06-1.23 | <0.000 | ||||||
| FSH | Late Premenopausal | 2.26 | 1.78- 2.88 | <0.001 | <0.001 | 2.56 | 2.27-2.89 | <0.001 | 0.536 |
| Early Transition | 2.92 | 2.26-3.77 | <0.001 | ||||||
| Late Transition | 2.66 | 2.15-3.28 | <0.001 | ||||||
| Post Menopausal | 2.41 | 1.79-3.23 | <0.001 | ||||||
| Inhibin B | Late Premenopausal | 1.74 | 1.49-2.02 | <0.001 | <0.001 | 0.54 | 0.49-0.59 | <0.001 | 0.075 |
| (decreasing) | Early Transition | 1.73 | 1.48-2.03 | <0.001 | |||||
| Late Transition | 2.38 | 1.94-2.92 | <0.001 | ||||||
| Post Menopausal | 1.90 | 1.20-3.00 | 0.006 | ||||||
| Estradiol | Late Premenopausal | 0.98 | 0.81-1.19 | 0.826 | 0.210 | 0.90 | 0.82-0.99 | 0.033 | 0.709 |
| Early Transition | 0.90 | 0.74-1.10 | 0.290 | ||||||
| Late Transition | 0.90 | 0.76-1.08 | 0.258 | ||||||
| Post Menopausal | 0.83 | 0.68-1.01 | 0.065 | ||||||
| Smoking | Late Premenopausal | 1.25 | 1.00-1.55 | 0.047 | 0.309 | 1.14 | 0.99-1.30 | 0.057 | 0.740 |
| Early Transition | 1.04 | 0.82-1.32 | 0.729 | ||||||
| Late Transition | 1.09 | 0.81-1.47 | 0.560 | ||||||
| Post Menopausal | 1.19 | 0.76-1.87 | 0.457 | ||||||
| Race (African American vs. Caucasian) | Late Premenopausal | 1.40 | 1.13- 1.74 | 0.002 | 0.018 | 1.07 | 0.94-1.22 | 0.312 | 0.012 |
| Early Transition | 0.89 | 0.71-1.13 | 0.349 | ||||||
| Late Transition | 0.85 | 0.63-1.13 | 0.259 | ||||||
| Post Menopausal | 1.10 | 0.72-1.68 | 0.665 | ||||||
| BMI | Late Premenopausal | 1.01 | 1.00- 1.03 | 0.041 | 0.027 | 1.00 | 0.99-1.01 | 0.980 | 0.012 |
| Early Transition | 1.00 | 0.99-1.02 | 0.862 | ||||||
| Late Transition | 0.98 | 0.97-1.01 | 0.167 | ||||||
| Post Menopausal | 0.96 | 0.94-0.99 | 0.034 | ||||||
| Alcohol | Late Premenopausal | 1.30 | 0.93-1.81 | 0.127 | 0.267 | 1.24 | 1.03-1.50 | 0.026 | 0.911 |
| Early Transition | 1.11 | 0.79-1.58 | 0.523 | ||||||
| Late Transition | 1.33 | 0.91-1.95 | 0.140 | ||||||
| Post Menopausal | 1.25 | 0.70-2.24 | 0.448 | ||||||
| Menarche | Late Premenopausal | 0.96 | 0.90-1.02 | 0.183 | 0.571 | 0.97 | 0.94-1.01 | 0.163 | 0.027 |
| Early Transition | 0.99 | 0.92-1.06 | 0.737 | ||||||
| Late Transition | 0.96 | 0.88-1.04 | 0.301 | ||||||
| Post Menopausal | 1.02 | 0.89-1.16 | 0.799 | ||||||
P value from the likelihood ratio test comparing common and distinct slopes.
Distinct slopes indicate the effect of the covariate at each menopausal stage. A significant common slope indicates the effect of the covariate is the same over all stages.
Race (P=0.018) and BMI (P=0.027) had different associations at different stages of the menopausal transition in unadjusted analysis African American women were more likely to enter the earliest stage compared to Caucasian women (P=0.002), but entrance into subsequent stages did not significantly differ between the two racial groups. Subjects with higher BMI were more likely to enter the earliest stage compared to those with lower BMI (P=0.041), although they were less likely to reach postmenopausal stage (P=0.034). Age at menarche had no significant association with the transition stages. Cycle length was not considered a predictor of transitions to menopausal stages because it was used to define the stages.
Six covariates remained significantly associated with the transition to menopause after adjusting for all other variables in the multivariable model (Table 4). The likelihood of moving into each transition stage was 2 times greater for each unit increase in log FSH (OR = 2.09, 95% CI 2.04, 2.15; P<0.001). The likelihood of moving into each transition stage was about 1 ½ times greater with each unit decrease in log inhibin b (OR= 1.57, 95% CI 1.14, 2.15, P<0.001). While higher estradiol levels significantly increased the odds of entering the earliest stage (OR 1.32, 95% CI 1.06-1.65; P=0.013), the probability of reaching postmenopause was associated with lower estradiol levels (Table 4). Current smoking increased the likelihood of moving into each stage of the transition by approximately 30% (OR=1.30, 95% CI 1.28, 1.32; P<0.001).
Table 4.
Odds Ratios (ORs) of Likelihood of Transitions to Menopausal Stages in the Final Multivariable Model
| Transition to Stage | Odds Ratio | 95% CI | Overall Significance (Wald P Value) | |
|---|---|---|---|---|
| Age | Late Premenopausal | 1.02 | 0.99-1.04 | <0.001 |
| Early Transition | 1.07 | 1.03-1.10 | ||
| Late Transition | 1.15 | 1.09-1.21 | ||
| Post Menopausal | 1.04 | 1.05-1.15 | ||
| Log FSH | Each Stage1 (Common slope) | 2.09 | 2.04-2.15 | <0.001 |
| Log Inhibin B (decreasing order) | Each Stage1 (Common slope) | 1.57 | 1.14- 2.15 | <0.001 |
| Log Estradiol | Late Premenopausal | 1.32 | 1.06-1.65 | 0.007 |
| Early Transition | 1.18 | 0.98-1.42 | ||
| Late Transition | 1.15 | 0.96-1.37 | ||
| Post Menopausal | 0.90 | 0.73-1.11 | ||
| Smoking | Each Stage1 (Common slope) | 1.30 | 1.28-1.32 | <0.001 |
| African American vs. Caucasian | Late Premenopausal | 1.32 | 1.06 - 1.64 | <0.001 |
The common slope assumes that the odds ratio of transition to the next menopausal stage is the same value at each transition.
To interpret these hormone changes clinically, Table 5 shows the mean observed value for each hormone at each menopausal stage. We also present the odds ratio with 95% confidence interval estimates for the mean change of the hormone level to each menopausal stage from the previous stage (back-transformed from the mean hormone changes on the log scale in the analysis). For example, the largest change in average FSH levels was comparing women in the late transition (stage 4), with an average of 24.78 miU/mL, to the early transition (stage 3), with an average of 10.38 miU/mL. Women experiencing this amount of change in FSH had an odds of transitioning from stage 3 to stage 4 of 1.90 (95% CI: 1.86-1.95). Average estradiol levels did not change dramatically between stages, and therefore the influence of estradiol on the odds of transition to the subsequent stage was relatively modest (odds ratios ranging from 1.01 in stages 2 and 3 to 1.05 for stages 4 and 5). Decreases in inhibin b resulted in odds ratios similar to the magnitude of changes in FSH.
Table 5.
Odds Ratio* for Transition by Hormone Level. Presented as observed mean (log mean±standard deviation).
| Estradiol | FSH | Inhibin B | ||||
|---|---|---|---|---|---|---|
| Observed Mean (log_mean±sd) | Odds Ratio* (95% CI)h | Observed Mean (log_mean±sd) | Odds Ratio* (95% CI) | Observed Mean (log_mean±sd) | Odds Ratio*1 (95% CI) | |
| Stage 1 | 38.09 (3.64±0.58) | ref | 7.69 (2.04±0.42) | ref | 69.41 4.24(0.67) | ref |
| Stage 2 | 39.25 (3.67±0.58) | 1.01 (1. 01-1.02) | 8.17 (2.10±0.50) | 1.05 (1.04-1.05) | 61.56 4.12(0.74) | 1.06 (1.00-1.10) |
| Stage 3 | 41.62 (3.73±0.81) | 1.01 (1. 00-1.01) | 10.38 (2.34±0.70) | 1.19 (1.19-1.20) | 40.04 3.69(0.80) | 1.21 (1.06-1.39) |
| Stage 4 | 33.45 (3.51±0.95) | 1.031 (0.99-1.07) | 24.78 (3.21±0.92) | 1.90 (1.86-1.95) | 20.29 3.01(0.53) | 1.36 (1.09-1.68) |
| Stage 5 | 20.70 (3.03±1.12) | 1.051 (0.95-1.16) | 44.26 (3.79±0.76) | 1.53 (1.51-1.56) | 16.78 2.82(0.49) | 1.09 (1.03-1.16) |
Adjusted Odds ratio (from Table 4) for the observed change in log-hormone levels from stage j-1 to j.
Odds ratio for the probability of transitioning from stage j-1 to j computed assuming a decrease in hormone values.
Increasing age was a significant predictor of entry into all but the earliest menopausal stage in multivariable analysis (P<0.001). As a predictor, the strongest association of age was with the Late Transition stage, with a 15% chance of entry into the Late Transition for each increased year of age (Table 4). This transition occurred more rapidly than transitions into other stages as implied by the odds ratios (OR=1.15 to the Late Transition compared to OR= 1.07 to the Early Transition and OR=1.04 to the Postmenopausal stage).
The effect of race remained significant for entry into the earliest transition stage in the multivariable analysis. African Americans were more likely to enter the earliest stage compared to Caucasians (OR=1.32, CI=1.06, 1.64; P<0.001), but entry into subsequent stages did not significantly differ between the two racial groups.
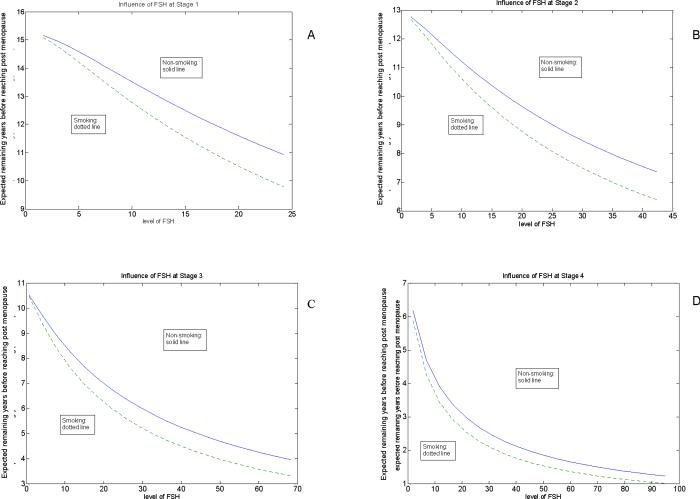
After adjusting for all risk factors in the multivariable model, the strongest predictors of entry into each menopausal stage were FSH levels, inhibin b levels and current smoking. We then examined the influence of FSH levels and smoking at each stage to estimate the predicted number of years to menopause (Figures 1A to 1D). Figure 1A shows that for women in Stage 1 (Premenopausal), non-smokers with an FSH level of 10 mIU/mL could expect to reach menopause in just under 14 years on average, while smokers could expect to reach menopause in just under 13 years. Non-smokers with an FSH level of 30 mIU/mL in Stages 2, 3, or 4 could expect to reach menopause in just under 9 years, 6 years, or 2 ½ years, respectively. Smokers consistently had a shorter time to menopause with estimates of just under 8 years in Stage 2, 5 years in Stage 3 and about 2 years in Stage 4.
Figure 1.
Influence of FSH and smoking on expected years to menopause at Stage 1 (A), Stage 2 (B), Stage 3 (C) and Stage 4 (D).
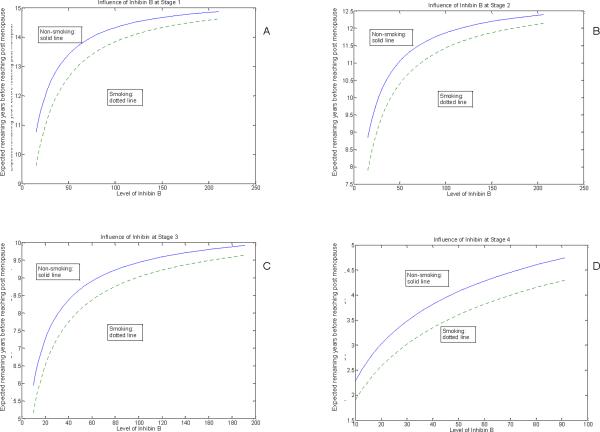
Figures 2A to 2D show the influence of inhibin b levels and smoking at each menopausal stage in estimates of the predicted number of years to menopause. The overall patterns of decreasing inhibin b are nearly identical to the FSH estimates, with the predicted time to menopause sightly shorter in the inhibin b estimates. Consistent with the FSH results, inhibin b levels indicate that smokers have a shorter time to menopause, with differences of approximately 1 to 1 ½ years compared to non-smokers.
Figure 2.
Influence of inhibin b and smoking on expected years to menopause at Stage 1 (A), Stage 2 (B), Stage 3 (C) and Stage 4 (D).
DISCUSSION
This study identified influential predictors of entry into each stage of the menopausal transition. The strongest predictors for the earliest transition stage, which was defined by >7 day shifts in cycle length, and all subsequent stages were increasing FSH levels, decreasing inhibin b levels and current smoking after adjusting for other risk factors. In contrast, estradiol levels and race were associated with entry only into the earliest stages, while BMI, alcohol use and age at menarche were not significant predictors of entry into menopausal stages after adjusting for other risk factors of the study.
While epidemiologic data have indicated that specific FSH cut points are not reliable predictors for the earliest transition stages, the present findings are consistent with a comprehensive study of endocrine features of menstrual cycles, where the earliest transition stages were associated with elevated FSH levels and erratic and often elevated levels of estradiol.13-15 These researchers then studied anti-mullerian hormone levels and concluded that an index of the FSH to inhibin b ratio together with AMH were the measures to clinically identify entry into the menopausal transition.14
This study indicates the major role of FSH in the estimated probabilities of entry into each transition stage in distinction to other associations of FSH levels that have been previously reported. FSH levels were associated with bleeding criteria proposed to identify the STRAW early transition stage and indicated that a persistent >6-day difference in cycle length was the earliest of the studied criteria.16 FSH levels predicted time to menopause, in contrast to the present study where FSH levels predict entry into each stage of the menopausal transition.17, 18 Examination of the rate of change in FSH levels demonstrated a rate shift at ages 40 and 42 years with major acceleration at age 45 years,19 findings that are consistent with our observations that the most rapid transition occurred in the late transition stage.
The associations of estradiol had different directions with different stages. Increased estradiol levels were significantly associated with entry into the earliest transition stage, while decreased estradiol levels were associated with entry into the postmenopausal stage. The different directions of the estradiol associations with menopausal stages suggest that there may be an interaction among the hormones, although this could not be determined in this study. A decrease in estradiol approximately 2 years before the final menstrual period has been consistently reported,20 but the causes of increased estradiol in the early transition are less well understood. A recent study of hormone patterns in the menopausal transition identified FSH rises in the luteal phase sufficient to recruit responsive follicles and a superimposition of follicular and luteal phases. These luteal out-of-phase (LOOP) events led to high and erratic estradiol levels in the menstrual phase and could result in the alternating normal, short and longer cycles that are observed in the early transition stage.21
Current smoking increased the likelihood of entry into each menopausal stage and has been consistently associated with an earlier age at menopause in other studies.22- 26 Some but not other studies demonstrated a dose response relationship between smoking and menopause,23, 26, 27 although whether smoking affects the timing of menopause through effects on estrogen metabolism or other mechanisms remains unclear.
Race was associated only with the earliest transition stage, with no racial differences in the transitions to subsequent stages in this study. It remains unclear whether an earlier entry into the transition results in an earlier menopause. One study reported an earlier age at menopause for African American compared to white women,23 another study reported a later menopause among African American women,26 while the SWAN study did not find a difference between African American and white women in age at menopause.22 Santoro et al reported that African American women had a greater likelihood of anovulatory cycles, greater progress to early perimenopause and greater progress to late perimenopause.28 These researchers also found a positive association of BMI with anovulatory cycles that ended with bleeding, although other reports indicate that high BMI is associated with increased age at menopause.25 We found a differential association of BMI only at entrance into the menopausal transition and not at later stages. This suggests that obesity may be associated with follicular dysfunction that alters the bleeding patterns that define menopausal stages rather than with diminished ovarian reserve.29, 30 Further studies are needed to clarify the associations between obesity, race and menopausal stages.
The menopausal stages in this study were adapted from STRAW stages, which are still in flux and require clinical validation. We divided the STRAW early transition stage (late premenopausal and early transition as defined above ) in order to detect significant associations and changes in the earliest stages of the menopausal transition and previously confirmed that significant and differential changes in FSH, inhibin b and LH levels could be observed in these earliest stages.5 We utilized the original 90-day amenorrhea criterion for entry into the late transition stage, but a recent study of 60-day and 90-day amenorrhea as markers of the STRAW stages demonstrated that 60 days amenorrhea was an acceptable criterion for entry into the late transition stage.31
Other possible limitations of the study should be considered. The menopausal stages were defined by menstrual bleeding patterns at each assessment period, but the statistical models assumed only forward transition through the stages. It is possible that menstrual cycle changes between assessment periods were missed, although time was not a variable in these analyses, and, if misclassification of menopausal stage occurred, it would not alter the associations of risk factors and the estimates of the likelihood of transition to the next menopausal stage. The hormone measures were obtained in the follicular phase when the measures are believed to be most reliable but do not address questions pertaining to the luteal phase or the dynamics of the full menstrual cycle. The behavioral and demographic risk factors were selected on the basis of evidence in previous studies, but other risk factors may be important. The current model assumes that time dependent covariates such as BMI and smoking are measured concurrently with menopausal stage, but we did not explicitly evaluate the impact of changes in these risk factors over time or how behavior modification might impact transition. Therefore, prediction from a longitudinal study such as this may not convert to practical clinical utility, and caution should be taken in using these data in counseling patients. While dropout over the 10-year follow-up period could bias results, we know of no indication that dropout influences entry into menopausal stages, which was the outcome variable of this study. All available data were included in the models, which assumed that missing data were missing at random and were non-differential with respect to the outcome. Our findings are from a population-based cohort of urban, generally healthy, African American and white women with no current hormone use and may not be generalizable to all menopausal women.
CONCLUSIONS
This prospective examination of menstrual bleeding patterns in late reproductive-age women indicates that changes in FSH, inhibin b and current smoking are the strongest predictors of entry into the earliest stages and all subsequent stages of the menopausal transition as defined by changes in bleeding patterns. Current smoking increased the likelihood of entry into each stage of the transition by approximately 30%. Estradiol levels and race were associated only with entry into the earliest transition stage. Further studies of the duration in each menopausal stage and the overall time to menopause from the earliest transition stage would strengthen these findings. Other studies of associations between the dynamic processes associated with menopause and health issues are needed to increase understanding of the role of reproductive aging in the health problems and symptoms of mid-life women.
Acknowledgments
Funding: This study was supported by grants from the National Institutes of Health, Bethesda, MD: #RO1 AG12745 (EWF), RR024134 (Clinical and Translational Research Center), RO1 CA84438 (WG), and T32 DK060455 (ZL).
APPENDIX 1
Those probabilities can be written in a matrix form, namely the transition probability matrix:
Covariates enter the model through those transition probabilities. Transition probabilities were modeled as where “z” stands for covariates. The form is similar as a logistic regression. While in logistic regression only transitions from one stage and in a given time interval can be modeled, here all the transitions can be modeled simultaneously and the time intervals are allowed to vary. If coming back are also allowed, the transition probabilities can be modeled as a proportional odds model, see [4].
The log-likelihood can be calculated as
here i stands for subject, j for visit, nij is the steps between two visits of a subject, and Si, j is the state of ith subject at jth visit. Note here all the observed transitions are assumed independent.
If the exact transition time is observed at the jth visit, then the corresponding likelihood contributed is
Now the likelihood is established, for a given data set, maximizing the likelihood over the parameter space gives out estimates and corresponding large sample variance. Inference can be made based on those estimates.
One further step is to restrict one covariate shares a common slope over different starting stages, and this assumption can be tested using likelihood ratio test. And one covariate can also be allowed to enter the model in some stages, but not in other stages.
REFERENCES
- 1.Pal L, Bevilacqua K, Zeitlian G, Shu J, Santoro N. Implications of diminished ovarian reserve (DOR) extend well beyond reproductive concerns. Menopause. 2008;15:1086–94. doi: 10.1097/gme.0b013e3181728467. [DOI] [PubMed] [Google Scholar]
- 2.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76:874–78. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 3.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007;110:230–40. doi: 10.1097/01.AOG.0000270153.59102.40. [DOI] [PubMed] [Google Scholar]
- 5.Freeman EW, Sammel MD, Gracia CR, et al. Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril. 2005;83:383–92. doi: 10.1016/j.fertnstert.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 6.Nelson DB, Sammel MD, Freeman EW, Liu L, Langan E, Gracia CR. Predicting participation in prospective studies of ovarian aging. Menopause. 2004;11:543–48. doi: 10.1097/01.gme.0000139770.14675.40. [DOI] [PubMed] [Google Scholar]
- 7.Gracia CR, Sammel MD, Freeman EW, et al. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12:128–35. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- 8.Pitteloud N, Dwyer AA, DeCruz S, et al. The relative role of gonadal sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab. 2008;93:2686–92. doi: 10.1210/jc.2007-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boepple PA, Hayes FJ, Dwyer AA, et al. Relative roles of inhibin b and sex steroids in feedback regulation of follicle-stimulating horjone in men across the full specrrum of seminiferous epithelium funciton. J Clin Endocrinol Metab. 2008;93:1809–14. doi: 10.1210/jc.2007-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall G, Jones RH. Multi-state models and diabetic retinopathy. Stat Med. 1995;14:1975–83. doi: 10.1002/sim.4780141804. [DOI] [PubMed] [Google Scholar]
- 11.Marshall G, Guo W. Ordinal discrete markov transition models, technical report. Department of Preventive Medicine and Biometrics, School of Medicine, University of Colorado Health Sciences Center; 1994. [Google Scholar]
- 12.Guo W, Marshall G. ORDMKV: a computer program fitting proportional odds model for multi-state Markov process. Comput Methods Programs Biomed. 1995;46:257–63. doi: 10.1016/0169-2607(95)01625-4. [DOI] [PubMed] [Google Scholar]
- 13.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92:3060–67. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 14.Robertson DM, Hale GE, Fraser IS, Hughes CL, Burger HG. A proposed classification system for menstrual cycles in the menopause transition based on changes in serum hormone profiles. Menopause. 2008;15:1139–44. doi: 10.1097/gme.0b013e3181735687. [DOI] [PubMed] [Google Scholar]
- 15.Randolph JF, Jr, Crawford S, Dennerstein L, et al. The value of follicle-stimulating hormone concentration and clinical findings as markers of the late menopausal transition. J Clin Endocrinol Metab. 2006;91:3034–40. doi: 10.1210/jc.2006-0243. [DOI] [PubMed] [Google Scholar]
- 16.Harlow DS, Mitchell ES, Crawford S, Nan B, Little R, Taffe J. The ReSTAGE collaboration: defining optimal bleeding criteria for onset of early menopausal transition. Fertil Steril. 2008;89:129–40. doi: 10.1016/j.fertnstert.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoro N, Brockwell S, Johnston J, et al. Helping midlife women predict the onset of the final menses: SWAN, the Study of Women's Health Across the Nation. Menopause. 2007;14:415–24. doi: 10.1097/gme.0b013e31802cc289. [DOI] [PubMed] [Google Scholar]
- 18.Taffe J, Dennerstein L. Time to the final menstrual period. Fertil Steril. 2002;78:397–403. doi: 10.1016/s0015-0282(02)03231-4. [DOI] [PubMed] [Google Scholar]
- 19.Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF., Jr Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab. 2008;93:3847–52. doi: 10.1210/jc.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burger HG, Dudley EC, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–30. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 21.Hale GE, Hughes CL, Burger HG, Robertson DM, Fraser IS. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16(1):50–9. doi: 10.1097/GME.0b013e31817ee0c2. [DOI] [PubMed] [Google Scholar]
- 22.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;53:865–74. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 23.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145:124–33. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 24.Fleming LE, Levis S, LeBlanc, et al. Earlier age at menopause, work, and tobacco smoke exposure. Menopause. 2008;15:1103–8. doi: 10.1097/gme.0b013e3181706292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol. 1998;51:1271–76. doi: 10.1016/s0895-4356(98)00119-x. [DOI] [PubMed] [Google Scholar]
- 26.Palmer JR, Rosenberg L, Wise LA, Horton NJ, Adams-Campbell LL. Onset of natural menopause in African American women. Am J Public Health. 2003;93:299–306. doi: 10.2105/ajph.93.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the Multiethnic Cohort Study. Am J Epidemiol. 2008;167:1287–94. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- 28.Santoro N, Crawford SL, Lasley WL, et al. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab. 2008;93:1711–21. doi: 10.1210/jc.2007-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartz AJ, Barboriak PN, Wong A, Katayama KP, Rimm AA. The association of obesity with infertility and related menstural abnormalities in women. Int J Obes. 1979;3:57–73. [PubMed] [Google Scholar]
- 30.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF., 3rd. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87:101–6. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 31.Harlow SD, Cain K, Crawford S, et al. Evaluation of four proposed bleeding criteria for the onset of late menopausal transition. J Clin Endocrinol Metab. 2006;91:3432–38. doi: 10.1210/jc.2005-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]