Abstract
The advent of HIV protease inhibitors has greatly extended the life span of AIDS patients. With an aging HIV+ population, the cardiometabolic side effects of these drugs are becoming increasingly important clinical concerns.
Objectives:
To test the hypothesis that inhibition of adipose lipolysis will retard atherogenic lesion development induced by the antiviral protease inhibitors.
Design:
LDLR null mice receiving ritonavir were compared with those receiving ritonavir plus lipolysis inhibitor acipimox or vehicle alone to determine how acipimox would affect ritonavir-induced atherogenesis. Intermittent high fat high cholesterol diet was used to facilitate optimal atheromatous lesion development.
Methods:
Drug effects were assessed as changes in aortic lesion score, plasma lipid and lipoprotein profile, body fat mass, and insulin-induced suppression of plasma fatty acid concentrations.
Results:
Ritonavir increased aortic lesions, in association with decreased body fat mass, impaired antilipolysis action of insulin, and increased proatherogenic plasma lipoproteins. All these adverse effects were attenuated by co-treatment with acipimox.
Conclusions:
Our results provide the first direct evidence that supports the hypothesis that dysregulation of adipose lipolysis is an important contributor to the proatherogenic role of selected HIV protease inhibitors.
Keywords: antiretroviral protease inhibitor, adipose tissue, insulin resistance, dyslipidemia, acipimox
Introduction
The advent of HIV protease inhibitors (PIs) such as ritonavir brought a remarkable transition in AIDS therapy. Together with selected nucleoside reverse transcriptase inhibitors (NRTIs), PIs greatly improved viral suppression and changed HIV/AIDS from a rapidly fatal disease to a controllable chronic illness. Substantially increased life expectancy of HIV-infected patients has shifted focus to drug-associated metabolic disorders, such as lipodystrophy, insulin resistance, and coronary artery disease 1-5. Some of these metabolic abnormalities have been reproduced in HIV-negative humans and animal models after administration of PIs 6-9, suggesting that a pro-atherogenic phenotype is more associated with antiviral therapy than with viral infection alone 10.
HAART patients with lipodystrophy generally display resistance to insulin regulation of lipolysis and glucose disposal 11-13. Direct binding of selected PIs to glucose transporter 4 (Glut4) acutely impairs insulin-stimulated glucose transport 14 . Repeated administration of PIs can cause sustained impairment of insulin signaling, resulting in failure to suppress adipose lipolysis and to promote lipogenesis during postprandial periods 15-16. These changes are predicted to reduce body fat mass and further exacerbate systemic insulin resistance, contributing to pro-atherogenic dyslipidemia and premature atherosclerosis. Indeed, HIV-infected patients with lipoatrophy often have elevated plasma fatty acids (FFA) concentrations and a more pro-atherogenic lipid profile 13, 17-19. This condition was found to be improved after inhibition of lipolysis by co-administration of acipimox 18-19; suggesting that lipolysis inhibitors may offer protection against HAART-induced premature atherosclerosis. For proof-of-concept, we tested how acipimox affects mouse aortic lesion development in LDLR null mice treated with ritonavir, a PI known to induce atherogenesis in mouse models 20.
Methods
To accelerate lesion development, mice were given an intermittent high fat high cholesterol diet (HFC) two days each week interspersed with standard chow. Ritonavir was provided orally twice daily (33 mg/kg/day), and acipimox was provided in drinking water (0.05% wt). For all other details, please see www.ahajournals.org.
Results
Ritonavir accelerates atherogenic lesion development: an effect that is attenuated by co-treatment with acipimox
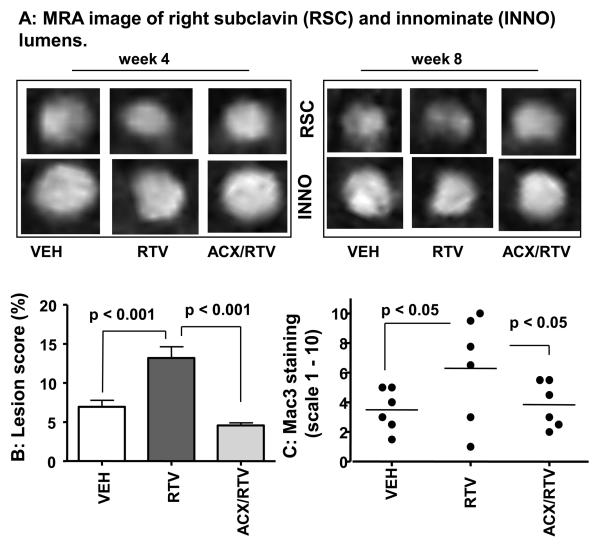
To determine the optimal treatment duration, we used high resolution magnetic resonance angiography (MRA) to longitudinally assess the progression of occlusive lesions in vivo in the innominate and right subclavian arteries (supplemental Figure IA, please see www.ahajournals.org). As shown in Figure 1A, LDLR −/− mice treated with ritonavir developed lumen asymmetry in these arteries at an earlier phase (week 4th) and had smaller luminal area for right subclavin (week 4th) and innominate (week 8th) than vehicle-treated mice. This effect was partially reversed by acipimox. In week 8, good concordance between occlusive vessels assessed by MRA and the presence of lipid-rich lesions was observed (Figure IB, www.ahajournals.org). Since en face analysis of the aortic vessel revealed only sparse lesions at this time (not shown), the experiment was extended by 6 more weeks.
Figure 1. Acipimox retards ritonavir-induced arterial lesions (please see supplemental data of Figures I-IV, www.ahajournals.org).
A. Cross-section of right subclavian and innominate arteries.
B. Aortic en face lesion score: the percentage of lesion-covered surface area.
C. Aortic-root lesion macrophage infiltration estimated by IHC staining for Mac3 (scaled 1 – 10).
After 14 weeks of treatment, atheromatous lesions were found throughout the aorta, with a greater density in the arch around the origin of its major branches (Figures II-III, www.ahajournals.org). Mice receiving ritonavir had an approximately 2-fold increase in the percentage of lesion-covered surface area (Figure 1B). This effect of ritonavir was reversed by acipimox. We also estimated macrophage levels within the aorta root as an index for atherosclerotic burden and also as an indicator of inflammatory responses. As shown Figure IV (please see www.ahajournals.org), lesions isolated from ritonavir-treated animals showed a remarkably greater staining intensity for Mac3, a macrophage marker commonly found in mouse atherosclerotic lesions 21. This effect was reversed by acipimox. The mean Mac3 staining intensity in the aortic root lesions (Figure 1C) closely correspond to the en face aortic artery lesion score (Figure 1B). This observation is in agreement with the in vitro findings that ritonavir stimulates macrophage activation 22. Further studies are required to determine whether increased macrophage infiltration is a direct effect of ritonavir on vascular wall inflammation or an indirect effect because of greater systemic lipid stress (see below).
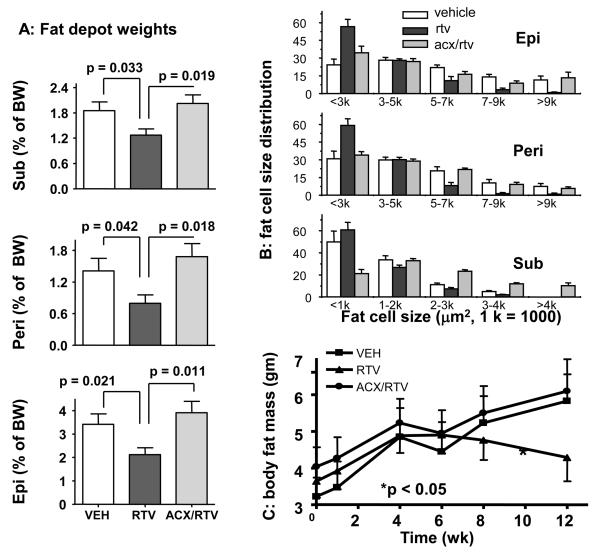
2. Ritonavir-associated loss of fat mass is blocked by co-treatment with acipimox
Consistent with previous studies that reported lipoatrophy in wild-type mice treated with protease inhibitors 7-8, we observed a significant reduction in all three fat depots measured in mice receiving ritonavir (Figure 2A). Analysis of fat cell size distribution revealed a shift towards smaller fat cells in ritonavir treated mice (Figure 2B). These changes were reversed by acipimox. NMR measurements show that body fat mass initially increased in all groups after HFC was introduced. However, 6 weeks later, mice receiving ritonavir began to lose fat mass, while mice receiving vehicle or ritonavir plus acipimox did not change (Figure 2C). By the end of treatment, whole body fat mass was lower in the ritonavir group (4.29 g vs 5.8 g, p = 0.031, compared to vehicle group) but not in the acipimox plus ritonavir group (5.8 g vs. 6.1 g, p = 0.791, compared to vehicle group).
Figure 2. Acipimox co-treatment blocks the ritonavir-induced lipoatrophy.
A. Fat mass from subtaneous (Sub) inguinal, epididymal (Epi) and perirenal (Peri) depots.
B. Fat cell size distribution for each depot (n= 3 for each treatment group).
C. A time-dependent change in body fat mass measured by NMR.
Ritonavir also reduced adipose mRNA expression of adipocyte transcription factor (PPARγ), fatty acid synthase (FAS), and acetyl CoA carboxylase (ACC), and the changes were reversed by acipimox (Figure V, please see www.ahajournals.org). It is of note that adipose tissue heme oxygenase-1 (HO-1), an enzyme that is critically involved in response to diverse types of cellular stress, was induced 2 fold by ritonavir (Figure V), in agreement with the previous in vitro findings 23-24. This effect was also reversed by acipimox (Figure V). Together, these results suggest that ritonavir induces profound adipose tissue malfunctions through a mechanism that is blocked by acipimox.
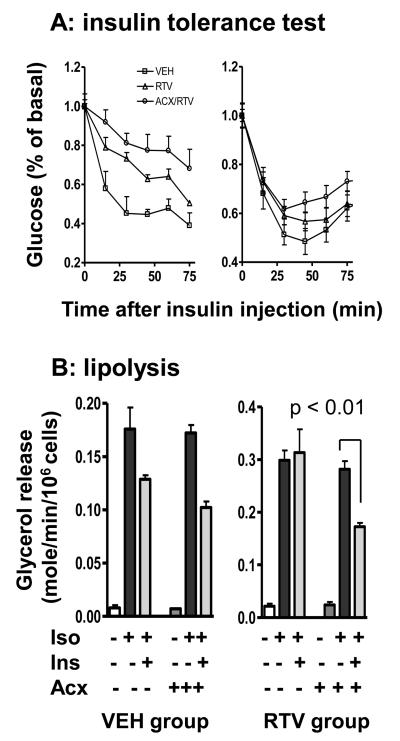
3. Ritonavir inhibits insulin-stimulated glucose disposal and the antilipolytic action of insulin: acipimox restores antilipolysis but not glucose disposal
Ritonavir induces acute and chronic insulin resistance. The acute effect is caused by direct interaction with Glut4 while the chronic effect is related to impaired insulin signaling 25-28. To determine the effects of acipimox on ritonavir-induced insulin resistance, we performed insulin tolerance tests in mice after 8 weeks of treatment. As expected, when mice were tested in 2 h after ritonavir dosing, there was a significant reduction in insulin-stimulated glucose disposal (Figure 3A, left panel), with AUC of 53.2 compared to 39.8 of the vehicle group (p <0.05, arbitrary unit). This effect was not reversed by acipimox (Figure 3A, AUC 61.8). We repeated the test in 16 h after the last ritonavir dosing. In this case, there was no difference in acute plasma glucose lowering within the first 15 min (Figure 3A, right panel). After 30 mins, the recovery of glucose concentration appears to be accelerated in ritonavir-treated mice with or without acipimox. These results suggest that the atheroprotective effect of acipimox is independent of immediate insulin-stimulated glucose disposal.
Figure 3. Acipimox rescues ritonavir-impaired insulin-induced antilipolysis but not insulin-stimulated glucose disposal.
A: insulin tolerance test in 2 h (left panel) and 16 h (right panel) after ritonavir dosing.
B. Lipolysis measured as glycerol release rate from fat cells treated with isoproterenol (iso, 0.5 μM) and insulin (1.7 nM).
HAART patients with lipodystrophy are reported to be less responsive to the antilipolytic action of insulin 13. Since lipolysis directly promotes hepatic VLDL secretion, we hypothesized that impairment of the anti-lipolytic action of insulin may be a relevant mechanism for ritonavir-induced atherogenesis. Accordingly, we compared glycerol release from adipocytes isolated from ritonavir and vehicle-treated mice, in response to a beta-agonist (isoproterenol, iso) and insulin. As shown in Figure 3B, insulin significantly suppressed iso-stimulated glycerol release in fat cells isolated from the vehicle group, but was not effective in fat cells isolated from the ritonavir group. Co-treatment with acipimox restored the cellular sensitivity to insulin (Figure 3B, right panel).
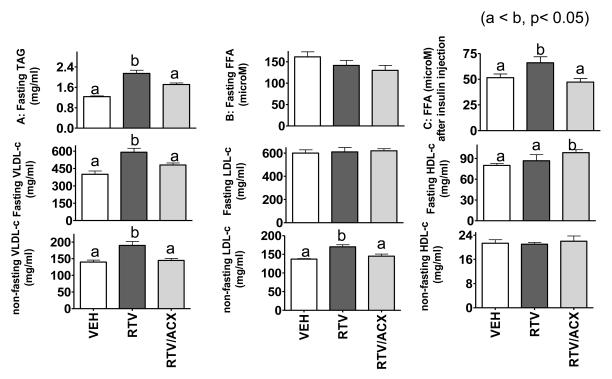
4. Acipimox ameliorates ritonavir-induced pro-atherogenic plasma lipid profile.
Increased plasma triglycerides (TAG) is typically found in HAART patients and PI treated rodents 7-8,19, 29. This was also found in the mice receiving ritonavir in our study, and the effect was largely reversed by acipimox (Figure 4A). However, no detectable differences in FFA was found among the three groups (Figure 4B). This was not too surprising because steady-state plasma FFA is regulated by multiple endogenous mechanisms minute-to-minute and the anti-lipolysis effect of acipimox can diminish after the drug (dissolved in drinking water) intake diminishes during the day. Even so, sustained lowering of plasma TAG implies that long-term acipimox intake still results in an overall better regulated lipid homeostasis 30-31. To determine whether acipimox improves insulin suppression of lipolysis in vivo, we measured plasma FFA after a moderate dose of insulin (0.6 U/kg, i.v.). As shown in Figure 4C, ritonavir dampened the insulin suppression of plasma FFA but the effect was reversed by acipimox.
Figure. 4. Acipimox attenuates ritonavir-induced dyslipidemia.
Upper panel: Fasting plasma triglycerides (A: TAG), fasting free fatty acids (B: FFA), and FFA measured post-insulin injection (C).
Middle panel: fasting cholesterol concentration associated with VLDL, LDL, and HDL.
Lower panel: non-fasting cholesterol concentration associated with VLDL, LDL, and HDL.
Dysregulation of plasma FFA and TAG predicts impaired VLDL metabolism. Indeed, as shown in Figure 4 (middle and lower panels), ritonavir increased VLDL-cholesterol. This effect was completely blocked by acipimox under non-fasting and partially blocked under fasting conditions. Note that anti-lipolysis effect of acipimox may decrease in the fasting period because the animals naturally drank less in the light cycle (8:00 am – 4:00 pm). In addition, ritonavir moderately increase non-fasting LDL-cholesterol. This effect was again reversed by acipimox. There was a slight but significant increase in fasting HDL-cholesterol in animals treated with acipimox plus ritonavir. This is likely secondary to reduced VLDL secretion, as acipimox alone does not affect HDL metabolism 31.
Discussion
This study provides the first in vivo evidence to support the hypothesis that the lipolysis inhibitor acipimox antagonizes the pro-atherogenic effect of HIV protease inhibitor ritonavir. The mechanistic framework of this hypothesis is shown in Figure VI (please see www.ahajournals.org). Briefly, this model envisions that HIV protease inhibitors impair insulin signaling for lipolysis regulation, causing increased FFA flux into the liver, which in turn increases VLDL output and promotes atherogenesis. This model predicts that atherogenesis will be attenuated if adipose lipolysis is suppressed by inhibitors that bypass the insulin signaling defects. While partial support for this hypothesis has come from previous studies 17-19, our work provides the first demonstration that ritonavir-induced atherogenesis is attenuated by acipimox, a lipolysis inhibitor that acts independent of insulin signaling.
Plasma lipoprotein concentrations largely depend on the coordinated metabolic regulation in liver, adipose tissue, and muscle. While many of the previous rodent studies documented strong drug effects on hepatic lipogenesis that can lead to VLDL hypersecretion, these findings were often confounded with high drug dosage and high-fat diet 8, 32-35. In this work, we show that, with a moderate drug dose and dietary fat content, ritonavir induced dyslipidemia and atherosclerosis in LDLR−/− mice without significantly damaging the liver itself (Figure VII, please see www.ahajournals.org). Based on the findings that acipimox did not correct the ritonavir effect on insulin tolerance test, we suggest that muscle is not a direct contributor either. Instead, our results suggest that the proatherogenic effect of ritonavir can be primarily explained by the drug effects on adipose tissue.
First, we showed that long-term ritonavir dosing caused fat tissue loss and resulted in smaller fat cell size; implying increased fat mobilization. We also presented the first in vivo evidence that ritonavir induced expression of a stress responsive gene HO-1 in adipose tissue. Because FFA stimulates stress response in preadipocytes36, we suggest that HO-1 may be induced as a result of increased adipocyte FFA release due to ritonavir-mediated insulin resistance. Second, we showed that ritonavir dampened in vivo plasma FFA response to insulin, which recapitulates the findings in HAART patients13. However, we also provided the first evidence that long-term drug treatment dampened ex vivo fat cells sensitivity to the antilipolysis action of insulin. Most importantly, we provided compelling evidence that acipimox largely reversed these ritonavir-induced fat tissue malfunctions, in parallel to its suppression on ritonavir-induced atherogenesis.
Increased VLDL is a hallmark of HAART associated dyslipidemia 10. Our results also show that VLDL-c is increased by ritonavir under both fasting and non-fasting conditions. Previous studies demonstrated that PI increases VLDL secretion without affecting VLDL catabolism 33, 37. If so, our results will suggest that insulin suppression of VLDL secretion is impaired by ritonavir under both fasting and non-fasting conditions. By increasing FFA flux into the liver, ritonavir may also increase secretion of TG-rich VLDL particles which may be more atherogenic than normal VLDL. Most importantly, we show that the effects of ritonavir on VLDL, fasting or non-fasting, were blocked by acipimox, in parallel to drug effects on aortic lesion development. This provides a clear link between adipose lipolysis and ritonavir-mediated dyslipidemia with drug-induced premature atherogenesis.
Finally, it is important to point out that while acipimox and niacin share the same receptor (GPR109A) pathway for lipolysis inhibition, niacin is, but acipimox is not, a precursor of NADH. For this reason, niacin is known to have profound atheroprotective effects beyond its function as a lipolysis inhibitor, most notably for its roles as a potent inhibitor for HDL catabolism and for hepatic lipid synthesis, as well as a general anti-oxidant 38-41. Such receptor-independent effects have not been reported for acipimox.
Overall, our observations have important clinical implications. We have recapitulated the major metabolic side effects of ritonavir in a mouse model and demonstrate that lipoatrophy, proatherogenic dyslipidemia, and accelerated atherogenesis can be alleviated by concomitant administration of a lipolysis inhibitor. Further studies to explore the therapeutic applications of lipolysis inhibitors in treating the metabolic complications of PIs are warranted.
Supplementary Material
Acknowledgements
(a) Source of funding: this research was supported by NIH grants RO1DK59261, 1RO1DK49296, R01DK64572 and 1R01DK078512-01. (b) Acknowledgements: all those who made substantial contributions to this work are listed as co-authors. (c) Disclosure: there is no conflict of interest among all the authors.
References
- 1.Sudano I, Spieker LE, Noll G, Corti R, Weber R, Lüscher TF. Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Thomas CM, Smart EJ. How HIV protease inhibitors promote atherosclerotic lesion formation. Curr Opin Lipidol. 2007;18:561–565. doi: 10.1097/MOL.0b013e3282ef604f. [DOI] [PubMed] [Google Scholar]
- 3.Calza L, Manfredi R, Pocaterra D, Chiodo F. Risk of premature atherosclerosis and ischemic heart disease associated with HIV infection and antiretroviral therapy. J Infect. 2008;57:16–32. doi: 10.1016/j.jinf.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Pao V, Lee GA, Grunfeld C. HIV therapy, metabolic syndrome, and cardiovascular risk. Curr Atheroscler Rep. 2008;10:61–70. doi: 10.1007/s11883-008-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein JH. Cardiovascular risks of antiretroviral therapy. N Engl J Med. 2007;356:1773–1775. doi: 10.1056/NEJMe078037. [DOI] [PubMed] [Google Scholar]
- 6.Shafran SD, Mashinter LD, Roberts SE. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med. 2005;6:421–425. doi: 10.1111/j.1468-1293.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 7.Goetzman ES, Tian L, Nagy TR, Gower BA, Schoeb TR, Elgavish A, Acosta EP, Saag MS, Wood PA. HIV protease inhibitor ritonavir induces lipoatrophy in male mice. AIDS Res Hum Retroviruses. 2003;19:1141–1150. doi: 10.1089/088922203771881248. [DOI] [PubMed] [Google Scholar]
- 8.Prot M, Heripret L, Cardot-Leccia N, Perrin C, Aouadi M, Lavrut T, Garraffo R, Dellamonica P, Durant J, Le Marchand-Brustel Y, Binétruy B. Long-term treatment with lopinavir-ritonavir induces a reduction in peripheral adipose depots in mice. Antimicrob Agents Chemother. 2006;50:3998–4004. doi: 10.1128/AAC.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee GA, Rao M, Mulligan K, Lo JC, Aweeka F, Schwarz JM, Schambelan M, Grunfeld C. Effects of ritonavir and amprenavir on insulin sensitivity in healthy volunteers. AIDS. 2007;21:2183–2190. doi: 10.1097/QAD.0b013e32826fbc54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddler SA, Li X, Otvos J, Post W, Palella F, Kingsley L, Visscher B, Jacobson LP, Sharrett AR. Multicenter AIDS Cohort Study. Antiretroviral therapy is associated with an atherogenic lipoprotein phenotype among HIV-1-infected men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2008;48:281–288. doi: 10.1097/QAI.0b013e31817bbbf0. [DOI] [PubMed] [Google Scholar]
- 11.Haugaard SB. Toxic metabolic syndrome associated with HAART. Expert Opin Drug Metab Toxicol. 2006;2:429–445. doi: 10.1517/17425255.2.3.429. [DOI] [PubMed] [Google Scholar]
- 12.van der Valk M, Bisschop PH, Romijn JA, Ackermans MT, Lange JM, Endert E, Reiss, PSH. Lipodystrophy in HIV-1-positive patients is associated with insulin resistance in multiple metabolic pathways. AIDS. 2001;15:2093–2100. doi: 10.1097/00002030-200111090-00004. [DOI] [PubMed] [Google Scholar]
- 13.Reeds DN, Mittendorfer B, Patterson BW, Powderly WG, Yarasheski KE, Klein S. Alterations in lipid kinetics in men with HIV-dyslipidemia. Am J Physiol Endocrinol Metab. 2003;285:E490–E497. doi: 10.1152/ajpendo.00118.2003. [DOI] [PubMed] [Google Scholar]
- 14.Hertel J, Struthers H, Horj CB, Hruz PW. A structural basis for the acute effects of HIV protease inhibitors on GLUT4 intrinsic activity. J Biol Chem. 2004;279:55147–55152. doi: 10.1074/jbc.M410826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Wijk JP, Cabezas MC, de Koning EJ, Rabelink TJ, van der Geest R, Hoepelman IM. In vivo evidence of impaired peripheral fatty acid trapping in patients with human immunodeficiency virus-associated lipodystrophy. J Clin Endocrinol Metab. 2005;90:3575–3582. doi: 10.1210/jc.2004-2343. [DOI] [PubMed] [Google Scholar]
- 16.Shahmanesh M, Das S, Stolinski M, Shojaee-Moradie F, Jackson NC, Jefferson W, Cramb R, Nightingale P, Umpleby AM. Antiretroviral treatment reduces very-low-density lipoprotein and intermediate-density lipoprotein apolipoprotein B fractional catabolic rate in human immunodeficiency virus-infected patients with mild dyslipidemia. J Clin Endocrinol Metab. 2005;90:755–760. doi: 10.1210/jc.2004-1273. [DOI] [PubMed] [Google Scholar]
- 17.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among human immunodeficiency virus-infected men receiving highly active antiretroviral therapy. Metabolism. 2002;51:1143–1147. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 18.Hadigan C, Rabe J, Meininger G, Aliabadi N, Breu J, Grinspoon S. Inhibition of lipolysis improves insulin sensitivity in protease inhibitor-treated HIV-infected men with fat redistribution. Am J Clin Nutr. 2003;77:490–494. doi: 10.1093/ajcn/77.2.490. [DOI] [PubMed] [Google Scholar]
- 19.Hadigan C, Liebau J, Torriani M, Andersen R, Grinspoon S. Improved triglycerides and insulin sensitivity with 3 months of acipimox in human immunodeficiency virus-infected patients with hypertriglyceridemia. J Clin Endocrinol Metab. 2006;91:4438–4444. doi: 10.1210/jc.2006-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dressman J, Kincer J, Matveev SV, Guo L, Greenberg RN, Guerin T, Meade D, Li XA, Zhu W, Uittenbogaard A, Wilson ME, Smart EJ. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J Clin Invest. 2003;111:389–397. doi: 10.1172/JCI16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in apoE-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 22.Lagathu C, Eustace B, Prot M, Frantz D, Gu Y, Bastard JP, Maachi M, Azoulay S, Briggs M, Caron M, Capeau J. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther. 2007;12:489–500. [PubMed] [Google Scholar]
- 23.Ben-Romano R, Rudich A, Etzion S, Potashnik R, Kagan E, Greenbaum U, Bashan N. Nelfinavir induces adipocyte insulin resistance through the induction of oxidative stress: differential protective effect of antioxidant agents. Antivir Ther. 2006;11:1051–1060. [PubMed] [Google Scholar]
- 24.Mühl H, Paulukat J, Höfler S, Hellmuth M, Franzen R, Pfeilschifter J. The HIV protease inhibitor ritonavir synergizes with butyrate for induction of apoptotic cell death and mediates expression of heme oxygenase-1 in DLD-1 colon carcinoma cells. Br J Pharmacol. 2004;143:890–898. doi: 10.1038/sj.bjp.0706023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Q, Hruz PW. Direct comparison of the acute in vivo effects of HIV protease inhibitors on peripheral glucose disposal. J Acquir Immune Defic Syndr. 2005;40:398–403. doi: 10.1097/01.qai.0000176654.97392.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudich A, Ben-Romano R, Etzion S, Bashan N. Cellular mechanisms of insulin resistance, lipodystrophy and atherosclerosis induced by HIV protease inhibitors. Acta Physiol Scand. 2005;183:75–88. doi: 10.1111/j.1365-201X.2004.01383.x. [DOI] [PubMed] [Google Scholar]
- 27.Hruz PW, Yan Q. Tipranavir without ritonavir does not acutely induce peripheral insulin resistance in a rodent model. J Acquir Immune Defic Syndr. 2006;43:624–625. doi: 10.1097/01.qai.0000245883.66509.b4. [DOI] [PubMed] [Google Scholar]
- 28.Hruz PW, Murata H, Qiu H, Mueckler M. Indinavir induces acute and reversible peripheral insulin resistance in rats. Diabetes. 2002;51:937–942. doi: 10.2337/diabetes.51.4.937. [DOI] [PubMed] [Google Scholar]
- 29.Riddler SA, Li X, Otvos J, Post W, Palella F, Kingsley L, Visscher B, Jacobson LP, Sharrett AR. Antiretroviral therapy is associated with an atherogenic lipoprotein phenotype among HIV-1-infected men in the multicenter AIDS cohort study. J. Acquir Immune Defic Syndr. 2008;48:281–288. doi: 10.1097/QAI.0b013e31817bbbf0. [DOI] [PubMed] [Google Scholar]
- 30.Hannah JS, Bodkin NL, Paidi MS, Anh-Le N, Howard BV, Hansen BC. Effects of Acipimox on the metabolism of free fatty acids and very low lipoprotein triglyceride. Acta Diabetol. 1995;32:279–283. doi: 10.1007/BF00576264. [DOI] [PubMed] [Google Scholar]
- 31.Davoren PM, Kelly W, Gries FA, Hubinger A, Whately-Smith C, Alberti KG. Long-term effects of a sustained-release preparation of acipimox on dyslipidemia and glucose metabolism in non-insulin-dependent diabetes mellitus. Metabolism. 1998;47:250–256. doi: 10.1016/s0026-0495(98)90252-9. [DOI] [PubMed] [Google Scholar]
- 32.Lenhard JM, Croom DK, Weiel JE, Winegar DA. HIV protease inhibitors stimulate hepatic triglyceride synthesis. Arterioscler Thromb Vasc Biol. 2000;20:2625–2629. doi: 10.1161/01.atv.20.12.2625. [DOI] [PubMed] [Google Scholar]
- 33.Riddle TM, Schildmeyer NM, Phan C, Fichtenbaum CJ, Hui DY. The HIV protease inhibitor ritonavir increases lipoprotein production and has no effect on lipoprotein clearance in mice. J Lipid Res. 2002;43:1458–1463. doi: 10.1194/jlr.m200129-jlr200. [DOI] [PubMed] [Google Scholar]
- 34.Lum PY, He YD, Slatter JG, Waring JF, Zelinsky N, Cavet G, Dai X, Fong O, Gum R, Jin L, Adamson GE, Roberts CJ, Olsen DB, Hazuda DJ, Ulrich RG. Gene expression profiling of rat liver reveals a mechanistic basis for ritonavir-induced hyperlipidemia. Genomics. 2007;90:464–473. doi: 10.1016/j.ygeno.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Riddle TM, Kuhel DG, Woollett LA, Fichtenbaum CJ, Hui DY. HIV protease inhibitor induces fatty acid and sterol biosynthesis in liver and adipose tissues due to the accumulation of activated sterol regulatory element-binding proteins in the nucleus. J Biol Chem. 2001;276:37514–37519. doi: 10.1074/jbc.M104557200. [DOI] [PubMed] [Google Scholar]
- 36.Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab. 2007;293:E576–E586. doi: 10.1152/ajpendo.00523.2006. [DOI] [PubMed] [Google Scholar]
- 37.Petit JM, Duong M, Florentin E, Duvillard L, Chavanet P, Brun JM, Portier H, Gambert P, Verges B. Increased VLDL-apoB and IDL-apoB production rates in nonlipodystrophic HIV-infected patients on a protease inhibitor-containing regimen: a stable isotope kinetic study. J Lipid Res. 2003;S44:1692–1697. doi: 10.1194/jlr.M300041-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005;258:94–114. doi: 10.1111/j.1365-2796.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 39.Airan-Javia SL, Wolf RL, Wolfe ML, Tadesse M, Mohler E, Reilly MP. Atheroprotective lipoprotein effects of a niacin-simvastatin combination compared to low- and high-dose simvastatin monotherapy. Am Heart J. 2009;157:687.e1–e8. doi: 10.1016/j.ahj.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamon-Fava S, Diffenderfer MR, Barrett PH, Buchsbaum A, Nyaku M, Horvath KV, Asztalos BF, Otokozawa S, Ai M, Matthan NR, Lichtenstein AH, Dolnikowski GG, Schaefer EJ. Extended-release niacin alters the metabolism of plasma apolipoprotein (Apo) A-I and ApoB-containing lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:1672–1678. doi: 10.1161/ATVBAHA.108.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101:20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.