Abstract
Although individuals born prematurely have subtle white matter abnormalities and are at risk for cognitive dysfunction, few studies have examined functional reorganization in these individuals. In this study we use magnetoencephalography (MEG) to examine cortical reorganization related to prematurity. Thirty-one adolescents systemically selected from a longitudinal study on child development based on gestational age, birth weight and neonatal complications (full term, low-risk premature, high-risk premature) and reading ability (good, average or poor) performed two reading-based rhyme tasks during MEG recording. Equivalent current dipoles were localized every 4ms during the 150ms to 550ms period following the onset of the word presentation. The association of the mean number of dipole (NOD) with birth risk, reading ability and latency was examined. During the real-word rhyme task, adolescents born at high-risk demonstrated a greater NOD in the left prefrontal area than those born at low-risk and term. During the non-word rhyme task, good and average readers born at high-risk demonstrated a greater NOD in the left prefrontal area than good and average readers born at low-risk and term. Time course analysis confirmed increased activation in the left prefrontal regions of those born at high-risk. This study suggests that adolescents born prematurely at high-risk, as compared to those born at low-risk and term, demonstrate increased prefrontal cortical activation during a reading task. These results suggest a reorganization of the prefrontal cortex in adolescents born prematurely at high-risk.
Keywords: Cortical reorganization, Prematurity, Reading, Magnetoencephalography
1. Introduction
Premature children have a unique pattern of cognitive deficits. For example, they have difficulties with executive function (Anderson and Doyle, 2004; Aylward, 2002; Bayless and Stevenson, 2007), visuospatial skills (Aylward, 2002; Waber and McCormick, 1995), and early language development (Sansavini et al., 2006; Sansavini et al., 2007; Stolt et al., 2007) and are also more likely to be diagnosed with a verbal or non-verbal learning disability, as compared to children born at term, despite adequate intelligence (Kirkegaard et al., 2006; Short et al., 2003; Wolke and Meyer, 1999). To better understand the relation between cognitive skills and reading disability in children born prematurely we recently investigated the cognitive profiles of premature and term children with good, average and poor reading ability (Frye et al., 2009a). We found that, reading ability and premature birth interacted such that children born prematurely at high-risk with poor reading ability were more likely than the other groups to perform poorly on executive function tasks, thus representing a unique subset of children with more severe cognitive deficits.
Weak executive function in high-risk, prematurely born children with poor reading ability suggests poor connectivity between frontal cortical areas responsible for executive control and other brain regions. Anatomic and functional neuroimaging support this notion. Diffusion tensor imaging (DTI) has demonstrated abnormal microstructure in white matter pathways connecting frontal area with the parietal, temporal and occipital areas in children born prematurely (Cheong et al., 2009; Constable et al., 2008; Skranes et al., 2007). Attention deficit disorder in premature children has been linked to microstructure differences in the longitudinal fasciculi (Skranes et al., 2007). Functional MRI (fMRI) activation during a passive auditory language listening task has demonstrated that prematurely born children, as compared to full-term children, modulated the parietal-temporal areas more and the frontal areas less, as compared to resting baseline (Ment et al., 2006). These results may represent a general failure in engagement of frontal systems during cognitive tasks in children born prematurely.
Poor white matter connectivity between the frontal and posterior brain regions may be detrimental to learning to read as connections between the temporal-parietal area and inferior frontal gyrus is especially important during reading (Pugh et al., 2000). This may be exceptionally detrimental to individuals with reading disability as neuroimaging studies suggest that full-term individuals with a history of reading disabilities overactivate frontal brain areas, presumably to compensate for dysfunctional cognitive reading networks (Pugh et al., 2000; Shaywitz et al., 2003). However, it is not known whether this applied to those born preterm. For example, our recent study suggests that only children born prematurely at high-risk with poor reading ability, as compared to those with average and good reading ability, are more likely to manifest executive function difficulties (Frye et al., 2009a). Thus we predict that cortical neural systems may reorganize to process language in children born prematurely at high-risk with average and good reading skills using frontal cortical areas for compensation, and poor readers born prematurely at high-risk fail to use frontal areas for compensatory processes. Identifying and understanding patterns of reorganization in this population can provide insight into the underlying mechanisms associated with their development and may provide a marker for cognitive dysfunction.
To identify patterns of functional reorganization we recorded neuromagnetic activity, using magnetoencephalograph (MEG), during a real-word and non-word reading-based rhyme task, in order to examine the function of the lexical and sublexical reading pathways individually. This is essential for several reasons. First, since phonological awareness is believed to be the basis of reading disability it is important to examine sublexical decoding, a processes that is highly dependent on phonological awareness. Second, previous studies have hypothesized that children born prematurely engage regions associated with semantic processing during phonological tasks (Ment et al., 2006). Contrasting brain activation during real-word and non-word tasks allows us to control for activation of brain areas related to semantic processing since non-words do not have meaning.
2. Results
Participant Characteristics
§
Neither the number of males nor females nor age was significantly different across reading or birth groups. Poor readers demonstrated a significantly lower Stanford-Binet quantitative skills score than good readers [F(4,22)=7.55,p<.01]. Birth weight [F(2,22)=222.91,p<.0001] and gestational age [F(2,22)=132.05 p<.0001] were significantly different across birth groups.
Woodcock-Johnson-III Word Attack [F(2,22)=9.47,p=.001] and Letter-Word Identification [F(2,22)=6.04,p<.01] and Comprehensive Test of Phonological Processing Phoneme Reversal [F(2,22)=9.42,p=.001] scores were different across reading groups. Poor readers demonstrated lower Word Attack, Phoneme Reversal and Letter-Word Identification scores than good readers and lower Word Attack and Phoneme Reversal scores than average readers. Comprehensive Test of Phonological Processing Rapid Naming scores were not difference across reading or birth groups. Continuous Performance Task Inattention scores were influenced a birth by reading group interaction [F(4,22)=4.02,p=.01] due to the fact that poor reader demonstrated higher Inattention scores than average and good readers for the high-risk [F(2,7)=25.79,p<.001] but not low-risk or full-term birth groups.
Real Word Rhyme Task
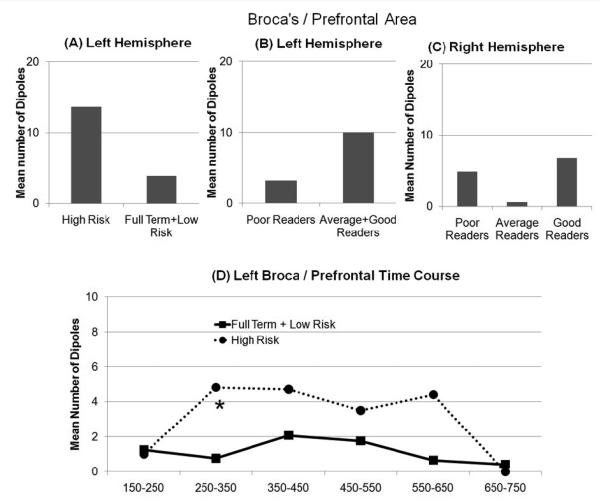
Sensitivity was not significantly different across birth or reading groups. The number of dipoles (NOD) in left Broca's and prefrontal areas combined (B/PFA) differed across birth [χ2(2)=7.11,p<.05] and reading [χ2(2)=6.02,p<.05] groups. NOD was greater for the high-risk group as compared to the other birth groups [χ2(1)=7.11,p<.01] but the term and low-risk groups did not differ from each other (Fig 1A). NOD was lower for poor readers as compared to the other reading groups [χ2(1)=6.00,p=.01] but the good and average readers were not different from each other (Fig 1B). NOD in the right B/PFA differed across reading groups [χ2(2)=8.54,p=.01]. Average readers demonstrated less NODs as compared to good [χ2(1)=9.57,p<.01] and poor [χ2(1)=8.15,p<.01] readers but good and poor readers did not differ from each other (Fig 1C).
Figure 1.
Mean number of dipoles in the left combined Broca's and prefrontal area during the real word rhyme task. (A,B,C) Mean number of dipoles over the 400ms (150ms-550ms) analysis period. (D) Time course analysis showing mean number of dipoles per 100ms for an extended period (* indicates a significant difference between the birth groups). Note that error bars are not included in the graphs since these data are derived from a discrete distribution in which the mean and variance are directly related to each other.
Nonword Rhyme Task
Sensitivity was different across reading groups [F(2,22)=13.08,p<.001] due to the fact poor readers [Mean(SE)=1.78(.31)] demonstrated a lower sensitivity than average readers [Mean(SE)=2.86(.19); t(22)=3.14, p<.01] and good readers [Mean(SE)=3.63(.24); t(22)=5.00,p<.0001].
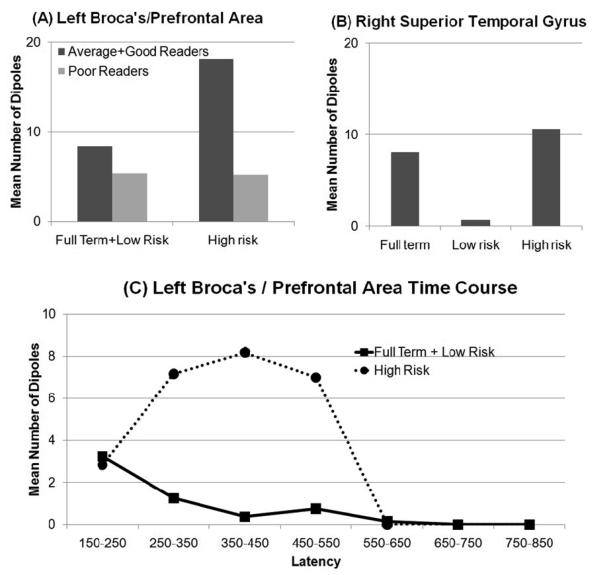
The NOD in the left B/PFA was dependent on a birth by reading group interaction [χ2(2)=14.152,p<0.05]. NOD was different across birth group for the good and average readers combined [χ2(2)=5.92,p=0.05]. High-risk good and average readers demonstrated significantly more NOD than low-risk and term good and average readers [χ2(1)=6.25,p=0.01] while the NOD did not differ between the latter two birth groups (Fig 2A). NOD was not different across birth groups for poor readers or across reading groups for high-risk or full-term and low-risk birth groups combined.
Figure 2.
Dipole activation in the (A) combined Broca's and prefrontal area and (B) right superior temporal gyrus, during the non-word rhyme task. (A,B) Mean number of dipoles over the 400ms (150ms-550ms) analysis period. (C) Time course analysis showing mean number of dipoles per 100ms for a extended period (* indicates a significant difference between the birth groups). Note that error bars are not included in the graphs since these data are derived from a discrete distribution in which the mean and variance are directly related to each other.
The NOD in the left superior temporal gyrus (STG) was dependent on a birth by reading group interaction [χ2(4)=12.75,p=0.01]. However, further examination using individual analyses did not demonstrate any consistent differences across birth or reading groups.
The NOD in the right STG differed across birth groups [χ2(2)=6.02,p<0.05]. The low-risk group demonstrated fewer NOD that the high-risk [χ2(1)=9.79,p<0.01] and full-term [χ2(1)=7.89,p<0.01] groups but the NOD did not differ between the high-risk and term groups (Fig 2B).
Time Course Analysis
The time courses in the left B/PFA during the two rhyme tasks were examined. The time course during the real word rhyme task revealed that the high risk group demonstrated a greater NOD than the other birth groups during the 250ms-350ms latency [χ2(1)=8.40,p<0.01] but not for any other time interval (Fig 1D). The time course during the non-word rhyme task revealed that the high-risk group demonstrated a greater NOD than the other birth groups during the 350ms-450ms latency [χ2(1)=6.41,p=0.01] but not for any other time interval (Fig 2C).
Dipole Localization
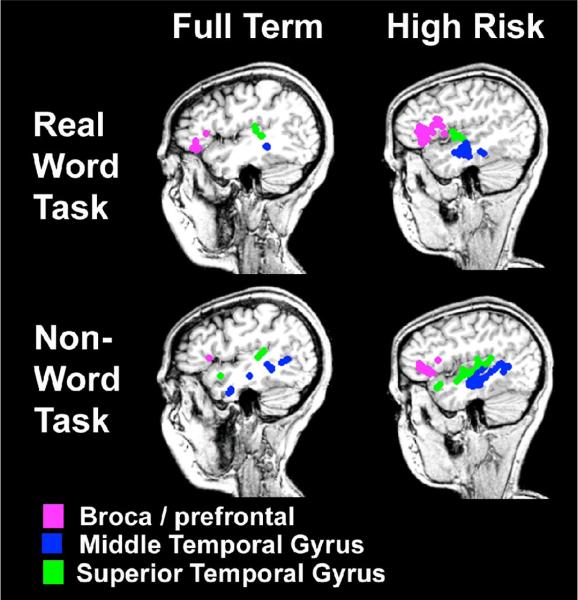
To determine if dipole localization differed across reading or birth groups we compared dipole localization between the groups that demonstrated significant differences in the analyses above. No significant differences were found. However, as an example of dipole localization for our participants we present case examples in Fig 3 for both the real word and non-word tasks and full term and high risk participants.
Figure 3.
Dipole localization for full term and high risk adolescents who were good readers for the two reading tasks. (Pink = Broca's / prefrontal area, blue = middle temporal gyrus, green= superior temporal gyrus).
3. Discussion
This is the first study to examine neuromagnetic responses in premature adolescents without obvious brain injury. In this study we examined cortical activation using MEG in term and high and low medical risk prematurely born adolescents with a wide range of reading skills during two reading-based rhyme tasks. The cohort of participants selected for this study paralleled our previous study. Overall, children previously categorized as poor readers, as compared to good and average readers, demonstrated poor sublexical decoding performance during adolescence, as measured by Word Attack skills and the non-word MEG language task, but only poor readers born prematurely at high-risk, as compared to good and average readers born prematurely at high-risk, demonstrated specific deficits in executive function, as measured by inattention on a continuous performance task during childhood.
Patterns of cortical activation were found to be different across reading groups for both the real-word and non-word tasks, with interactions between reading and birth groups for the non-word task. Interestingly, we found an atypical pattern of cortical activation for adolescents born prematurely at high-risk that was consistent across both language tasks. In both language tasks, adolescents born prematurely at high-risk demonstrate a particularly high NOD in the left B/PFA as compared to the other birth groups, with this effect seen primarily in the average and good readers for the non-word task. A time course analysis suggested that this difference was particularly greater during the 250ms-350ms latency for the real-word task and 350ms-450ms for the non-word task. The greater left B/PFA activation for the adolescents born prematurely at high-risk was limited to average and good readers, not poor readers, during the non-word rhyme task, thereby paralleling performance. This suggests that the pattern of frontal overactivation in average and good readers born prematurely at high-risk may represent a compensatory mechanism similar to that seen in full-term individuals with reading disability (Pugh et al., 2000; Shaywitz et al., 2003). This is evidence that poor-readers born prematurely at high-risk may fail to engage frontal brain areas to compensate for weak phonological processes.
Significant differences in NOD across reading groups were found primarily for the real-word rhyme task. Poor readers appear to have a lower NOD in typical left hemisphere language pathways, specifically in the left B/PFA, as compared to other readers and a greater NOD in homologous cortical regions in the right hemisphere, as compared to average readers. This is consistent with research that shows reduced activation of the left hemisphere language network in children with reading disabilities and a compensatory use of homologous right hemisphere areas (Simos et al., 2000; Simos et al., 2007). These data are consistent with neuroanatomical imaging on individuals with reading disability that demonstrate selective disturbance in left temporoparietal white matter oriented along the superior longitudinal fasciculus, the key tract connecting anterior and posterior language areas (Klingberg et al., 2000). Interestingly, like poor readers, good readers demonstrated greater NOD in the right B/PFA as compared to average readers during word reading, suggesting that, like poor readers, good readers may also utilize right hemisphere pathways to process words. This might suggest that good readers developed better reading skills compare to average readers as children because they recruited more cortical areas when reading. Since this is the first study to differentiate between average and good readers, future studies will be needed to verify this activation pattern.
Given the evidence of abnormal microstructure in white matter pathways connecting frontal areas with posterior brain regions (Cheong et al., 2009; Constable et al., 2008; Skranes et al., 2007) and fMRI evidence of decreased modulation of frontal areas during a language task in children born prematurely (Ment et al., 2006), one might have expected decreased activation of frontal regions during our reading tasks. However, in the present study, adolescents born prematurely at high-risk demonstrated a greater NOD as compared to low-risk and term adolescents in the left B/PFA for all readers during the real-word task and for the average and good readers during the non-word task. Since our dependent variable is a temporal measure of maximal activation, these data suggested that B/FPA activity is ongoing for a longer period of time in individuals born premature at high-risk. Such a prolongation of activity is also suggested by examination of the time course graphs comparing the birth groups.
The disparity between these MEG results and the previously reported fMRI results are unclear, but others have shown a disassociation between MEG and fMRI activation during equivalent language tasks in the past with normal adults, although with fMRI demonstrating greater frontal activation (Billingsley-Marshall et al., 2007). Differences in neuroplasticity of the frontal lobe (as discussed below) or in the neurovascular coupling between full-term and premature children could change the underlying assumptions of imaging methods. Clearly further studies are needed to determine whether the differences between this study and fMRI studies are due to differences in the tasks or other factors, such as MEG analysis methodology. For example, distributed source modeling may be more sensitivity to the complex patterns of cortical activation that occur during reading. Nevertheless, if the findings of this study are validated, they may provide insight into the neuropathology associated with prematurity and serve as a biological marker of cortical reorganization in premature children.
Both animal models and human studies suggest white matter injury, whether severe or subtle, is the most common brain injury of prematurity (Khwaja and Volpe, 2008; Mewes et al., 2006). Many studies have confirmed that premyelinating oligodendrocytes loss underlies this white matter injury (Back et al., 2007; Khwaja and Volpe, 2008). Oligodendrocytes loss could result in temporal dispersion of the neural impulse which would desynchronize the neural transmission to the frontal lobes and spread out the activation over time. Although this might decrease the total number of neurons firing synchronously, resulting in the release of neurotransmitters at fewer synapses at any particular time, upregulation of post-synaptic receptors could compensate for this phenomenon. In addition, a mouse model of white matter damage in prematurity suggests that decreased levels of the oligodendrocyte regulatory protein Nogo-A is associated with axonal outgrowth (Weiss et al., 2004). Either one of these latter mechanisms of neuroplasticity could increase the number of connections from afferent axons to the frontal lobes.
Unfortunately, neuropathology studies and animal models of prematurity that manifest subtle brain injury are lacking. The survival of premature neonates has increased over the last two decades and improvements in perinatal care have reduced the severity of periventricular white matter injury, resulting in more children with subtle white matter injury (Back and Rivkees, 2004; Fanaroff et al., 2003). Thus, it is important to understand the underlying neuropathology and mechanisms of reorganization associated with prematurity in order to better target remedial and medical therapies. In addition, identifying the neuroimaging signature associated with cognitive function and dysfunction in children born prematurely may provide a biomarker for identifying premature children at risk for cognitive difficulties. An MEG signature of cognitive dysfunction may be especially helpful in adolescents born prematurely as MRI findings of white matter abnormalities may be limited in the ability to predict cognitive dysfunction (Rushe et al., 2001).
4. Experimental Procedure
Participants in the Longitudinal Study
The participants were selected from a longitudinal cohort of 360 children recruited from three hospitals in the Houston area. Term children had no significant prenatal, perinatal or neonatal complications. Children born preterm had a gestational age ≤36 weeks and a birth weight <=1600g and were demographically similar to those born term. The high-risk cohort had more severe medical neonatal complications as compared to the low-risk cohort, primarily bronchopulmonary dysplasia. Participants were excluded if they had nervous system abnormalities, symptomatic syphilis, short bowel syndrome, positive HIV antibody, the mother was <16 years of age or tested positive for drugs at the time of the child's birth or if English was not the primary language at home. Most participants were African-American (63.0%) with fewer being of Caucasian (20.1%) and Hispanic (15.0%) ethnicity. The sample contained predominately lower social economic status (SES) participants. There were slightly more females than males. Quality of schooling, SES, gender and ethnicity were not different across birth groups. Sixteen (5%) of the original cohort were eliminated due to two or more Stanford-Binet 4th Ed. quantitative skill scores below 85 during the 3rd, 5th or 7th grades. This subscale was used due to its relatively low language load.
In our previous study (Frye et al., 2009a) we clustered participants into poor, average, and good readers by analyzing the growth of phonological word decoding as indexed by Word Attack (McGrew et al., 2007) during the 3rd, 5th and 7th grades. Cluster analysis using Ward's technique (Ward, 1963) determined the number of clusters and a K-means clustering algorithm reassigned participants into clusters. Prior to clustering 91 participants (25%) were eliminated due to attrition, leaving a total of 253 participants.
Selection of adolescents for the current study
We selected five participants prototypic of each reading and birth combination (Word Attack level and growth near the group centroid) evenly distributed across gender (Table 1). Of these, 9 declined to participate, one demonstrated ventriculomegally, one could not perform the tasks, one had unilateral deafness, and technical problems arose with two. Right-handedness was confirmed by a laterality index as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971) score greater than 50 (Dragovic, 2004). After description of the study, written informed consent was obtained in accordance with our Institutional Review Board regulations. Participants were tested on reading-related skills to verify their reading group assignments.
Table 1.
Participant characteristics. Number of male and female participants and mean (standard error) age, Stanford-Binet quantitative skills score, birth weight and gestational age across reading and birth groups. Significant differences between groups are indicated.
| Male:Female | Poor | Average | Good | All Reading |
| Full-Term | 3:2 | 4:0 | 0:2 | 7:4 |
| Low Risk | 1:3 | 1:1 | 3:1 | 5:5 |
| High Risk | 3:0 | 1:3 | 1:2 | 5:5 |
| All Birth Groups | 7:5 | 6:4 | 4:5 | 17:14 |
| Age | Poor | Average | Good | All Reading |
| Full-term | 16.3 (.17) | 16.6 (.29) | 16.9 (.21) | 16.6 (.14) |
| Low Risk | 16.3 (.30) | 17.0 (.04) | 16.2 (.25) | 16.4 (.17) |
| High Risk | 15.6 (.32) | 16.8 (.19) | 15.9 (.36) | 16.2 (.22) |
| All Birth Groups | 16.1 (.16) | 16.8 (.13) | 16.3 (.20) | 16.4 (.11) |
| Quantitative skills | Poor | Average | Good | All Reading |
| Full-term | 97.1 (2.8) | 98.8 (1.0) | 100.6 (5.1) | 98.3 (1.5) |
| Low Risk | 90.6 (2.0) | 98.3 (8.7) | 98.5 (.7) | 95.3 (2.0) |
| High Risk | 89.9 (4.4) | 96.1 (5.9) | 106.7 (5.4) | 97.4 (3.6) |
| All Birth Groups | 93.1 (1.9) | 97.6 (2.6) | 101.7 (2.2)* | 97.1 (1.4) |
| Birth Weight | Poor | Average | Good | All Reading |
| Full-term | 3362 (139) | 3607 (251) | 3582 (158) | 3491 (110) |
| Low Risk | 1265 (96) | 1185 (175) | 1408 (43) | 1306 (55)** |
| High Risk | 825 (208) | 929 (17) | 960 (187) | 907 (75)**,*** |
| All Birth Groups | 2028 (352) | 2051 (435) | 1742 (360) | 1952 (216) |
| Gestational Age | Poor | Average | Good | All Reading |
| Full-term | 40.0 (0.0) | 40.0 (0.0) | 40.0 (0.0) | 40.0 (0.0) |
| Low Risk | 30.5 (1.2) | 30.5 (1.5) | 32.3 (1.0) | 31.2 (0.7)** |
| High Risk | 27.0 (1.7) | 28.5 (0.6) | 28.0 (1.0) | 29.7 (1.2)** ,*** |
| All Birth Groups | 34.5 (1.5) | 34.1 (1.9) | 32.6 (1.6) | 33.3 (1.0) |
Significantly different (p<=0.01) than poor readers;
Significantly different (p<0.001) than full-term;
Significantly difference (p<0.01) than low-risk
Performance of adolescents on reading-related tasks
Adolescents were tested on carefully selected reading-related skills (Table 2). Orthographic lexical and sublexical decoding was measured with Letter-Word Identification and Word Attack, respectively. Auditory phonological awareness and phonological speed-of-processing was assessed using Phoneme Reversal and Rapid Naming, respectively. We also present the average Stanford-Binet 4th Ed. quantitative skill scores (Table 1) and Inattention scores during the 3rd, 5th or 7th grades.
Table 2.
Reading and executive function characteristics. Mean (standard error) for Woodcock-Johnson III Word Attack and Letter-Word Identification and Comprehensive Test of Phonological Processing Phoneme Reversal and Rapid Naming Composite tested during adolescence, and Continuous Performance Task Inattention scale tested during childhood. Significant differences between groups are indicated.
| Word Attack | Poor | Average | Good | All Groups |
| Full-Term | 87.3 (2.6) | 99.0 (3.2) | 105.5 (9.5) | 95.9 (3.2) |
| Low Risk | 81.5 (4.8) | 103.5 (11.5) | 108.5 (6.2) | 96.7 (5.6) |
| High Risk | 91.3 (7.2) | 97.8 (4.4) | 106.7 (4.8) | 98.5 (3.4) |
| All Birth Groups | 87.3 (2.7) | 99.4 (2.9)* | 107.2 (3.3)* | 97.0 (2.2) |
| Letter-Word ID | Poor | Average | Good | All Groups |
| Full-term | 93.8 (2.8) | 97.6 (5.3) | 104.0 (9.0) | 97.6 (2.7) |
| Low Risk | 84.5 (6.4) | 112.5 (8.5) | 103.0 (4.0) | 97.8 (5.1) |
| High Risk | 84.7 (5.2) | 96.8 (4.4) | 109.7 (6.9) | 97.0 (4.8) |
| All Birth Groups | 89.8 (3.3) | 99.0 (4.3) | 105.4 (3.2) * | 97.3 (2.3) |
| Phoneme Reversal | Poor | Average | Good | All Groups |
| Full-Term | 5.5 (.9) | 9.6 (1.5) | 9.5 (2.5) | 8.2 (.6) |
| Low Risk | 4.8 (1.3) | 8.5 (2.5) | 10.5 (.9) | 7.8 (1.2) |
| High Risk | 6.3 (.9) | 8.3 (.8) | 11.3 (.9) | 8.6 (.8) |
| All Birth Groups | 5.8 (.6) | 8.9 (.9)* | 10.6 (.7)* | 8.2 (.5) |
| Rapid Naming | Poor | Average | Good | All Groups |
| Full-term | 89.5 (2.6) | 93.4 (5.8) | 91.0 (3.0) | 91.9 (2.9) |
| Low Risk | 90.3 (9.9) | 101.5 (22.5) | 99.3 (8.1) | 94.3 (6.1) |
| High Risk | 83.0 (15.5) | 94.0 (6.8) | 103.0 (3.0) | 93.4 (5.4) |
| All Birth Groups | 88.5 (4.6) | 95.2 (5.1) | 98.7 (3.8) | 93.6 (2.7) |
| Inattention | Poor | Average | Good | All Groups |
| Full-term | 5.6 (2.4) | 5.7 (1.6) | 2.0 (.5) | 5.0 (1.2) |
| Low Risk | 6.4 (2.6) | 3.3 (2.3) | 7.2 (1.9) | 6.1 (1.3) |
| High Risk | 15.9 (0.8) | 4.5 (1.6)* | 2.8 (1.1)* | 7.4 (2.0) |
| All Birth Groups | 8.5 (1.8) | 4.8 (1.0) | 4.6 (1.2) | 6.1 (0.9) |
Significantly different (p<=0.01) than poor readers
MEG Language Task
Thirty-one participants performed six minute real-word and non-word rhyme tasks during the MEG recording session with a 3 minute break separating tasks. Participants were required to determine if two consecutively presented words rhymed. Real words were either high frequency non-exception (42%) or exception (58%) words while non-words were non-pseudohomophone pronounceable letter strings. Real words and nonwords had similar orthographic characteristics, including similar mean length and unconstrained bigram frequency. Each word was displayed in the center of the screen for 700ms and the words were separated by a 500ms interstimulus interval. The participant responded using a response pad. The intertrial interval lasted from 2 to 3 seconds. A cross was displayed in the center of the screen when the words were not displayed. Each task contained 68 trials with words rhyming in half of the trials. Tasks were controlled by Presentation™ (Neurobehavioral Systems, Albany, CA).
Performance Measurements
To obtain a pure measurement of performance without regard to performance bias, a signal detection paradigm was used. Using such a paradigm, rhyme trials were considered the signal+noise trials while the non-rhyme trials were considered the noise trials. Sensitivity (d-prime) was calculated using standard signal detection paradigm method from the hit and false alarm rates assuming an equal variance model (i.e., z(Hit Rate) – z(False Alarm Rate)).
MEG Data Acquisition
Recordings were made in a magnetically shielded room with a WH3600 (4D Neuroimaging, San Diego, CA) whole-head neuromagnetometer that consisted of 248 axial gradiometers in a cryogenic dewar. The signal was continuously sampled at 500Hz and filtered on-line with a bandpass filter between 0.1 and 50 Hz. Event-related fields (ERFs) were extracted and averaged after removing trials during which eye movement or blink occurred. The averaged epochs were digitally lowpass filtered at 20 Hz.
Magnetic Resonance Imaging (MRI)
Two sets of structural T1 MRIs were acquired for each participant using a 3T Siemens Sonata scanner (Malvern, PA). The best quality of the two images was used for dipole localization. All scans were reviewed by a board-certified radiologist and/or neurologist. Adolescents with any cortical or significant subcortical abnormality were eliminated.
Magnetic Source Imaging
A detailed account of the magnetic source imaging is provided elsewhere (Frye et al., 2009b; Papanicolaou et al., 1999). The intracranial sources of the average ERFs were modeled as single equivalent current dipoles (ECDs). An ECD was fitted at successive 4ms intervals using a non-linear fitting algorithm. The algorithm was applied to an automatically selected group of 34-38 magnetometers that included both magnetic flux extrema. ECD solutions were considered satisfactory if the predicted and observed magnetic field distribution correlated with r > 0.90. The ECDs were coregistered with the structural MRI (Papanicolaou et al., 1999).
Cortical Regions of Interest
We selected regions of interest (ROI) described to be activated during reading. These areas included Broca's and prefrontal areas combined, the frontal and supplementary motor areas combined (F/SMA), the middle temporal gyrus, superior temporal and Hescl's gyri combined and the supramarginal and angular gyri combined (SM/AG). Areas were combined due to their proximity and coactivation.
Statistical Analysis
Participant characteristics, scores on reading and cognitive tasks and performance during the MEG tasks were analyzed using a two-way analysis of variance as implemented by the ‘genmod’ procedure in SAS 9.1 (SAS Institute Inc., Cary, NC).
NOD in ROIs over a specific time interval was the dependent measure (Papanicolaou et al., 2006). A dipole represents the maximal brain activation over a 4ms period and NOD, our dependent variable, is a measure on the number of 4ms periods that a particular ROI has such activity over a particular latency. Since NOD was quite skewed we modelled the data using a log link function and a negative binomial as implemented with the ‘genmod’ procedure in SAS 9.1 (SAS Institute Inc., Cary, NC) (Papanicolaou et al., 2006; Zeger and Liang, 1986). In order for this statistical model to be valid the mean NOD across participants needed to be greater than two. F/SMA and SM/AG did not reach this criterion.
We conducted a two stage analysis. First, we identified whether the NOD differed in a particular ROI across reading ability (good, average, poor) and/or risk level (full-term, low-risk premature, high-risk premature) from 150ms to 550ms following the onset of the stimulus. This model was computed for each ROI, task and hemisphere separately. Orthogonal contrasts were used for planned post-hoc comparison. For birth risk, we compared high-risk vs the other groups and low-risk vs full-term and for the reading group we compared poor readers vs the other groups and average vs good readers. If both contrasts for each effect were significant, additional contrasts comparing all levels of an effect were conducted. For reading by risk group effects we conducted separate analyses broken down by each effect.
For the second step, we selected particular analyses in which NOD differed significantly between the high-risk and the other risk groups for a more detailed time course analysis. In the time course analysis we determined the difference in the NOD across risk groups every 100ms from 150ms to 550ms. Additionally, we provide examples of dipole localization.
Acknowledgement
This study was supported by grant NS046565 to Dr. Richard E. Frye and HD25128 to Dr. Susan Landry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study has not been presented or published previously.
Financial disclosure: None
Conflict of interest: None
References
- Anderson PJ, Doyle LW. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114:50–7. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Cognitive and neuropsychological outcomes: more than IQ scores. Ment Retard Dev Disabil Res Rev. 2002;8:234–40. doi: 10.1002/mrdd.10043. [DOI] [PubMed] [Google Scholar]
- Back SA, et al. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–30. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28:405–14. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Bayless S, Stevenson J. Executive functions in school-age children born very prematurely. Early Hum Dev. 2007;83:247–54. doi: 10.1016/j.earlhumdev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Billingsley-Marshall RL, et al. A comparison of functional MRI and magnetoencephalography for receptive language mapping. J Neurosci Methods. 2007;161:306–13. doi: 10.1016/j.jneumeth.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Cheong JL, et al. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. AJNR Am J Neuroradiol. 2009;30:623–8. doi: 10.3174/ajnr.A1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable RT, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306–16. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Dragovic M. Categorization and validation of handedness using latent class analysis. Neuropsychiatrica. 2004;16:212–218. doi: 10.1111/j.0924-2708.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Fanaroff A, et al. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Seminar in Perinatology. 2003;27:281–287. doi: 10.1016/s0146-0005(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Frye RE, et al. Executive dysfunction in poor readers born prematurely at high risk. Dev Neuropsychol. 2009a;34:254–71. doi: 10.1080/87565640902805727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, et al. Functional Neuroimaging of language using magnetoencephalography. Physics of Life Reviews. 2009b;6:1–10. doi: 10.1016/j.plrev.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–61. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard I, et al. Gestational age and birth weight in relation to school performance of 10-year-old children: a follow-up study of children born after 32 completed weeks. Pediatrics. 2006;118:1600–6. doi: 10.1542/peds.2005-2700. [DOI] [PubMed] [Google Scholar]
- Klingberg T, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- McGrew K, et al. Technical Manual. Woodcock-Johnson III Normative Update., vol. Riverside Publishing; Rolling Meadows, IL: 2007. [Google Scholar]
- Ment LR, et al. Cortical recruitment patterns in children born prematurely compared with control subjects during a passive listening functional magnetic resonance imaging task. J Pediatr. 2006;149:490–8. doi: 10.1016/j.jpeds.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewes AU, et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118:23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, et al. Functional neuroimaging with MEG: normative language profiles. Neuroimage. 2006;33:326–42. doi: 10.1016/j.neuroimage.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, et al. Magnetoencephalographic mapping of the language-specific cortex. J Neurosurg. 1999;90:85–93. doi: 10.3171/jns.1999.90.1.0085. [DOI] [PubMed] [Google Scholar]
- Pugh KR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment Retard Dev Disabil Res Rev. 2000;6:207–13. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Rushe TM, et al. Neuropsychological outcome at adolescence of very preterm birth and its relation to brain structure. Dev Med Child Neurol. 2001;43:226–33. doi: 10.1017/s0012162201000433. [DOI] [PubMed] [Google Scholar]
- Sansavini A, et al. Early relations between lexical and grammatical development in very immature Italian preterms. J Child Lang. 2006;33:199–216. doi: 10.1017/s0305000905007208. [DOI] [PubMed] [Google Scholar]
- Sansavini A, et al. Are early grammatical and phonological working memory abilities affected by preterm birth? J Commun Disord. 2007;40:239–56. doi: 10.1016/j.jcomdis.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, et al. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Short EJ, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112:e359. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, et al. Brain activation profiles in dyslexic children during non-word reading: a magnetic source imaging study. Neurosci Lett. 2000;290:61–5. doi: 10.1016/s0304-3940(00)01322-7. [DOI] [PubMed] [Google Scholar]
- Simos PG, et al. Altering the brain circuits for reading through intervention: a magnetic source imaging study. Neuropsychology. 2007;21:485–96. doi: 10.1037/0894-4105.21.4.485. [DOI] [PubMed] [Google Scholar]
- Skranes J, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–66. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Stolt S, et al. Size and composition of the lexicon in prematurely born very-low-birth-weight and full-term Finnish children at two years of age. J Child Lang. 2007;34:283–310. doi: 10.1017/s0305000906007902. [DOI] [PubMed] [Google Scholar]
- Waber DP, McCormick MC. Late neuropsychological outcomes in preterm infants of normal IQ: selective vulnerability of the visual system. J Pediatr Psychol. 1995;20:721–35. doi: 10.1093/jpepsy/20.6.721. [DOI] [PubMed] [Google Scholar]
- Ward J. Hierarchical grouping to optimize an objective function. Journal of American Statistical Society. 1963;77:841–847. [Google Scholar]
- Weiss J, et al. Neonatal hypoxia suppresses oligodendrocyte Nogo-A and increases axonal sprouting in a rodent model for human prematurity. Exp Neurol. 2004;189:141–9. doi: 10.1016/j.expneurol.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Wolke D, Meyer R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: the Bavarian Longitudinal Study. Dev Med Child Neurol. 1999;41:94–109. doi: 10.1017/s0012162299000201. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]