Abstract
Neurologic and psychiatric (NP) manifestations are severe complications of systemic lupus erythematosus (SLE). As commonly seen in patients, spontaneous disease onset in the MRL/MpJ-Faslpr/ J (MRL-lpr) mouse model of NP-SLE is accompanied by increased autoantibodies, proinflammatorycytokines and behavioral dysfunction which precede neuroinflammation and structural brain lesions. The role of purinergic receptors in the regulation of immunity and behavior remains largely unexplored in the field of neuropsychiatry. To examine the possibility that purinoception is involved in the development of affective behaviors, the P2X purinoceptor antagonist, suramin, was administered to lupus-prone mice from 5 to 14 weeks of age. In addition to food and water measures, novel object and sucrose preference tests were performed to assess neophobic anxiety- and anhedonic-like behaviors. Enzyme-linked immunosorbant assays for anti-nuclear antibodies (ANA) and pro-inflammatory cytokines were employed in immunopathological analyses. Changes in dendritic morphology in the hippocampal CA1 region were examined by a Golgi impregnation method. Suramin significantly lowered serum ANA and prevented behavioral deficits, but did not prevent neuronal atrophy in MRL-lpr animals. In a new batch of asymptomatic mice, systemic administration of corticosterone was found to induce aberrations in CA1 dendrites, comparable to the “stress” of chronic disease. The precise mechanism(s) through which purine receptor inhibition exerted beneficial effects is not known. The present data supports the hypothesis that activation of the peripheral immune system induces nociceptive-related behavioral symptomatology which is attenuated by the analgesic effects of suramin. Hypercortisolemia may also initiate neuronal damage, and metabolic perturbations may underlie neuro-immuno-endocrine imbalances in MRL-lpr mice.
Keywords: P2X purine receptors, suramin, neophobia, anhedonia, nociception, pain-related behaviors, analgesia, corticosterone, neuronal atrophy, metabolic perturbations
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic, multisystemic autoimmune/inflammatory disease characterized by a broad spectrum of clinical and immunological manifestations (Isenberg et al., 1989). In addition to serological manifestations (e.g. an imbalanced cytokine network and increased production of autoreactive antibodies), a significant number of SLE patients develop various neurologic and psychiatric (NP) symptoms, ranging from seizures and strokes to depression and psychosis (Denburg and Denburg 2003; Moskowitz, 1989). Frequent psychiatric symptoms, heralding the clinical manifestations of disease, include anxiety and anhedonia (Wekking, 1993; van Dam et al., 1994).
Similar to humans, MRL/MpJ-Faslpr/J (MRL-lpr) mice spontaneously develop an accelerated form of lupus-like disease accompanied by CNS involvement. In comparison to age-matched congenic MRL/MpJ (MRL +/+) controls, these animals also develop deficits reflective of anxiety and blunted hedonic responsiveness (Sakic et al., 1992, 1996). The constellation of all behavioral deficiencies has been operationally termed “autoimmunity-associated behavioral syndrome”, or AABS, and coincides with a profound divergence in the immune statuses of the two MRL substrains around 8 weeks of age (Szechtman et al., 1997). In MRL-lpr mice, brain growth is retarded (Sakic et al., 1998; Ballok et al., 2004a) and ventricles increase in size along with the accelerated emergence of autoimmune manifestations (Denenberg et al. 1992). Reduced dendritic complexity and density of pyramidal neurons (Sakic et al., 1998, 2000), and evidence of degenerating axon terminals (Ballok et al. 2004b) are also common observations in brains of diseased animals by 14 weeks of age. The severe disruption of the blood-brain barrier, or BBB(Vogelweid et al., 1991), accompanies increased neurodegeneration and neuroinflammation observed between 5 and 6 months of age (Ballok et al., 2003a, 2006). No lesions are observed within the CNS of asymptomatic, behaviorally unimpaired congenic MRL +/+ controls. Few MRL-lpr animals survive beyond 6 months of age (Dixon et al., 1978; Fernandes and Good, 1984), which is likely due to fatal immune complex type glomerulonephritis and pernicious autoimmune/inflammatory encephalopathy.
Widespread neuronal injury and demyelination are proposed to form the structural basis of CNS manifestations at later stages of NP-SLE (Brooks et al., 1997). In MRL-lpr brains, while brain lesions in dopaminergic-rich regions can account for behavioral deficits in older animals with progressive disease (Ballok, 2007), early peripheral disease manifestations (prior to appreciable CNS damage) have also been associated with dysfunctional behavior (Szechtman et al., 1997). In fact, affective behaviors provide the earliest sign of disease onset, and aberrant performance in the novel object and sucrose preference tests are detected at the earliest stages of the disease process (Sakic et al., 1994, 1996). Evidence that immunosuppressive treatment, which also suppresses proinflammatory cytokines (Ballok et al., 2004a), abolishes early impairments in both of these tasks (Sakic et al., 1995, 1996) supports the notion that spontaneous autoimmune/inflammatory disease is linked to the pathophysiology of affective dysfunction in MRL-lpr animals. Therefore, in addition to autoantibodies, performance deficits may be mediated by increases in peripheral cytokines (Szechtman et al., 1997; Ballok et al., 2003b), similar to “sickness behavior” (Dantzer, 2001).
The biological significance of systemic purines in vivo as intercellular signaling molecules is only just beginning to be understood (Rathbone et al., 1999; Schmmidt et al., 2007). Extracellular purines (such as adenosine) activate P2X receptors, a family of ligand-gated ion channels (Burnstock, 2007). Purinergic signaling not only regulates numerous organ systems, it is also involved in embryonic development, CNS injury, and pain (Koles et al., 2007). Moreover, there is evidence that purinergic receptors play a role in the regulation of behavior and immunity. For example, motor and reward-related behaviors appear to be regulated through central purine and dopamine interactions (Kim and Palmiter, 2008), and recent studies examining immune mediators of behavioral depression in rats have revealed that drug and cytokine-induced depressive-like behavior is reversed by systemic injection of purine receptor antagonists (Minor et al., 2003, 2006). Similar antagonism has been found to suppress autoimmunity and signs of clinical disease in experimental allergic encephalomyelitis (EAE) afflicted mice (Novales-Li, 1996). To test the possibility that extracellular purinergic signaling plays a role in the spontaneous emergence of autoimmune/inflammatory disease, neuronal atrophy, and behavioral deficits in MRL-lpr animals, the P2X purinoceptor antagonist (Humphrey et al., 1995), suramin, was employed in the present study. An imbalanced neuro-immuno-endocrine network is also proposed to play a key role in the etiology of brain damage in lupus-like disease (Sakic et al., 1997a; Ballok, 2007), but whether central neurons can be initially damaged by an autoimmune-driven upregulation in corticosterone production (Shanks et al., 1999; Lechner et al., 2000) has not previously been examined in MRL mice. Therefore, the overall expectation was that chronic treatment with suramin would suppress the onset of peripheral disease, thereby preventing behavioral impairments, as well as early signs of neurodegeneration in NP-SLE mice. Chronic administration of corticosterone was also expected to produce atrophy of CA1 hippocampal dendrites in asymptomatic mice.
2. Materials and Methods
2.1 Animals
Twenty male MRL-lpr and 16 male MRL +/+ mice were purchased from Jackson Laboratories (Bar Harbor, ME). Animals arrived at four weeks of age and were housed 4 or 5 per cage in isolated rooms with a 12 hour light cycle (8:00 to 20:00). They habituated to the laboratory environment for one week before suramin treatment began. A second cohort of group housed MRL +/+ mice (20 males, 5 per cage) were obtained to assess whether glucocorticoids directly contribute to the altered dendritic morphology which characteristically develops along the progression of disease in autoimmune animals (Sakic et al., 1998, 2000). All experimental protocols were approved by the McMaster Animal Care Committee and were carried out in accordance with rules and regulations of the Canadian Council of Animal Care.
2.2 Drug administration
Half the mice in each substrain of the first cohort received either a 60 mg/kg intraperitoneal (i.p.) injection of suramin (Sigma Chemical Co., St. Louis, MO) while others received phosphatebuffered saline. Mice were injected once weekly, from 5 weeks of age until behavioral testing began at 14 weeks. All mice were separated into single cages several days prior to behavioral testing. In a second batch of MRL +/+ mice, the effects of hypercortisolemia on CA1 neurons was examined. A procedure similar to the well-established, noninvasive corticosterone treatment method was followed (Fairchild et al., 2003; Magarinos et al., 1998), which mimics the effects of chronic stress in rats (Magarinos et al., 1997; Donohue et al., 2006). In brief, corticosterone (Sigma, St. Louis, MO) was dissolved in ethanol and administered via the drinking water (n = 10) at a final concentration of 200 µg/ml, similar to a previously published protocol for mice (Pauly et al., 1988). The control group (n = 10) received the same percentage of ethanol in drinking water (1.2%) ad libitum over 18 days. Both corticosterone and vehicle solutions were made fresh each day. Testing began at 8 weeks of age, and postmortem dendritic complexity in the hippocampus was examined following euthanasia.
2.3 Body weight and food/water intake
In the first batch of mice, body weight was measured once a week from five weeks of age until the time animals were killed at 15 weeks. To measure food consumption beginning at 14 weeks, each mouse was restricted to pre-weighed pellets. Twenty-four hour consumption was assessed as a difference in the weight of three food pellets (initial weight ~10–13 g) placed on the cage floor. Daily water intake was determined by a difference in the bottle weight on two consecutive days over a 4-day testing period.
2.4 Behavioral tests
Two of the earliest and most profound behavioral deficits reflecting “emotional reactivity” in lupus-prone animals are less contact with a novel object and blunted responsiveness to sweet solutions (Szechtman et al., 1997). Therefore, these two tests were employed at age fourteen weeks, similar to protocols described before (Sakic et al., 1995, 2002). Briefly, when symptoms of disease are relatively mild, MRL-lpr mice explore novel objects less well than do their congenic controls (Sakic et al., 1995). This test serves to discriminate between approach and avoidance tendencies of animals toward novel stimuli and is postulated to reflect anxiety or neophobic-like behavior. In the present study, the test was conducted in a brightly lit room and filmed with a camera mounted to the ceiling and attached to a time code generator. Each mouse was placed into a square Plexiglas box (40 × 40 × 35 cm) with the sides and floor fully covered by black cardboard to occlude vision of the outer environment. After 10 minutes, a white plastic funnel (cylindrical diameter 25 cm and 50 cm in height) was placed, with the large open end down, into the center of the field. The animal’s behavior was then videotaped for an additional 10 min. After each session, the field was cleaned with standard glass cleaner and dried with paper towels. The following two measures were obtained from video tapes by an independent observer unaware of the group assignment of the mouse: 1) latency to make contact with the funnel, or touch latency, and 2) contact duration, assessed as time spent sniffing, biting, touching (with the paws or snout), during the session. MRL animals were also given access to a sucrose solution over three consecutive nights. More specifically, in addition to a standard tap water bottle, a plastic 10-cc syringe filled with 4% sucrose solution was added onto the cage lid, allowing a mouse to drink from it over a 60-min period (9:30–10:30 PM). Being choice paradigms with minimal activity demands, the 1-hour sucrose test has revealed that diseased MRL-lpr animals show a blunted concentration-intake response in comparison to less symptomatic MRL +/+ controls (Sakic et al., 1996; Ballok et al., 2003b). This impairment is proposed to represent anhedonia (i.e. loss of the capacity to experience pleasure), which is one of two core symptoms of depression (Monleon et al., 1995).
2.5 Golgi-impregnation method assessing dendritic complexity in the hippocampus
Hippocampal neurons, and those cultured from the CA1 area in particular (Mattson and Kater, 1989), are among the most sensitive to metabolic insults (Sapolsky, 1996). Given this fact, the CA1 region was selected to assess early disease-associated changes in neuron morphology. A detailed protocol is described elsewhere (Sakic et al., 1998). In brief, following immersion in Golgi- Cox solution (i.e. a mixture of K2 Cr2 O7, HgCl2 and K2CrO4 water solutions), fixed tissue was sectioned at 200 µm, and morphological analysis was performed by a technician blinded to the experimental design (Gibb and Kolb, 1998). In the CA1 region of the hippocampus, basilar fields were measured for branching based on criteria previously defined (Sakic et al., 2000). Briefly, the dendrite was traced (1000x) using a camera lucida drawing tube and the exact length of the dendritic segment was calculated by placing a thread along the drawing and measuring (µm) the thread length. As a result of incomplete perfusion of tissue, eight brains were excluded from subsequent analyses in the following groups: MRL-lpr mice (2 suramin, 4 saline); MRL +/+ mice (1 suramin, 1 saline).
2.6 Indices of autoimmunity
Eight days after the final suramin injection, animals were killed and spleens were weighed immediately upon extraction using a scientific digital scale (Sartorius 2024 MP). Given that high levels of anti-nuclear antibodies (ANA) are a typical serologic manifestation of the lupus-like disease (Dixon et al., 1978), this marker was used to differentiate immune statuses between the groups. Terminal bleeding from the vena cava was performed under Somnotol anesthesia (i.p. 60 mg/kg body weight;MTC Pharmecuticals, Cambridge, ON). Blood samples (~1 ml) were left to coagulate in small plastic vials before centrifugation for 10 min at 3000 rpm. Serum were separated from the clot and stored at −20 °C until further analysis could be performed. ANA concentration was measured using the Mouse Anti-Nuclear Antibodies (ANA) total immunoglobulins (Ig's)ELISA kit (Cat. #5200), according to the manufacturer’s instructions (Alpha Diagnostic International, San Antonio, TX) and the protocol previously described (Ballok et al., 2003b). This kit is specific for detecting ANA Ig’s (IgG, IgA and IgM) in mouse serum.
Given the importance of pro-inflammatory cytokines in the induction of sickness behavior (Dantzer, 2001) and their association with the appearance of some behavioral deficits in MRL-lpr mice (Ballok et al., 2003b; Ballok et al., 2004a), serum levels of tumor necrosis factor (TNF)-alpha and interleukin (IL)-1 beta were measured in the present study. In particular, the synthesis of TNF-alpha coincides with the initial onset of autoimmunity in the MRL-lpr substrain, and IL-1 beta synthesis arises later in disease (Ballok et al., 2003b). Serum samples were analyzed for these cytokines by murine ELISA kits from R & D Systems (Minneapolis, MN). The procedure was performed according to recommendations from the manufacturer, as previously described (Ballok et al., 2003b).
2.7 Statistical analysis
Variance in the data was analyzed by a one-way ANOVA with Substrain (MRL-lpr vs. MRL+/+) and Treatment (suramin vs. saline) as between group factors, followed by Tukey’s HSD post-hoc analysis to make pair-wise comparisons. Tukey’s multiple comparisons were performed at 0.05 significance level. In neuromorphological analyses, where differences among only two normally distributed groups of data were compared, a Student’s t-test was employed. Significance level was set at p ≤ 0.05 and all computations (including Pearson’s correlation) were performed using the SPSS 15 statistical package. Graphs show means ± S.E.M., and p ≤ 0.05, p ~ 0.01 and p ~ 0.001 are represented as *, **, and ***, respectively.
3. Results
3.1 Effects of suramin on body weight and food/water intake
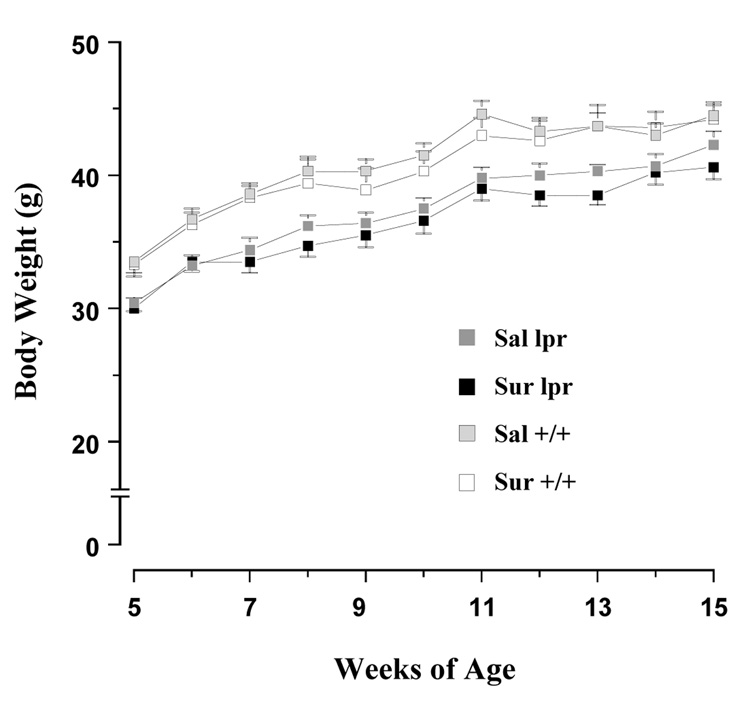
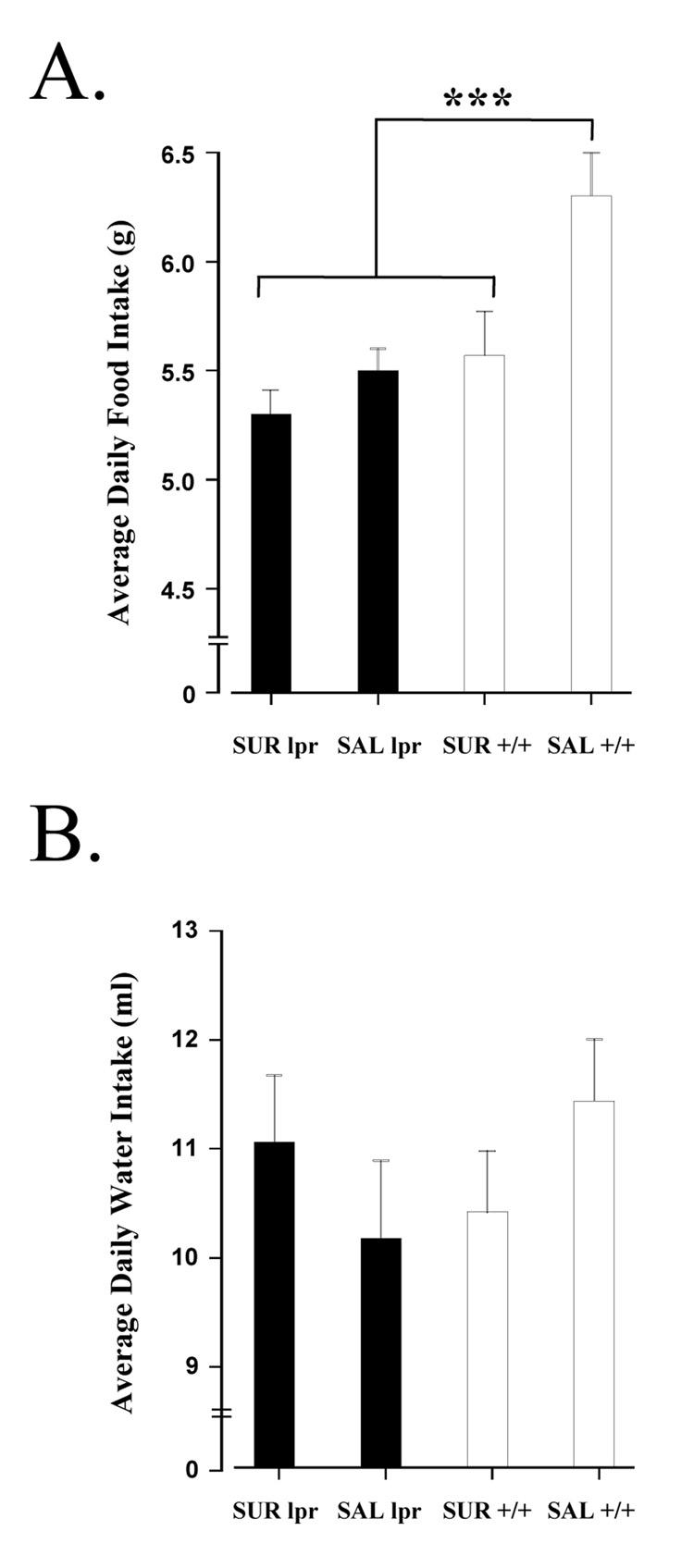
As shown in Figure 1, all groups gained weight between 5 and 15 weeks of age. Analyses using one-way ANOVA followed by Tukey HSD tests indicated that MRL-lpr mice weighed significantly less than the MRL +/+ controls at the onset of the experiment (at 5 weeks: F(3, 31) = 5.020, p = .006), and this difference persisted throughout the study. When examining the amount of food consumed, a difference was detected between groups (F(3, 31) = 18.027, p < 0.001; Fig. 2A). Tukey’s multiple comparison test found that this difference existed between the MRL+/+ saline and other three groups (p < 0.001). The observation that MRL +/+ control animals consumed more food than MRL-lpr mice is consistent with previous reports (Ballok et al., 2003b, 2006). Suramin treatment reduced food intake in only the MRL +/+ group (MRL+/+ saline vs. MRL+/+ suramin, p < 0.001; MRL-lpr saline vs. MRL-lpr suramin, p = 0.082). When assessing average daily water intake, no differences were detected among the groups (F(3, 31) = 1.199, n.s.; Fig. 2B).
Fig. 1.
The time course of body weight changes. Similar to a previous report (Strassmann et al., 1993), suramin treatment did not affect body growth rates of mice in the present study. Although MRL-lpr animals weighed less than MRL +/+ mice at the outset of the experiment, all animals continued to gain weight throughout the testing period.
Fig. 2.
Measures of relative food and water intake. (A) Unrestricted daily food consumption was measured prior to brief sucrose preference tests. As previously reported, autoimmune MRL-lpr mice consumed less food than untreated congenic controls (Ballok et al., 2003b). Suramin, however, significantly reduced food intake in MRL +/+ animals which is likely related to metabolic differences between the substrains. (B) Unrestricted daily water intake was measured prior to brief sucrose preference tests. No difference in the amount of water ingested was found in comparisons between the groups.
3.2 Effects of suramin on measures of neophobic- and anhedonic-like behavior
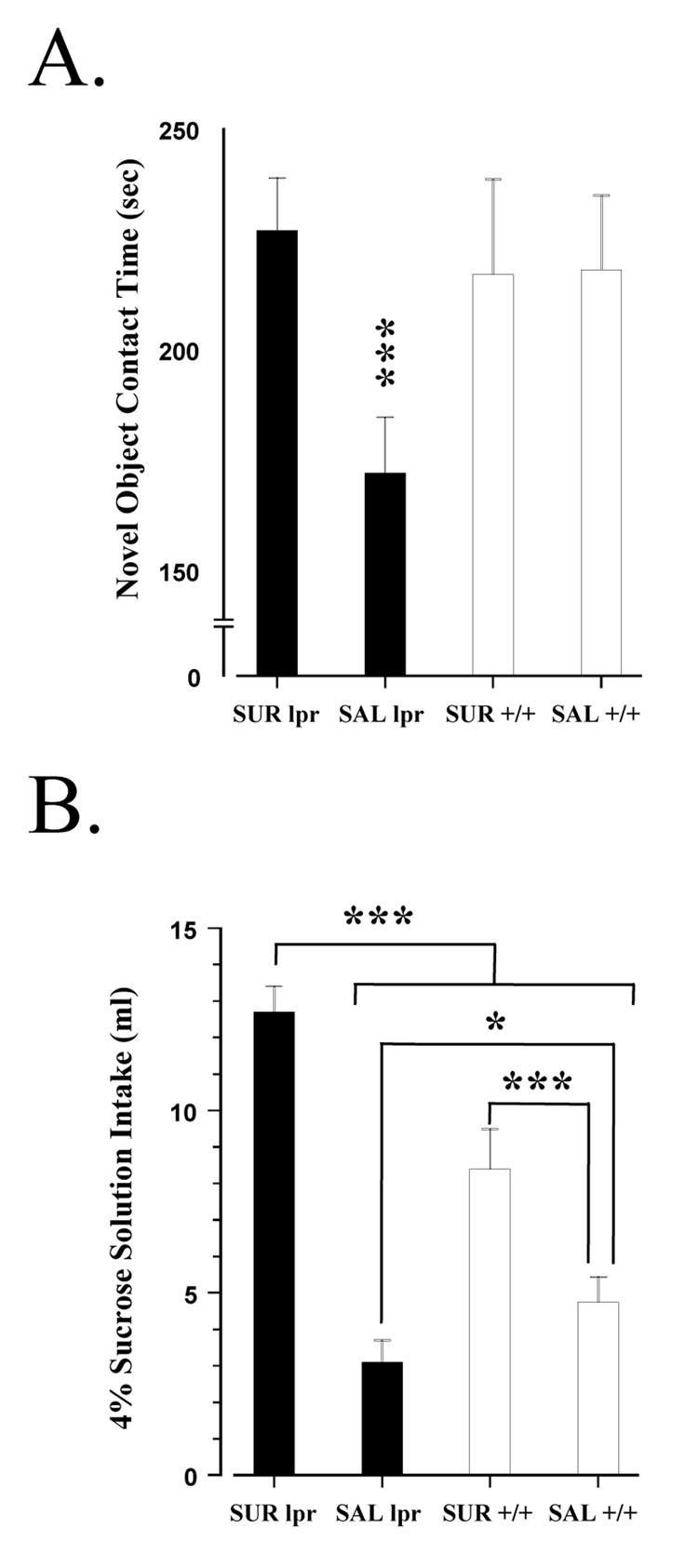
In comparison to congenic controls, MRL-lpr mice appeared to take longer to make initial contact with a novel object (MRL-lpr: 7.8 ± 1.0 vs.MRL+/+: 5.1 ± 1.2 sec), however, no differences were detected among the groups (F(3, 31) = 0.997, n.s.; data not shown). When examining total time spent in contact with the object, a group difference was detected (F(3, 31) = 5.155, p < 0.005; Fig. 3A). More specifically, post-hoc analysis revealed that contact time of suramin treated MRL-lpr mice did not differ from either MRL +/+ group, but that the MRL-lpr saline group differed from the other three (p < 0.001). In the analysis of the sucrose preference test (i.e. total intake of a 4% sucrose solution across three 60-min tests), a significant difference was also found (F(3,34) = 35.800, p < 0.001; Fig. 3B). Post-hoc analysis showed that these differences existed between the two MRL-lpr groups (p < 0.001) and between the MRL +/+ groups (p = 0.008). Furthermore, a difference was detected between the saline groups (MRL-lpr vs. MRL +/+, p = 0.03), with consumption being lowest in the 14-week-old MRL-lpr group. This result illustrates the anhedonic-like deficit that characteristically emerges in lupus-prone mice, relative to congenic controls (Sakic et al., 1996; Maric et al., 2001; Ballok et al., 2004a).
Fig. 3.
Behavioral performance between MRL substrains. (A) In the novel object test, untreated MRL-lpr mice exhibited increased anxiety-like behavior (i.e. distress to novelty), consistent with previous reports (Sakic et al., 1994, 1995). Suramin treatment, however, prevented the emergence of “neophobia” in MRL-lpr animals. (B) Total intake of sucrose in 60-min preference tests given over three consecutive nights. MRL-lpr saline controls consumed significantly less of the 4% sucrose solution (ml) in comparison to the other three groups. The difference between MRL-lpr saline and MRL+/+ saline animals has previously been reported (Sakic et al., 1996; Maric et al., 2001; Ballok et al., 2004a), and is proposed to reflect “anhedonia”. Suramin treatment, however, resulted in a 3-fold increase in sucrose consumption by MRL-lpr animals (relative to the MRL-lpr saline group). This increase could not be attributed to caloric requirements or water deprivation, and suggests a reversal of anhedonic-like behavior. The MRL +/+ drug group also showed elevated sucrose intake, but when considering the significant reduction in food consumption of this group (prior to sucrose testing), the caloric value of solution may account for the observed 2-fold increase. Alternatively, suramin treatment may have increased craving for carbohydrates in both MRL groups.
3.3 Neuromorphological changes in the CA1 region of the hippocampus
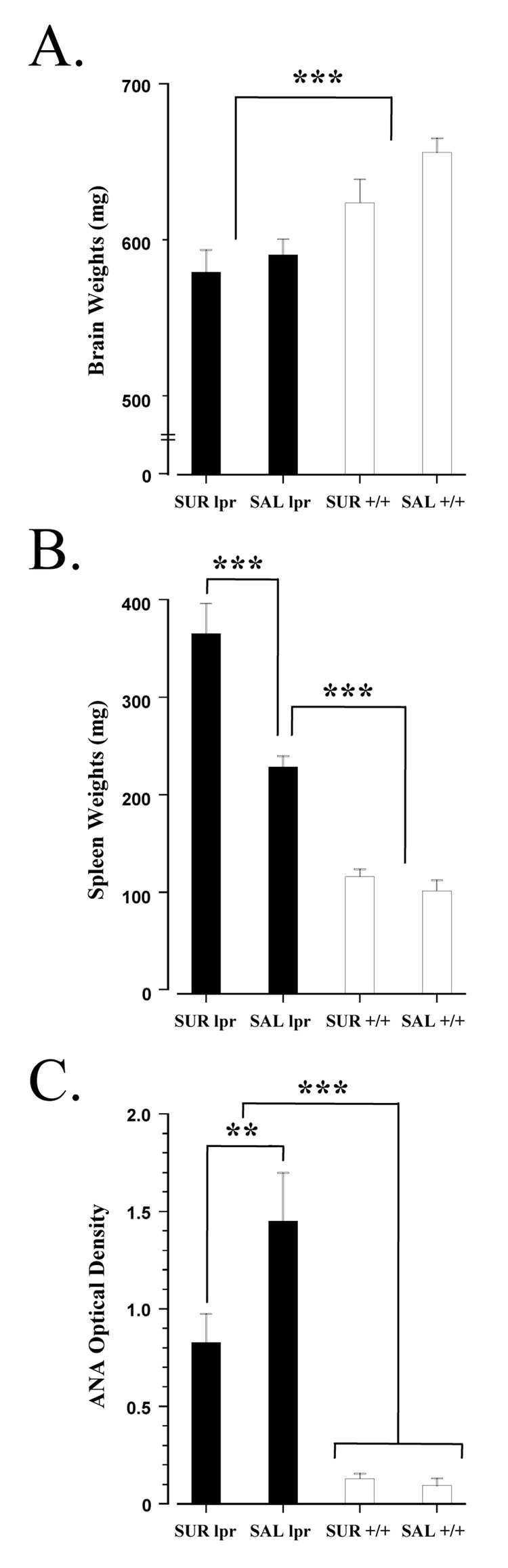
Interestingly, suramin did not alter the neuromorphological measures in MRL-lpr animals and the data were combined in subsequent statistical analyses (i.e. comparisons between the two substrains). Measures of dendrites were reduced in all MRL-lpr brains (for total number of crossings, substrain: t27= 60.023, p < 0.001; for total number of branches, substrain: t27= 33.213, p < 0.001; Table 1). These observations complement previous findings in mice of a similar age (Sakic et al., 1998, 2000). In the second cohort of MRL +/+ animals, chronic corticosterone treatment resulted in markedly deteriorated dendritic morphology, relative to mice receiving vehicle (Table 2). More specifically, comparisons between the corticosterone and vehicle groups revealed a significant difference between hypercortisolemic and non-hypercortisolemic mice (for total number of crossings, treatment: t19 = 52.033, p < 0.001; for total number of branches, treatment: t19 = 54.294, p < 0.001). Consistent with previous reports (Sakic et al., 1998; Ballok et al., 2004a), comparisons between MRL-lpr and MRL +/+ substrains revealed lower brain weights in the MRL-lpr group (t34 = 78.381, p < 0.001; Fig. 4A).
Table 1. Measures of dendritic neurons expressed as the mean length (µm) of basilar crossings and basilar branches in MRL mice at 15 weeks of age.
Neuromorphological measures in the CA1 region of the hippocampus following suramin treatment. Since suramin treated MRL-lpr animals did not differ from saline controls, these data were combined in subsequent analysis. In comparison to MRL +/+ animals, the lengths of Sholl crossings and dendritic basilar branches were reduced in MRL-lpr mice at 15 weeks of age. Considering that suramin does not cross the BBB or the blood-CSF barrier (Sanderson et al., 2007), the observed neuronal atrophy may be a result of aberrant autoimmune and endocrine mechanisms, that jointly contribute to early neuron damage in MRL-lpr brains (Ballok, 2007).
| Hippocampus | MRL-lpr | MRL +/+ |
|---|---|---|
| Crossings | 95.44 ± 1.99* | 105.26 ± 1.98 |
| Branches | 38.34 ± 2.28† | 42.79 ± 0.45 |
In comparison to MRL +/+ brains mean total length of dendritic crossings (t27=60.023, p < 0.001).
The mean total length of dendritic branches was also lower in lupus-prone MRL-lpr mice, relative to controls (t27= 33.213, p < 0.001).
Note: N = 14 areas per mouse for total number of crossings; N = 6 areas per mouse for total number of branches.
Table 2. Measures of dendritic neurons expressed as the mean length (µm) of basilar crossings and basilar branches in chronic corticosterone treated MRL+/+ mice.
Neuromorphological measures in the CA1 region of the hippocampus following corticosterone treatment. By inducing a state of hypercortisolemia in MRL +/+ mice, similar to the innate condition documented in autoimmune diseased MRL-lpr animals (Shanks et al., 1999), an exacerbated deterioration of neuronal dendrites was observed. However, the overall mean lengths of CA1 dendrites in both corticosterone and control groups were notably reduced in comparison to the first cohort of MRL +/+ mice (seen in Table 1). This can likely be attributed to the well-known effects of chronic ethanol consumption (i.e. drinking water contained 1.2% ethanol) on hippocampal fields (Pawlak et al., 2002). Regardless of the adverse effects of ethanol, the present results demonstrate that early neuronal damage can be caused by sustained upregulation of glucocorticoids in MRL mice. While corticosterone-induced atrophy of neurons may be reversible, it is also indicative of an early stage of neurodegeneration (McEwen, 1999).
| Hippocampus | MRL +/+ CORT | MRL +/+ VEH |
|---|---|---|
| Crossings | 81.15 ± 1.89* | 90.88 ± 1.75 |
| Branches | 34.22 ± 0.78† | 39.32 ± 0.39 |
In comparison to MRL +/+ VEH treated mice mean total length of dendritic crossings (t19 = 52.033, p < 0.001)
The mean total length of dendritic branches was significantly lower in MRL +/+ CORT animals relative to VEH (t19 = 54.294, p < 0.001).
Note: N = 14 areas per mouse for total number of crossings; N = 6 areas per mouse for total number of branches.
Fig. 4.
Brain weights and immune status of MRL mice at 15 weeks of age. (A) Regardless of treatment, all MRL-lpr mice had comparable brain masses that were significantly lighter then congenic controls (Sakic et al., 1998; Ballok et al., 2004a). (B) As expected, MRL-lpr animals had the largest spleens (Dixon et al., 1978), and suramin treatment exacerbated the splenomegaly of MRL-lpr mice. This finding is similar to previous reports on amphetamine and quinpirole’s effects on potentiating splenomegaly in MRL-lpr animals (Anderson et al., 2006; Chun et al., 2007). Much like suramin-induced splenomegaly in a rat model mucopolysaccharidosis (Rees et al., 1982), the natural enlargement of spleens in diseased mice (regardless of treatment), points to inherited metabolic deficiencies. (C) Serum levels of ANA were highest in MRL-lpr mice, as previously reported (Sakic et al., 1998; Ballok et al., 2003a). While treatment significantly reduced ANA levels in MRL-lpr animals, it did not abolish autoimmunity. Given that lupus-prone mice are hypergammaglobulinemic, an examination of kidney pathology (not performed in this study) would have better determined the magnitude of disease in autoimmune animals. All MRL +/+ mice were “negative” for this marker of disease.
3.4 Effects of suramin on indices of autoimmunity and inflammation
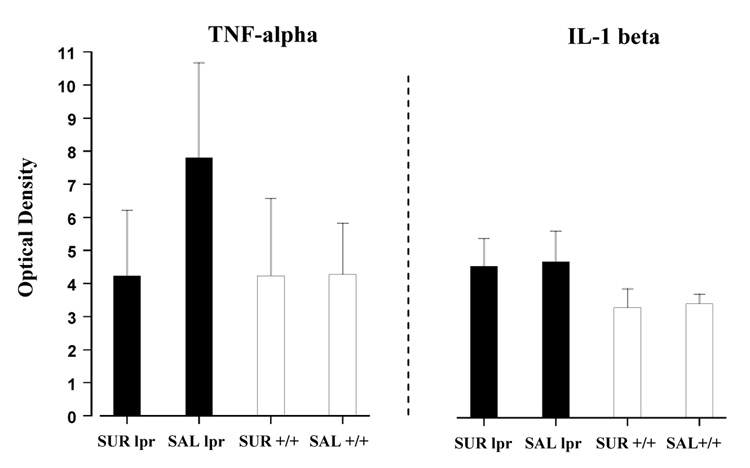
Differences were detected when analyzing spleen weights between the four groups (F(3, 31) = 44.847, p < 0.001; Fig. 4B). All MRL-lpr animals normally develop enlarged spleens along the course of disease (Fernandes and Good, 1984). Post-hoc analysis, however, revealed that suramin treatment exacerbated spleen size in MRL-lpr animals compared to the MRL-lpr saline group (p < 0.001). No difference in spleen weights were detected between the MRL +/+ groups (p = 0.778). Differences were also found in the levels of serum ANA between groups (F(3, 31) = 33.527, p < 0.001; Fig. 4C). More specifically, the difference between the MRL-lpr groups was significant (p = 0.010), indicating that suramin modulated autoimmunity in MRL-lpr mice, similar to its effects in EAE mice (Novales-Li, 1996). Suramin treated MRL-lpr animals, however, were still considered to be “autoimmune” since serum levels of ANA remained significantly elevated in comparison to both MRL+/+ groups (MRL-lpr suramin vs. MRL+/+ suramin, p < 0.001; MRL-lpr suramin vs. MRL +/+ saline, p < 0.001). No differences in ANA were detected between the asymptomatic MRL+/+ groups. Although suramin treatment appeared to decrease TNF-alpha serum concentrations in MRL-lpr animals (comparable to the lower levels of MRL+/+ groups), no significant differences were found (F(3, 31) = 2.497, n.s.; Fig. 5). Within the MRL-lpr saline group, however, TNF-alpha levels correlated with total contact time in the novel object test (r(1,9) = 0.718, p < 0.001; data not shown). No difference in serum levels of IL-1 beta were found between MRL groups at 15 weeks of age (F(3, 31) = 0.901, n.s.; Fig. 5).
Fig. 5.
Serum levels of pro-inflammatory cytokines, as measured by ELISA at 15 weeks of age. Although TNF-alpha appeared to be elevated in untreated MRL-lpr mice, this difference was not significant in comparison to the other groups. Significant differences in concentrations of IL-1 beta were also not detected between MRL groups. A previous study has reported that differences in serum cytokines become apparent by 19 weeks of age, with MRL-lpr mice showing significantly higher levels compared to MRL +/+ controls (Ballok et al., 2003b).
4. Discussion
The present results support the hypothesis that aberrant behaviors in MRL-lpr mice are associated with the activation of peripheral immunity, and that deficits in affective behaviors can be ameliorated by analgesic effects of a purine receptor antagonist. This assumption is based on several lines of evidence. First, dysfunctional affective behaviors were not observed in suramin treated lupus-prone animals and these changes coincided with a significant reduction in circulating autoantibody levels. Second, MRL-lpr animals treated with suramin showed a profound 3-fold increase in their responsiveness to a palatable solution (i.e. a reversal of anhedonia) that could not be attributed to caloric need. Thirdly, there was a strong negative correlation between total time spent in exploration of a novel object and serum TNF-alpha levels within the MRL-lpr saline group. This correlation suggests that increases in peripheral pro-inflammatorycytokines are associated with neophobic-like behavior (i.e. autoimmune mice with the highest TNF-alpha levels are also the most distressed). When considering that suramin does not significantly cross the BBB or the blood-CSF barrier (Sanderson et al., 2007), and that neuronal atrophy did not arise in drug treated MRL +/+ animals, it was unlikely that dendritic aberrations in the hippocampus of MRL-lpr animals were a by-product of treatment. Since chronic administration of corticosterone in drinking water, however, severely deteriorated neuronal morphology of asymptomatic animals, CA1 atrophy may be induced by an autoimmunity-sustained upregulation of corticosterone production in MRL-lpr animals (Shanks et al., 1999). Given that a low-calorie diet completely prevents splenomegaly and autoimmunity in MRL-lpr animals (Fernandes and Good, 1984), inborn errors in metabolism appear to underlie the dysregulation of neuro-immuno-endocrine networks that manifest disease. Overall, suramin’s modulating effects on affective behaviors may in part be due to a correction of an undefined enzymatic deficiency or P2X receptor antagonism of peripheral nociceptive pathways.
The so-called “autoimmunity-associated behavioral syndrome” in MRL-lpr mice resembles, in many aspects, sickness behavior of infected animals (Sakic et al., 1997a). Sickness behavior appears to be the expression of a central motivational state that reorganizes the organism’s priorities to cope with infectious pathogens (Hart, 1990). Although considered a normal response of the host’s immune system, emerging evidence suggests that mechanisms operational in sickness behavior may play a role in the pathophysiology of behavioral deficits, including anxiety and anhedonia (Brebner et al., 2000). In the present study, reduced exploration of novel objects correlated with elevated concentrations of serum TNF-alpha in MRL-lpr control mice. These observations are consistent with other experimental models (Fiore et al., 1998; Yamada et al., 2000; Silverman et al., 2007). Specifically, poor performance in tests postulated to measure anxiety were associated with elevated endogenous TNF-alpha levels. Although no correlations were found between sucrose consumption and TNF-alpha concentrations, previous studies have shown this cytokine to be associated with reductions in sweet solution intake (Brebner et al., 2000; Ballok et al., 2003b; Grippo et al., 2005). While not examined in the present study, other pro-inflammatory cytokines, such as IL-6, is reportedly inhibited by suramin (Strassmann et al., 1993) and may have contributed to the reversal of “anhedonia” observed in MRL-lpr animals (Sakic et al., 1996, 1997b, 2001). In comparison to the relatively acute presentation of cytokine-driven sickness behavior, however, additional pathogenic events likely take place in chronic systemic autoimmune disease that account for changes in behavior.
Persistent pain is associated with neuronal hyperexcitability within nociceptive pathways (Attal and Bouhassira 1999). In rodents, this manifests behaviorally as an increased expression of anxiety- and anhedonic- like symptoms (Munro et al., 2007; de Vasconcellos et al., 2006). Changes in affective behaviors of mice may represent an adaptive coping mechanism to pain (Siegfried et al., 1990; Bolles and Fanselow, 1980). In the present study, suramin treatment prevented the development of both of these behavioral impairments which characteristically emerge in diseased MRL-lpr mice. When considering suramin’s antagonism of P2X purine receptors (Nakazawa et al., 1991), important in peripheral “pain” signaling (Waldron and Sawynok, 2004), it is possible that the drug indirectly altered affective behaviors in lupus animals through its analgesic properties (Lakshmi and Joshi, 2005). More specifically, while many factors may induce sensory pain or neuropathy (Zimmermann, 1991; Attal and Bouhassira, 1999; Wuttke et al., 2007), pathogenic autoantibody binding maybe involved in nociception in autoimmune disorders (Newsom-Davis et al., 2003). Past studies have shown that spleen cells from MRL-lpr animals produced a potent antibody response to purines (Phipps et al., 1988), and in vivo evidence has linked aberrant purine receptor activities with serum anti-DNA antibodies in MRL-lpr mice (Colburn et al., 1990). Moreover, the presence of specific anti-purine IgGautoantibodies has been documented in the serum of both MRL-lpr mice and lupus patients, and these autoantibodies were found to correlate with acute disease, and exacerbations in disease symptoms (Colburn et al., 1990, 2003). Therefore, it is proposed that peripheral anti-purine autoantibodies (binding to P2X purine receptors) may induce neuronal hyperexcitability within nociceptive pathways of MRL-lpr animals. Due to the chronicity of autoimmune disease in lupus-prone mice, sustained stimulation of these pathways may induce the observed deficits in affective behaviors. Thus, alleviation of these behavioral deficits in autoimmune mice may be due to suramin’s ability to block binding (Nakazawa et al., 1991) of extracellular factors (i.e. purines and/or pathogenic IgGs) to peripheral P2X purinoceptors. The finding that the immunosuppressive drug cyclophosphamide also prevented anxiety- and anhedonic-like manifestations in MRL-lpr animals (Sakic et al. 1995, 1996) lends support to this novel hypothesis.
Earlier studies have revealed that prolonged immunosuppression suppresses the appearance of systemic autoimmunity and inflammation, prevents initial dendritic atrophy and terminal neurodegeneration (Sakic et al., 2000; Ballok et al., 2004a) and ameliorates behavioral deficits in lupus-prone mice (Sakic et al., 1995, 1996; Ballok et al., 2004b). In this study, suramin attenuated autoimmunity in MRL-lpr animals, but it did not suppress disease. Additionally, suramin did not prevent neuronal atrophy in these mice. It is likely that, despite treatment, characteristic immune-endocrine imbalances (Shanks et al., 1999) continued to emerge in MRL-lpr animals which produced the observed dendritic aberrations. More specifically, it is proposed that the onset of autoimmunity activates the hypothalmic-pituitary-adrenal (HPA) axis in lupus-prone mice (Hu et al., 1993; Shanks et al., 1998). When the HPA axis is chronically stimulated, excessive circulating levels of corticosterone can cross an intact BBB and initiate neuronal damage (Ballok, 2007). Although not significantly increased in the present study, pro-inflammatory cytokines have been shown to alter the HPA axis, leading to hypercortisolemia (Eskay et al., 1990). The complex phenomenon of pain (involving immune and centrally mediated responses) can also lead to activation of the HPA axis (Wolka et al., 2003). Regardless of the cause of dendritic atrophy, suramin’s ability to prevent the emergence of affective dysfunctions suggests that these behavioral impairments are not associated with initial CNS damage. Other behavioral manifestations, however, may be related to neurodegeneration and disruption of the BBB. More specifically, autoantibodies that gained access to the CNS through a breached BBB were shown to cross-react with NR2 and NMDA receptors, leading to neuronal death and cognitive deficits (DeGiorgio et al., 2001; Kowal et al., 2004, 2006). Interestingly, mediators released in response to pain have been shown to affect the structure and function of the BBB in vivo (Huber et al., 2001, 2002), which may contribute to alterations in BBB permeability and cytoarchitecture in MRL-lpr animals.
When delineating the progressive stages leading to spontaneous CNS disease in MRL-lpr mice, an autoimmune-associated hypercortisolemic state (Shanks et al., 1999) may be an important factor ultimately leading to midbrain degeneration (Ballok et al., 2004a). For example, prolonged corticosterone exposure has been found to evoke upregulation of dopamine receptors in the substantia nigra and ventral tegmental area of experimental animals (Czyrak et al., 2003), and similar dopamine neuron receptor upregulation/hypersensitivity develops in MRL-lpr mice by 15 weeks of age (Landau et al., 2005; Chun et al., 2007). Together with increases in brain endothelial adhesion molecules, important in lymphocyte/endothelial cell adhesion during the initial stages of CNS disease (McHale et al., 1999; Raine et al., 1990), deposition of complement proteins (Alexander et al., 2005) appears to facilitate the breakdown of the BBB (Alexander et al., 2003; Sidor et al., 2005; Ma et al., 2006). The BBB becomes compromised in all MRL-lpr mice by 6 months of age (Vogelweid et al., 1991). Once the integrity of the BBB is lost, supersensitized dopaminergic neurons become “unprotected” from peripheral cross-reactive autoantibodies (Hoffman et al., 1987, Ballok et al., 2004a) which bind cells to induce excitotoxic cell death (Sakic et al., 2005; Ballok et al., 2006). Disease-induced hypercortisolemia may then accelerate the loss of dopaminergic cells in midbrains of autoimmune MRL-lpr mice (Ballok et al., 2004a) as seen in a rat model of Parkinson’s (Smith et al., 2008). Thus, fixed structural lesions likely account for impaired emotionality and cognition at later stages of lupus-like disease (Huerta et al., 2006; Ballok et al., 2004a, 2004b, 2007; Kowal et al., 2006).
Recent evidence suggests that peripheral purinergic transmission and cytokines both play an important role in regulating immunity and behavior (Liu and Tracey, 2000; Minor et al., 2006). In the present study, while the purine receptor antagonist suramin attenuated autoimmune/inflammatory disease in MRL-lpr mice, it was not immunosuppressive and did not prevent brain atrophy. Suramin treatment did, however, prevent neophobic- and anhedonic-like deficits in MRL-lpr mice. When considering all evidence, there are two possible explanations (which are not mutually exclusive) for suramin’s beneficial effects on affective behaviors. First, the drug may have corrected an inherited metabolic deficiency in the MRL-lpr substrain (similar to mucopolysaccharidoses) which left untreated leads to the evolution of behavioral impairments (Dorfman and Matalon, 1976). Although MRL-lpr mice share more than 99.9% of their genome with MRL +/+ congenic controls (Carlsten and Tarkowski, 1989), genetic interactions between each locus is highly complex (Vidal et al.,1998). Additional linkage analyses are required to identify novel loci and to enhance understanding of the genes effecting metabolism in MRL animals (Srivastava et al., 2006; Gu et al., 1999; Gilkeson et al., 1997; Keng et al., 2000). Studies assessing interactions between enzyme activity perturbations, autoimmune disease, and splenomegaly in lupus-prone mice are also needed. The second possibility (when considering the extracellular actions of suramin and its restricted passage into the CNS) is that the drug blocked pathogenic autoantibodies from binding and exciting peripheral P2X “pain” receptors (i.e. nociceptive pathways). Suramin’s analgesic effects could have resulted in reduced pain-related affective behavior deficits. Although chronic corticosterone treatment in congenic MRL +/+ animals produced dendritic aberrations similar to that observed in autoimmune mice, future studies should determine if corticosterone alone is sufficient to cause this manifestation in MRL-lpr neurons. As each organism is greater than the sum of its parts, this neuro-immuno-endocrine model of NP-SLE promises to provide insights into novel therapeutic interventions, in multiple systems, along the progression of autoimmune/inflammatory conditions with both peripheral and CNS involvement.
Acknowledgments
This work was supported by funds from the Canadian Institutes of Health Research doctoral award grant (MOP 38065) to D.A. Ballok, and the National Institute of Health (1R21 AR49163-01) to B. Sakic. Special thanks to Grazyna Gorny and Bryan Kolb from The University of Lethbridge for their expertise in performing the dendrite analyses. Thanks also to Jason Millward for his technical assistance throughout the study, and to Dr. Bruce McEwen for his helpful input. D.A. Ballok is a student fellow of the Father Sean O’Sullivan Research Centre (FSORC), and recipient of the Parkinson Society Canada National Research Fellowship. B. Sakic is a recipient of the FSORC career development award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander JJ, Jacob A, Bao L, Macdonald RL, Quigg RJ. Complement-dependent apoptosis and inflammatorygene changes in murine lupus cerebritis. J. Immunol. 2005;175:8312–8319. doi: 10.4049/jimmunol.175.12.8312. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Bao L, Jacob A, Kraus DM, Holers VM, Quigg RJ. Administration of the soluble complement inhibitor, Crry-Ig, reduces inflammation and aquaporin 4 expression in lupus cerebritis. Biochim. Biophys. Acta. 2003;1639:169–176. doi: 10.1016/j.bbadis.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Anderson KK, Ballok DA, Prasad N, Szechtman H, Sakic B. Impaired response to amphetamine and neuronal degeneration in the nucleus accumbens of autoimmune MRL-lpr mice. Behav. Brain Res. 2006;166:32–38. doi: 10.1016/j.bbr.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal N, Bouhassira D. Mechanisms of pain in peripheral neuropathy. Acta. Neurol. Scand. Suppl. 1999;173:12–24. doi: 10.1111/j.1600-0404.1999.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Ballok DA. Neuroimmunopathology in a murine model of neuropsychiatric lupus. Brain Res. Rev. 2007;54:67–79. doi: 10.1016/j.brainresrev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballok DA, Earls AM, Krasnik C, Hoffman SA, Sakic B. Autoimmune-induced damage of the midbrain dopaminergic system in lupus-prone mice. J. Neuroimmunol. 2004a;152:83–97. doi: 10.1016/j.jneuroim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Ballok DA, Ma X, Denburg JA, Arsenault L, Sakic B. Ibuprofen fails to prevent brain pathology in a model of neuropsychiatric lupus. J. Rheumatol. 2006;33:2199–2213. [PMC free article] [PubMed] [Google Scholar]
- Ballok DA, Millward JM, Sakic B. Neurodegeneration in autoimmune MRL-lpr mice as revealed by Fluoro Jade B staining. Brain Res. 2003a;964:200–210. doi: 10.1016/s0006-8993(02)03980-x. [DOI] [PubMed] [Google Scholar]
- Ballok DA, Szechtman H, Sakic B. Taste responsiveness and diet preference in autoimmune MRL mice. Behav. Brain Res. 2003b;140:119–130. doi: 10.1016/s0166-4328(02)00276-0. [DOI] [PubMed] [Google Scholar]
- Ballok DA, Woulfe J, Sur M, Cyr M, Sakic B. Hippocampal damage in mouse and human forms of systemic autoimmune disease. Hippocampus. 2004b;14:649–661. doi: 10.1002/hipo.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model of fear and pain. Behav. Brain Sci. 1980;3:291–323. [Google Scholar]
- Brebner K, Hayley S, Zacharko R, Merali Z, Anisman H. Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacol. 2000;22:566–580. doi: 10.1016/S0893-133X(99)00166-9. [DOI] [PubMed] [Google Scholar]
- Brooks WM, Sabet A, Sibbitt WL, Barker PB, van Zijl PC, Duyn JH, Moonen CT. Neurochemistry of brain lesions determined by spectroscopic imaging in systemic lupus erythematosus. J. Rheumatol. 1997;24:2323–2329. [PubMed] [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol. Life Sci. 2007;64:147–183. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten H, Tarkowski H. Expression of Heterozygous lpr Gene in MRL Mice. Scand. J. Immunol. 1989;30:457–462. doi: 10.1111/j.1365-3083.1989.tb02450.x. [DOI] [PubMed] [Google Scholar]
- Chun S, McEvilly R, Foster JA, Sakic B. Proclivity to self-injurious behavior in MRL-lpr mice: implications for autoimmunity-induced damage in the dopaminergic system. Mol.Psychiatry. 2007 doi: 10.1038/sj.mp.4002078. [DOI] [PubMed] [Google Scholar]
- Colburn K, Boucek R, Gusewitch G, Wong A, Wat P, Weeks D. B-adrenergic receptor stimulation increases anti-DNA antibody production in MRL/1 pr mice. J. Rheumatol. 1990;17:138–141. [PubMed] [Google Scholar]
- Colburn K, Wong AL, Weisbart RH, Green LM. Antiguanosine antibodies in murine and human lupus have the internal image of G-binding proteins. J. Rheumatol. 2003;30:993–997. [PubMed] [Google Scholar]
- Czyrak A, Mackowiak M, Chocyk A, Fijal K, Wedzony K. Role of glucocorticoids in the regulation of dopaminergic neurotransmission. Pol. J. Pharmacol. 2003;55:667–674. [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann. N.Y. Acad. Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- Denburg SD, Denburg JA. Cognitive dysfunction and antiphospholipid antibodies in systemic lupus erythematosus. Lupus. 2003;12:883–890. doi: 10.1191/0961203303lu497oa. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Sherman GF, Rosen GD, Morrison L, Behan PO, Galaburda AM. A behavior profile of the MRL/Mp lpr/lpr mouse and its association with hydrocephalus. Brain Behav. Immun. 1992;6:40–49. doi: 10.1016/0889-1591(92)90058-v. [DOI] [PubMed] [Google Scholar]
- de Vasconcellos AP, Nieto FB, Fontella FU, da Rocha ER, Dalmaz C. The nociceptive response of stressed and lithium-treated rats is differently modulated by different flavors. Physiol. Behav. 2006;88:382–388. doi: 10.1016/j.physbeh.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Dixon FJ, Andrews BS, Eisenberg RA, McConahey PJ, Theofilopoulos AN, Wilson CB. Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis Rheum. 1978;21:S64–S67. doi: 10.1002/art.1780210909. [DOI] [PubMed] [Google Scholar]
- Donohue HS, Gabbott PL, Davies HA, Rodríguez JJ, Cordero MI, Sandi C, Medvedev NI, Popov VI, Colyer FM, Peddie CJ, Stewart MG. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: a stereological and three-dimensional ultrastructural study. Neurosci. 2006;140:597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- Dorfman A, Matalon R. The mucopolysaccharidoses (a review) Proc. Natl. Acad. Sci. USA. 1976;73:630–637. doi: 10.1073/pnas.73.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskay RL, Grino M, Chen HT. Interleukins, signal transduction, and the immune system-mediated stress response. Adv. Exp. Med. Biol. 1990;274:331–343. doi: 10.1007/978-1-4684-5799-5_21. [DOI] [PubMed] [Google Scholar]
- Fairchild GM, Leitch M, Ingram CD. Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharm. 2003;45:925–934. doi: 10.1016/s0028-3908(03)00269-7. [DOI] [PubMed] [Google Scholar]
- Fernandes G, Good RA. Inhibition by restricted-calorie diet of lymphoproliferative disease and renal damage in MRL/lpr mice. Proc. Natl. Acad. Sci. USA. 1984;81:6144–6148. doi: 10.1073/pnas.81.19.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Alleva E, Probert L, Kollias G, Angelucci F, Aloe L. Exploratory and displacement behavior in transgenic mice expressing high levels of brain TNF-alpha. Physiol. Behav. 1998;63:571–576. doi: 10.1016/s0031-9384(97)00514-3. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J. Neurosci. Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Gilkeson GS, Mudgett JS, Seldin MF, Ruiz P, Alexander AA, Misukonis MA, Pisetsky DS, Weinberg JB. Clinical and Serologic Manifestations of Autoimmune Disease in MRL-lpr/lpr Mice Lacking Nitric Oxide Synthase Type 2. J. Exp. Med. 1997;186:365–373. doi: 10.1084/jem.186.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol. Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Gu L, Johnson MW, Lusis AJ. Quantitative Trait Locus Analysis of Plasma Lipoprotein Levels in an Autoimmune Mouse Model. Interactions Between Lipoprotein Metabolism, Autoimmune Disease, and Atherogenesis. Arterioscl. Thromb. Vasc. Biol. 1999;19:442–453. doi: 10.1161/01.atv.19.2.442. [DOI] [PubMed] [Google Scholar]
- Hart BL. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 1990;14:273–294. doi: 10.1016/s0149-7634(05)80038-7. [DOI] [PubMed] [Google Scholar]
- Hoffman SA, Arbogast DN, Ford PM, Shucard SW, Harbeck RJ. Brain-reactive autoantibody levels in the sera of ageing autoimmune mice. Clin. Exp. Immunol. 1987;70:74–83. [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Dietrich H, Herold M, Heinrich PC, Wick G. Disturbed immuno-endocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune disease. Int. Arch. Allergy Immunol. 1993;102:232–241. doi: 10.1159/000236531. [DOI] [PubMed] [Google Scholar]
- Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- Huber JD, Hau VS, Borg L, Campos CR, Egleton RD, Davis TP. Blood-brain barrier tight junctions are altered during a 72-h exposure to lambda-carrageenan-induced inflammatory pain. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1531–H1537. doi: 10.1152/ajpheart.00027.2002. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc. Natl. Acad. Sci. USA. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey PP, Buell G, Kennedy I, Khakh BS, Michel AD, Surprenant A, Trezise DJ. New insights on P2X purinoceptors. Naunyn. Schmiedebergs Arch. Pharmacol. 1995;352:585–596. doi: 10.1007/BF00171316. [DOI] [PubMed] [Google Scholar]
- Isenberg D, Bacon P, Bombardier C, Gladman D, Goldsmith CH, Kalunian K, Liang M, Maddison P, Nived O, Richter M. Criteria for assessing disease activity in systemic lupus erythematosus. J. Rheumatol. 1989;16:1395–1396. [PubMed] [Google Scholar]
- Keng T, Privalle CT, Gilkeson GS, Weinberg JB. Peroxynitrite formation and decreased catalase activity in autoimmune MRL-lpr/lpr mice. Mol. Med. 2000;6:779–792. [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Palmiter RD. Interaction of dopamine and adenosine receptor function in behavior: studies with dopamine-deficient mice. Front. Biosci. 2008;13:2311–2318. doi: 10.2741/2845. [DOI] [PubMed] [Google Scholar]
- Koles L, Furst S, Illes P. Purine ionotropic (P2X) receptors. Curr. Pharm. Des. 2007;13:2368–2384. doi: 10.2174/138161207781368747. [DOI] [PubMed] [Google Scholar]
- Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, Volpe BT. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Kowal C, DeGiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, Diamond B. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl. Acad. Sci. USA. 2006;103:19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi S, Joshi PG. Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol. Neurobiol. 2005;25:819–832. doi: 10.1007/s10571-005-4936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau AM, Luk KC, Jones ML, Siegrist-Johnstone R, Young YK, Kouassi E, Rymar VV, Dagher A, Sadikot AF, Desbarats J. Defective Fas expression exacerbates neurotoxicity in a model of Parkinson's disease. J. Exp. Med. 2005;202:575–581. doi: 10.1084/jem.20050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner O, Dietrich H, Oliveira dos SA, Wiegers GJ, Schwarz S, Harbutz M, Herold M, Wick G. Altered circadian rhythms of the stress hormone and melatonin response in lupus-prone MRL/MP-fas(Ipr) mice. J. Autoimmun. 2000;14:325–333. doi: 10.1006/jaut.2000.0375. [DOI] [PubMed] [Google Scholar]
- Liu T, Tracey DJ. ATP P2X receptors play little role in the maintenance of neuropathic hyperalgesia. Neuroreport. 2000;11:1669–1672. doi: 10.1097/00001756-200006050-00015. [DOI] [PubMed] [Google Scholar]
- Ma X, Foster J, Sakic B. Distribution and prevalence of leukocyte phenotypes in brains of lupus-prone mice. J. Neuroimmunol. 2006;179:26–36. doi: 10.1016/j.jneuroim.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Res. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. USA. 1997;94:14002–14009. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric D, Millward JM, Ballok DA, Szechtman H, Denburg JA, Barkera JL, Sakic B. Neurotoxic properties of cerebrospinal fluid from behaviorally impaired autoimmune mice. Brain Res. 2001;920:183–193. doi: 10.1016/s0006-8993(01)03060-8. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Kater SB. Development and selective neurodegeneration in cell cultures from different hippocampal regions. Brain Res. 1989;490:110–125. doi: 10.1016/0006-8993(89)90436-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McHale JF, Harari AO, Marshall D, Haskard DO. TNF-alpha and IL-1beta Sequentially Induce endothelial ICAM-1 and VCAM-1 expression in MRL/lpr lupus-prone mice. J. Immunol. 1999;163:3993–4000. [PubMed] [Google Scholar]
- Minor TR, Huang Q, Foley EA. Cytokine-purine interactions in behavioral depression in rats. Integr. Physiol. Behav. Sci. 2003;38:189–202. doi: 10.1007/BF02688853. [DOI] [PubMed] [Google Scholar]
- Minor TR, Huang Q, Witt AE. Cytokine-purine interactions in traumatic stress, behavioral depression, and sickness. CNS. Neurol. Disord. Drug Targets. 2006;5:547–560. doi: 10.2174/187152706778559282. [DOI] [PubMed] [Google Scholar]
- Monleon S, D’Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl.) 1995;117:453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- Moskowitz N. Systemic lupus erythematosus of the central nervous system: Molecular theories and models for mental disease. Mt. Sinai J. Med. 1989;56:23–29. [PubMed] [Google Scholar]
- Munro G, Erichsen HK, Mirza NR. Pharmacological comparison of anticonvulsant drugs in animal models of persistent pain and anxiety. Neuropharmacol. 2007;53:609–618. doi: 10.1016/j.neuropharm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Inoue K, Fujimori K, Takanaka A. Effects of ATP antagonists on purinoceptor-operated inward currents in rat phaeochromocytoma cells. Pflugers Arch. 1991;418:214–219. doi: 10.1007/BF00370517. [DOI] [PubMed] [Google Scholar]
- Newsom-Davis J, Buckley C, Clover L, Hart I, Maddison P, Tuzum E, Vincent A. Autoimmune disorders of neuronal potassium channels. Ann. N.Y. Acad. Sci. 2003;998:202–210. doi: 10.1196/annals.1254.022. [DOI] [PubMed] [Google Scholar]
- Novales-Li P. Suramin exerts in vivo cytokine modulatory properties on splenocytes from experimental allergic encephalomyelitis-induced SJL mice: implications for autoimmune disease therapy. Immunopharmacol. 1996;35:155–162. doi: 10.1016/s0162-3109(96)00141-5. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Ullman EA, Collins AC. Adrenocortical hormone regulation of nicotine sensitivity in mice. Physiol. Behav. 1988;44:109–116. doi: 10.1016/0031-9384(88)90353-8. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Skrzypieca A, Sulkowskib S, Buczkoa W. Ethanol-induced neurotoxicity is counterbalanced by increased cell proliferation in mouse dentate gyrus. Neurosci. Lett. 2002;327:83–86. doi: 10.1016/s0304-3940(02)00369-5. [DOI] [PubMed] [Google Scholar]
- Phipps RP, Spaulding M, Szakos J. DNA is a potent immunogen for spleen cells and for guanosine-binding B lymphocytes. Cell Immunol. 1988;113:202–213. doi: 10.1016/0008-8749(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Raine CS, Canella B, Duijvestijn AM, Cross AH. Homing to central nervous system vasculature by antigen-specific lymphocytes. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab. Invest. 1990;63:476–489. [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss c, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog. Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Rees S, Constantopoulos G, Barranger JA, Brady RO. Organomegaly and histopathology in an animal model of mucopolysaccharidosis induced by suramin. Naunyn. Schmiedebergs Arch. Pharmacol. 1982;319:262–270. doi: 10.1007/BF00495876. [DOI] [PubMed] [Google Scholar]
- Sakic B, Denburg JA, Denburg SD, Szechtman H. Blunted sensitivity to sucrose in autoimmune MRL-lpr mice: a curve-shift study. Brain Res. Bull. 1996;41:305–311. doi: 10.1016/s0361-9230(96)00190-6. [DOI] [PubMed] [Google Scholar]
- Sakic B, Kolb B, Whishaw IQ, Gorny G, Szechtman H, Denburd JA. Immunosuppression prevents neuronal atrophy in lupus-prone mice: evidence for brain damage induced by autoimmune disease? J. Neuroimmunol. 2000;111:93–101. doi: 10.1016/s0165-5728(00)00364-7. [DOI] [PubMed] [Google Scholar]
- Sakic B, Lacosta S, Denburg JA, Szechtman H. Altered neurotransmission in brains of autoimmune mice: pharmacological and neurochemical evidence. J. Neuroimmunol. 2002;129:84–96. doi: 10.1016/s0165-5728(02)00171-6. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Denburg JA. Neurobehavioral alterations in autoimmune mice. Neurosci. Biobehav. Rev. 1997a;21:327–340. doi: 10.1016/s0149-7634(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Braciak T, Richards C, Gauldie J, Denburg JA. Reduced preference for sucrose in autoimmune mice: a possible role of interleukin-6. Brain Res. Bull. 1997b;44:155–165. doi: 10.1016/s0361-9230(97)00107-x. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Denburg JA, Gorny G, Kolb B, Whishaw IQ. Progressive atrophy of pyramidal neuron dendrites in autoimmune MRL-lpr mice. J. Neuroimmunol. 1998;87:162–170. doi: 10.1016/s0165-5728(98)00085-x. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Denburg SD, Denburg JA. Immunosuppressive treatment prevents behavioral deficit in autoimmune MRL-lpr mice. Physiol. Behav. 1995;58:797–802. doi: 10.1016/0031-9384(95)00135-6. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Keffer M, Talangbayan H, Stead R, Denburg JA. A behavioral profile of autoimmune lupus-prone MRL mice. Brain Behav. Immun. 1992;6:265–285. doi: 10.1016/0889-1591(92)90048-s. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Talangbayan H, Denburg SD, Carbotte RM, Denburg JA. Disturbed emotionality in autoimmune MRL-lpr mice. Physiol. Behav. 1994;56:609–617. doi: 10.1016/0031-9384(94)90309-3. [DOI] [PubMed] [Google Scholar]
- Sakic B, Gauldie J, Denburg JA, Szechtman H. Behavioral effects of infection with IL-6 adenovector. Brain Behav. Immun. 2001;15:25–42. doi: 10.1006/brbi.1999.0576. [DOI] [PubMed] [Google Scholar]
- Sakic B, Kirkham DL, Ballok DA, Mwanjewe J, Fearon IM, Macri J, Yu G, Sidor MM, Denburg JA, Szechtman H, Lau J, Ball AK, Doering LC. Proliferating brain cells are a target of neurotoxic CSF in systemic autoimmune disease. J. Neuroimmunol. 2005;169:68–85. doi: 10.1016/j.jneuroim.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson L, Khan A, Thomas S. Distribution of suramin, an antitrypanosomal drug, across the blood-brain and blood-cerebrospinal fluid interfaces in wild-type and P-glycoprotein transporter-deficient mice. Antimicrob. Agents Chemother. 2007;51:3136–3146. doi: 10.1128/AAC.00372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, Glucocorticoids, and Damage to the Nervous System: The Current State of Confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Schmidt AP, Lara DR, Souza DO. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol. Ther. 2007;16:401–416. doi: 10.1016/j.pharmthera.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Shanks N, Harbuz MS, Jessop DS, Perks P, Moore PM, Lightman SL. Inflammatory disease as chronic stress. Ann. N.Y. Acad. Sci. 1998;840:599–607. doi: 10.1111/j.1749-6632.1998.tb09599.x. [DOI] [PubMed] [Google Scholar]
- Shanks N, Moore PM, Perks P, Lightman SL. Alterations in hypothalamic-pituitary-adrenal function correlated with the onset of murine SLE in MRL +/+ and lpr/lpr mice. Brain Beh. Immun. 1999;13:348–360. doi: 10.1006/brbi.1998.0535. [DOI] [PubMed] [Google Scholar]
- Sidor MM, Sakic B, Malinowski PM, Ballok DA, Oleschuk CJ, Macri J. Elevated immunoglobulin levels in the cerebrospinal fluid from lupus-prone mice. J. Neuroimmunol. 2005;165:104–113. doi: 10.1016/j.jneuroim.2005.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried B, Frischknecht HR, Nunes de Souza RL. An ethological model for the study of activation and interaction of pain, memory and defensive systems in the attacked mouse. Role of endogenous opioids. Neurosci. Biobehav. Rev. 1990;14:481–490. doi: 10.1016/s0149-7634(05)80071-5. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Macdougall MG, Hu F, Pace TW, Raison CL, Miller AH. Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice. Mol. Psychiatry. 2007;12:408–417. doi: 10.1038/sj.mp.4001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LK, Jadavji NM, Colwell KL, Katrina Perehudoff S, Metz GA. Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson's disease. Eur. J. Neurosci. 2008;27:2133–2146. doi: 10.1111/j.1460-9568.2008.06177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AK, Mohan S, Masinde GF, Masinde L, Yu H, Baylink DJ. Identification of quantitative trait loci that regulate obesity and serum lipid levels in MRL/MpJ x SJL/J inbred mice. J. Lip. Res. 2006;47:123–133. doi: 10.1194/jlr.M500295-JLR200. [DOI] [PubMed] [Google Scholar]
- Strassmann G, Fong M, Freter CE, Windsor S, D'Alessandro F, Nordan RP. Suramin interferes with interleukin-6 receptor binding in vitro and inhibits colon-26-mediated experimental cancer cachexia in vivo. J. Clin. Invest. 1993;92:2152–2159. doi: 10.1172/JCI116816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechtman H, Sakic B, Denburg JA. Behaviour of MRL mice: an animal model of disturbed behaviour in systemic autoimmune disease. Lupus. 1997;6:223–229. doi: 10.1177/096120339700600302. [DOI] [PubMed] [Google Scholar]
- van Dam AP, Wekking EM, Callewaert JA, Schipperijn AJ, Oomen HA, de Jong J, Swaak AJ, Smeenk RJ, Feltkamp TE. Psychiatric symptoms before systemic lupus erythematosus is diagnosed. Rheumatol. Int. 1994;14:57–62. doi: 10.1007/BF00300248. [DOI] [PubMed] [Google Scholar]
- Vidal S, Kono DH, Theofilopoulos AN. Loci predisposing to autoimmunity in MRL-Fas lpr and C57BL/6-Faslpr mice. J. Clin. Invest. 1998;101:696–702. doi: 10.1172/JCI1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelweid CM, Johnson CG, Besch-Williford CL, Basler J, Walker SE. Inflammatory central nervous system disease in lupus-prone MRL/lpr mice: comparative histologic and immunohistochemical findings. J. Neuroimmunol. 1991;35:89–99. doi: 10.1016/0165-5728(91)90164-3. [DOI] [PubMed] [Google Scholar]
- Waldron JB, Sawynok J. Peripheral P2X receptors and nociception: interactions with biogenic amine systems. Pain. 2004;110:79–89. doi: 10.1016/j.pain.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Wekking EM. Psychiatric symptoms in systemic lupus erythematosus: an update. Psychosom. Med. 1993;55:219–228. doi: 10.1097/00006842-199303000-00011. [DOI] [PubMed] [Google Scholar]
- Wolka AM, Huber JD, Davis TP. Pain and the blood-brain barrier: obstacles to drug delivery. Adv. Drug Deliv. Rev. 2003;55:987–1006. doi: 10.1016/s0169-409x(03)00100-5. [DOI] [PubMed] [Google Scholar]
- Wuttke TV, Jurkat-Rott K, Paulus W, Garncarek M, Lehmann-Horn F, Lerche H. Peripheral nerve hyperexcitability due to dominant-negative KCNQ2 mutations. Neurology. 2007;69:2045–2053. doi: 10.1212/01.wnl.0000275523.95103.36. [DOI] [PubMed] [Google Scholar]
- Yamada K, Iida R, Miyamoto Y, Saito K, Sekikawa K, Seishima M, Nabeshima T. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-alpha gene: implications for emotional behavior. J. Neuroimmunol. 2000;111:131–138. doi: 10.1016/s0165-5728(00)00375-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Pathophysiological mechanisms of fibromyalgia. Clin. J. Pain. 1991;7:S8–S15. [PubMed] [Google Scholar]