Abstract
Drosophila melanogaster is an ideal model organism for various types of aging studies. They are easy to maintain, relatively inexpensive, have short life cycles, provide large sample sizes, and can be genetically manipulated via various methods for testing. The 49th Annual Drosophila Research Conference, held in San Diego, CA (April 2-6, 2008), had over 30 poster presentations and eight platform talks devoted to physiology and aging, and seven presentations in a longevity and functional senescence workshop. The data presented via these avenues included life span manipulation, physiological related genes, candidate aging genes, gene expression, signaling, and using D. melanogaster as a model for age related disease, to name a few. This report provides highlights of some of the information presented in the poster, platform and workshop presentations.
Keywords: life span manipulation, physiological related genes, candidate aging genes, gene expression, signaling, Drosophila melanogaster, model for age related diseases
Introduction
Throughout history, scientists and philosophers have pondered the questions of why and how organisms age. A major historical event for aging research was the United States Congress granting the authority to form the National Institute on Aging (NIA) in 1974. The focus of the institute was to provide support and research human biological aging and age-related diseases.1 In 1993, the NIA initiated a program to identify genes involved in the regulation of longevity of nonhumans. Several species were targeted by the program, including Drosophila melanogaster, with the goal to transfer the results to human age-related problems.2 The United States was not alone in its interest of aging. An Internet search revealed several organizations or foundations all over the world interested in this biological process.
Life Span Manipulation
In recent years, life span manipulation of D. melanogaster has been studied via several methods including dietary restrictions (DR),3 exposure to nutrient-derived odorants,4 and changes in metabolism.5 Both life span increases and decreases have been demonstrated depending on the study conditions. Benzer and Ja (Caltech) along with Brummel (Long Island University) studied the role of ad libitum water on fruit fly life span. Their work indicated an increase in longevity of the flies when providing an ad libitum water supply especially at high food concentrations. These results suggest that better hydration of the flies may also play a role in increasing life span.
DR has been shown to increase life span in several species and also to delay the incidence and onset of age-related diseases. During the longevity and functional senescence workshop, Kabil (Pletcher lab, Baylor College of Medicine; Harshman lab, University of Nebraska-Lincoln) proposed a hypothesis of DR changing life span of flies via the role of reduced glutathione (GSH) levels and the transsulfuration pathway. It is hypothesized that DR reduces levels of reactive oxygen species (ROS) by the action of anti-oxidants such as GSH, which leads to a life span extension. The results of this study indicated that DR led to higher GSH levels in flies and attenuated age-related decline in GSH levels. DR flies were shown to survive better under oxidative stress and life span increases gained by DR were lost by inhibition of the transsulfuration pathway. This understanding of diet and anti-oxidant levels has many implications for human health and aging.
Another presentation from the Pletcher lab (by Skorupa) concentrated on a model of specific dietary nutrients and their impact on longevity. Flies were placed on a surface that varied the nutrient levels of sugar and yeast, and subsequent consumption rates were detected by using a blue dye in the food. It was found that flies ate more food, but had decreased fecundity when the sugar amount increased in the food. An increase in yeast concentrations led to increased protein storage and fecundity. Also, obese flies obtained more calories from sugar, while lean flies acquired more calories from protein. An overall message from the talk was that a balanced diet promotes longevity.
Physiology and Aging Genes
Over the past ten years, there have been various genes discovered in D. melanogaster that extend life span (methuselah,6 chico,7 Indy,8 etc.) or are responsible for fly physiology. Several poster presentations and workshop talks studied the effects of genes on various processes or gene functions. A physiology gene to take note of is lot's wife (lwf). The Blumenthal lab (Marquette University) studied the effects of lwf expression on muscle contractions that move food from the crop into the midgut, on triglyceride levels, and feeding behavior. A mutation to lwf resulted in adult lethality presumably via starvation. Female flies homozygous for the recessive null mutation, lwf1, had twice the rate of crop duct contractions and three times the rate of crop lobe contractions as flies heterozygous for lwf1. But, the lobe/duct contraction ratio in the mutants vs. the heterozygotes was different, suggesting an organ-level muscle coordination problem. Canton S males and males hemizygous for lwf1 were used to look at triglyceride levels and feeding behavior. Mutant flies showed smaller triglyceride stores on the third day post-eclosion and for feeding behavior had high levels of printing, which was measured by a proboscis print assay, when they normally begin to die. Additional studies will continue looking into the function of this gene.
The effects of the couch potato (cpo) gene have been extensively studied since it was first reported.9 The Eanes (SUNY Stony Brook) and Schmidt labs (University of Pennsylvania) have now studied cpo and its relationship to longevity and aging. Expression levels of cpo were experimentally altered by several methods and flies were tested against multiple stressors. The results showed that reduced function of cpo resulted in decreased longevity and stress resistance. Increased expression of the gene led to increased life span and stress resistance. These findings may be pointing to another aging gene in Drosophila.
Another gene, ponchik, was being studied at Baylor College of Medicine (Pletcher lab). The gene was identified after microarrays were done comparing decreased adiposity to increased adiposity. Ponchik mutant flies had high levels of triglycerides, increased fecundity, higher feeding rates, and were long-lived. Overall, this gene has been implicated to modulate energy balance and life span. By studying genes involved in energy balance, scientists hope to have a better understanding of the effects of diet on obesity and health.
Tower (University of Southern California) gave a workshop talk over the nuclear-mitochondrial signaling of heat shock protein 70 (hsp70) and hsp22 and life span. A circadian rhythm exists between oxidative metabolism resulting in ROS and nuclear-mitochondrial ROS signaling and repair. It is hypothesized that a disruption of the nuclear-mitochondrial ROS signaling can lead to aging. Both genes had been shown to be induced in response to heat and oxidative stress.10,11 Gene expression was linked to green fluorescent protein (GFP) output, and in addition to fly movement, was tracked via a 3D system using video cameras to take silhouettes of flies.12 Using this technology, hsp22 expression was shown to increase and became circadian as flies aged, while hsp70 expression only became circadian. There was a rhythmic expression of both hsp22 and hsp70, suggesting that these genes may be predictive biomarkers of life span.
A collaborative effort between our lab (Carlson, University of Nebraska at Kearney) and the Harshman lab (University of Nebraska-Lincoln) is studying the duration of aging in a cohort and gene expression changes. The approach was to study the transcriptome related to mortality in a large caged population (10,000) of D. melanogaster. Microarray data revealed 205 significant genes differentially expressed across all time points tested. Gene ontology analysis revealed that developmental, neuronal and apoptotic gene expression changed over time. This study demonstrated the utility of using large populations, which provides an opportunity for taking many samples and testing when flies are very old.
Signaling
The target of rapamycin (TOR) and insulin/IGF (insulin growth factor) pathways continue to be actively researched areas. These two pathways are important for growth and development. The TOR pathway is nutrient-responsive and regulates growth through the prothoracic gland (PG). The PG secretes the steroidal prohormone, ecdysone, which plays a role in moulting.13 The effect of nutrition changes (amounts of yeast) in fly food and regulation of the ecdysone pathway on developmental timing of fly larvae was studied in the Leopold lab (CNRS/University of Nice). Food with less yeast caused a decrease in the regulation of TOR in the PG and increased the length of larvae development, while leading to an increase in adult size. This downregulation was also associated with the inability to regulate ecdysone levels. Results indicated that TOR inhibition before 72 or after 80 hours had no effect on development, therefore, revealing some sort of larval developmental checkpoint. These findings point to TOR acting as a link between nutritional information and ecdysone in larval development.
Work has continued with the role of falafel in D. melanogaster aging (Tatar lab, Brown University). The falafel gene is a D. melanogaster homolog to SMK-1 (Caenorhabditis elegans), which is responsible for longevity extension upon reduced insulin/IGF-1 signaling.14 Studies showed that falafel overexpression increased fly life span, while strong falafel overexpression decreased it. When DR was added as a variable, it was found that falafel is not regulated by changes in nutrients. Some results suggested a role of falafel having a cardiac protective effect and a role with dFOXO (Drosophila fork-head box, sub-group O) in apoptosis. Falafel was overexpressed in the heart of adult flies, which lead to life span extension. Flies had increased apoptosis when falafel was overexpressed with dFOXO in the eye compared to flies with overexpression of dFOXO only. This suggests that falafel and dFOXO may work together in regulating longevity, however this relationship is still unclear.
Age-Related Diseases
The use of D. melanogaster as a model to study human diseases is becoming more common. The Drosophila Research Conference even had two platform sessions devoted to this topic due to the increase in this area of focus. There was also interest in looking at age-related diseases using the fruit fly.
Schulze (Western Washington University) studied lamin processing and Progeria using Drosophila. Lamins are filaments that provide structural support for the nuclear envelope and are present in the nucleoplasm and inner lining of the nuclear envelope. There are A-type and B-type lamins in humans and loss of function mutations in the A-type lamin gene can lead to one of many laminopathies. One laminopathy, Hutchinson-Gilford Progeria syndrome, results in premature aging.15 D. melanogaster possess both types of lamins, and Schulze made transgenic flies that expressed human A-type and B-type lamins. If human B-type lamins were expressed in flies, they died. However, human A-type lamins were stably expressed in the fly nuclear envelope. The life span of the fly model of Progeria was about 3-5 days and will be utilized for future studies.
The quality of life is more important to most people compared to the length of life, therefore, several areas of research are focusing on quality of life. Functional declines with aging are seen in several species (including flies) and age-related locomotor impairment (ARLI) is one decline being studied at Virginia Commonwealth University (Grotewiel). ARLI in flies was measured by using a rapid interactive negative geotaxis (RING) method. Methuselah mutants were studied and showed no change in geotaxis with age while other ARLI mutant flies (PDK1 [Phosphoinositide dependent kinase 1] and Doc3 [Drosocross 3]) had a decrease in ARLI and an increase in life span. Temperature and DR were also applied to the model. Increases in temperature led to accelerated ARLI and shortened life span while DR resulted in no change in locomotion behavior.
Another area studied was the mechanisms of cardiac functional decline (Wessells lab, University of Michigan). Both heart rate and the response to cardiac stress are age dependent. Results showed that a reduction of the Drosophila insulin like peptide extended fly life span and cardiac performance. Therefore, cardiac specific inhibition of insulin signaling impeded cardiac aging without life span changes. It was proposed that TOR and insulin signaling might converge on 4EBP and regulate cardiac function. Continued work and understanding in declines in cardiac function linked to aging mechanisms in flies could prove to be very useful in the future when looking at human cardiac function.
Conclusions
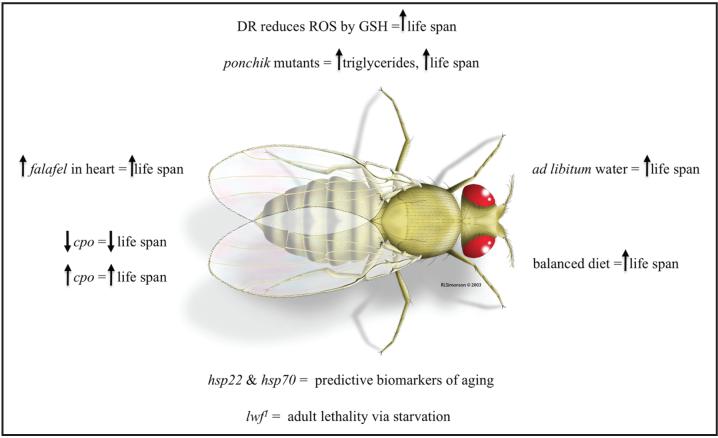
Research into aging and age-related diseases has increased to study this biological process. The presentations covered in this report, in addition to several other thought provoking posters and talks at the 49th Annual Drosophila Research Conference, provided more insight into this area of research (Fig. 1). The bad news is that questions of why and how organisms age are still unanswered. The good news is that many laboratories specializing in various biological disciplines (genetics, molecular biology, etc.) are searching for the answers using D. melanogaster as their model organism.
Figure 1.
Cartoon depiction of genes and manipulations that affect life span in Drosophila. The recent developments described in the text are shown in the figure. Abbreviations: DR (dietary restriction), ROS (reactive oxygen species), GSH (reduced glutathione), cpo (couch potato), hsp22 (heat shock protein 22), hsp70 (heat shock protein 70) and lwf1 (lot's wife null mutation). A ↑ indicates an increase and a ↓ indicates a decrease. Female D. melanogaster reprinted with permission from Rick Simonson of RLSimonson Studios.
Acknowledgements
The authors thank Larry Harshman for useful editorial comments and the following sources for funding: NIH grant number P20 RR016469 from the INBRE Program of the National Center for Research Resources (all authors), University of Nebraska (UNK) College of Natural and Social Sciences (Kimberly A. Carlson and Darby J. Carlson), UNK Research Services Council (Anjeza Pashaj), UNK Undergraduate Research Council (Kylee Gardner), and UNK Student Talent Development funds (Anjeza Pashaj and Kylee Gardner).
References
- 1.Marx JL. Aging research (i): cellular theories of senescence. Science. 1974;186:1105–7. doi: 10.1126/science.186.4169.1105. [DOI] [PubMed] [Google Scholar]
- 2.Warner HR. Subfield history: use of model organisms in the search for human aging genes. Sci Aging Knowledge Environ. 2003:6–1. doi: 10.1126/sageke.2003.6.re1. [DOI] [PubMed] [Google Scholar]
- 3.Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mech Ageing Dev. 2005;126:938–50. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Libert S, Zwiener J, Chu X, VanVoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–37. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- 5.Palmer MR, Sackton TB. The effects of dietary coenzyme Q on Drosophila lifespan. Aging Cell. 2003;2:335–39. doi: 10.1046/j.1474-9728.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 6.Lin YJ, Seroude L, Benzer S. Extended lifespan and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–6. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 7.Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by chico, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–75. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- 8.Rogina B, Reenan RA, Nelsen SP, Helfand SL. Extended life-span conferred by cotransporter gene mutations in Drosophila. Science. 2000;290:2137–40. doi: 10.1126/science.290.5499.2137. [DOI] [PubMed] [Google Scholar]
- 9.Bellen HJ, Vaessin H, Bier E, Kolodkin A, D'Evelyn D, Kooyer S, Jan YN. The Drosophila couch potato gene: an essential gene required for normal adult behavior. Genetics. 1992;131:365–75. doi: 10.1093/genetics/131.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler JC, Bieschke ET, Tower J. Muscle-specific expression of Drosophila hsp70 in response to aging and oxidative stress. Proc Natl Acad Sci USA. 1995;92:10408–12. doi: 10.1073/pnas.92.22.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhole D, Allikian MJ, Tower J. Doxycycline-regulated overexpression of hsp22 has negative effects on stress resistance and life span in adult Drosophila melanogaster. Mech Ageing Dev. 2004;125:651–63. doi: 10.1016/j.mad.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Grover D, Tower J, Tavare S. O fly, where art thou? J R Soc Interface. 2008 doi: 10.1098/rsif.2007.1333. E-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hafen E. Interplay between growth factor and nutrient signaling: lessons from Drosophila TOR. Curr Top Microbiol Immunol. 2004;279:153–67. doi: 10.1007/978-3-642-18930-2_10. [DOI] [PubMed] [Google Scholar]
- 14.Wolff S, Ma H, Burch D, Maciel GA, Hunter T, Dillin A. SMK-1, an essential regulator of DAF-16-mediated longevity. Cell. 2006;124:1039–53. doi: 10.1016/j.cell.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Schulze SR, Curio-Penny B, Li Y, Imani RA, Rydberg L, Geyer PK, Wallrath LL. Molecular genetic analysis of the nested Drosophila melanogaster lamin c gene. Genetics. 2005;171:185–96. doi: 10.1534/genetics.105.043208. [DOI] [PMC free article] [PubMed] [Google Scholar]