Abstract
Many individuals who sustain a cervical spinal cord injury are unable to maintain adequate ventilation due to diaphragm muscle paralysis. These patients become dependent on mechanical ventilators and this situation is associated with ongoing problems with pulmonary clearance, infections, and lung injury leading to significant morbidity and reduced life expectancy. Therefore, functional recovery of rhythmic phrenic activity and the ability to generate expulsive forces would dramatically affect the quality of life of patients with cervical spinal cord injury. Neurotrophins are very promising in that they have been shown to play an important role in modulating functional neuroplasticity. Specifically, brain-derived neurotrophic factor (BDNF) acting via the tropomyosin-related kinase receptor type B (TrkB) has been implicated in neuroplasticity following spinal cord injury. Our central hypothesis is that functional recovery of rhythmic phrenic activity after cervical spinal cord injury is enhanced by an increase in BDNF/TrkB signaling in phrenic motoneurons, providing a novel therapeutic target for patients.
Keywords: Respiratory muscles, diaphragm, skeletal muscle, neurotrophin, neuregulin
1. Introduction
Each year, about 6,000 people in the USA suffer cervical spinal cord injury that results in partial or complete diaphragm muscle paralysis with an annual expense of more than $3 billion (National Spinal Cord Injury Statistical Center, 2008). Individuals who are unable to maintain adequate ventilation due to diaphragm muscle paralysis become dependent on mechanical ventilators, a situation that is associated with ongoing problems with pulmonary clearance, infections, and lung injury leading to significant morbidity and mortality (Brown et al., 2006; Linn et al., 2000). Therefore, functional recovery of rhythmic phrenic activity and the ability to generate expulsive forces would dramatically affect the quality and life expectancy of patients with cervical spinal cord injury. Neurotrophins are very promising in that they have been shown to play an important role in modulating functional neuroplasticity (Kang & Schuman, 1995; Poo, 2001). Specifically, brain-derived neurotrophic factor (BDNF) acting via the tropomyosin-related kinase receptor type B (TrkB) has been implicated in neuroplasticity following spinal cord injury (Bregman et al., 2002; Coumans et al., 2001). Our central hypothesis is that functional recovery of rhythmic phrenic activity after cervical spinal cord injury is enhanced by an increase in BDNF/TrkB signaling in phrenic motoneurons.
2. Recovery of rhythmic phrenic activity after cervical spinal cord injury
By disrupting descending premotor excitatory drive to phrenic motoneurons, upper cervical spinal cord injury causes phrenic inactivity and diaphragm muscle paralysis (Mantilla et al., 2007; Miyata et al., 1995; Prakash et al., 1999). More than a century ago, it was observed that rhythmic phrenic activity is eventually restored after upper cervical spinal cord hemisection. In a series of studies, (Porter, 1895) demonstrated functional recovery of phrenic activity after unilateral hemisection of the C2 cervical spinal cord segment (C2HS). This is one of the first and likely one of the best examples of neuroplasticity and recovery of motor function after spinal cord injury. Subsequently, the term “crossed-phrenic phenomenon” was coined to describe these observations (Rosenblueth & Ortiz, 1936), which were replicated by a number of other investigators (c.f., (Goshgarian, 1981; Rosenbaum & Renshaw, 1949; Seligman & Davis, 1941)).
There is controversy regarding the timing and conditions necessary for recovery of rhythmic phrenic activity after C2HS (Fuller et al., 2008; Golder et al., 2001a; Goshgarian et al., 1989; Nantwi et al., 1999; O’Hara & Goshgarian, 1991; Vinit et al., 2006; Zhou et al., 2001). One problem is that the C2 hemisection may be incomplete, resulting in varying amounts of residual phrenic activity. If C2 hemisection includes the anterior and lateral columns (predominant descending bulbospinal pathways to the phrenic motor nucleus), phrenic nerve and diaphragm muscle activity completely disappear on the ipsilateral side (Fuller et al., 2008; Golder et al., 2001a; Miyata et al., 1995; Nantwi et al., 1999; Vinit et al., 2006; Zhan et al., 1997). Functional recovery of rhythmic phrenic activity occurs anywhere from a few hours (most likely due to incomplete C2HS) to 30 days or more after C2HS (Golder et al., 2001a; Goshgarian, 1981; Nantwi et al., 1999; Vinit et al., 2006). Unfortunately, most of these studies did not perform a longitudinal analysis of phrenic activity; therefore, the timing of restored activity was not actually determined. The C2HS model we employ involves transection of the anterior and lateral columns as well as the central core region of the spinal cord, thereby disrupting any ipsilateral direct or indirect (polysynaptic, interneuron mediated) input to phrenic motoneurons. In adult male rats, we routinely verify the absence of ipsilateral diaphragm muscle EMG activity at the time of C2HS and at 3 days post-C2HS (Fig. 1). Using these a priori inclusion criteria we observed functional recovery of rhythmic phrenic activity (diaphragm EMG activity) in only ~10% of unanesthetized animals at 7 days after C2HS, ~30% at 14 days after C2HS, and ~70% at 28 days after C2HS (unpublished observations). The extent of recovery at each time point was increased by increasing respiratory drive. For example, by exposing animals to 5% CO2 and 10% O2, we observed functional recovery in ~20% of animals at 7 days after C2HS, ~50% at 14 days after C2HS and in all animals at 28 days after C2HS. Thus, there appears to be a progressive increase in the extent of functional recovery of rhythmic phrenic activity after C2HS, which most likely reflects the process of neuroplasticity and strengthening of contralateral premotor respiratory drive to phrenic motoneurons.
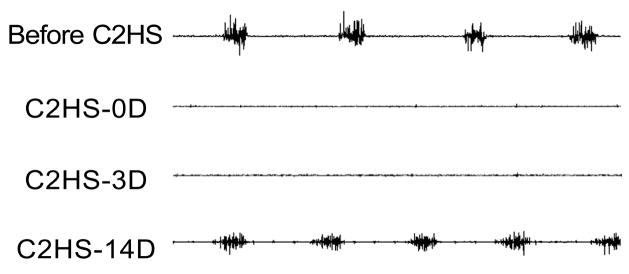
Figure 1.
Representative diaphragm muscle EMG recordings from a single rat where rhythmic eupneic activity recovered by 14 days after a C2 spinal hemisection (C2HS). Chronically-implanted EMG electrodes were placed prior to C2HS surgery and were used to verify complete C2HS at the time of surgery and 3 days post-C2HS.
3. Diaphragm motor unit recruitment
In the classic model of neuromotor control proposed by Sherrington (Liddell & Sherrington, 1925), neural control of muscle force generation results from a combination of an orderly recruitment of motor units together with changes in the frequency of motoneuron discharge (frequency coding). Henneman proposed that intrinsic size-related electrophysiological properties of motoneurons underlie the orderly recruitment of motor units (“size principle”) (Henneman et al., 1965). Accordingly, with similar synaptic input currents, smaller motoneurons are recruited before larger motoneurons (Burke, 1981). Support for the “size principle” has come from comparisons of motor unit recruitment order in relation to axonal conduction velocities (Berger, 1979; Clamann & Henneman, 1976). Motor units comprising smaller motoneurons, smaller axons, and thus slower axonal conduction velocities are recruited before units comprising larger motoneurons with faster axonal conduction velocities (Dick et al., 1987; Henneman et al., 1965). In addition, relationships exist between recruitment order, motor unit (muscle fiber) type, and force-frequency responses (Burke, 1981). Smaller motoneurons (type S motor units) innervate slow-twitch muscle fibers (i.e., type I fibers) that generate lower maximum tetanic force with leftward-shifted force-frequency response curves. Larger motoneurons innervate fast twitch muscle fibers that generate greater maximum tetanic forces with rightward-shifted force-frequency response curves. Among fast twitch motor units, fatigue resistant (FR – innervating type IIa fibers) units have the lowest recruitment threshold, followed in rank order by fatigue intermediate (FInt – innervating type IIx) and highly fatigable (FF – innervating type IIb fibers) units. Thus, size-related motor unit recruitment order is directly related to mechanical and fatigue properties of muscle unit fibers.
Several studies have provided evidence for an orderly recruitment of diaphragm muscle motor units based on the intrinsic electrophysiological properties of phrenic motoneurons. (Dick et al., 1987) found that, during spontaneous breathing in cats (eupnea), those phrenic motoneurons recruited first had slower axonal conduction velocities compared to later recruited motoneurons. In another study (Jodkowski et al., 1987), two subpopulations of phrenic motoneurons were reported in cats, distinguished by differences in membrane input resistance and rheobase. Phrenic motoneurons with higher input resistance and lower rheobase were recruited earlier during spontaneous breathing, while phrenic motoneurons with lower input resistance and higher rheobase were rarely recruited during inspiration, even with end tidal CO2 at 7%. In previous studies (Sieck, 1991b; Sieck, 1991a; Sieck & Fournier, 1989), we assumed an orderly size-related recruitment of diaphragm motor units based on their fatigue-resistance: type S and FR motor units recruited first, followed by type FInt and FF motor units. Based on measurements of the forces generated by different motor unit types, we then estimated the proportion of the phrenic motor unit pool in the cat and hamster diaphragm muscle that would be recruited during different ventilatory and non-ventilatory (e.g., sneezing, coughing) behaviors. We measured transdiaphragmatic pressures (Pdi) to estimate diaphragm muscle forces generated during these different behaviors and compared them to the maximum Pdi (Pdi max) evoked by bilateral phrenic nerve stimulation. We found that the Pdi generated during eupnea in cats was ~12% of Pdi max (Sieck & Fournier, 1989). In contrast, in the hamster diaphragm muscle, eupneic Pdi was ~27% of Pdi max (Sieck, 1991b; Sieck, 1991a). In both species, we concluded that eupneic Pdi could be accomplished by the activation of type S and FR motor units alone. When cats and hamsters were exposed to a hypercapnic/hypoxic (5% CO2 and 10% O2) ventilatory drive, Pdi increased (to ~28% and 55% of Pdi max in cats and hamsters, respectively). This diaphragm muscle force level could be generated by the additional recruitment of some FInt units. Even during total airway occlusion, diaphragm muscle forces generated by cats (49% of Pdi max) and hamsters (63% of Pdi max) can be accomplished without recruitment of FF units. Only during expulsive non-ventilatory behaviors (e.g., coughing, sneezing) were maximum forces generated in the cat and hamster diaphragm muscle.
Implicit in the size principle of motor unit recruitment is the concept that premotor excitatory drive to phrenic motoneurons is widely-distributed and that the timing of phrenic motoneuron activation is attributable primarily to the intrinsic, size-related properties. The predominant descending premotor drive to phrenic motoneurons is ipsilateral with a smaller contralateral input (Ellenberger et al., 1990). Following cervical spinal cord hemisection at C2 (C2HS), the predominant ipsilateral descending premotor drive to phrenic motoneurons is removed, and as a result phrenic motoneurons no longer reach threshold for activation and the diaphragm is paralyzed on that side (Mantilla et al., 2007; Miyata et al., 1995; Prakash et al., 1999; Zhan et al., 1997). However, there is a latent contralateral excitatory premotor input to phrenic motoneurons that can be strengthened with time after C2HS (neuroplasticity) leading to functional recovery of rhythmic phrenic activity - crossed phrenic phenomenon (Fuller et al., 2008; Golder et al., 2001a; Goshgarian et al., 1991; Nantwi et al., 1999; Vinit et al., 2006; Zhou et al., 2001). Functional recovery of diaphragm muscle activation is dependent on strengthening this residual contralateral premotor input to phrenic motoneurons. It is presently unknown whether the contralateral synaptic input is widely distributed or selective onto specific phrenic motoneuron types, e.g., small vs. large motoneurons. However, we found that the same pattern of orderly diaphragm motor unit recruitment occurred after C2HS and was associated with increasing ventilatory demands (unpublished observations).
Based on measurements of diaphragm muscle fiber strength following C2HS, it can be assumed that the mechanical properties of diaphragm motor units remains largely unchanged for at least 2 weeks after C2HS, and likely much longer (Zhan et al., 1997). Thus, for this initial time period, the maximum force contributed by each motor unit would remain unchanged after C2HS. Once recruited, the contribution of each motor unit to diaphragm force depends only on the discharge frequency of phrenic motoneurons (frequency coding of motor unit force). In a previous study in the cat, we found that type S and type FR motor units have lower onset discharge frequencies than type FInt and type FF motor units (8 vs. 15 Hz), and once recruited, discharge rate increases to a peak frequency of 20 and 40 Hz, respectively (Sieck et al., 1984). This corresponds with the steepest portion of the force-frequency response curve for each motor unit type; thus indicating a significant role of frequency coding in neuromotor control of diaphragm force (Sieck, 1991a). Currently there is no information on whether frequency-coding changes after spinal cord injury.
Up to 3 months after C2HS, the pattern of breathing in rats is altered, with higher respiratory rates and lower tidal volumes compared to controls (Fuller et al., 2008; Golder et al., 2001b). These investigators also reported that after the restoration of phrenic activity, hypercapnic responses were attenuated. Thus, these results suggest that even though diaphragm muscle activity is restored after C2HS, the extent of motor unit recruitment is reduced even with increasing neural drive. As mentioned above, motor unit recruitment depends on motoneuron size and motor unit type. Although the contralateral premotor drive to phrenic motoneurons is strengthened after C2HS, it may be insufficient to recruit a comparable number of motor units during ventilatory and non-ventilatory behaviors of the diaphragm muscle. If it is assumed that neuroplasticity after C2HS results in a general strengthening of all pre-existing descending synaptic drive, the normal orderly recruitment of motor units (type S and FR first followed by FInt and FF) will be unchanged, although fewer motor units may be recruited.
We found that the forces generated with functional recovery of rhythmic diaphragm activity after 2 weeks of C2HS were consistently lower across a full range of motor behaviors. For example, during spontaneous breathing instead of generating 20% of maximum force the diaphragm generated only 5% of maximum. Since measuring force is difficult, we used EMG as a surrogate and referenced integrated EMG responses to the maximum generated during fictive sneezing before injury and on the contralateral side (Dow et al., 2006). Across a 4 week period following C2HS, integrated diaphragm EMG responses increased progressively. In the small number of animals that showed recovery at 7 days after C2HS, the diaphragm EMG generated during spontaneous breathing was only ~30% of that generated before injury. This is also true for diaphragm EMG generated during fictive sneezing. Thus, it appears that the diaphragm motor unit recruitment curve is shifted to the right and that it may be impossible to recruit all diaphragm motor units based on contralateral synaptic input only unless it is considerably strengthened over time after C2HS.
Another very important factor to consider is that the size of phrenic motoneurons is affected by C2HS and by 2 weeks after C2HS, the heterogeneity of size within the phrenic motoneuron pool is greatly reduced (Mantilla & Sieck, 2003), most likely as a result of a decrease in size of the larger phrenic motoneurons. It is possible that this results from a removal of a trophic influence onto phrenic motoneurons (e.g., neuregulin), inactivity of the motoneurons per se and/or retrograde trophic influences emanating from diaphragm muscle fibers. Regardless of the underlying cause, this trend towards uniformity of phrenic motoneuron size would affect motor unit recruitment based on the size principle, and may be a compensatory mechanism by which functional recovery is optimized. The opposite trend is seen during postnatal development where there is initially relative uniformity in motor unit size that then progresses to greater heterogeneity of motoneuron size (Prakash et al., 2000). This coincides with increasing functional diversity of ventilatory and non-ventilatory behaviors. In early postnatal development, because of lung and chest wall compliance there is a need to recruit a greater fraction of the diaphragm motor unit pool to accomplish resting breathing (Mantilla & Sieck, 2008b). If the longer term effects of diaphragm muscle inactivity following spinal cord injury results in diaphragm muscle atrophy and weakness, recruitment of a greater fraction of the motor pool would be required to sustain ventilation. Regardless of the mechanism, strengthening of synaptic input via a presynaptic mechanism, or an increase in motoneuron excitability via a postsynaptic mechanism would result in increased motor unit recruitment and/or altered frequency coding.
4. Neurotrophins and their receptors
There are several members of the neurotrophin family including nerve growth factor (NGF), BDNF, neurotrophin-3 (NT-3) and NT-4 (Huang & Reichardt, 2001). These neurotrophins signal via interactions with either a lower affinity p75 receptor or a family of high affinity Trk receptors that are neurotrophin specific: TrkA for NGF, TrkB for BDNF and NT-4, and TrkC for NT-3.
Most studies to date have focused on the role of neurotrophins and Trk receptors during development, and in particular, on their influence on survival of specific neuronal populations (Timmusk et al., 1993). However, neurotrophins have additional important effects, even in the adult (Ermilov et al., 2007; Lu, 2003; Mantilla & Sieck, 2003; Mantilla & Sieck, 2008a; Widenfalk et al., 2001; Zhan et al., 2003). Neurotrophins and their receptors are expressed by motoneurons (Koliatsos et al., 1993; Lu, 2003; Widenfalk et al., 2001), including phrenic motoneurons (Mantilla & Sieck, 2008a).
Several Trk receptor isoforms result form alternative mRNA splicing (Reichardt, 2006). Full-length Trk receptors (e.g., TrkB.FL) typically display kinase activity and thus are capable of intracellular signaling via phosphorylation of targeted proteins. In contrast, truncated Trk isoforms (e.g., TrkB.T1 and TrkB.T2) lack the intracellular kinase domain and are therefore incapable of neurotrophin signaling. Thus, truncated Trk receptors can act in a dominant-negative fashion to competitively inhibit neurotrophin/Trk signaling. Unfortunately, no other study has unambiguously discriminated between TrkB isoforms, nor have they assessed differences in relative expression of full-length vs. truncated Trk receptor isoforms. Such a distinction is very important since BDNF signaling in phrenic motoneurons can be either enhanced or inhibited by increasing and/or decreasing the relative expression of TrkB.FL compared to TrkB.T1 or TrkB.T2 expression; thereby, altering the relative capability for neurotrophin-mediated TrkB tyrosine kinase activation. Recently, we explored whether spinal cord injury affects the ratio of full-length to truncated TrkB isoform expression in phrenic motoneurons. We found that by 3 days post C2HS, the ratio of TrkB.FL to truncated TrkB receptor isoforms increased. Such a change could be important in enhancing the overall extent of neurotrophin signaling.
4.1. Role of neurotrophin signaling in functional recovery following cervical spinal cord injury
Based on considerable evidence (Bennett et al., 1999; Dougherty et al., 2000; King et al., 2000; Widenfalk et al., 2001), it appears that expression of neurotrophins increases following spinal cord injury. Most of these studies examined mRNA expression rather than protein expression with limited temporal resolution. For example, Widenfalk and colleagues reported that after midthoracic spinal cord injury in rats, there is a generalized increase in p75 mRNA expression in neurons, as well as an increase in truncated TrkB receptor mRNA expression (Widenfalk et al., 2001). In unpublished observations, we found that both BDNF and NT-4 mRNA and protein levels increase in the cervical ventral horn of rats by 3 days (mRNA) and 14 days (protein) after C2HS. We also found that there is an increase in BDNF immunoreactivity in retrogradely-labeled phrenic motoneurons and surrounding microglia at 14 days post-C2HS. Contrary to the study by Widenfalk and colleagues, we found an increase in TrkB.FL mRNA and protein expression in phrenic motoneurons after C2HS (not truncated TrkB). Our results support the concept that an increase in BDNF and NT-4 expression occurs post-spinal cord injury together with an increase in TrkB.FL to truncated isoform ratio would enhance neurotrophin/TrkB signaling in phrenic motoneurons after C2HS (Fig. 3).
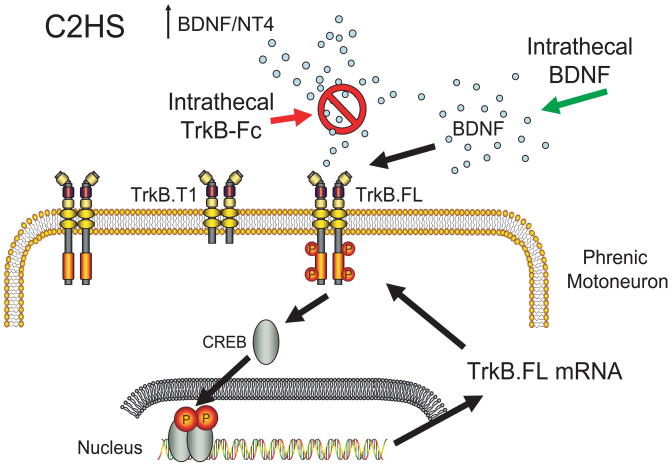
Figure 3.
Conceptually, we propose that after C2HS, there is an increase in BDNF and neurotrophin-4 (NT-4) levels that triggers activation of the catalytically-active full-length TrkB receptor (TrkB.FL) in phrenic motoneurons with downstream transcriptional activation (e.g., cAMP response element binding protein, CREB) resulting in increased motoneuron excitability, possibly via increased TrkB.FL expression – a positive feedback effect. By therapeutically increasing TrkB.FL expression in phrenic motoneurons, functional recovery can be accelerated.
It is possible that BDNF and NT-4 autoregulate further expression of BDNF, NT-4 and TrkB receptor in phrenic motoneurons – positive feedback control. In agreement, in PC12 and cultured neocortical cells neurotrophins induce further expression of neurotrophins (Canossa et al., 1997; Kruttgen et al., 1998; Xiong et al., 2002), and in PC12 cells, neurotrophins induce an upregulation of their receptors (Rankin et al., 2008). To the best of our knowledge, no studies have examined neurotrophin-induced receptor upregulation in vivo.
Previous studies examined changes in expression of neurotrophins or Trk receptors in the thoracic or lumbar spinal cord after spinal cord injury, usually at the level of injury rather than distant motor pools (Frisen et al., 1992; King et al., 2000; Widenfalk et al., 2001). In addition, most studies limited their analyses to changes in neurotrophin expression using homogenates of entire spinal cord (Nakamura & Bregman, 2001; Widenfalk et al., 2001). Some studies used in situ hybridization (Frisen et al., 1992; King et al., 2000; Liebl et al., 2001; Widenfalk et al., 2001) or immunohistochemistry (Gulino et al., 2004) to localize changes in neurotrophin expression to larger neurons within the ventral horn (assumed to be motoneurons), but these approaches were semi-quantitative at best. Detailed, systematic examinations of motoneuron expression of neurotrophin receptors are not available, let alone changes induced by spinal cord injury. Such studies would be particularly important given the role of neurotrophins and their receptors in neuroplasticity and functional recovery.
5. Mechanisms underlying neurotrophin influence on synaptic plasticity
A number of studies implicate neurotrophins and their receptors in synaptic plasticity (Kang & Schuman, 1995; Poo, 2001). Baker-Herman and colleagues examined the role of BDNF/TrkB signaling in intermittent hypoxia-induced long-term facilitation of phrenic activity, a form of neuroplasticity in adults (Baker-Herman et al., 2004). In rats that were vagotomized, paralyzed and mechanically ventilated, they showed that BDNF applied to the dorsal surface of the cervical spinal cord was sufficient to elicit enhancement in phrenic nerve activity that was comparable to the long-term facilitation induced by intermittent hypoxia output. Furthermore, they demonstrated that non-specific tyrosine kinase inhibition using K252a blocked intermittent hypoxia-induced long-term facilitation of phrenic activity. In light of this evidence for a key role of neurotrophins in spinal cord neuroplasticity, it is surprising that no previous study examined whether BDNF and/or NT-4 treatment affects functional recovery of rhythmic phrenic activity after C2HS. In unpublished studies, we found that the proportion of rats displaying restored rhythmic phrenic activity progressively increases from 7 days to 28 days post-C2HS (~10% to ~70%, respectively). We also found that intrathecal infusion of BDNF (or NT-4) significantly enhanced functional recovery after C2HS (~50% of all animals at day 7), whereas quenching BDNF and NT-4 by intrathecal infusion of TrkB-Fc markedly delayed functional recovery (no animals displaying recovery by day 28). Thus, local neurotrophins appear to play an important role in neuroplasticity and functional recovery following C2HS.
5.1. Role of neurotrophin/Trk signaling in pre-synaptic plasticity
Regardless of their source, neurotrophins may enhance synaptic input from descending premotor pathways by increasing the strength of pre-existing synaptic connections and/or increasing sprouting and generation of new synaptic connections. Indeed, it has been shown that BDNF and NT-4 treatment can directly modulate synaptic efficacy (Kang & Schuman, 1995; Mantilla et al., 2004b). Furthermore, BDNF promotes sprouting of serotonergic axons (Mamounas et al., 2000; Mamounas et al., 1995), and both BDNF and NT-4 increase serotonergic, noradrenergic, and corticospinal axonal ingrowth into transplanted fetal spinal cord after spinal cord injury (Bregman et al., 2002; Bregman et al., 1997; Coumans et al., 2001; Lynskey et al., 2006).
In the hippocampus, it was shown that BDNF stimulates synapsin I phosphorylation in a mitogen activated protein kinase-dependent manner (Jovanovic et al., 2000), and therefore, regulates neurotransmitter release. In the rat diaphragm muscle-phrenic nerve preparation, we demonstrated that acute BDNF and NT-4 treatment improved neuromuscular transmission (Mantilla et al., 2004b; Zhan et al., 2003). In these studies, diaphragm muscle force was measured during repeated phrenic nerve stimulation (0.1. ms stimuli in 330-ms 40-Hz trains) and every 15 s, the diaphragm muscle was directly stimulated and the resulting force measured. The difference between forces generated during nerve and muscle stimulation reflects the extent of neuromuscular transmission failure. In control animals, the extent of neuromuscular transmission failure increases progressively reaching ~40% after 2-min. Surprisingly, after 2 weeks of C2HS, the extent of neuromuscular transmission failure in the rat diaphragm muscle was markedly attenuated, with only 10% neuromuscular transmission failure after 2-min of stimulation (Prakash et al., 1999). We hypothesize that this was due, at least in part, to enhanced BDNF/TrkB signaling at the presynaptic terminal. To address this hypothesis, we conducted a series of studies to determine the effect of BDNF on neuromuscular transmission. In control animals, exogenous BDNF or NT-4 treatment induced improvement in neuromuscular transmission that could be blocked by the non-specific tyrosine kinase inhibitor K252a (Mantilla et al., 2004b) or more specifically by quenching endogenous neurotrophins using the fusion protein TrkB-Fc (unpublished observations). Electrophysiological assessment of quantal release at diaphragm neuromuscular junctions demonstrated that with repeated phrenic nerve stimulation there was depletion of readily-releasable and reserve synaptic vesicle pools that could be responsible for reductions in neuromuscular transmission with repeated activation (Mantilla et al., 2004a; Rowley et al., 2007). We found that BDNF treatment actually mitigated the rate of depletion of these synaptic vesicle pools and could actually improve quantal release (Ermilov et al., 2007). While consistent with a pres-synaptic effect, these results did not provide direct evidence for a role of TrkB signaling at the presynaptic terminal. Toward this end, in more recent studies, we used a TrkBF616A knock-in mouse model developed by David Ginty and colleagues (Chen et al., 2005) to explore the role of TrkB signaling in neuromuscular transmission. These mice show specific, rapid, and reversible inhibition of TrkB kinase activity when treated with soluble protein protease inhibitors such as 1NMPP1. In these mice, 1NMPP1-treatment reversibly impaired neuromuscular transmission in diaphragm muscle-phrenic nerve preparations. What remains unresolved is whether BDNF/TrkB signaling has the same presynaptic effects at phrenic motoneurons, especially with respect to descending premotor excitatory input, e.g., glutamatergic release.
There is evidence that afferent feedback to phrenic motoneurons can influence expression of neurotrophins and enhance synaptic plasticity. For example, bilateral cervical dorsal rhizotomy involving the C3-C6 roots has been shown to increase immunoreactivity for neurotrophins (BDNF and NT-3) in large neurons in the cervical ventral horn (presumed phrenic motoneurons) and increased BDNF and NT-3 protein expression in the cervical ventral horn (Johnson et al., 2000). It has also been shown that cervical dorsal rhizotomy enhances phrenic responses to intermittent hypoxia (Kinkead et al., 1998) and the amplitude of evoked phrenic responses elicited by contralateral intraspinal stimulation of the putative crossed phrenic pathway (Fuller et al., 2002). It is unclear whether such effects are mediated pre- or post-synaptically, or what mechanisms might be involved. Combining these results suggest that an increase in neurotrophin signaling after cervical dorsal rhizotomy may enhance the efficacy of crossed phrenic pathways.
5.2. Role of neurotrophin/Trk signaling in post-synaptic plasticity
The primary direct synaptic input to phrenic motoneurons is glutamatergic (Glu) acting via non-NMDA receptors, likely AMPA receptors (Liu et al., 1990). However, other excitatory Glu receptors (GluR) are also expressed in phrenic motoneurons including NMDA and the metabotropic receptors mGluR1 and mGluR5 (Dong & Feldman, 1999; McCrimmon et al., 1989), which given their neuromodulatory effect may play a more prominent role in neuroplasticity. In the hippocampus, changes in NMDA and mGluR1 are associated with long-term potentiation – a form of neuroplasticity (Nicoll & Malenka, 1999) and both BDNF and NT4 may enhance NMDA-mediated Glu neurotransmission (Kang & Schuman, 1995; Lessmann et al., 1994). To the best of our knowledge, no previous study has explored changes in GluR expression in phrenic motoneurons following C2HS. In contrast, there is evidence for a role of changes in serotonergic receptor (5-HTR) expression in recovery of rhythmic diaphragm muscle activity following C2HS. For example, Fuller et al. (2005) found an increase in 5-HTR2A protein expression in the cervical ventral horn using Western analysis as well as increased 5-HTR2A immunoreactivity in purported phrenic motoneurons (not retrogradely labeled). Blocking 5-HTR2A using ketanserin delayed functional recovery post-C2HS (Fuller et al., 2005). The role of neurotrophins in such postsynaptic remodeling of excitatory neurotransmitter receptor expression was not explored. Studies by the Mitchell group have primarily focused on the postsynaptic effects of BDNF/TrkB signaling in enhancing intermittent hypoxia-induced long-term facilitation at phrenic motoneurons (Baker-Herman et al., 2004). They propose an interaction between BDNF/TrkB signaling and serotonergic signaling that results in overall increase in phrenic motoneuron excitability associated with intermittent hypoxia. These effects are dependent on protein synthesis, which is consistent with the need to synthesize new receptors, although considerable amount of detail remains to be explored. These results are indicative of a role for neurotrophins in the functional recovery associated with neuroplasticity. In addition to changes in protein synthesis, changes in the distribution of receptors as a result of changes in phrenic motoneuron size can also influence motoneuron excitability by changes in the summation of synaptic currents.
6. Strategies for enhancing respiratory function after spinal cord injury
Converging evidence suggests that BDNF acting through TrkB.FL plays an important role in the neuroplasticity required for functional recovery of rhythmic respiratory activity following spinal cord injury. Importantly, only TrkB.FL is capable of signaling via phosphorylation, whereas truncated TrkB isoforms (TrkB.T1 and TrkB.T2) act in a dominant-negative fashion to inhibit TrkB signaling (Reichardt, 2006). Our initial studies indicate that intrathecal BDNF treatment enhances functional recovery of rhythmic phrenic activity, whereas infusion of TrkB-Fc, a fusion protein that quenches extracellular BDNF (Yajima et al., 2005), delays functional recovery (Fig. 2). Unfortunately, exogenous intrathecal neurotrophin treatment is associated with significant negative adverse effects that preclude its therapeutic use (Bregman et al., 1997; Iarikov et al., 2007; Koda et al., 2004; Lu et al., 2003; Lu et al., 2005; Lynskey et al., 2006; Novikova et al., 2000; Novikova et al., 2002; Ye & Houle, 1997). We have recently proposed a targeted approach to promote recovery of rhythmic respiratory activity following cervical spinal cord injury involving enhanced TrkB.FL expression and signaling in phrenic motoneurons, while avoiding undesirable adverse effects of neurotrophin treatment. Our central hypothesis is that functional recovery of rhythmic phrenic activity after C2HS is enhanced by increasing TrkB.FL signaling in phrenic motoneurons (Fig. 3). An alternative hypothesis has been recently proposed by Golder et al. (2008) in which an immature, deglycosylated form of TrkB receptor is transphosphorylated by an adenosine-dependent intracellular signaling pathway (Golder et al., 2008). This proposal is based on previous in vitro work by Chao and colleagues in PC12 cells where they demonstrated activation of TrkB receptors in the absence of their natural ligands (Lee & Chao, 2001; Lee et al., 2002). Certainly, these two hypotheses are not mutually exclusive. Unfortunately, identification of immature TrkB receptor is problematic since it is based on indirect evidence for the presence of a low molecular weight TrkB isoform detected by non-specific Western blot analysis using a single antibody moiety.
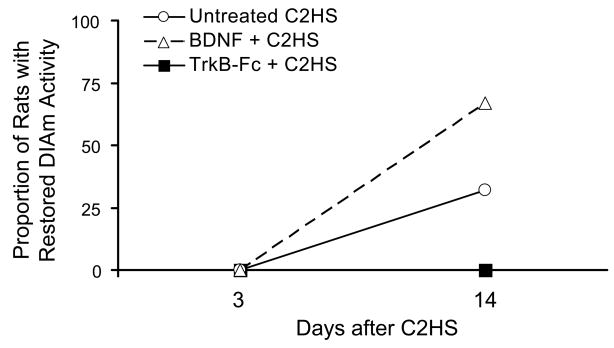
Figure 2.
Proportion of rats (%) displaying recovery of eupneic diaphragm EMG activity after C2HS. Intrathecal brain-derived neurotrophic factor (BDNF; 180 ng/day) accelerated recovery of rhythmic diaphragm activity, while quenching local, endogenously-released neurotrophins using the fusion protein TrkB-Fc (1500 ng/day) blocked recovery.
Given available information on neurotrophins and their receptors, it is very reasonable to suspect a positive therapeutic benefit of neurotrophin treatment on functional recovery. A number of animal studies have shown increased neuron survival and axonal sprouting following neurotrophin treatment via intrathecal infusion (Bregman et al., 1997; Novikova et al., 2000; Novikova et al., 2002; Ye & Houle, 1997), adenovirus (Koda et al., 2004), or stem cell transplants (Iarikov et al., 2007; Lu et al., 2003; Lu et al., 2005; Lynskey et al., 2006). The possibility of the therapeutic use of systemic (A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III), 1999) and intrathecal (Beck et al., 2005; Ochs et al., 2000) neurotrophins was also explored in humans, albeit for neuronal survival in amyotrophic lateral sclerosis patients. Unfortunately, human studies were disappointing in that significant adverse events mainly related to sensory disturbances, agitation and behavioral responses were induced at low doses of BDNF. Recent studies highlight the importance of BDNF in pain processing following spinal cord injury (Coull et al., 2005; Groth & Aanonsen, 2002; Yajima et al., 2005). In addition, bioavailability of BDNF is limited even with intrathecal delivery, it is possible that BDNF treatment may adversely affect inflammatory responses to injury. Thus, it appears that neurotrophin treatment per se may be fraught with problems. Alternatively, we are proposing a targeted approach of specifically enhancing TrkB expression and signaling in phrenic motoneurons by intrapleural delivery of viral constructs that enhance TrkB.FL expression following SCI. The feasibility of this intrapleural approach to specifically target phrenic motoneurons has been recently demonstrated (Mantilla et al., 2009). Once developed, this novel approach will obviate the problems associated with exogenous neurotrophins while enhancing the response in motoneurons, the final output of motor control.
Acknowledgments
Supported by NIH grant AR051173.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III) Neurology. 1999;52:1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Beck M, Flachenecker P, Magnus T, Giess R, Reiners K, Toyka KV, Naumann M. Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:100–103. doi: 10.1080/14660820510028412. [DOI] [PubMed] [Google Scholar]
- Bennett AD, Taglialatela G, Perez-Polo R, Hulsebosch CE. NGF levels decrease in the spinal cord and dorsal root ganglion after spinal hemisection. Neuroreport. 1999;10:889–893. doi: 10.1097/00001756-199903170-00040. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol. 1979;42:76–90. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- Bregman BS, McAtee M, Dai HN, Kuhn PL. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol. 1997;148:475–494. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Coumans JV, Dai HN, Kuhn PL, Lynskey J, McAtee M, Sandhu F. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog Brain Res. 2002;137:257–273. doi: 10.1016/s0079-6123(02)37020-1. [DOI] [PubMed] [Google Scholar]
- Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–868. discussion 869–870. [PMC free article] [PubMed] [Google Scholar]
- Burke RE. Motor units: anatomy, physiology and functional organization. In: Peachey LD, editor. Handbook of Physiology. The Nervous System. Motor Control. Vol. 3. Am Physiol Soc; Bethesda, MD: 1981. pp. 345–422. [Google Scholar]
- Canossa M, Griesbeck O, Berninger B, Campana G, Kolbeck R, Thoenen H. Neurotrophin release by neurotrophins: implications for activity-dependent neuronal plasticity. Proc Natl Acad Sci U S A. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Clamann HP, Henneman E. Electrical measurement of axon diameter and its use in relating motoneuron size to critical firing level. J Neurophysiol. 1976;39:844–851. doi: 10.1152/jn.1976.39.4.844. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Correlation of recruitment order with axonal conduction velocity for supraspinally driven diaphragmatic motor units. J Neurophysiol. 1987;57:245–259. doi: 10.1152/jn.1987.57.1.245. [DOI] [PubMed] [Google Scholar]
- Dong XW, Feldman JL. Distinct subtypes of metabotropic glutamate receptors mediate differential actions on excitability of spinal respiratory motoneurons. J Neurosci. 1999;19:5173–5184. doi: 10.1523/JNEUROSCI.19-13-05173.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Dow DE, Mantilla CB, Zhan WZ, Sieck GC. EMG-based detection of inspiration in the rat diaphragm muscle. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1204–1207. doi: 10.1109/IEMBS.2006.260688. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, Goshgarian HG. Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. J Comp Neurol. 1990;302:707–714. doi: 10.1002/cne.903020403. [DOI] [PubMed] [Google Scholar]
- Ermilov LG, Sieck GC, Zhan WZ, Mantilla CB. Neurotrophins improve synaptic transmission in the adult rodent diaphragm. Neurophysiology. 2007;39:327–337. [Google Scholar]
- Frisen J, Verge VM, Cullheim S, Persson H, Fried K, Middlemas DS, Hunter T, Hokfelt T, Risling Mg. Increased levels of trkB mRNA and trkB protein-like immunoreactivity in the injured rat and cat spinal cord. Proc Natl Acad Sci USA. 1992;89:11282–11286. doi: 10.1073/pnas.89.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Johnson RA, Mitchell GS. Chronic cervical spinal sensory denervation reveals ineffective spinal pathways to phrenic motoneurons in the rat. Neurosci Lett. 2002;323:25–28. doi: 10.1016/s0304-3940(02)00121-0. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001a;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Davenport PW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol. 2001b;91:2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72:211–225. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Yu XJ, Rafols JA. Neuronal and glial changes in the rat phrenic nucleus occurring within hours after spinal cord injury. J Comp Neurol. 1989;284:519–533. doi: 10.1002/cne.902840404. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei: a possible substrate for the crossed-phrenic phenomenon. Exp Neurol. 1991;111:135–139. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- Groth R, Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100:171–181. doi: 10.1016/s0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- Gulino R, Lombardo SA, Casabona A, Leanza G, Perciavalle V. Levels of brain-derived neurotrophic factor and neurotrophin-4 in lumbar motoneurons after low-thoracic spinal cord hemisection. Brain Res. 2004;1013:174–181. doi: 10.1016/j.brainres.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iarikov DE, Kim BG, Dai HN, McAtee M, Kuhn PL, Bregman BS. Delayed transplantation with exogenous neurotrophin administration enhances plasticity of corticofugal projections after spinal cord injury. J Neurotrauma. 2007;24:690–702. doi: 10.1089/neu.2006.0172. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol. 1987;58:105–124. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Okragly AJ, Haak-Frendscho M, Mitchell GS. Cervical dorsal rhizotomy increases brain-derived neurotrophic factor and neurotrophin-3 expression in the ventral spinal cord. J Neurosci. 2000;20:RC 77. doi: 10.1523/JNEUROSCI.20-10-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- King VR, Bradbury EJ, McMahon SB, Priestley JV. Changes in truncated trkB and p75 receptor expression in the rat spinal cord following spinal cord hemisection and spinal cord hemisection plus neurotrophin treatment. Exp Neurol. 2000;165:327–341. doi: 10.1006/exnr.2000.7480. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda M, Hashimoto M, Murakami M, Yoshinaga K, Ikeda O, Yamazaki M, Koshizuka S, Kamada T, Moriya H, Shirasawa H, Sakao S, Ino H. Adenovirus vector-mediated in vivo gene transfer of brain-derived neurotrophic factor (BDNF) promotes rubrospinal axonal regeneration and functional recovery after complete transection of the adult rat spinal cord. J Neurotrauma. 2004;21:329–337. doi: 10.1089/089771504322972112. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- Kruttgen A, Moller JC, Heymach JV, Jr, Shooter EM. Neurotrophins induce release of neurotrophins by the regulated secretory pathway. Proc Natl Acad Sci U S A. 1998;95:9614–9619. doi: 10.1073/pnas.95.16.9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Rajagopal R, Chao MV. Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002;13:11–17. doi: 10.1016/s1359-6101(01)00024-7. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. Neuroreport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- Liddell EGT, Sherrington CS. Recruitment and some other factors of reflex inhibition. Proc Roy Soc Lond (Biol) 1925;97:488–518. [Google Scholar]
- Liebl DJ, Huang W, Young W, Parada LF. Regulation of Trk receptors following contusion of the rat spinal cord. Exp Neurol. 2001;167:15–26. doi: 10.1006/exnr.2000.7548. [DOI] [PubMed] [Google Scholar]
- Linn WS, Adkins RH, Gong H, Jr, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch Phys Med Rehabil. 2000;81:757–763. doi: 10.1016/s0003-9993(00)90107-2. [DOI] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005;191:344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Lynskey JV, Sandhu FA, Dai HN, McAtee M, Slotkin JR, MacArthur L, Bregman BS. Delayed intervention with transplants and neurotrophic factors supports recovery of forelimb function after cervical spinal cord injury in adult rats. J Neurotrauma. 2006;23:617–634. doi: 10.1089/neu.2006.23.617. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Invited Review: Mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol. 2003;94:1230–1241. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Rowley KL, Fahim MA, Zhan WZ, Sieck GC. Synaptic vesicle cycling at type-identified diaphragm neuromuscular junctions. Muscle Nerve. 2004a;30:774–783. doi: 10.1002/mus.20173. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve. 2004b;29:381–386. doi: 10.1002/mus.10558. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neurosci. 2007;146:178–189. doi: 10.1016/j.neuroscience.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Trophic factor expression in phrenic motor neurons. Respir Physiol Neurobiol. 2008a;164:252–262. doi: 10.1016/j.resp.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J Appl Physiol. 2008b;104:1818–1827. doi: 10.1152/japplphysiol.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods. 2009 doi: 10.1016/j.jneumeth.2009.06.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Smith JC, Feldman JL. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J Neurosci. 1989;9:1910–1921. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Bregman BS. Differences in neurotrophic factor gene expression profiles between neonate and adult rat spinal cord after injury. Exp Neurol. 2001;169:407–415. doi: 10.1006/exnr.2001.7670. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Repair. 1999;13:225–234. [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury: Facts and Figures at a Glance. University of Alabama; Birmingham: Jan, 2008. [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann N Y Acad Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Novikova LN, Novikov LN, Kellerth JO. BDNF abolishes the survival effect of NT-3 in axotomized Clarke neurons of adult rats. J Comp Neurol. 2000;428:671–680. doi: 10.1002/1096-9861(20001225)428:4<671::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Novikova LN, Novikov LN, Kellerth JO. Differential effects of neurotrophins on neuronal survival and axonal regeneration after spinal cord injury in adult rats. J Comp Neurol. 2002;452:255–263. doi: 10.1002/cne.10381. [DOI] [PubMed] [Google Scholar]
- O’Hara TEJ, Goshgarian HG. Quantitative assessment of phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals after spinal cord injury. Exp Neurol. 1991;111:244–250. doi: 10.1016/0014-4886(91)90012-2. [DOI] [PubMed] [Google Scholar]
- Ochs G, Penn RD, York M, Giess R, Beck M, Tonn J, Haigh J, Malta E, Traub M, Sendtner M, Toyka KV. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Porter J. The path of the respiratory impulse from the bulb to the phrenic nuclei. J Physiol (Lond) 1895;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol. 2000;89:563–572. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- Rankin SL, Guy CS, Rahimtula M, Mearow KM. Neurotrophin-induced upregulation of p75NTR via a protein kinase C-delta-dependent mechanism. Brain Res. 2008;1217:10–24. doi: 10.1016/j.brainres.2008.03.076. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum H, Renshaw B. Descending respiratory pathways in the cervical spinal cord. Am J Physiol. 1949;157:468–476. doi: 10.1152/ajplegacy.1949.157.3.468. [DOI] [PubMed] [Google Scholar]
- Rosenblueth A, Ortiz T. The crossed respiratory impulses to the phrenic. Am J Physiol. 1936;117:495–513. [Google Scholar]
- Rowley KL, Mantilla CB, Ermilov LG, Sieck GC. Synaptic vesicle distribution and release at rat diaphragm neuromuscular junctions. J Neurophysiol. 2007;98:478–487. doi: 10.1152/jn.00251.2006. [DOI] [PubMed] [Google Scholar]
- Seligman AM, Davis WA. The effects of some drugs on the crossed phrenic phenomenon. Am J Physiol. 1941;143:102–106. [Google Scholar]
- Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Exp Neurol. 1984;85:316–335. doi: 10.1016/0014-4886(84)90143-2. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Sieck GC. Neural control of the inspiratory pump. NIPS. 1991a;6:260–264. [Google Scholar]
- Sieck GC. Diaphragm motor units and their response to altered use. Sem Respir Med. 1991b;12:258–269. [Google Scholar]
- Timmusk T, Belluardo N, Metsis M, Persson H. Widespread and developmentally regulated expression of neurotrophin-4 messenger RNA in rat brain and peripheral tissues. Eur J Neurosci. 1993;5:605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma. 2006;23:1137–1146. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Futamura T, Jourdi H, Zhou H, Takei N, Diverse-Pierluissi M, Plevy S, Nawa H. Neurotrophins induce BDNF expression through the glutamate receptor pathway in neocortical neurons. Neuropharmacol. 2002;42:903–912. doi: 10.1016/s0028-3908(02)00043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Yamaguchi T, Tamaki H, Wachi H, Seyama Y, Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem. 2005;93:584–594. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]
- Ye JH, Houle JD. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp Neurol. 1997;143:70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol. 1997;82:1145–1153. doi: 10.1152/jappl.1997.82.4.1145. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Mantilla CB, Sieck GC. Regulation of neuromuscular transmission by neurotrophins. Sheng Li Xue Bao. 2003;55:617–624. [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2001;91:2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]