Abstract
Dietary supplements containing preparations of ginger roots/rhizomes (Zingiber officinale Roscoe) are being used by consumers, and clinical trials using ginger dietary supplements have been carried out to evaluate their anti-inflammatory or anti-emetic properties with inconsistent results. Chemical standardization of these products is needed for quality control and to facilitate the design of clinical trials and the evaluation of data from these studies. To address this issue, methods based on liquid chromatography-tandem mass spectrometry (LC-MS-MS) were developed for the detection, characterization and quantitative analysis of gingerol-related compounds in botanical dietary supplements containing ginger roots/rhizomes. During negative ion electrospray with collision induced-dissociation, the cleavage of the C4-C5 bond with a neutral loss of 194 u and benzylic cleavage leading to the neutral loss of 136 u were found to be class characteristic fragmentation patterns of the pharmacologically active gingerols or shogaols, respectively. Based on these results, an assay using LC-MS-MS with neutral loss scanning (loss of 194 u or 136 u) was developed that is suitable for the fingerprinting of ginger dietary supplements based on the selective detection of gingerols, shogaols, paradols, and gingerdiones. In addition, a quantitative assay based on LC-MS-MS with selected reaction monitoring was developed for the quantitative analysis of 6-gingerol, 8-gingerol, 10-gingerol, 6-shogaol, 8-shogaol, and 10-shogaol in ginger dietary supplements. After method validation, the quantities of these compounds in three commercially available ginger dietary supplements were determined. This assay showed excellent sensitivity, accuracy and precision and may be used to address the need for quality control and standardization of ginger dietary supplements.
Keywords: LC-MS-MS, constant neutral loss, gingerol, shogaol, ginger dietary supplements
INTRODUCTION
Botanical dietary supplements containing preparations of roots/rhizomes of ginger, Zingiber officinale Roscoe, are being used by consumers for the relief of nausea (1, 2) or for the relief of inflammation associated with arthritis (3)._Although the labels on these dietary supplements usually specify the amount (by weight) of ginger rhizome in each capsule, these products are rarely chemically standardized to specific active ingredients. Clinical trials evaluating various crude ginger extracts and powdered ginger rhizomes for the treatment of arthritis (3-6) or pregnancy-related nausea (2,7-9) have provided mixed results which might be due, at least in part, to the lack of standardization of the ginger dietary supplements being tested. Therefore, the standardization of active constituents in ginger products is an essential step in the process of ensuring the safety and in determining the efficacy of this or any botanical dietary supplement (6).
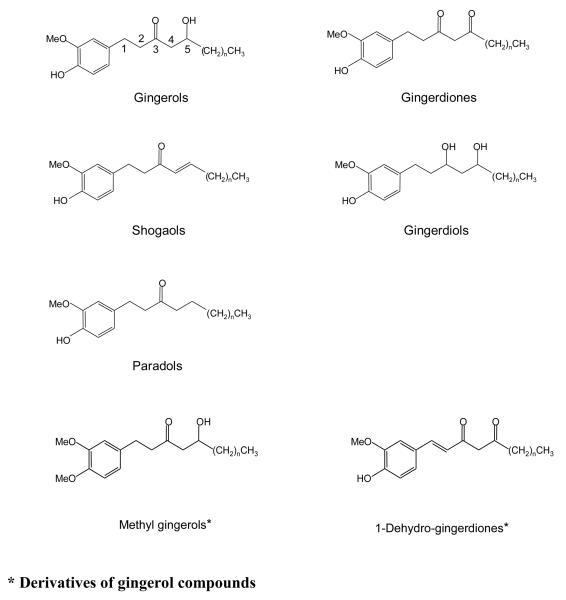
Sesquiterpene hydrocarbons, diarylheptanoids and gingerol-related compounds are major constituents of ginger (10-15); and among these, the gingerol-related compounds (Figure 1) are believed to exhibit anti-inflammatory and anti-nausea effects (11). Furthermore, the gingerol-related compounds can be further classified into five major groups as follows: gingerols, which are homologues of 1-(3-methoxy-4-hydroxyphenyl)-3-keto-5-hydroxyhexane and include the sub-group methyl gingerols (14); shogaols, which are dehydration products of the gingerols; paradols, β-ketone hydroxyl deoxygenation products of gingerols; gingerdiones, which are β-ketone hydroxyl dehydrogenation products of gingerols and include the sub-group 1-dehydro gingerdiones; and gingerdiols, which are ketone-reduction products of gingerols (10-14) (Figure 1). Each group of ginger homologues contains an unbranched alkyl chain of varying length and range in mass between 300 and 500 Da. For example, gingerol homologues include 4-, 6-, 8-, 10- and 12-gingerol, and shogaol homologues include 4-, 6-, 8-, 10- and 12-shogaol.
Figure 1.
Structures of the major gingerol-related compounds and their possible derivatives in ginger (the rhizome of Zingiber officinale). In each group of compounds, the carbon atom on the alkyl side chain next to the phenyl ring is defined as C1. Using gingerol as an example, if n=2, 4, 6, 8, or 10, then the compound is called 4-, 6-, 8-, 10-, or 12-gingerol.
Methods reported for the characterization or quantitative analysis of ginger constituents in botanical extracts have included gas chromatography-mass spectrometry (GC-MS) (12,13), high-performance thin layer chromatography (16), HPLC with UV absorbance detection (17), HPLC coupled with mass spectrometry ((LC-MS) (18,19), or HPLC coupled with tandem mass spectrometry (LC-MS-MS) (14,15,20). Due to the complexity of botanical extracts, many nongingerol-related compounds will be detected during HPLC-UV, high-performance thin layer chromatography, GC-MS, or LC-MS analysis, which may complicate data interpretation. Since thermal degradation of gingerol-related compounds has been reported to occur during GC-MS (12), liquid phase separations such as HPLC-based methods are preferred for the analysis of gingerols and related compounds. In addition, most quantitative methods address only standard commercially available compounds, such as 6-, 8-, 10-gingerol and 6-shogaol (3,17), and we are unaware of any validated methods for the quantitative analysis of other compounds such as 8-shogaol or 10-shogaol in botanical dietary supplements.
We report here that class-characteristic fragmentation patterns of 6-, 8-, 10-gingerol and 6-, 8-, 10-shogaol formed during product ion MS-MS using negative ion electrospray with collision-induced dissociation (CID) may be used during LC-MS-MS with constant neutral loss scanning to detect these and other gingerol-related compounds in extracts of ginger rhizomes or in ginger dietary supplements such as 12-gingerol, 12-shogaol, paradols, and gingerdiones. Using this approach, most gingerol-related species (except for gingerdiols) may be detected selectively and rapidly to fingerprint and authenticate a ginger extract or ginger-containing dietary supplement. In addition, to address the need for quality control and standardization of ginger dietary supplements, a sensitive, accurate and precise assay based on LC-MS-MS with negative ion electrospray and selected reaction monitoring (SRM) was developed and validated for the quantitative analysis of 6-, 8-, 10-gingerol and 6-, 8-, 10-shogaol. Finally, this quantitative assay was applied to the analysis of three commercial dietary supplements containing ginger rhizomes.
MATERIALS AND METHODS
Materials
Reference standards of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol were purchased from Chromadex (Santa Ana, CA). The internal standard, paeonol (2′-hydroxy-4′-methoxyacetophenone), was purchased from Sigma-Aldrich (St. Louis, MO). All solvents (HPLC grade) were purchased from Fisher Scientific (Fair Lawn, NJ). Sephadex LH-20 was purchased from Amersham Biosciences (Piscataway, NJ). Ginger dietary supplements for analysis were purchased from local pharmacies in Chicago, IL and included products from General Nutrition Corp. (Pittsburgh, PA), Nature's Resource Products (Mission Hills, CA) and Twinlab Division (American Fork, Utah).
Isolation and identification of 8-shogaol and 10-shogaol
A 400 g sample of powdered ginger root (General Nutrition Corp.) was divided into three parts and extracted three times with 1000 mL portions of methanol. Each extract was filtered, and evaporated to dryness under vacuum, resuspended in 250 mL water, and then extracted three times with 750 mL portions of chloroform. The combined extracts were filtered and evaporated to dryness under vacuum to yield ~25 g ginger oleoresin.
Approximately 24 g of ginger oleoresin was fractionated by using silica gel column chromatography with a 60 cm × 10 cm i.d. column packed with activated silica gel, 60-140 mesh, using a 40-step gradient from 100% hexane to 100% ethyl acetate. The fractions containing abundant shogaols (determined using electrospray mass spectrometry) were combined and evaporated to yield a single shogaol-rich fraction. 8-Shogaol and 10-shogaol were isolated from this shogaol fraction by using semi-preparative reversed-phase HPLC followed by Sephadex LH-20 column chromatography. Semi-preparative HPLC was carried out using a Shimadzu (Columbia, MD) preparative HPLC-8A system with an SPD-10A VP UV-VIS detector set at 210 nm and an Agilent (Palo Alto, CA) 250 mm × 9.4 mm i.d., 5 μm, Eclipse XDB-C18 column. The mobile phase consisted of a 20-min linear gradient from 50 to 90% acetonitrile in water containing 1.0% formic acid at a flow rate of 5 mL/min. Purification using Sephadex LH-20 column chromatography was carried out using a 25 cm × 5 cm column eluted with methanol. Purities of both compounds were greater than 97.5% by HPLC-UV.
The structures of 8-shogaol and 10-shogaol were verified by comparing their 1H-NMR, 13C-NMR and high resolution mass spectrometry with accurate mass measurement with those reported in the literature (21). NMR spectra were acquired using a Bruker (Rheinstettern, Germany) DPX-400 MHz spectrometer with deuteromethanol as solvent and tetramethylsilane as the internal standard. Accurate mass measurements were carried out using a Micromass (Manchester, UK) Q-TOF-2 mass spectrometer equipped with negative ion electrospray. The purities of both compounds were greater than 97.5% as determined by HPLC-UV.
Sample preparation
Standard stock solutions and quality control (QC) stock solutions were prepared separately by dissolving weighed amounts of 6-, 8- and 10-gingerol and 6-, 8- and 10-shogaol in methanol so that the final concentration of each gingerol and shogaol was 0.1 mg/mL. Calibration working solutions from 0.2 to 200 ng/mL of each standard were prepared containing paeonol as an internal standard at 10 ng/mL. Three QC solutions of 5, 10 and 100 ng/mL were prepared from the QC stock solution and were stored at −20 °C until use.
For each of the ginger dietary supplements to be tested, the contents of three capsules were weighed and dissolved in 15.0 mL of methanol. The mixture was sonicated at 25 °C for 60 min, cooled to room temperature and filtered through Whatman (San Francisco, CA) #40 filter paper. The residue on the filter paper was washed with 15 mL methanol three times, and the combined methanol extracts were evaporated to dryness under vacuum at 45 °C. After reconstitution in methanol, the extract was transferred to a 10-mL volumetric flask and made up to the volume with methanol. The sample solution was then filtered (0.2 μm pore size) to remove particulates and diluted 10-, 100- or 1000-fold with the introduction of the internal standard paeonol at a final concentration of 10 ng/mL. It should be noted that three samples of each commercial dietary supplement (each with same lot number) were analyzed and that each of the three different sets of diluted samples from each individual capsule was measured three times.
LC-MS-MS
For detection and identification of gingerol-related species, aliquots of 10 μL of each gingerol-related standard or ginger extract (100 μg/mL) were analyzed using a Micromass Quattro II triple quadrupole mass spectrometer equipped with negative ion electrospray and CID and interfaced to a Waters (Milford, MA) 2690 Alliance HPLC system. A Waters Xterra MS C18 column (100 mm × 2.1 mm i.d., 3.5 μm) was used for HPLC separations at a flow rate of 0.2 mL/min. The gradient solvent system consisted of a 45 min linear gradient from 20 to 80% acetonitrile in water, followed by 80 to 95% acetonitrile over 5 min, and then isocratic 95% acetonitrile for the next 20 min. The column was re-equilibrated at the initial mobile phase composition for at least 10 min between injections. The range m/z 80-500 was scanned during constant neutral loss analysis of ions formed by the loss of 194 u or 136 u, which were characteristic of gingerols and shogaols, respectively. During subsequent product ion analysis with CID of the deprotonated molecules of gingerol-related compounds, the range m/z 80-400 was scanned also at a rate of 1 scan/s. Instrument conditions were optimized for the MS-MS analysis of the deprotonated molecules of the standards 6-, 8- 10-gingerol and 6-, 8-, 10-shogaols. The negative ion electrospray source was operated at 140 °C with a desolvation temperature of 320 °C, a capillary voltage of −3.0 kV, and a cone voltage of −35 V. Argon at 1 × 10−3 mBar was used for CID at a collision energy of 15 eV.
For quantitative analysis, an Applied Biosystems (Foster City, CA) API 4000 triple quadrupole mass spectrometer equipped with a Shimadzu 10A VP HPLC system was used with CID and selected reaction monitoring (SRM) instead of the Micromass Quattro II instrument. The SRM transition pairs for each gingerol or shogaol were chosen according to the most abundant fragment ions observed during product ion analysis and are shown in Table 1. During SRM of gingerols, loss of a neutral molecule of 194 was monitored, [M-H-194]−, as well as the formation of the characteristic gingerol ion of m/z 193. As indicated in Table 1, shogaols formed only one abundant fragment ion, [M-H-136]−, the formation of which was monitored during quantitative analysis. Since three gingerol standards and three shogaol standards were available, SRM was used to monitor these six compounds with a dwell time of 50 ms per transition. The HPLC separations for quantitative analysis were carried out using an Agilent ZORBAX SB-C18 column (100 mm × 2.1 mm i.d., 3.5 μm) at a flow rate of 0.25 mL/min. The gingerol-related species were eluted from the column using a linear gradient from 40 to 85% acetonitrile in water over 12 min followed by a 3 min gradient from 85 to 100% acetonitrile. After 3 min at 100% acetonitrile, the column was re-equilibrated at 40% acetonitrile and 60% water for 5 min before the next injection. The negative ion electrospray source was operated at 350 °C and −4.2 kV. Nitrogen was used as the nebulizing gas, auxiliary gas, curtain gas, and collision gas at 20, 20, 20, and 4 psig, respectively, and as the collision gas at 2.28 × 1015 molecules/cm3. The declustering potential and collision energy were −80 V and 20 V, respectively, for all species except for the internal standard standard paeonol (m/z 165→ 122) which were −60 V and 10 eV, respectively. The mass spectrometer was operated at unit resolution corresponding to a full width of half maximum (FWHM) value of 0.7. Peaks in the chromatograms were integrated using Applied Biosystems Analyst 1.2 software, and a weighted 1/concentration2 linear regression was used to generate calibration curves from the standards.
Table 1.
LC-MS-MS Product Ion Tandem Mass Spectra with CID of the Deprotonated Molecules of the Gingerol-Related Species Detected During LC-MS-MS with Constant Neutral Loss Scanning in Figure 4.
| Retention time (min) |
[M-H]− | Major product ions (Relative abundance) |
Compound |
|---|---|---|---|
| 25.2 | 293 | 293.1 (45); 193.1 (60); 99.2 (100); 56.9 (28) | 6-gingerol |
| 33.2 | 321 | 320.9 (95); 193.1 (100);127.0 (88) | 8-gingerol |
| 40.8 | 349 | 349.1 (52); 193.1 (95); 155.1 (100); 179.1(15) | 10-gingerol |
| 47.7 | 377 | 377.2 (5); 192.8 (100); 182.9 (95); 177.8 (10) | 12-gingerol |
| 35.4 | 275 | 275.0 (50); 138.9 (100) | 6-shogaol |
| 42.9 | 303 | 303.1(20); 167.1 (100) | 8-shogaol |
| 50.2 | 331 | 331.0 (35); 195.0 (100) | 10-shogaol |
| 38.2 | 277 | 276.9 (40); 261.9 (10); 140.9 (100) | 6-paradol |
| 45.8 | 305 | 305.1 (30); 290.2 (20); 169.1 (100) | 8-paradol |
| 52.6 | 333 | 332.9 (60); 317.9 (10); 196.9 (100) | 10-paradol |
| 21.2 | 291 | 290.9 (20); 192.8 (40); 154.9 (100); 96.9 (20) | 6-dehydro-6-gingerol |
| 28.1 | 290.9 (20); 154.9 (100); 112.9 (15) | 6-hydroxy-6-shogaol* | |
| 38.1 | 290.9 (20); 154.9 (100); 112.9 (15) | 6-gingerdione* | |
| 28.6 | 319 | 319.1 (25); 192.8 (40); 182.9 (100);125.9 (20) | 6-dehydro-8-gingerol |
| 36.0 | 319.1 (25); 182.9 (100); 141.0 (15) | 6-hydroxy-8-shogaol* | |
| 45.6 | 319.1 (25); 182.9 (100); 141.0 (15) | 8-gingerdione* | |
| 37.0 | 347 | 347.0 (80); 210.9 (20);192.8 (85); 152.9 (100) | 6-dehydro-10-gingerol |
| 43.9 | 347.0 (98); 210.9 (100); 168.9 (25) | 6-hydroxy-10-shogaol* | |
| 52.4 | 347.0 (98); 210.9 (100); 168.9 (25) | 10-gingerdione* | |
Proposed compound assignments
RESULTS and DISCUSSION
Product ion tandem mass spectra of 6-, 8-, and 10-gingerol and 6-, 8- and 10-shogaol
After isolation from ginger oleoresin, the structures of purified 8-shogaol, (E)-1-(4-hydroxy-3-methoxyphenyl)dodec-4-en-3-one, and 10-shogaol, (E)-1-(4-hydroxy-3-methoxyphenyl)tetradec-4-en-3-one, were confirmed using 1H-NMR, 13C-NMR and accurate mass measurement using high resolution mass spectrometry. The NMR data were consistent with those in the literature (21). The accurate mass calculated for 8-shogaol, [M-H]− C19H27O3, was 303.1960, and the measured m/z value was 303.1958 (−0.7 ppm). For 10-shogaol, [M-H]− C21H31O3, the calculated mass was 331.2273, and the measured value was 331.2258 (−4.5 ppm).
Abundant deprotonated molecules of 6-, 8-, and 10-gingerol and 6-, 8- and 10-shogaol were formed during negative ion electrospray. As shown in Figure 2A, CID of the deprotonated molecule of 6-gingerol (m/z 293) produced abundant product ions of m/z 99 and 193 probably due to cleavage of the C4-C5 bond and minor fragment ions of m/z 178, 235 and 275. The base peak at m/z 99 was formed by neutral loss of 194 u (probably 4-(4-hydroxy-3-methoxyphenyl)-butan-2-one) with transfer of a hydrogen atom, and the ion of m/z 193 probably corresponded to the elimination of hexanal (100 u). The less abundant product ion of m/z 275 most likely corresponded to the elimination of a water molecule from the deprotonated molecule of m/z 293, and the product ion of m/z 178 was probably formed by loss of a methyl radical from the ion of m/z 193. Other minor product ions were observed at m/z 235 and m/z 57 most likely corresponded to hydroxyl-β-cleavage at C6-C7.
Figure 2.
Negative ion electrospray product ion tandem mass spectra of the deprotonated molecules of A) 6-gingerol; B) 8-gingerol; and C) 10-gingerol. The product ion of m/z 193 and neutral loss of 194 u were observed as abundant fragmentation patterns in the tandem mass spectra of all gingerols due to characteristic fragmentation at C4-C5.
The fragmentation patterns of the deprotonated molecules of 8-gingerol and 10-gingerol (m/z 321 and m/z 349, respectively) were similar to those of 6-gingerol and also appeared to be dominated by cleavage at the C4-C5 bond (Figure 2B and 2C). In particular, the product ions of m/z 127 and 155 were formed by the neutral loss of 194 u from the deprotonated molecules of 8-gingerol and 10-gingerol, respectively, and the other abundant product ion of m/z 193 in each tandem mass spectrum (Figure 2B and 2C) most likely resulted from cleavage at the C4-C5 bond with the loss of an aldehyde. It should be noted that these gingerol fragmentation patterns are similar to those reported by Jiang et al. (14). Therefore, CID of deprotonated gingerols appears to produce abundant cleavage at C4-C5 with either abundant neutral loss of 194 u or formation of the corresponding [M-H-193]− ion as class characteristic product ions.
The deprotonated molecules of 6-, 8- and 10-shogaol were observed at m/z 275, m/z 303 and m/z 331 during negative ion electrospray, and their product ion tandem mass spectra with CID are shown in Figure 3A, 3B and 3C, respectively. Unlike the fragmentation at C4-C5 occurring in gingerols, benzylic cleavage of the C1-C2 bond probably produced base peaks of m/z 139, 167 and 195 for 6-, 8- and 10-shogaol, respectively, and a class-characteristic neutral loss of 136 u (2-methoxy-4-methylenecyclohexa-2,5-dienone). The double bond at C4-C5 and the absence of a hydroxyl group at C5 of shogaols prevented fragmentation at this position during CID. Therefore, benzylic cleavage at C1-C2 instead of fragmentation at C4-C5 most likely distinguishes shogaols from gingerols during CID and tandem mass spectrometry.
Figure 3.
Negative ion electrospray product ion tandem mass spectra of the deprotonated molecules of A) 6-shogaol; B) 8-shogaol; and C) 10-shogaol. Neutral loss of 136 u due to benzylic cleavage of 6-, 8- and 10-shogaol resulted in the formation of the base peaks at m/z 139, 167 and 195, respectively.
Detection and identification of gingerol-related compounds in ginger dietary supplements
Gingerols, shogaols, gingerdiones, gingerdiols, and paradols share the same 1, 2, 4-trisubstituted benzene ring consisting of a 3-methoxy group, a 4-hydroxyl group and an unbranched 1-alkyl chain. Within each of these classes of ginger compounds, the unbranched alkyl chain varies in length from 4, 6, 8, 10, or 12 carbons. Based on the product ion tandem mass spectra of the gingerols and shogaols shown in Figures 2 and 3, the C4-C5 bond in the gingerols and the C1-C2 bond in the shogaols were probably the most labile bonds during negative ion electrospray MS-MS with CID; and changes in the length of the alkyl side chain did not appear to alter the lability of these bonds.
Although a reference standard of 12-gingerol was not available, its fragmentation pattern is predicted to be same as other gingerols. Similarly, the fragmentation of 12-shogaol, whose reference standard was also not available, should be same as the other shogaols. In consideration of the structures of paradols and gingerdiones, their fragmentation patterns could be expected to be similar to those of shogaols rather than gingerols. In particular, benzylic cleavage might be characteristic of paradols and gingerdiones. In summary, the product ions of m/z 193 and [M-H-194]− appear to be class characteristic of gingerols, and the formation of [M-H-136]−, corresponding to benzyl cleavage, is predicted to be class characteristic of of shogaols, paradols, and gingerdiones.
Based on these data for authentic standards of gingerols and shogaols, a LC-MS-MS assay with constant neutral loss scanning of 194 u or 136 u during negative ion electrospray was developed for the selective detection of gingerol-related compounds in ginger dietary supplements. The LC-MS-MS chromatograms of a chloroform extract of a ginger dietary supplement obtained using constant neutral loss scanning of 194 u and 136 u are shown in Figure 4A and 4B, respectively. During neutral loss scanning, the m/z values of the deprotonated molecules that fragmented to eliminate 194 u or 136 u were determined. Product ion scanning of these deprotonated molecules was then carried out using CID for further characterization, and the tandem mass spectra data are summarized in Table 1.
Figure 4.
Negative ion electrospray LC-MS-MS chromatograms of a chloroform extract of ginger roots/rhizomes using constant neutral loss analysis of A) 194 u showing the detection of gingerols; and B) 136 u showing the detection of shogaols, gingerdiones, hydroxyl-shogaols, dehydrogingerols, and paradols.
Detection of gingerols and 6-dehydro-10-gingerol during the constant neutral loss scanning of 194 u
As shown in Figure 4A, the peaks eluting at 25.2, 33.2 and 40.8 min were identified as 6-gingerol, 8-gingerol and 10-gingerol based on the comparison of retention times and tandem mass spectra with those of authentic standards. The peak eluting at 47.7 min was tentatively identified as 12-gingerol based on its m/z value at 377 and similar fragmentation pattern as those of other gingerols (Table 1). The peak eluting at 16.4 min (Figure 4) showed loss of 194 u from the deprotonated molecule of m/z 265, which corresponded to 4-gingerol. Unfortunately, the product ion tandem mass spectrum could not be acquired due to the low abundance of the deprotonated molecule. It should be noted that there is an abundant peak co-eluting with 4-gingerol, and its tandem mass spectrum could not be interpreted. The peak eluting at 37.0 min showed a deprotonated molecule of m/z 347, which is 2 amu less than the deprotonated molecule of 10-gingerol. By comparing the product ion tandem mass spectrum of this peak with that of 10-gingerol, the peak eluting at 37.0 min was tentatively identified as the dehydrogenation product of 10-gingerol. The abundant product ions of m/z 193 and m/z 153 suggested that dehydrogenation might have occurred at C6 on the alkyl side chain. According to Jolad et al. (12), this dehydrogenation product of 10-gingerol has been named 6-dehydro-10-gingerol.
Detection of shogaols and paradols during the constant neutral loss scanning of 136 u
Comparing the LC-MS-MS chromatograms of a ginger root/rhizome chloroform extract for constant neutral loss of 194 u (Figure 4A) to loss of 136 u (Figure 4B), more peaks were detected showing loss of 136 u. This suggests that most gingerol-related species exhibit benzyl cleavage during negative ion electrospray. The peaks eluting at 35.3, 42.9 and 50.1 min in Figure 4B were identified as 6-, 8- and 10-shogaol based on the comparison of the retention times and tandem mass spectra with those of authentic standards. The peaks eluting at 38.2, 45.8 and 52.5 min were assigned as 6-, 8- and 10-paradol based on their m/z values of 277, 305 and 333, respectively, and their product ion tandem mass spectra (Table 1). It was observed that all paradols have longer retention times than shogaols during reverse phase-HPLC; this is consistent with a literature report (14). In addition, the retention times of these compounds during reverse phase-HPLC increased with each additional –CH2CH2– unit, which is consistent with increasing hydrophobicity as the alkyl chain length increases. No peak corresponding to the deprotonated molecule of 12-shogaol or 12-paradol (m/z 359 and 361) was detected during constant neutral loss scanning of 136 u, possibly due to the low concentration of these compounds in or absence from the chloroform ginger extract.
Detection of gingerdiones during the constant neutral loss scanning of 136 u
According to Jolad et al. (12), there are two classes of homologues, namely 6-dehydro-gingerols and 6-hydroxy-shogaols, with the same molecular masses as gingerdiones in commercial extracts of ginger rhizomes. These were also observed in the present study. As indicated in Table 1, the peaks eluting at 37.0 min, 43.9 min and 52.5 min were detected at m/z 347, which corresponds to the same m/z value as deprotonated 10-gingerdione. The peak eluting at 37.0 min was detected during the neutral loss scanning of 194 u instead of 136 u, and its tandem mass spectrum was different from those of the peaks eluting at 43.9 min and 52.5 min. Therefore, the peak eluting at 37.0 was tentatively identified as 6-dehydro-10-gingerol.
The peaks eluting at 43.9 min and 52.5 min (Table 1 and Figure 4B) corresponded to isomeric compounds that formed deprotonated molecules of m/z 347 and abundant product ions of m/z 211 (base peak) corresponding to a loss of 136) and m/z 169. The deprotonated molecule of m/z 347 is 16 u higher than 10-shogaol. In addition, the base peaks of m/z 211 are 16 mass units higher than the base peak of 10-shogaol and correspond to a neutral loss of 136 u, which is class characteristic of shogaols, paradols and gingerdiones. Since the masses of these compounds preclude them from being gingerols or paradols, they are probably 6-hydroxy-10-shogaol and 10-gingerdione. The peak eluting at 43.9 min and prior to the 10-shogaol peak at 50.2 min (Figure 4B) was probably 6-hydroxy-10-shogaol, since hydroxylation of 10-shogaol should increase the polarity of the molecule and reduce its retention time during reverse phase HPLC. Therefore, the isomeric peak eluting at 52.5 min is proposed to be 10-gingerdione based on its mass, fragmentation pattern and HPLC retention time.
Using similar reasoning and based on mass, tandem mass spectra and HPLC retention times, the structures of several 6-dehydro-gingerols, 6-hydroxy-shogaols, 6-gingerdione, and 8-gingerdione were tentatively assigned as indicated in Table 1. It should be noted that not all members of the 6-dehydro-gingerol series shown in Table 1 fragmented during CID to eliminate 194 u and 136 u, which indicated that 6-dehydro-gingerol series should be detected in both LC-MS-MS chromatograms of the neutral loss scanning of 194 u and 136. However, 6-dehydro-6-gingerol and 6-dehydro-8-gingerol were only detected in the LC-MS-MS chromatogram of neutral loss scanning of 136 u (Figure 4B), and 6-dehydro-10-gingerol was only observed in LC-MS-MS with neutral loss scanning of 194 u (Figure 4A).
Peaks from gingerdiols were not detected in neutral loss scanning of either 194 u or 136 u, which indicated that gingerdiols did not fragment via C4-C5 cleavage or benzylic cleavage. This observation also suggested that the ketone group on C3 is critical for the negative ion electrospray CID fragmentation patterns discussed above for gingerol-related compounds. In addition, derivatives from gingerol-related compounds such as methyl gingerols or 1-dehydro-gingerdiones (Figure 1) were not detected using the current method. For methyl gingerols, cleavage at C4-C5 bond could be their characteristic fragmentation pattern. However, this homolog lacks the 2-methoxy-4-methylphenol moiety and thus can not be detected during the neutral loss scan of 194 u. For 1-dehydro-gingerdiones, the double bond at C1-C2 would possibly prevent the benzyl cleavage, and therefore, this homolog can not be detected during the neutral loss scan of 136 u.
In summary, despite the complexity of the composition of ginger extract, neutral loss scanning of 194 u or 136 u can filter out the isobaric compounds without the 4-(4-hydroxy-3-methoxyphenyl) butan-2-one moiety or 2-methoxy-4-methylphenol moiety to selectively detect most gingerol-related species (except for gingerdiols). When compared with the LC-MS in Q1 scan mode, the current LC-MS-MS constant neutral loss scanning method provided a rapid and simple procedure for peak identification. This neutral loss scanning method can also be extended and applied to identify other compounds such as diarylheptanoids since characteristic fragmentation patterns were also observed during positive or negative ion electrospray with CID (15).
Quantitative analysis of 6-, 8- 10-gingerol and 6-, 8- 10-shogaol in ginger dietary supplements
Since standards of 6-, 8- and 10-gingerol and 6-, 8- and 10-shogaol were available, standard curves were prepared, and the LC-MS-MS based method for the quantitative analysis of these compounds in ginger dietary supplements was validated. Although shogaols fragmented to form only one abundant product ion for SRM analysis, [M-H-136]− (Figure 3 and Figure 5), gingerols formed abundant [M-H-194]− ions as well as product ions of m/z 193 (Figure 2). The formation of either gingerol product ion was suitable for quantitative analysis using SRM (Figure 5). However, monitoring both species is useful for gingerol identification and for the detection of possible interference, since the ratio of both of these abundant gingerol product ions should be constant.
Figure 5.
Quantitative analysis of the most abundant gingerols and shogaols in a methanolic extract of the ginger dietary supplement B using negative ion electrospray LC-MS-MS with CID and SRM.
The linear regression coefficients of determination (R2) for the standard curves of all analytes exceeded 0.997 indicating excellent linearity. The limits of detection, defined as a signal-to-noise of 3:1, of 6-, 8- and 10-gingerol were 0.2, 0.2 and 0.6 ng/mL, respectively, and the limit of detection values for 6-, 8- and 10-shogaol were 0.5, 0.4, 0.2 ng/mL, respectively. The limits of quantitation, defined as a signal-to-noise of 10:1, were 0.5, 0.5 and 1.6 ng/mL for 6-, 8- and 10-gingerol, respectively, and 1.5, 1.3 and 0.6 ng/mL for 6-, 8-, and 10-shogaol, respectively.
The accuracy and precision of the method were evaluated by analyzing 6-, 8- and 10-gingerol and 6-, 8- and 10-shogaol in three sets of quality control samples (5, 10, 100 ng/mL each) on three consecutive days (n=3). For all analytes, the intra-day relative standard deviation (RSD) (%), relative error (RE) (%), and the inter-day RSD (%) values were less than 9.6%, 9.8% and 8.0%, respectively, and within acceptable limits (additional validation data are available in the on-line Supporting Information). The recoveries of the analytes were determined by spiking 0.1 mg of each compound into three sets of accurately weighed ginger dietary supplements. By comparing the concentrations of gingerols and shogaols between the spiked and non-spiked samples, the recovery values were found to range from 90.1% to 110.8%, and the corresponding RSD (%) values were < 6.7%. These recovery studies indicated that the extraction method was suitable for the quantitative analysis of the ginger dietary supplements.
Next, the levels of 6-, 8- and 10-gingerol and 6-, 8- and 10-shogaol were measured in three commercially available ginger dietary supplements using this LC-MS-MS quantitative assay. Negative ion electrospray LC-MS-MS chromatograms of these six gingerols and shogaols in a mixture of standards (50 ng/mL each) and in a methanolic extract of a ginger dietary supplement are shown in Figure 5. The quantities of the gingerols and shogaols in these supplements were calculated, and the results are shown in Table 2. 6-Gingerol and 6-shogaol were the most abundant among the analytes tested in all three products followed in decreasing order by 8-gingerol, 10-gingerol, 8-shogaol, and 10-shogaol. The ginger dietary supplement A and B were similar to each other in ginger composition and contained more gingerols and shogaols (by weight) than supplement C. The weight contents of gingerols and shogaols in capsules from the same lot of each product were found to be consistent.
Table 2.
Weight Content (%) of Gingerols and Shogaols in Ginger Dietary Supplements
| Commercial product |
Sample | 6- gingerol (wt%) |
8- gingerol (wt%) |
10- gingerol (wt%) |
6- shogaol (wt%) |
8- shogaol (wt%) |
10- shogaol (wt%) |
|---|---|---|---|---|---|---|---|
| Ginger Dietary Supplement A |
Meana | 0.2619 | 0.0482 | 0.0468 | 0.1685 | 0.0074 | 0.0015 |
| RSD%b | 8.8 | 6.1 | 4.3 | 6.0 | 7.5 | 6.7 | |
| Ginger Dietary Supplement B |
Mean | 0.3132 | 0.0621 | 0.0620 | 0.1891 | 0.0107 | 0.020 |
| RSD% | 8.8 | 6.1 | 4.3 | 6.0 | 7.5 | 6.7 | |
| Ginger Dietary Supplement C |
Mean | 0.1354 | 0.0220 | 0.0215 | 0.1085 | 0.0033 | 0.0006 |
| RSD% | 1.9 | 8.9 | 8.8 | 6.7 | 7.7 | 9.1 | |
Mean value was obtained by the analysis of three capsules of each commercial dietary supplement with same lot number.
SD % -- Relative Standard Deviation
The fragmentation patterns of the major gingerol-related compounds in ginger roots/rhizomes were investigated using negative ion electrospray tandem mass spectrometry with CID. The assay using LC-MS-MS with neutral loss scanning (loss of 194 u or 136 u) can be suitable for the fingerprinting of ginger dietary supplements based on the selective detection of gingerols, shogaols, paradols, and gingerdiones. Not limited to the detection of the gingerol-related compounds, the assay could be extended to the identification of other ginger constituents, such as diarylheptanoids, considering that characteristic fragmentation patterns may also exist for these compounds. The obtained results with respect to the characteristic fragmentation patterns of these gingerol-related compounds can be used for their future quantitative or metabolic transformation studies. In support of bioassays and clinical trials, the developed LC-MS-MS method for the quantitation of gingerols and shogaols would be suitable for routine quality control and standardization of ginger dietary supplements.
Supplementary Material
Negative electrospray LC-MS-MS chromatogram of the product ion scanning of the deprotonated molecules of A) m/z 377; B) m/z 331; C) m/z 277; and D) m/z 347. Tandem mass spectra of major peaks in Figure 1A, 1B, and 1C are inserted. Tandem mass spectra of the major peaks in Figure 1D are shown in Figure 2.
Negative electrospray LC-MS-MS product ion analysis of the deprotonated molecule of m/z 347. The tandem mass spectrum of the peak eluting at 36.7 min in Figure 1D is shown in Figure 2A, and the peak was tentatively identified as 6-dehydro-10-gingerol. The tandem mass spectra corresponding to the peaks eluting at 43.7 min and 52.3 min in Figure 1D were similar and are shown in Figure 2B. The peaks eluting at 43.7 min and 52.3 min were tentatively identified as 6-hydroxy-10-shogaol or 10-gingerdione, respectively.
Precision and Accuracy of LC-MS-MS Analysis of Gingerols and Shogaols.
ACKNOWLEDGEMENTS
Supported by research grant P50 AT000155 funded jointly by the Office of Dietary Supplements (ODS), the National Center for Complementary and Alternative Medicine (NCCAM), the Office for Research on Women's Health (ORWH) and the National Institute for General Medical Sciences (NIGMS).
Footnotes
This material is available free of charge via the internet at http://pubs.acs.org.
LITERATURE CITED
- 1.Boone SA, Shields KM. Treating pregnancy-related nausea and vomiting with ginger. Ann. Pharmacother. 2005;39:1710–1713. doi: 10.1345/aph.1G086. [DOI] [PubMed] [Google Scholar]
- 2.Gregory PJ, Sperry M, Wilson AF. Dietary supplements for osteoarthritis. Am. Fam. Physician. 2008;77:177–84. [PubMed] [Google Scholar]
- 3.Schwertner HA, Rios DC, Pascoe JE. Variation in concentration and labeling of ginger root dietary supplements. Obstet. Gynecol. 2006;107:1337–1343. doi: 10.1097/01.AOG.0000217697.33787.8c. [DOI] [PubMed] [Google Scholar]
- 4.Bliddal H, Rosetzsky A, Schlichting P, Weidner MS, Andersen LA, Ibfelt HH, Christensen K, Jensen ON, Barslev J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthritis Cartilage. 2000;8:9–12. doi: 10.1053/joca.1999.0264. [DOI] [PubMed] [Google Scholar]
- 5.Altman RD, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001;44:2461–2462. doi: 10.1002/1529-0131(200111)44:11<2531::aid-art433>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Wigler I, Grotto I, Caspi D, Yaron M. The effects of Zintona EC (a ginger extract) on symptomatic gonarthritis. Osteoarthritis Cartilage. 2003;11:783–789. doi: 10.1016/s1063-4584(03)00169-9. [DOI] [PubMed] [Google Scholar]
- 7.Keating A, Chez RA. Ginger syrup as an antiemetic in early pregnancy. Altern. Ther. Health Med. 2002;8:89–91. [PubMed] [Google Scholar]
- 8.Willetts KE, Ekangaki A, Eden JA. Effect of a ginger extract on pregnancy-induced nausea: a randomised controlled trial. Aust. N. Z. J. Obstet. Gynaecol. 2003;43:139–144. doi: 10.1046/j.0004-8666.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- 9.Borrelli F, Capasso R, Aviello G, Pittler MH, Izzo AA. Effectiveness and safety of ginger in the treatment of pregnancy-induced nausea and vomiting. Obstet. Gynecol. 2005;105:849–856. doi: 10.1097/01.AOG.0000154890.47642.23. [DOI] [PubMed] [Google Scholar]
- 10.Charles R, Garg SN, Kumar S. New gingerdione from the rhizomes of Zingiber officinale. Fitoterapia. 2000;71:716–718. doi: 10.1016/s0367-326x(00)00215-x. [DOI] [PubMed] [Google Scholar]
- 11.Afzal M, Al-Hadidi D, Menon M, Pesek J, Dhami MS. Ginger: an ethnomedical, chemical and pharmacological review. Drug Metabol. Drug Interact. 2001;18:159–190. doi: 10.1515/dmdi.2001.18.3-4.159. [DOI] [PubMed] [Google Scholar]
- 12.Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN. Commercially processed dry ginger (Zingiber officinale): composition and effects on LPS-stimulated PGE2 production. Phytochemistry. 2005;66:1614–1635. doi: 10.1016/j.phytochem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Jolad SD, Lantz RC, Solyom AM, Chen GJ, Bates RB, Timmermann BN. Fresh organically grown ginger (Zingiber officinale): composition and effects on LPS-induced PGE2 production. Phytochemistry. 2004;65:1937–1954. doi: 10.1016/j.phytochem.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Solyom AM, Timmermann BN, Gang DR. Characterization of gingerol-related compounds in ginger rhizome (Zingiber officinale Rosc.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:2957–2964. doi: 10.1002/rcm.2140. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Timmermann BN, Gang DR. Characterization and identification of diarylheptanoids in ginger (Zingiber officinale Rosc.) using high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:509–518. doi: 10.1002/rcm.2858. [DOI] [PubMed] [Google Scholar]
- 16.Rai S, Mukherjee K, Mal M, Wahile A, Saha BP, Mukherjee PK. Determination of 6-gingerol in ginger (Zingiber officinale) using high-performance thin-layer chromatography. J. Sep. Sci. 2006;29:2292–2295. doi: 10.1002/jssc.200600117. [DOI] [PubMed] [Google Scholar]
- 17.Schwertner HA, Rios DC. High-performance liquid chromatographic analysis of 6-gingerol, 8-gingerol, and 6-shogaol in ginger-containing dietary supplements, species, teas, and beverages. J. Chromatogr. B. 2007;856:41–47. doi: 10.1016/j.jchromb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 18.He X, Bernart MW, Lian L, Lin L. High-performance liquid chromatography-electrospray mass spectrometric analysis of pungent constituents of ginger. J. Chromatogr. A. 1998;796:327–334. [Google Scholar]
- 19.Li X, Lou Z, Zhang H, Zhao L, Wu H, Zhang G, Wu Y, Chai Y. Rapid LC-TOFMS separation and identification of diarylheptanoids and gingerol-related compounds in dried ginger. Chromatographia. 2009;69:531–536. [Google Scholar]
- 20.Jiang H, Somogyi A, Timmermann BN, Gang DR. Instrument dependence of electrospray ionization and tandem mass spectrometric fragmentation of the gingerols. Rapid Commun. Mass Spectrom. 2006;20:3089–3100. doi: 10.1002/rcm.2699. [DOI] [PubMed] [Google Scholar]
- 21.Fleming SA, Dyer CW, Eggington J. A convenient one-step gingerol synthesis. Syn. Commun. 1999;29:1933–1939. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Negative electrospray LC-MS-MS chromatogram of the product ion scanning of the deprotonated molecules of A) m/z 377; B) m/z 331; C) m/z 277; and D) m/z 347. Tandem mass spectra of major peaks in Figure 1A, 1B, and 1C are inserted. Tandem mass spectra of the major peaks in Figure 1D are shown in Figure 2.
Negative electrospray LC-MS-MS product ion analysis of the deprotonated molecule of m/z 347. The tandem mass spectrum of the peak eluting at 36.7 min in Figure 1D is shown in Figure 2A, and the peak was tentatively identified as 6-dehydro-10-gingerol. The tandem mass spectra corresponding to the peaks eluting at 43.7 min and 52.3 min in Figure 1D were similar and are shown in Figure 2B. The peaks eluting at 43.7 min and 52.3 min were tentatively identified as 6-hydroxy-10-shogaol or 10-gingerdione, respectively.
Precision and Accuracy of LC-MS-MS Analysis of Gingerols and Shogaols.