Summary
Purpose
To investigate the impact of IFN-γ-mediated upregulation of MHC Class I expression on tumor-specific T cell cytotoxicity and T cell trafficking into neuroblastoma tumors in vivo.
Experimental design
Restoration of MHC Class I expression by IFN-γ treatment enhances killing of neuroblastoma cells. To understand the potential of this approach in vivo, we developed a novel model of neuroblastoma in which NOD/scid/IL2Rgammanull immunodeficient mice are engrafted with both human T cells and tumor cells.
Results
Here we show enhanced killing of neuroblastoma cells by patient-derived, tumor-specific T cells in vitro. In addition, IFN-γ treatment in vivo induces efficient upregulation of MHC Class I expression on neuroblastoma tumor cells, and this is accompanied by significantly enhanced infiltration of T cells into the tumor. In a pilot clinical trial in patients with high-risk neuroblastoma, we similarly observed augmented T cell trafficking into neuroblastoma nests in tumor biopsy specimens obtained from patients after 5 days of systemic IFN-γ therapy.
Conclusions
IFN-γ overcomes critical obstacles to the killing of human neuroblastoma cells by specific T cells. Together, these findings provide a rationale for the further testing of IFN-γ as an approach for improving the efficacy of T cell-based therapies for neuroblastoma and other MHC Class I-deficient malignancies. In addition, we describe a model that may expedite the pre-clinical screening of approaches aimed at augmenting T cell trafficking into human tumors.
Introduction
Although it is now well-established that cellular immunity plays a critical role in tumor immune surveillance, attempts to harness the potential of anti-tumor T cells for effective clinical responses have been mixed and often negative, with meaningful clinical results obtained only in a minority of patients treated on early-phase clinical trials. A significant contributing factor to the poor clinical responses is the presence of tumor evasion mechanisms in patients with pre-existing disease that reduce the effectiveness of anti-tumor immunotherapy. The development of strategies to overcome the influence of these immune evasion mechanisms may significantly improve the efficacy of immune-based therapies for cancer.
One of the most widely reported mechanisms by which solid tumor cells evade immune effectors is the down-regulation of major histocompatibility complex (MHC) Class I antigen expression (1). Given that CD8+ cytotoxic T lymphocytes (CTL) are a primary source of anti-tumor activity in the immune system (2, 3), the reduction of MHC Class I expression may profoundly effect immunosurveillance. This hypothesis is supported by the correlation between MHC Class I expression deficiency on tumor cells and prognosis for several malignancies (4–8). While the down-regulation of MHC Class I expression can be the result of loss of function of components of the Class I antigen presentation machinery (APM), in the majority of tumors the low Class I expression is caused by non-structural, transcriptional changes and can be restored by upregulating expression of the APM genes (9). Such up-regulation may be efficiently achieved by IFN-γ, which enhances expression of several components of the MHC Class I processing pathway, including proteasome sub-units, β2-microglobulin and TAP transporters (10–12). Restoration of MHC Class I expression by IFN-γ has been shown to increase CTL killing of human tumor cells and correlate with improved survival in mouse tumor models (13–15).
Neuroblastoma (NB) is the most frequent solid extra-cranial tumor in children (16). Despite aggressive conventional therapy, NB remains a major cause of cancer mortality in young children, with limited improvements in event-free survival seen over the past 2 decades. Thus, new treatment strategies are urgently needed. We have recently demonstrated that many NB patients harbor functional CTL specific for the tumor-associated antigen survivin at presentation (17). However, despite these cellular responses to NB, the presence of tumor-infiltrating CTL is rare, suggesting a block in T cell trafficking that may protect the tumor from CTL-mediated cytotoxicity. The profound deficiency in MHC Class I expression that is characteristic of NB cells may represent a fundamental mechanism for this immune evasion (18–20).
While it has been shown in a variety of models that IFN-γ treatment increases MHC Class I expression on human tumor cells, the impact of this increase on human T cell activity in vivo remains largely untested. In this study, we investigated the ability of IFN-γ treatment to achieve three goals that will be central to the design of better immunotherapeutics for NB: enhancement of MHC Class I expression on human NB cells in vivo, restoration of infiltration of human T cells into established human NB tumors, and augmentation of killing of NB cells by tumor-specific T cells.
Methods and Materials
Patient samples, normal T cells and cell lines
For preclinical studies, peripheral blood leukocytes were obtained by leukapheresis from patients after informed consent on IRB-approved Children's Hospital of Philadelphia protocols for the treatment of high risk NB (patients with stage 3 or 4 disease with unfavorable biology features). Leukapheresis was performed at the time of diagnosis. Paraffin-embedded tumor samples were obtained at the time of diagnosis. Human CD3+ T cells were derived from normal donor blood obtained by phlebotomy or leukapheresis following informed consent on protocols approved by the University of Pennsylvania IRB. CD3+ T cells that had undergone co-stimulated, activated expansion in culture using anti-CD3/CD28 beads (21) were kindly provided by Dr. Bruce Levine (University of Pennsylvania, Philadelphia, PA). The NB cell lines SY5Y, NLF and CHP-134 were obtained from Dr. Garrett Brodeur (Children's Hospital of Philadelphia), the NSJ3 line was obtained from Dr. Richard Carroll (University of Pennsylvania). Other NB lines were from ATCC (Manassas, VA). All cell lines were maintained in RPMI supplemented with 10% fetal bovine serum.
Clinical trial of IFN-γ in neuroblastoma
In a previously reported clinical trial (22), patients with relapsed NB were enrolled after informed consent on the clinical protocol NCI 90-C-0210 with approval of the Institutional Review Board of the NCI to investigate the safety and immunological impact of IFN-γ in patients. Patients were treated subcutaneously every day for 5 days with recombinant human IFN-γ (0.1 mg/m2 per day). Tumor biopsies were obtained prior to treatment and immediately, or up to 4 days after the last day of INF-γ therapy, and were evaluated by immunohistochemistry.
IFN-γ treatment of NB cells
For in vitro studies, NB cell lines were incubated in the presence or absence of 100U/ml human IFN-γ (Actimmune, InterMune, Brisbane, CA) for 48 hours prior to analysis by flow cytometry. For in vivo studies, NOD/scid/IL2Rgammanull (NOG) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen-free conditions in micro-isolators. All experiments were performed in accordance with protocols approved by the University of Pennsylvania IACUC. Mice were injected subcutaneously in the flank with 5×106 NB tumor cells in Matrigel (BD Biosciences, San Jose, CA). Tumor growth was monitored and recombinant human IFN-γ injections were initiated when tumors measured >200mm3. Mice were injected with 30,000 IU IFN-γ subcutaneously every day for 3 days in peri-tumoral region and tumors were harvested on day 4.
Human T cell tumor infiltration assay
NOG mice were first injected subcutaneously in the flank with 5×106 SY5Y tumor cells. One week later, mice were injected via the lateral tail vein with 50×106 human CD3+ T cells. Mice were then monitored weekly for T cell engraftment by flow cytometric detection of human CD45+/CD3+ cells in mouse peripheral blood. Mice with SY5Y tumors of >100mm3 in which peripheral T cells were detected were randomized to PBS or IFN-γ experimental arms. Treatment consisted of a regimen of 3 days of treatment (30,000 IU IFN-γ or PBS) followed by 3 days rest, repeated three times. Upon completion of treatment, the mice were sacrificed and spleen and tumor harvested for analysis.
Flow cytometry
Single cell suspensions were prepared from cell cultures, spleens and tumors by passage through a 40μM filter. For analysis of cell surface molecules, all samples were labeled with directly conjugated fluorescent antibodies at 4°C for 20 minutes. The samples were then washed and resuspended in 1.5% paraformaldehyde and analyzed within 24 hours. Antibodies to human CD3, CD4, CD8, CD45 and HLA-A,-B,-C were obtained from BD Biosciences. The relevant labeled isotype control antibodies were included in all experiments.
Expansion and evaluation of survivin-specitifc T cells
Patient-derived CD40-activated B cells lines were established and RNA electroporation was performed as previously described (17, 23). Survivin mRNA and eGFP mRNA was used at 2–5 μg/sample. Electroporated CD40-B were washed in T cell media (RPMI with 10% human AB serum, 2mM glutamine, 20 mM HEPES, and 15 ug/ml gentamicin) and plated at 5×105 cells/well with 2×106 PBMC/well in 24-well plates. Cultures were supplemented with 500 U/ml rhIL-4 (R&D Systems, Minneapolis, MN) and 10 ng/ml rhIL-7 (Sigma, St. Louis, MO) on day 1 and 20 U/ml IL-2 (Chiron, Emeryville, CA) on days 2 and 5. T cell cultures were restimulated with RNA-transfected CD40-B on day 7.
Chromium release assays were performed as previously described (23). Standard deviation was <5%. Target cells were evaluated for HLA-A2 and MHC class I expression by FACS analysis (BB7.2, Dako NorthAmerica, Carpinteria, CA; and anti-HLA-ABC, BD Biosciences). In some cases NB cells were pretreated with 100 IU/ml rhIFN-γ (R&D systems) for 48 hours.
Immunohistochemistry
For immunoperoxidase studies of primary human tissue, paraffin sections of formalin-fixed tissue samples were used. Sections were deparaffinized and antigen retrieval performed using heat-induced epitope retrieval. Sections were incubated with polyclonal antibody to CD3 (1:400; Dako NorthAmerica), or CD8 (1:100; C8/144B Dako NorthAmerica) or mAb specific for MHC class I (1:200, clone HC-10, kind gift of Dr. Soldano Ferrone, University of Pittsburgh). Sections were incubated with peroxidase-labeled secondary antibodies and signal localized using 3,3'-diaminobenzidine tetrahydrochloride as the chromogen. Hematoxylin was used as a counterstain. For immunoperoxidase studies of engrafted tissue from NOG mice, paraformaldehyde-fixed paraffin embedded tumor sections were incubated with antibody specific to human CD3 (1:400; Dako NorthAmerica), or human CD8 (1:100; Novocastra, Bannockburn, IL). Sections were incubated with peroxidase-labeled secondary antibodies and signal localized using 3,3'-diaminobenzidine tetrahydrochloride as the chromogen. Hematoxylin was used as a counterstain.
For dual CD3 and Ki67 immunofluorescence staining, tumor sections were incubated with antibody to CD3, followed by Texas red goat anti-rabbit IgG (1:200, Invitrogen). Proliferating cells were recognized by a mouse anti-human Ki67 Ab (1:50, Dako NorthAmerica), followed by a biotinylated anti-mouse IgG (1:250, Vector labs), and FITC-streptavidin (1:300, Vector labs). Sections were counterstained with hematoxylin, and 4',6'-diamidino-2-phenylindole (DAPI). Multispectral images (at 10 nm spectral resolution) of stained slides were captured using a multispectral camera (Nuance®, CRi Inc., Woburn, MA) mounted on an Olympia epifluorescence microscope. Nuance software was used to unmix different fluorophores in the data cube, separating each into a different channel based on reference spectra of the pure fluorophore. Image data including individual stain channels were entered into FARSIGHT software* (Rensselaer Polytechnic Institute, Troy, NY), which segmented individual nuclei using the DAPI channel, typed cells by their association/non-association with CD3 staining and associated nuclei with Ki67 staining. Eight 400x fields were analyzed for each tumor.
Statistical analysis
Comparisons between experimental groups were performed using unpaired t-tests, assuming equal standard deviations, and significance of p < 0.05. Two-tailed p values are shown.
Results
MHC Class I expression on NB tumors
To evaluate expression of MHC Class I in NB tumors, we performed immunohistochemistry for MHC class I expression. In diagnostic tumor biopsies from 26 patients presenting with high risk NB, we found that NB tumor cells in all cases were negative for MHC class I, consistent with previous observations (18–20). Two representative patients are shown in Figure 1. MHC class I expression was readily detectable on benign cells, including lymphocytes, macrophages, and endothelial, mesothelial, and epithelial (e.g. adrenal) cells (Figure 1). Rare, large MHC class I-positive cells within the tumor appeared to be tumor-associated macrophages.
Figure 1.
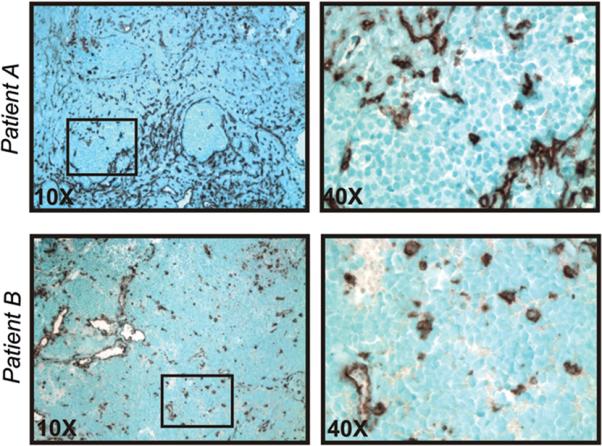
MHC class I expression in NB tumors. Paraffin sections of high-risk NB tumors at diagnosis were stained with anti-MHC class I mAb. Results for Patient A (stroma-rich tumor) and Patient B (stroma-poor tumor) are shown. Black boxes in 10x panels (left) indicate areas of MHC class I-negative tumor nests (magnified in right panels); certain stromal elements, blood vessels, and tumor-associated macrophages are MHC class I-positive. Similar results were obtained for a total of 26 patients with HRNBL.
Enhanced T cell killing of IFN-γ treated neuroblastoma cells
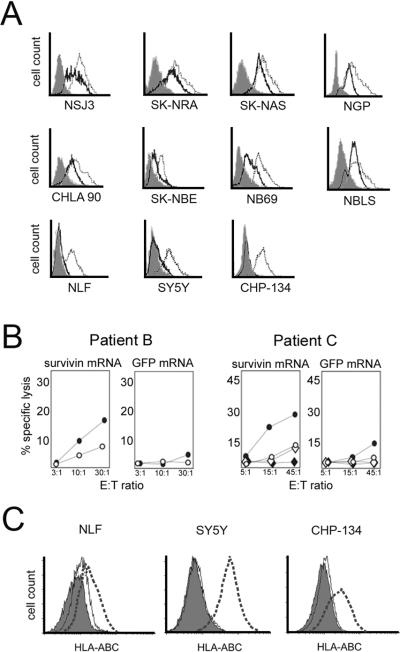
To determine whether IFN-γ upregulation of MHC class I expression on NB cells enhances specific CTL responses, we first screened a panel of NB cell lines for MHC Class I expression. In contrast to primary tumors, the majority of NB cell lines that we examined (8/11, 73%) were found by flow cytometry to express medium or high levels of MHC class I (Figure 2A). Three of 11 NBL cell lines (NLF, SY5Y, and CHP-134) were negative for MHC class I by this analysis, but 48 hrs of treatment with IFN-γ in vitro upregulated MHC class I expression by each cell line. IFN-γ further upregulated MHC class I expression on MHC class I-positive NBL lines (Figure 2A).
Figure 2.
IFN-γ-mediated activities in vitro. A) Eleven NB cell lines were tested for expression of MHC class I by flow cytometry. Solid black lines, anti-MHC class I on untreated cells; gray lines, anti-MHC class I on cells treated with IFN-γ (100 U/ml for 48 hrs); filled histograms, isotype control antibody. B) Diagnostic PBMC from 2 HLA-A2+ patients were stimulated with CD40-B cells loaded with survivin mRNA or GFP mRNA, and analyzed after 14 days in culture. Tumor targets were MHC class I negative NLF cells (circles) or MHC class I negative SY5Y (diamonds). Tumor targets were either untreated (open symbols) or treated with IFN-γ (filled symbols). Both cell lines upregulated MHC class I following IFN-γ but only NLF cells were HLA-A2+. Both cell lines were survivin positive by RT-PCR. C) Three MHC Class I negative NB cell lines were tested for upregulation of expression of MHC class I by IFN-γ in vivo. NOG mice bearing established NB cell line tumors were injected subcutaneously adjacent to the tumor with recombinant human IFN-γ or PBS daily for 3 injections (30,000 IU/injection) and tumors harvested on day 4. Single cell suspensions were prepared and examined by flow cytometry for expression of HLA-A,-B,-C. Solid black lines, anti-MHC class I on untreated cells; dashed lines, anti-MHC class I on cells treated with IFN-γ; filled histogram, isotype control antibody on untreated cells; solid gray lines, isotype control antibody with IFN-γ.
Using MHC class I negative cell lines as targets, we then compared the cytolytic activity of survivin-specific CTL from two NB patients against untreated NB tumor cells or NB cells treated with IFN-γ in vitro. For targets, we used the MHC class I negative NB lines, NLF and SY5Y, both of which upregulated MHC class I after 48hrs of treatment with IFN-γ. NLF cells express HLA-A2 but SY5Y cells are HLA-A2-negative; both cell lines express survivin (data not shown). HLA-A2+ patient T cells stimulated with survivin mRNA demonstrated minimal lysis of untreated NLF cells, but efficiently lysed NLF following treatment with IFN-γ and upregulation of MHC class I (Figure 2B). These T cells failed to lyse the HLA-A2-negative SY5Y, either untreated or after IFN-γ treatment. Patient T cells stimulated with GFP mRNA demonstrated only minimal lysis of these tumor targets (Figure 2B). These data support the notion that survivin-expressing NB cells may escape immunosurveillance by failing to express MHC class I, but that class I expression and susceptibility to T cell lysis may be restored by exposure to inflammatory cytokines.
To investigate the ability of IFN-γ to enhance MHC Class I expression in vivo, we established explants of human NB tumor cells in immunodeficient NOG mice. This highly immunodeficient mouse strain (T cell, B cell and NK cell deficient) efficiently accepts human tumor xenografts and also has the potential to allow the engraftment and expansion of normal human T cells. NOG mice were injected with MHC class I-negative SY5Y, NLF or CHP-134 NB tumor cells, and when tumors measured >200 mm3, recombinant human IFN-γ or PBS was injected subcutaneously around the tumor bed daily for 3 days. On day 4, the tumors were harvested, processed to single cell suspensions and evaluated by flow cytometry for expression of HLA-A,-B,-C. While no differences in morphology were observed for tumors from PBS- and IFN-γ-treated mice, MHC class I expression was markedly elevated on all 3 cell line tumors after IFN- γ treatment compared to tumors from mice given PBS (Figure 2C).
T cell trafficking to tumors in IFN-γ treated mice
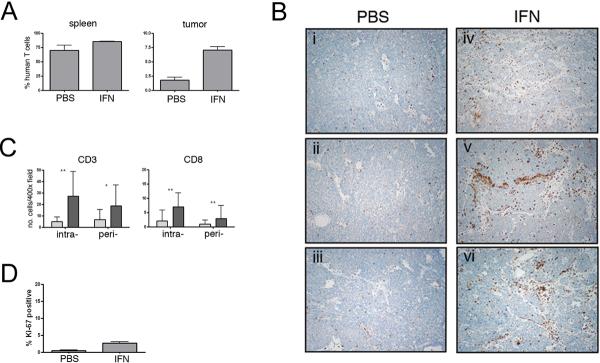
Given the ability of IFN-γ to efficiently enhance MHC Class I expression on NB tumors in vivo, we hypothesized that the use of this cytokine may increase the infiltration of tumors by T cells recognizing the newly expressed MHC Class I antigens. To test this hypothesis we established explants of SY5Y tumors subcutaneously in NOG mice. One week later, the mice were given 50 × 106 in vitro expanded allogeneic human CD3+ T cells from a healthy donor. Mice were then followed for T cell engraftment, measured by the presence of human CD45+/CD3+ cells in peripheral blood, and tumor size. Mice that achieved detectable peripheral human T cells and tumors of >100 mm3 were then treated with PBS or IFN-γ for 18 days on a thrice repeated regimen of 3 days of treatment followed by 3 days rest. Upon completion of treatment, mice were sacrificed and spleen and tumor removed for analysis. There was no significant difference in the sizes of tumors in the two groups at the completion of treatment (data not shown). While all mice achieved peripherally detectable human T cells, a more accurate measure of systemic engraftment levels was obtained by flow cytometric analysis of the spleen from each animal, showing very high levels of T cell engraftment in the NOG animals. Of the 8 animals used in the study, 3 PBS-treated mice and 4 IFN-γ-treated mice achieved >50% human T cells in the spleen, with no significant difference in T cell engraftment levels between these groups (70+/−15.2% vs 85+/−1.4% CD3+ cells among total splenocytes, respectively, p=0.09 (Figure 3A). One PBS-treated mouse achieved significantly less than 50% engraftment and for this reason was excluded from the analysis of T cell trafficking.
Figure 3.
IFN-γ induces T cell tumor infiltration. A) Spleen and tumors from NOG mice treated with IFN-γ or PBS were evaluated for the presence of CD3+ human T cells by flow cytometry. While T cell engraftment measured in the spleen was not significantly different between the two groups (p=0.09), the percentage of T cells in tumor preparations was significantly higher in IFN-γ treated mice (p=0.0013). B) Representative 10X magnification fields of tumors from 3 PBS-treated mice (panels i-iii) and 3 IFN-γ-treated mice (panels iv-vi) showing infiltration of CD3+ T cells. C) Cumulative counts of intra-and peri-tumoral CD3+ and CD8+ T cells. At least ten 400X magnification fields were counted for each tumor from 3 PBS (light bars) and 4 IFN-γ (dark bars) treated mice. (* indicates p<0.005, ** p<0.0005). D) Ki-67 expression by tumor infiltrating T cells. Following dual-labeling with anti-CD3 and anti-Ki-67 antibodies, the percentage of double positive T cells was calculated from eight 400x fields for each tumor (3 PBS treated, 4 IFN-γ treated). Significantly more double positive T cells were observed in IFN-γ treated tumors (p<0.0001), but <3% of all CD3+ T cells were proliferating in any case.
Single cell suspensions prepared from tumor specimens from each animal were analyzed by flow cytometry for the presence of human T cells. A significantly higher percentage of human CD3+ T cells, calculated as percentage of total cells to account for variation in tumor size, was detected in tumors from IFN-γ-treated mice compared to PBS-treated mice (7.0%+/−1.2 vs. 1.8%+/−0.8, p=0.0013) (Figure 3A). Immunohistochemistry was then performed on tumor sections from PBS- and IFN-γ-treated mice to determine the localization of the human T cells (Figure 3B). Consistent with the flow cytometric data, the total number of CD3+ T cells detected by immunohistochemistry was increased in IFN-γ treated tumors compared to PBS-treated tumors (45.9+/−26 per 400x field vs. 11.6+/−10.5, p<0.0001). Infiltrating T cells were then evaluated histologicially as peri-tumoral or intra-tumoral. Increased numbers of peri-tumoral CD3+ T cells were observed in IFN-γ-treated tumors compared to PBS-treated tumors (18.7+/−18.3 vs. 6.6+/−8.9, p=0.0012) (Figure 3C). Strikingly, greater increases in the number of intra-tumoral CD3+ T cells were achieved in IFN-γ-treated tumors (27.2+/−21.5 vs. 4.9+/−3.9, p<0.0001) (Figure 3C). Although CD8+ T cells accounted for only about 25% of the T cells detected by immunohistochemistry, the number of intra- and peri-tumoral CD8+ cells was also increased by IFN-γ treatment (Figure 3C).
As the increase in of intra-tumoral T cell numbers could be the result of either increased trafficking into or proliferation within the tumor, we examined the infiltrating CD3+ T cells for expression of the proliferation marker Ki-67 (Figure 3D). While significantly more Ki-67 expressing CD3+ T cells were present in IFN-γ treated than in PBS treated tumors (2.66+/−0.4 vs 0.44+/−0.25, p<0.0001), only a small fraction of the T cells from either tumor source were proliferating.
IFN-γ effects in a neuroblastoma clinical trial
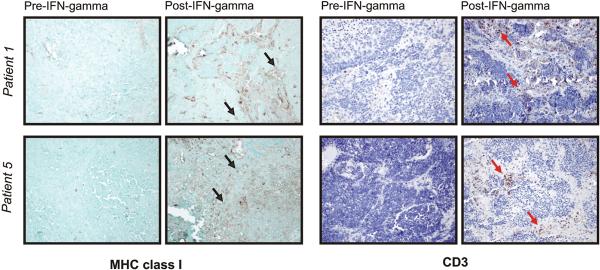
We hypothesized that, given the ability of IFN-γ to enhance human T cell numbers in NB tumors in our mouse model, this cytokine may be useful clinically as a means to improve MHC class I expression and T cell infiltration. To determine whether systemic administration of IFN-γ to patients with NB modulates tumor expression of MHC class I, we studied tumor biopsies from 5 high risk NB patients obtained before and after administration of IFN-γ per National Cancer Institute (NCI) protocol NCI-90-C0210. Patients on this study were treated subcutaneously every day for 5 days with recombinant human IFN-γ (0.1 mg/m2 (2 million IU) per day) (22). Tumor biopsies were obtained prior to treatment and immediately after the 5-day treatment (Figure 4). As expected, MHC class I expression was not detected on tumor cells at baseline in any patient; however, in two patients, MHC class I expression was upregulated following IFN-γ treatment, with a subset of NB tumor cells clearly expressing MHC class I. Upregulation of MHC class I correlated with the presence of infiltrating CD3+ T cells. At baseline in each patient, CD3+ T cells were rare or absent. After IFN-γ treatment, there was a notable increase in CD3+ T cell infiltration in the biopsies of the same two patients, both within the tumor nests and in the fibrovascular bundles. In three other patients, IFN-γ administration failed to upregulate MHC class I expression and only very rare CD3+ Tcells were observed before and after treatment.
Figure 4.
Patients with relapsed NB were treated subcutaneously every day for 5 days with recombinant human IFN-γ (0.1 mg/m2 per day). Tumor biopsies obtained prior to treatment and immediately after the 5-day treatment were evaluated by immunohistochemistry for expression of MHC class I and CD3. Results from 2 patients are shown (20X). Black arrows note examples of MHC class I-positive tumor cells; red arrows note CD3+ T cells.
Discussion
Lack of MHC Class I expression in cancer, particularly neuroblastoma, is a well recognized mechanism of escape from cellular immune surveillance. In this study we demonstrate that exposure of human NB tumor cells to IFN-γ upregulates MHC Class I expression both in vitro and in vivo, augments killing of NB cells by specific T cells in vitro, and induces significantly enhanced infiltration of human T cells into both NB tumor-bearing mice and patients with the disease.
IFN-γ-dependent induction of MHC Class I expression on NB cells in vivo was comparable to that achieved in vitro. Among the multiple defects in the antigen processing machinery that have been well-characterized in NB (20), none is considered structural but rather reflects abnormalities in regulatory mechanisms involved in the expression of these molecules, including MHC class I heavy chain itself. IFN-γ induces or upregulates MHC class I expression on most NB tumor cell lines in vitro by enhancing the transcription of critical APM components (24, 25). It seems most likely that the up-regulation of MHC Class I achieved on NB cells in NOG mice and in patient tumors on the clinical trial is achieved through a similar influence on transcription of APM components.
We have previously reported that functional CTL recognizing the tumor-associated antigen survivin can be isolated from NB patients, despite the lack of T cell infiltration into tumors from these patients (17). Here, we observed that IFN-γ treatment of MHC class I-negative NB cells resulted in tumor lysis by patient-derived survivin-specific CTL which otherwise did not kill such NB cells. This finding indicates that IFN-γ can augment the presentation of the peptide repertoire that is relevant for these CTL. Thus, the ability of IFN-γ to restore MHC class I expression by NB cells in vivo shown in this study may provide a clinical strategy to enhance the impact of endogenous NB-specific CTL in patients.
Increasing the susceptibility of NB cells to killing by CTL is, however, irrelevant if the CTL never gain access to the tumor. In this regard, our observation that in vivo administration of IFN-γ in mice engrafted with human T cells leads to enhanced infiltration of total CD3+ and CD8+ lymphocytes into established NB tumors is encouraging. The expression of Ki-67 by only a small minority of intra-tumoral T cells supports our conclusion that enhanced trafficking, rather than in situ proliferation in response to allogeneic tumor cells, is responsible for the increased lymphocyte numbers. The modest number of T cells infiltrating NB tumors in patients treated for 5 days with IFN-γ is consistent with observations made in our mouse xenograft model. While significantly enhanced T cell infiltration was obtained after our 18 day IFN-γ treatment, such effects were not observed following 3 and 5 day treatment regimens, despite the enhanced MHC Class I expression on NB cells achieved with the shorter IFN-γ treatments (data not shown). These results suggest that longer courses of treatment may be needed to achieve clinical enhancement of T cell therapy by IFN-γ. Alternatively, the different outcomes may be associated with the proximity of the tumor to the site of IFN-γ administration in the human and mouse experiments. The model described in this study will provide a useful tool for the evaluation of such variables in the optimization of this approach.
The lack of T cell trafficking into patient NB tumors that failed to upregulate MHC Class I in response to IFN-γ suggests that the increased antigen expression is a critical component for the T cell infiltration. This hypothesis is consistent with observation that restoration of MHC Class I restricted antigen presentation by murine tumor cells via APM gene transfer is sufficient to enhance tumor infiltration by T cells, perhaps through increased cross-presentation of tumor antigens by dendritic cells (26). However, IFN-γ has been shown to exert several additional effects on the tumor micro-environment that may contribute to enhanced T cell infiltration, including stimulation of chemokine production and activation of the vasculature (27, 28). The observation that many of the infiltrating T cells in our model were CD8-negative suggests that additional influences of IFN-γ may contribute to the enhanced trafficking. We did not detect any expression of MHC Class II antigens on NB cells after IFN-γ treatment (data not shown). The xenograft tumor model for human T cell infiltration developed in this study, with the potential for high-level human T cell engraftment in these NOG mice, will provide a powerful tool to evaluate the role of each of the IFN-γ-mediated micro-environment changes. This mouse model will be useful for the development and testing of novel approaches to enhance lymphocyte trafficking into human tumors. Such approaches have been reported to improve the efficiency of immune-based therapies in mouse tumor models (14, 15).
In summary, our results demonstrate that IFN-γ enhances trafficking of human T cells into NB cell tumors in vivo, providing further rationale for using this approach to overcome immune evasion mediated by down-regulated MHC Class I expression in this tumor. These observations are particularly relevant to NB given that recombinant human IFN-γ (Actimmune) has FDA-approved indications for use in children (chronic granulomatous disease and osteopetrosis), with a defined pediatric dose. Further clinical study in NB is warranted, particularly because treatment courses of Actimmune longer than that used in the NCI study are tolerated in children.
Acknowledgements
We thank William Lee (University of Pennsylvania, Philadelphia, PA) and Badrinath Roysam (Rensselaer Polytechnic Institute, Troy, NY) for advice and assistance with multispectral microscopy and histocytometry analysis using FARSIGHT software (development supported by S-IDEA award W81XWH-07-1--0325 from the US Army and grant R01 EB005157 from the NIH/NIBIB. We also thank Dr Mark Fleming for helpful discussions and Junior Hall for assistance with the in vivo experiments.
Grant support: Supported by grants from the Alliance for Cancer Gene Therapy (R.H.V); and R21 CA110516, The WW Smith Charitable Trust, and the Foerderer-Murray, Weinberg and Sanford Funds of the Children's Hospital of Philadelphia. (S.A.G).
Footnotes
Statement of Translational Relevance Lack of MHC Class I expression in cancer, particularly neuroblastoma, is a well recognized mechanism of escape from cellular immune surveillance. In this study we demonstrate that exposure of human NB tumor cells to IFN-γ upregulates MHC Class I expression both in vitro and in vivo, augments killing of NB cells by specific T cells in vitro, and induces significantly enhanced infiltration of human T cells into both NB tumor-bearing mice and patients with the disease. This work demonstrates the need for further clinical testing of IFN-γ as an approach to augment immune therapy for neuroblastoma and other MHC Class I deficient tumors.
References
- 1.Garcia-Lora A, Algarra I, Collado A, Garrido F. Tumour immunology, vaccination and escape strategies. Eur J Immunogenet. 2003;30:177–83. doi: 10.1046/j.1365-2370.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–84. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 3.Van Der Bruggen P, Zhang Y, Chaux P, et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065x.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 4.Meissner M, Reichert TE, Kunkel M, et al. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11:2552–60. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 5.Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. Am J Pathol. 1999;154:745–54. doi: 10.1016/S0002-9440(10)65321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao J, Li Y, Liu Y, et al. Expression of transporters associated with antigen processing and human leucocyte antigen class I in malignant melanoma and its association with prognostic factors. Br J Dermatol. 2008;158:88–94. doi: 10.1111/j.1365-2133.2007.08294.x. [DOI] [PubMed] [Google Scholar]
- 7.Ogino T, Shigyo H, Ishii H, et al. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66:9281–89. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 8.Mehta AM, Jordanova ES, Kenter GG, Ferrone S, Fleuren GJ. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother. 2008;57:197–206. doi: 10.1007/s00262-007-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother. 2008;57:1719–1726. doi: 10.1007/s00262-008-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Restifo NP, Esquivel F, Kawakami Y, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–72. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seliger B, Hohne A, Knuth A, et al. Analysis of the major histocompatibility complex class I antigen presentation machinery in normal and malignant renal cells: evidence for deficiencies associated with transformation and progression. Cancer Res. 1996;56:1756–60. [PubMed] [Google Scholar]
- 12.Johnsen A, France J, Sy MS, Harding CV. Down-regulation of the transporter for antigen presentation, proteasome subunits, and class I major histocompatibility complex in tumor cell lines. Cancer Res. 1998;58:3660–67. [PubMed] [Google Scholar]
- 13.Weidanz JA, Nguyen T, Woodburn T, et al. Levels of specific peptide-HLA class I complex predicts tumor cell susceptibility to CTL killing. J Immunol. 2006;177:5088–97. doi: 10.4049/jimmunol.177.8.5088. [DOI] [PubMed] [Google Scholar]
- 14.Merritt RE, Yamada RE, Crystal RG, Korst RJ. Augmenting major histocompatibility complex class I expression by murine tumors in vivo enhances antitumor immunity induced by an active immunotherapy strategy. J Thorac Cardiovasc Surg. 2004;127:355–64. doi: 10.1016/j.jtcvs.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–39. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 16.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin CM, Fleming MD, Carroll RG, et al. Immunosurveillance and survivin-specific T-cell immunity in children with high-risk neuroblastoma. J Clin Oncol. 2006;24:5725–34. doi: 10.1200/JCO.2005.05.3314. [DOI] [PubMed] [Google Scholar]
- 18.Main EK, Lampson LA, Hart MK, Kornbluth J, Wilson DB. Human neuroblastoma cell lines are susceptible to lysis by natural killer cells but not by cytotoxic T lymphocytes. J Immunol. 1985;135:242–46. [PubMed] [Google Scholar]
- 19.Wolfl M, Jungbluth AA, Garrido F, et al. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother. 2005;54:400–6. doi: 10.1007/s00262-004-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raffaghello L, Prigione I, Bocca P, et al. Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene. 2005 Jul 7;24:4634–44. doi: 10.1038/sj.onc.1208594. [DOI] [PubMed] [Google Scholar]
- 21.Levine BL, Bernstein WB, Connors M, et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–30. [PubMed] [Google Scholar]
- 22.Yang X, Merchant MS, Romero ME, et al. Induction of caspase 8 by interferon gamma renders some neuroblastoma (NB) cells sensitive to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but reveals that a lack of membrane TR1/TR2 also contributes to TRAIL resistance in NB. Cancer Res. 2003;63:1122–29. [PubMed] [Google Scholar]
- 23.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–54. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 24.Raffaghello L, Prigione I, Airoldi I, et al. Mechanisms of immune evasion of human neuroblastoma. Cancer Lett. 2005;228:155–61. doi: 10.1016/j.canlet.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 25.Lampson LA, George DL. Interferon-mediated induction of class I MHC products in human neuronal cell lines: analysis of HLA and beta 2-m RNA, and HLA-A and HLA-B proteins and polymorphic specificities. J Interferon Res. 1986;6:257–65. doi: 10.1089/jir.1986.6.257. [DOI] [PubMed] [Google Scholar]
- 26.Zhang QJ, Li XL, Wang D, et al. Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses. PLoS ONE. 2008;3:e3097. doi: 10.1371/journal.pone.0003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunz M, Toksoy A, Goebeler M, Engelhardt E, Brocker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J Pathol. 1999;189:552–58. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Tay SS, McCormack A, Lawson C, Rose ML. IFN-gamma reverses the stop signal allowing migration of antigen-specific T cells into inflammatory sites. J Immunol. 2003;170:3315–22. doi: 10.4049/jimmunol.170.6.3315. [DOI] [PubMed] [Google Scholar]