Abstract
The biological attack conducted through the U.S. postal system in 2001 broadened the threat posed by anthrax from one pertinent mainly to soldiers on the battlefield to one understood to exist throughout our society. The expansion of the threatened population placed greater emphasis on the reexamination of how we vaccinate against Bacillus anthracis. The currently-licensed Anthrax Vaccine, Adsorbed (AVA) and Anthrax Vaccine, Precipitated (AVP) are capable of generating a protective immune response but are hampered by shortcomings that make their widespread use undesirable or infeasible. Efforts to gain U.S. Food and Drug Administration (FDA) approval for licensure of a second generation recombinant protective antigen (rPA)-based anthrax vaccine are ongoing. However, this vaccine's reliance on the generation of a humoral immune response against a single virulence factor has led a number of scientists to conclude that the vaccine is likely not the final solution to optimal anthrax vaccine design. Other vaccine approaches, which seek a more comprehensive immune response targeted at multiple components of the B. anthracis organism, are under active investigation. This review seeks to summarize work that has been done to build on the current PA-based vaccine methodology and to evaluate the search for future anthrax prophylaxis strategies.
Keywords: Bacillus anthracis, anthrax, vaccine
Introduction
Bacillus anthracis is a highly virulent organism of intense interest within the biodefense field. The natural characteristics of B. anthracis, particularly the persistence and transmissibility of anthrax spores and the high rates of morbidity and mortality that result from spore exposure, make the bacterium an ideal candidate for weaponization. During the Cold War, the principal perceived threat of an anthrax-based weapon was its use as part of a military campaign. In the post-Cold War world this remains a concern, but following the attacks conducted through the U.S. postal system in 2001 (Jernigan et al., 2001; Jernigan et al., 2002) this scenario has been eclipsed by the worry that anthrax spores might be used against the general population. These and other developments have led to a renewed interest in anthrax vaccines.
The vaccine currently used in the U.S. has its origins in research dating back more than 50 years (Wright et al., 1951; Wright and Slein, 1951; Belton and Strange, 1954; Puziss and Wright, 1954; Wright et al., 1954; Auerbach and Wright, 1955; Henderson et al., 1956). Human field trials conducted with an earlier version of the vaccine demonstrated effectiveness in reducing rates of cutaneous, and perhaps inhalational, anthrax among those exposed to B. anthracis (Brachman et al., 1962). However, despite the tremendous step forward that the United States' anthrax vaccine, adsorbed (AVA) and the United Kingdom's anthrax vaccine, precipitated (AVP) represent, numerous reports have questioned the safety, practicality and long-term efficacy of the vaccine regimens (Turnbull, 2000; Baillie, 2001; Sever et al., 2004; Brey, 2005; Grabenstein, 2008). These concerns have resulted in the search for new and improved anthrax vaccines, including the possible development of a vaccine that incorporates multiple antigens presented by B. anthracis and targets different aspects of the organism's pathogenicity (Tournier et al., 2009). This review will attempt to summarize and assess research into new approaches to vaccinate against B. anthracis.
Toxin-Based Anthrax Vaccines
AVA
The currently-licensed AVA vaccine used in the United States and the closely-related AVP vaccine used in the United Kingdom strive to protect vaccinees from the lethal effects of toxins elaborated by B. anthracis following spore germination (Mock and Fouet, 2001). The key component of the vaccine is protective antigen (PA), the element common to lethal toxin (LT) and edema toxin (ET). These toxins play critical roles in B. anthracis pathogenicity (Keppie et al., 1955; Smith et al., 1955a; Smith et al., 1955b; Molnar and Altenburn, 1963; Pezard et al., 1991). Early work demonstrating the protective efficacy of an antibody-based immune response to PA, along with the subsequent development of PA-based anthrax vaccines, is the subject of numerous reviews (Hambleton et al., 1984; Nass, 1999; Baillie, 2001; Little, 2005; Brey, 2005; Wang and Roehrl, 2005; Grabenstein, 2008). Studies performed by Brachman and colleagues (Brachman et al., 1962) demonstrated the capacity of a PA-based vaccine to lower the incidence of anthrax among exposed human populations and provided a strong rationale for administration to certain at-risk populations within the military and health-care communities. It is worth noting that differences exist between the AVA and AVP vaccines even though both are supernatant-based preparations designed to generate a protective response to PA. Compared to AVA, the British AVP contains lower levels of PA and higher concentrations of additional B. anthracis antigens, such as lethal factor (LF), edema factor (EF), and certain bacillus surface proteins (Turnbull, 1991; Baillie et al., 2003; Whiting et al., 2004). These additional or enriched components in the British AVP anthrax vaccines, which reflect the production strain used and/or the vaccine preparation techniques employed, may impart a slight enhancement in protection (Baillie et al., 2004), and may also be the source of the increased transient reactogenicity seen in comparison to AVA (Turnbull, 2000).
In recent times, events such as the Persian Gulf War of 1991 and the anthrax attacks of 2001 caused the perceived “at-risk” population to grow. As the target population grew, so too did concerns over the efficacy, cumbersome regimen (6 shots over 18 months and an annual booster), and possible side effects of the AVA vaccine (Pittman et al., 2001; Swanson-Biearman and Krenzelok, 2001; Geier and Geier, 2002; Greidanus and Honl, 2002; Hoffman et al., 2003; Lange et al., 2003; Wasserman et al., 2003; Geier and Geier, 2004; Hunter et al., 2004; Pittman et al., 2004; Grabenstein et al., 2006; Vasudev and Zacharisen, 2006; McNeil et al., 2007; Payne et al., 2007; Smith et al., 2007). Much energy has been devoted to investigating and addressing these concerns (Friedlander et al., 1999; Joellenbeck et al., 2002; Niu et al., 2009). Recent studies demonstrated efficacious vaccination schedules that require fewer shots to achieve necessary antibody titers (Pittman et al., 2000; Pittman et al., 2002; Pittman et al., 2006; Marano et al., 2008; Singer et al., 2008). However, questions persist regarding variability of AVA production batches and reactogenicity, a common yet variable side effect of repeated vaccination (Brachman et al., 1962) that may be attributable to the less well-defined elements of the vaccine (Turnbull, 2000). Moreover, the AVA vaccine fails to fully protect against certain geographic isolates of B. anthracis (Little and Knudson, 1986; Fellows et al., 2001). Taken together, these questions about the tolerability and efficacy of AVA as administered in the current regimen have provided the impetus for the development of a 2nd generation vaccine.
Recombinant PA
In part to address the existing concerns over issues such as the safety and lot-to-lot variability of AVA, any 2nd generation anthrax vaccine needed to contain well-defined and controlled components. Given the essentiality of protection against intoxication, and the knowledge that an immune response to PA blocks the action of both key anthrax toxins, it was clear that this vaccine would be based on a recombinantly expressed PA (rPA) molecule. Studies with guinea pigs (McBride et al., 1998), rabbits (Little et al., 2004) and monkeys (Ivins et al., 1998) have demonstrated the capacity of rPA to protect against aerosol challenge. Like AVA, protection afforded by rPA-based vaccines is primarily antibody-based (Williamson et al., 2005). Titers of toxin-neutralizing antibodies, which are the most reliable predictor of protection against lethal challenge (Weiss et al., 2006), can be induced to similar levels with an rPA-based vaccine as with AVA (Gorse et al., 2006; Campbell et al., 2007). The rPA molecule can also be truncated (Hepler et al., 2006) or modified (Ramirez et al., 2002; Ribot et al., 2006; Rhie et al., 2005) while still maintaining the capacity to induce a protective humoral response. As with AVA, results with rPA-based vaccines consistently indicate the requirement for annual boosters to maintain levels of toxin-neutralizing antibodies sufficient to ensure protection (Ivins et al., 1998; Pittman et al., 2002; Little et al., 2006). Human clinical trials meant to investigate the safety and immunogenicity of the rPA-based vaccine PA102 are currently underway (Gorse et al., 2006; Keitel, 2006).
Alternative Approaches to PA-based Vaccines
Modified Recombinant PA
Despite the efficacy of the rPA-based anthrax vaccine currently under development, many researchers strive to develop improved rPA vaccines that demonstrate more rapid humoral induction, more durable efficacy and/or broader utility. One approach with potentially broad application involves the use of Dominant Negative Inhibitors (DNIs), mutated forms of PA that can undergo heptamerization at the cell surface but not the conformational changes necessary for membrane translocation. In the absence of that PA translocation step, anthrax toxin trafficking and function cease (Sellman et al., 2001a; Sellman et al., 2001b; Singh et al., 2001). These DNIs are protective against intoxication when administered with active toxin (Sellman et al., 2001b; Singh et al., 2001). They are also capable of inducing antibodies that successfully neutralize the activity of normal rPA (Aulinger et al., 2005; Yan et al., 2008). Unlike fully-functional rPA, DNIs might be given to a patient post-exposure without risk of enhancing intoxication during an infection. Hence, DNIs could potentially serve as both a pre-exposure prophylactic vaccine and a post-exposure therapeutic.
Alternate Adjuvants
Much research has been devoted to improving anti-PA humoral responses through modified delivery of rPA, rather than modification of rPA itself. One strategy involves the investigation of new adjuvants that enhance the host response when compared with the alum adjuvants currently approved for human use (Edelman, 2000). Adjuvants capable of evoking a more rapid or more durable response offer hope of a reduced number of shots during initial vaccination, or extension of the period between sustaining boosts. Early studies that used certain microbial products and/or oil chemical compounds, such as saponin, achieved antibody levels and protection equivalent to that achieved with approved alum adjuvants in a variety of animal models (Ivins et al., 1992; Ivins et al., 1995; Ivins et al., 1998). Experiments with the Ribi adjuvant (a mixture of squalene, Tween-80, mycobacterial products and the bacterial compound monophosphoryl lipid A) demonstrated greater protection of guinea pigs against inhalational anthrax despite lower doses of immunogen (McBride et al., 1998).
CpG oligodeoxynucleotide molecules (ODMs) have also been evaluated as possible adjuvants for anthrax vaccines. CpG ODMs work as an adjuvant by stimulating the immune response through the induction of Toll-Like Receptor 9 (TLR9) (Takeshita et al., 2004). These molecules induced a humoral response that was more rapid, more avid, and longer lasting than when rPA (or AVA) was administered with alum (Klinman et al., 2004). This stronger, broader response led to protection that was more rapidly generated and longer in duration for animal models ranging from mice (Coeshott et al., 2004) to macaques (Klinman et al., 2004). Given some of the afore-mentioned limitations of the AVA regimen, an additive capable of increasing both the speed and the endurance of a protective humoral response warrants further investigation.
Needle-free Delivery
Aside from attempts to augment the response generated to rPA when administered through the traditional intramuscular route, numerous investigators have sought to use alternate delivery routes to achieve more relevant or robust immune responses. Intradermal injection shows evidence of inducing toxin-neutralizing antibodies whose levels correlate well with protection in rabbits (Mikszta et al., 2005). Some results (Mikszta et al., 2006) suggest that these intradermal injectors produce a more rapid humoral induction than that achieved through classic intramuscular injection. Skin patches that contained rPA adjuvanted with E. coli heat-labile toxin conferred protection onto A/J mice subsequently challenged with a toxigenic, unencapsulated anthrax strain (Kenney et al., 2004; Matyas et al., 2004). This model system (Welkos and Friedlander, 1988), which involves a complement-deficient mouse (The Jackson Laboratory, 2008) and an attenuated B. anthracis strain, is commonly used for preliminary research but does not always accurately reflect an infection with fully-virulent anthrax. Indeed, further mouse experimentation demonstrated that upon challenge with fully virulent B. anthracis the protection was only partial (Peachman et al., 2006). The patches nonetheless do induce significant levels of IgA in the lungs of vaccinated animals (Kenney et al., 2004). New variations in the intradermal delivery of PA (Knockenhauer et al., 2008) offer the continued possibility of successful anthrax vaccination through this approach.
Mucosal Vaccination
Perhaps the most likely scenario for a biological attack involving anthrax entails the inhalation of aerosolized spores. Because of the known persistence of spores in the lung following such exposure (Barnes, 1947; Henderson et al., 1956), the presence of anti-PA IgA molecules in the lung may be valuable in the prevention of disease. A reasonable approach for stimulating anti-PA IgA in the lung would be to intranasally administer a vaccine, installing the immunogen into the same sites likely to be exposed during inhalational infection. Intranasal administration of rPA to mice not only yielded higher anti-PA IgA titers than did subcutaneous administration but also generated similar levels of anti-PA IgG and toxin-neutralizing antibodies (Gaur et al., 2002). However, later studies that examined the immunogenicity and protection afforded to mice (Sloat and Cui, 2006a; Sloat and Cui, 2006b; Zeng et al., 2007; Baillie et al., 2008), guinea pigs (Bielinska et al., 2007) and rabbits (Huang et al., 2007) yielded mixed results. The technique appears to be most effective when rPA is provided in powder form, rather than as a liquid (Wimer-Mackin et al., 2006; Klas et al., 2008). Intramuscular priming of rPA following by an intranasal boost may prove to be the most effective strategy (Flick-Smith et al., 2002).
An alternate approach to inducing mucosal immunity involves oral administration of PA in the form of PA-expressing bacterial vectors. Efforts thus far have mostly focused on either nontoxigenic, unencapsulated B. anthracis strains expressing PA or attenuated, PA-producing Salmonella enterica. Oral administration of live, unencapsulated, nontoxigenic B. anthracis spores expressing rPA yielded significant anti-PA IgA and toxin-neutralizing levels but afforded protection to guinea pigs that was less than that provided by subcutaneous administration of the same vector (Aloni-Grinstein et al., 2005). Meanwhile, after less than encouraging early results (Garmory et al., 2003), S. enterica-based vaccines show greater promise. Efforts to improve PA expression led to improved immune responses (Galen et al., 2004; Osorio et al., 2009). As a consequence, enhanced induction of toxin-neutralizing antibodies has resulted in substantial protection against aerosol exposure in experiments involving A/J mice (Stokes et al., 2007; Osorio et al., 2009). Recent results (Mohamadzadeh et al., 2008; Mohamadzadeh et al., 2009) with PA-encoding Lactobacillus vehicles offer to further expand the possibilities of this approach.
Live, attenuated bacilli injection
Another approach to generating a protective anti-toxin response is through the direct injection of attenuated B. anthracis. The injection of rats and guinea pigs with a toxigenic, nonencapsulated strain generated a robust antibody response to PA, LF and EF and resulted in protection against subsequent challenge with virulent anthrax (Ivins et al., 1986). A more recent approach afforded protection in mice, guinea pigs and rabbits via the delivery of nonfunctional but immunogenic PA, LF and EF through a killed but metabolically-active (KBMA) strain. This unencapsulated B. anthracis construct is capable of secretion despite its photochemically-inactivated and asporogenic state (Skoble et al., 2009). This most recent approach succeeds in achieving immunogenicity and protection while exhibiting less reactogenicity than rPA adjuvanted with alum (Skoble et al., 2009).
DNA Vaccination
By introducing immunogen-encoding genetic material into a host cell, DNA vaccinations offer the prospect of maintaining levels of target antigen within a vaccinated individual for a longer period than with protein injection. The potential utility of this approach is illustrated by available evidence which suggests that while PA-encoding DNA vaccines might not achieve the same intensity in the initial immune response as is seen with a protein-based vaccine, the resulting immunological memory is longer-lasting (Hahn et al., 2006a). Work toward a DNA vaccine for anthrax has included demonstration of the generation of an anti-PA immune response in mice, rats and rabbits (Gu et al., 1999; Luxembourg et al., 2008). However, reports of successful protection of hosts through the administration of PA-encoding DNA alone have been limited (Riemenschneider et al., 2003; Midha and Bhatnagar, 2009). In an attempt to enhance the intensity of the immune response to the antigen targets, several groups have pursued a DNA prime/protein boost strategy (Williamson et al., 1999; Price et al., 2001; Galloway et al., 2004). These researchers achieved higher anti-PA IgG and toxin neutralizing antibody levels through this approach than they did with a series of DNA injections alone. Other investigators found that the co-administration of LF-encoding DNA alongside the PA-encoding DNA yielded higher IgG and toxin-neutralizing antibody responses than when either target was administered alone (Price et al., 2001; Hermanson et al., 2004; Liu et al., 2009). Still other have succeeded in generating anti-PA responses (Lee et al., 2003; Shivachandra et al., 2007; Liu et al., 2009) and protection from challenge (Hashimoto et al., 2005) through application of PA-encoding viral vectors. Thus far, almost all positive findings with viral vectors and other DNA vaccines have been achieved with mouse models. The ability to translate these successes into demonstrated efficacy in more advanced model systems remains to be seen.
New Vaccine Targets
Despite the potential improvements in PA-based vaccine strategies, there is reason to remain uneasy about continued reliance upon antibodies to PA as the sole protector against anthrax. Titers of total anti-PA IgG (Turnbull et al., 1988; Pitt et al., 1999; Pitt et al., 2001; Pittman et al., 2005; Little et al., 2004), or more specifically, toxin-neutralizing antibodies (McBride et al., 1998; Reuveny et al., 2001; Marcus et al., 2004; Pittman et al., 2005; Weiss et al., 2006; Hewetson et al., 2008), are generally considered to be good predictors of a protective immune response. However, certain studies suggest that the anti-PA humoral response generated by PA-based vaccines and the level of protection conferred by antibodies to PA are more variable than was first thought (Welkos and Friedlander, 1988a; Ivins et al., 1992; Ivins et al., 1995; Ivins et al., 1998; Fellows et al., 2001). In situations where patients face infection from anthrax in the midst of immunocompromise, a robust response to PA may be insufficient to confer complete protection (Welkos and Friedlander, 1988b; Peachman et al., 2006). Also, the need to subject a patient to extended vaccination regimens in order to reach acceptable levels of anti-PA antibodies remains a major drawback to the PA-based approach. Furthermore, the potential exists for development of a genetically-engineered B. anthracis strain in which PA has been supplanted by an alternative virulence factor. Certain naturally-occurring isolates which are at least partially refractile to AVA, through molecular bases not currently understood (Little and Knudson, 1986; Fellows et al., 2001), raise concerns over the PA-based approach.
Undoubtedly, the generation of a robust response to PA will remain a critical component of any future anthrax vaccine. The absence of PA negates any potential benefits from other vaccine components (Ivins et al., 1986; Pezard et al., 1995; Cohen et al., 2000; Brossier et al., 2002; Brahmbhatt et al., 2007; Cybulski, Jr. et al., 2008). However, it seems altogether wise to consider a comprehensive, multi-targeted approach to anthrax vaccination that mirrors successful approaches taken with other bacterial pathogens such as Bordetella pertussis. The existence of distinct life stages and virulence factors, each of which is essential to bacterial virulence and immunogenic during the course of a natural infection (Sirisanthana et al., 1988; Harrison et al., 1989; Zhang et al., 2008), provides vaccine researchers with an array of potential targets.
Capsular Antigens
By virtue of its position as the outermost surface of the bacilli, the antiphagocytic (Ezzell and Welkos, 1999) poly-γ-D-glutamic acid (PGGA) capsule is the face presented to the host immune system upon spore germination. Examination of the sera from survivors of anthrax infection demonstrates an almost universal response to the B. anthracis capsule (Sirisanthana et al., 1988; Harrison et al., 1989), though this information alone does not necessarily ensure that such a response has protective benefits. Early investigations (Goodman and Nitecki, 1966) demonstrated the relatively poor immunogenicity of PGGA administered alone. Therefore, a common approach to investigating the possible benefit of PGGA has involved conjugation of capsular polypeptides to protein carriers. Vaccinating with a PA:PGGA conjugate holds the potential for a dual benefit, with the PA protein enhancing the immune response to the capsule while simultaneously immunizing against intoxication. This approach successfully induced IgG responses to both PA and PGGA in mice (Schneerson et al., 2003; Rhie et al., 2003; Wang et al., 2004; Sloat and Cui, 2006a). Simultaneous administration of the conjugated components did not deleteriously affect the response to PA compared to PA administered alone (Sloat and Cui, 2006a). Enhanced protection against spore challenge was demonstrated in mice (Chabot et al., 2004; Joyce et al., 2006) and rabbits (Wimer-Mackin et al., 2006) with PA:PGGA conjugates compared to PA alone or PA plus unconjugated PGGA. Anti-PGGA antibodies show a capacity in vitro to induce opsonophagocytosis (Schneerson et al., 2003; Wang et al., 2004) and complement-mediated killing (Rhie et al., 2003).
The spore
When considering possible approaches to the development of more comprehensive protection against anthrax, one must also consider research involving attenuated strains of B. anthracis administered in spore form. Pasteur and Greenfield's original vaccine strains (Tigertt, 1980; Sternbach, 2003), as well as the strain isolated by Sterne in the 1930's and still in use today as a veterinary vaccine (Sterne, 1939; Turnbull, 2000), relied on administration of whole spores. An attenuated, live spore anthrax vaccine, developed by scientists in the former Soviet Union, is still in use in Russia and China (Dong, 1990; Shlyakhov and Rubinstein, 1994). Spore vaccines constructed from toxigenic, unencapsulated strains have repeatedly demonstrated the capacity to confer protection to mice and guinea pigs against anthrax challenge (Klein et al., 1962; Ivins et al., 1986; Little and Knudson, 1986; Welkos and Friedlander, 1988a; Ivins et al., 1990; Brossier et al., 2000). Certain studies indicate that these attenuated spore vaccines are more protective than existing PA-based vaccines (Little and Knudson, 1986; Ivins et al., 1986; Welkos and Friedlander, 1988a). However, one must always bear in mind the key role for protection against intoxication in any vaccination scheme. Spores made from unencapsulated, nontoxigenic strains fail to protect against subsequent challenge (Ivins et al., 1986; Pezard et al., 1995; Cohen et al., 2000), whereas the reintroduction of PA into such toxin-minus strains via rPA-encoding plasmids restores protective efficacy (Barnard and Friedlander, 1999; Cohen et al., 2000; Aloni-Grinstein et al., 2005; Mendelson et al., 2005). Effective generation of protective immunity can also be achieved by the administration of spores made from an unencapsulated strain encoding for nonfunctional but immunogenic toxin molecules (Brossier et al., 2000). However, utilization of live spores for vaccination has been hampered in the U.S. and Europe, despite its possible benefit, due in part to concerns over residual virulence, safety and the efficacy of the approach. In order to harness the protective benefit of the spore, an alternative strategy must be identified.
Studies involving the use of formalin-inactivated spores (FIS) administered as a vaccine in conjunction with PA offer an alternate approach to harnessing the spore to generate enhanced protective immunity (Brossier et al., 2002; Gauthier et al., 2009). These investigations demonstrated enhanced protection of both mice and guinea pigs by vaccination with FIS plus PA compared to PA alone. This work provided one potential strategy to achieve a broader immune response in the absence of a live B. anthracis strain. A later study demonstrated that immunization with spores induced a cellular immune response that might contribute to protective immunity against anthrax (Glomski et al., 2007). Demonstrations that protective anti-spore responses were not dependent on the presence of a live organism raise the possibility that individual spore parts might, in association with PA, augment a protective immune response.
One enticing target from among the long list of spore components is Bacillus Collagen-like antigen (BclA), the immunodominant glycoprotein responsible for the hair-like protrusions present on the outermost surface of B. anthracis (Hachisuka et al., 1966; Sylvestre et al., 2002; Steichen et al., 2005). Studies that examined the efficacy of BclA delivered with PA in either DNA (Hahn et al., 2006a; Hahn et al., 2006b) or protein (Brahmbhatt et al., 2007) form showed enhanced protection compared to PA alone. Enhancement correlated with the appearance of an anti-BclA humoral response. More recently, a screen of a list of proteins known to be visible to the host and immunogenic in the context of whole spores broadened the inventory of potentially-protective spore antigens (Cybulski, Jr. et al., 2008). This study identified the structural protein BxpB and the previously-undescribed antigen p5303 as immunogens capable of enhancing the protection afforded by PA when A/J mice are challenged with toxigenic, unencapsulated B. anthracis. Each of these investigations also reaffirmed the importance of PA, as they failed to observe any significant protection when the spore antigens were administered alone.
Passive Antibody Therapy
Our growing understanding of protective immune responses to anthrax can be used not only to develop and improve prophylactic vaccines but also to design post-exposure therapies. One approach involves the use of those same vaccines, which have been shown to improve the outcome of exposure in previously-unvaccinated individuals when combined with antibiotic therapy (Friedlander et al., 1993; Fowler et al., 2005; Peterson et al., 2006; Baccam and Boechler, 2007). Another tactic, valuable perhaps in situations where vaccination is precluded by limited supply or host immunocompromise, is the passive administration of protective antibodies. Protective benefits of the passive administration of AVA-induced antisera seem to be derived primarily from the toxin-neutralizing activity of anti-PA antibodies (Little et al., 1997; Beedham et al., 2001; Reuveny et al., 2001; Kobiler et al., 2002; Hewetson et al., 2008), though studies by Welkos and colleagues indicate that antibodies raised against PA also have anti-spore characteristics (Welkos et al., 2001; Welkos et al., 2002).
Much of the recent work in the field of anti-anthrax passive antibody therapeutics has been devoted to the identification and development of human monoclonal antibodies (mAbs). The identification of human mAbs with the capacity to neutralize PA has come primarily through the screening of sera belonging to AVA-vaccinated individuals (Wild et al., 2003; Sawada-Hirai et al., 2004; Zhou et al., 2008). While these reagents carry the potential benefit of better characterization and standardized production compared to polyclonal sera, the challenge lies in the identification of mAbs that match the efficacy of the polyclonal approach (Casadevall, 2002) and do not have the unintended effect of enhancing infection (Mohamed et al., 2004). Anti-PA mAbs have demonstrated the capacity to protect a number of animal models, including mice (Peterson et al., 2006), guinea pigs (Mabry et al., 2005; Peterson et al., 2006) and rabbits (Mohamed et al., 2005; Peterson et al., 2006; Peterson et al., 2007; Wild et al., 2007). Protective efficacy of mAbs against anthrax upon subcutaneous administration of antibodies (Peterson et al., 2007; Wild et al., 2007) offers the possibility of a less cumbersome regimen than the intravenous antibody administration utilized to date. Evidence of a synergistic effect when antibodies are administered alongside antibiotics (Peterson et al., 2006) is also promising. This latter point may prove crucial given that the effectiveness of passive therapy declines as time passes between exposure and mAb administration (Kozel et al., 2004; Mohamed et al., 2005; Peterson et al., 2007; Wild et al., 2007).
PA is an obvious target upon which to focus efforts for development of a therapeutic human mAb. However, as in the case of vaccination, mounting evidence supports the value of pursuing additional or alternate targets for antibody therapy. One such target is the LF molecule, whose interaction with PA is essential for the intoxication that PA-based therapies aim to block (Ascenzi et al., 2002). Several studies (Albrecht et al., 2007; Staats et al., 2007) indicate that anti-LF mAbs are perhaps a superior therapeutic to anti-PA mAbs. In vitro evidence indicates that, unlike anti-PA mAbs, anti-LF mAbs are able to protect cell lines from intoxication despite delayed administration after exposure to toxin (Staats et al., 2007). A mouse protection study suggested that anti-LF mAbs are retained in the bloodstream longer, and protect hosts at a lower concentration, than do anti-PA mAbs (Albrecht et al., 2007). An additional advantage of a potential anti-LF mAb is that it might be administered alongside a PA-based vaccine with minimal risk of interference with the development of an anti-PA response (Pelat et al., 2007).
Aside from major virulence factors such as PA and LF, the targeting of other bacterial components may also hold protective potential. For example, monoclonal antibodies directed against the poly-D-γ-glutamic capsule afforded mice protection against anthrax (Kozel et al., 2007). Moreover, a recent study demonstrated that mAbs to anthrolysin O provided partial protection of A/J mice against infection with B. anthracis Sterne strain (Nakouzi et al., 2008). Our laboratory found that antibodies to certain spore antigens promoted the opsonophagocytosis of spores (Brahmbhatt et al., 2007; Cybulski, Jr. et al., 2008), a step which helps confer partial protection by promoting spore clearance and reducing the effective infectious load. As is the case with vaccination, it is hard to conceive of a passive therapeutic approach that does not incorporate protection against intoxication. However, these results suggest that it may be possible to find ways to enhance any protection afforded by a PA- or LF-based approach.
Conclusion
The threat of an intentional anthrax outbreak, and the search for improvements in the available anthrax prophylaxis tools, will likely remain a subject of great interest within the realm of biodefense for the foreseeable future. The AVA and AVP vaccines represented a significant step forward in an era before modern molecular biological techniques and provided a major defense mechanism for key populations at risk for anthrax exposure. However, the 1991 Persian Gulf War and the 2001 anthrax attacks led to the massive expansion of the population of potential vaccinees, which, in turn, led to a focus on the limitations of these vaccines. The development of a defined, recombinant PA-based vaccine promises to address some of concerns, but questions remain. Without a firm understanding of how important the “undefined” components of AVA and AVP are in the levels of protection afforded by that vaccine, it remains unclear whether or not rPA will entirely recapitulate the efficacy of these vaccines in non-human primates and humans. While numerous strategies that involve molecular alteration, improved adjuvants, and novel delivery offer the possibility of enhancing the effectiveness of a PA-based approach, there is ample reason to remain skeptical that a vaccine relying on PA alone can be a final answer. The surge of research interest that resulted from the biological attacks of 2001 has led to tremendous progress in the understanding of the basis molecular processes of the B. anthracis pathogenesis. As has recently been discussed elsewhere (Tournier et al., 2009), these new insights have broadened, and will likely continue to broaden, the array of possible vaccine targets that might enhance the current PA-based protection scheme. Consequently, a multi-pronged approach targeting distinct aspects of the B. anthracis organism seems like a logical goal for future anthrax vaccines.
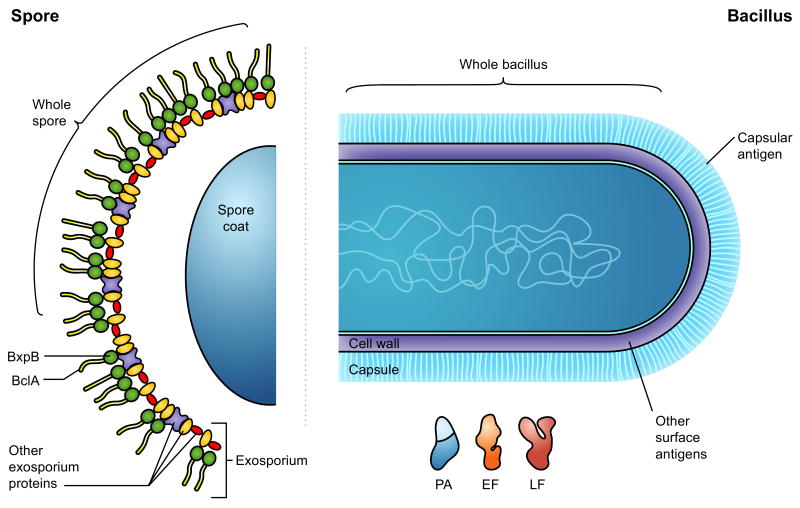
Figure 1.
Potential anthrax vaccination targets. Protective or partially-protective immune responses can be directed at either phase of the Bacillus anthracis life cycle: the spore or the vegetative bacilli. Aside from the administration of live, attenuated spores, an anti-spore humoral response can be elicited via the delivery of individual spore components such as BclA or BxpB. Prime targets from the vegetative life cycle include the anthrax toxin components protective antigen (PA), lethal factor (LF) and edema factor (EF) as well as the antigen that comprises the antiphagocytic poly-γ-D-glutamic acid capsule. Direct administration of live, attenuated bacilli, primarily as a vehicle for delivery of PA, has also been explored as a vaccination strategy.
Table 1. Potential Anthrax Vaccine Strategies.
| PA-based Vaccination Strategies |
| First Generation (AVA) |
| 2nd Generation (PA102) |
| 3rd Generation (alternative rPA-based) |
| Modified rPA |
| Alternative adjuvants |
| Needle-free delivery |
| Intradermal injection |
| Transcutaneous administration |
| Mucosal administration |
| Intranasal delivery |
| Oral delivery (attenuated bacterial vector) |
| Live, attenuated bacilli injection |
| DNA vaccination |
| Viral vector |
| Plasmid injection |
| Alternative vaccination strategies |
| Bacilli capsular antigens |
| Attenuated whole spores |
| Spore antigens |
| BclA |
| BxpB |
| other |
| Passive antibody therapy |
| Anti-PA |
| Anti-LF |
| Anti-capsule |
| other |
Acknowledgments
We thank Ms. Sophia Echelmeyer and Mr. Patrice Bolté for help with illustration production. This work was supported by NIH/NIAID Middle Atlantic Regional Center of Excellence grant U54 AI057168 and research funds from the United States Navy through Uniformed Services University grant # G173HS. Special thanks also to the United States Army Long-Term Health Education and Training Program for support of one of us (R. Cybulski). The opinions and assertions in this paper are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Navy or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Albrecht MT, Li H, Williamson ED, LeButt CS, Flick-Smith HC, Quinn CP, Westra H, Galloway D, Mateczun A, Goldman S, Groen H, Baillie LW. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect Immun. 2007;75:5425–5433. doi: 10.1128/IAI.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni-Grinstein R, Gat O, Altboum Z, Velan B, Cohen S, Shafferman A. Oral spore vaccine based on live attenuated nontoxinogenic Bacillus anthracis expressing recombinant mutant protective antigen. Infect Immun. 2005;73:4043–4053. doi: 10.1128/IAI.73.7.4043-4053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascenzi P, Visca P, Ippolito G, Spallarossa A, Bolognesi M, Montecucco C. Anthrax toxin: a tripartite lethal combination. FEBS Lett. 2002;531:384–388. doi: 10.1016/s0014-5793(02)03609-8. [DOI] [PubMed] [Google Scholar]
- Auerbach S, Wright GG. Studies on immunity in anthrax. VI. Immunizing activity of protective antigen against various strains of Bacillus anthracis. J Immunol. 1955;75:129–133. [PubMed] [Google Scholar]
- Aulinger BA, Roehrl MH, Mekalanos JJ, Collier RJ, Wang JY. Combining anthrax vaccine and therapy: a dominant-negative inhibitor of anthrax toxin is also a potent and safe immunogen for vaccines. Infect Immun. 2005;73:3408–3414. doi: 10.1128/IAI.73.6.3408-3414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccam P, Boechler M. Public health response to an anthrax attack: an evaluation of vaccination policy options. Biosecur Bioterror. 2007;5:26–34. doi: 10.1089/bsp.2006.0001. [DOI] [PubMed] [Google Scholar]
- Baillie L. The development of new vaccines against Bacillus anthracis. J Appl Microbiol. 2001;91:609–613. doi: 10.1046/j.1365-2672.2001.01498.x. [DOI] [PubMed] [Google Scholar]
- Baillie L, Hebdon R, Flick-Smith H, Williamson D. Characterisation of the immune response to the UK human anthrax vaccine. FEMS Immunol Med Microbiol. 2003;36:83–86. doi: 10.1016/S0928-8244(03)00085-3. [DOI] [PubMed] [Google Scholar]
- Baillie L, Townend T, Walker N, Eriksson U, Williamson D. Characterization of the human immune response to the UK anthrax vaccine. FEMS Immunol Med Microbiol. 2004;42:267–270. doi: 10.1016/j.femsim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Baillie LW, Rodriguez AL, Moore S, Atkins HS, Feng C, Nataro JP, Pasetti MF. Towards a human oral vaccine for anthrax: the utility of a Salmonella Typhi Ty21a-based prime-boost immunization strategy. Vaccine. 2008;26:6083–6091. doi: 10.1016/j.vaccine.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard JP, Friedlander AM. Vaccination against anthrax with attenuated recombinant strains of Bacillus anthracis that produce protective antigen. Infect Immun. 1999;67:562–567. doi: 10.1128/iai.67.2.562-567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JM. The development of anthrax following the administration of spores by inhalation. Br J Exp Pathol. 1947;28:385–394. [Google Scholar]
- Beedham RJ, Turnbull PC, Williamson ED. Passive transfer of protection against Bacillus anthracis infection in a murine model. Vaccine. 2001;19:4409–4416. doi: 10.1016/s0264-410x(01)00197-9. [DOI] [PubMed] [Google Scholar]
- Belton FC, Strange RE. Studies on a protective antigen produced in vitro from Bacillus anthracis: medium and methods of production. Br J Exp Pathol. 1954;35:144–152. [PMC free article] [PubMed] [Google Scholar]
- Bielinska AU, Janczak KW, Landers JJ, Makidon P, Sower LE, Peterson JW, Baker JR., Jr Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect Immun. 2007;75:4020–4029. doi: 10.1128/IAI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman PS, Gold H, Plotkin SA, Fekety FR, Werrin M, Ingraham NR. Field Evaluation of a Human Anthrax Vaccine. Am J Public Health Nations Health. 1962;52:632–645. doi: 10.2105/ajph.52.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmbhatt TN, Darnell SC, Carvalho HM, Sanz P, Kang TJ, Bull RL, Rasmussen SB, Cross AS, O'Brien AD. Recombinant exosporium protein BclA of Bacillus anthracis is effective as a booster for mice primed with suboptimal amounts of protective antigen. Infect Immun. 2007;75:5240–5247. doi: 10.1128/IAI.00884-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey RN. Molecular basis for improved anthrax vaccines. Adv Drug Deliv Rev. 2005;57:1266–1292. doi: 10.1016/j.addr.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Brossier F, Levy M, Mock M. Anthrax spores make an essential contribution to vaccine efficacy. Infect Immun. 2002;70:661–664. doi: 10.1128/iai.70.2.661-664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossier F, Weber-Levy M, Mock M, Sirard JC. Role of toxin functional domains in anthrax pathogenesis. Infect Immun. 2000;68:1781–1786. doi: 10.1128/iai.68.4.1781-1786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JD, Clement KH, Wasserman SS, Donegan S, Chrisley L, Kotloff KL. Safety, reactogenicity and immunogenicity of a recombinant protective antigen anthrax vaccine given to healthy adults. Hum Vaccin. 2007;3:205–211. doi: 10.4161/hv.3.5.4459. [DOI] [PubMed] [Google Scholar]
- Casadevall A. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg Infect Dis. 2002;8:833–841. doi: 10.3201/eid0808.010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot DJ, Scorpio A, Tobery SA, Little SF, Norris SL, Friedlander AM. Anthrax capsule vaccine protects against experimental infection. Vaccine. 2004;23:43–47. doi: 10.1016/j.vaccine.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Coeshott CM, Smithson SL, Verderber E, Samaniego A, Blonder JM, Rosenthal GJ, Westerink MA. Pluronic F127-based systemic vaccine delivery systems. Vaccine. 2004;22:2396–2405. doi: 10.1016/j.vaccine.2003.11.064. [DOI] [PubMed] [Google Scholar]
- Cohen S, Mendelson I, Altboum Z, Kobiler D, Elhanany E, Bino T, Leitner M, Inbar I, Rosenberg H, Gozes Y, Barak R, Fisher M, Kronman C, Velan B, Shafferman A. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect Immun. 2000;68:4549–4558. doi: 10.1128/iai.68.8.4549-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski RJ, Jr, Sanz P, McDaniel D, Darnell S, Bull RL, O'Brien AD. Recombinant Bacillus anthracis spore proteins enhance protection of mice primed with suboptimal amounts of protective antigen. Vaccine. 2008;26:4927–4939. doi: 10.1016/j.vaccine.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong SL. Progress in the control and research of anthrax in China. Salisb Med Bull. 1990;68:104. [Google Scholar]
- Edelman R. An Overview of Adjuvant Use. In: O'Hagan DT, editor. Vaccine Adjuvants. Totowa, N.J.: Humana Press, Inc.; 2000. pp. 1–27. [Google Scholar]
- Ezzell JW, Welkos SL. The capsule of Bacillus anthracis, a review. J Appl Microbiol. 1999;87:250. doi: 10.1046/j.1365-2672.1999.00881.x. [DOI] [PubMed] [Google Scholar]
- Fellows PF, Linscott MK, Ivins BE, Pitt ML, Rossi CA, Gibbs PH, Friedlander AM. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine. 2001;19:3241–3247. doi: 10.1016/s0264-410x(01)00021-4. [DOI] [PubMed] [Google Scholar]
- Flick-Smith HC, Eyles JE, Hebdon R, Waters EL, Beedham RJ, Stagg TJ, Miller J, Alpar HO, Baillie LW, Williamson ED. Mucosal or parenteral administration of microsphere-associated Bacillus anthracis protective antigen protects against anthrax infection in mice. Infect Immun. 2002;70:2022–2028. doi: 10.1128/IAI.70.4.2022-2028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler RA, Sanders GD, Bravata DM, Nouri B, Gastwirth JM, Peterson D, Broker AG, Garber AM, Owens DK. Cost-effectiveness of defending against bioterrorism: a comparison of vaccination and antibiotic prophylaxis against anthrax. Ann Intern Med. 2005;142:601–610. doi: 10.7326/0003-4819-142-8-200504190-00008. [DOI] [PubMed] [Google Scholar]
- Friedlander AM, Pittman PR, Parker GW. Anthrax vaccine: evidence for safety and efficacy against inhalational anthrax. JAMA. 1999;282:2104–2106. doi: 10.1001/jama.282.22.2104. [DOI] [PubMed] [Google Scholar]
- Friedlander AM, Welkos SL, Pitt ML, Ezzell JW, Worsham PL, Rose KJ, Ivins BE, Lowe JR, Howe GB, Mikesell P. Postexposure prophylaxis against experimental inhalation anthrax. J Infect Dis. 1993;167:1239–1243. doi: 10.1093/infdis/167.5.1239. [DOI] [PubMed] [Google Scholar]
- Galen JE, Zhao L, Chinchilla M, Wang JY, Pasetti MF, Green J, Levine MM. Adaptation of the endogenous Salmonella enterica serovar Typhi clyA-encoded hemolysin for antigen export enhances the immunogenicity of anthrax protective antigen domain 4 expressed by the attenuated live-vector vaccine strain CVD 908-htrA. Infect Immun. 2004;72:7096–7106. doi: 10.1128/IAI.72.12.7096-7106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D, Liner A, Legutki J, Mateczun A, Barnewall R, Estep J. Genetic immunization against anthrax. Vaccine. 2004;22:1604–1608. doi: 10.1016/j.vaccine.2003.09.043. [DOI] [PubMed] [Google Scholar]
- Garmory HS, Titball RW, Griffin KF, Hahn U, Bohm R, Beyer W. Salmonella enterica serovar typhimurium expressing a chromosomally integrated copy of the Bacillus anthracis protective antigen gene protects mice against an anthrax spore challenge. Infect Immun. 2003;71:3831–3836. doi: 10.1128/IAI.71.7.3831-3836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur R, Gupta PK, Banerjea AC, Singh Y. Effect of nasal immunization with protective antigen of Bacillus anthracis on protective immune response against anthrax toxin. Vaccine. 2002;20:2836–2839. doi: 10.1016/s0264-410x(02)00207-4. [DOI] [PubMed] [Google Scholar]
- Gauthier YP, Tournier JN, Paucod JC, Corre JP, Mock M, Goossens PL, Vidal DR. Efficacy of a vaccine based on protective antigen and killed spores against experimental inhalational anthrax. Infect Immun. 2009;77:1197–1207. doi: 10.1128/IAI.01217-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, Geier MR. Anthrax vaccination and joint related adverse reactions in light of biological warfare scenarios. Clin Exp Rheumatol. 2002;20:217–220. [PubMed] [Google Scholar]
- Geier MR, Geier DA. Gastrointestinal adverse reactions following anthrax vaccination: an analysis of the Vaccine Adverse Events Reporting System (VAERS) database. Hepatogastroenterology. 2004;51:762–767. [PubMed] [Google Scholar]
- Glomski IJ, Fritz JH, Keppler SJ, Balloy V, Chignard M, Mock M, Goossens PL. Murine splenocytes produce inflammatory cytokines in a MyD88-dependent response to Bacillus anthracis spores. Cell Microbiol. 2007;9:502–513. doi: 10.1111/j.1462-5822.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- Goodman JW, Nitecki DE. Immunochemical studies on the poly- -D-glutamyl capsule of Bacillus anthracis. I. Characterization of the polypeptide and of the specificity of its reaction with rabbit antisera. Biochemistry. 1966;5:657–665. doi: 10.1021/bi00866a036. [DOI] [PubMed] [Google Scholar]
- Gorse GJ, Keitel W, Keyserling H, Taylor DN, Lock M, Alves K, Kenner J, Deans L, Gurwith M. Immunogenicity and tolerance of ascending doses of a recombinant protective antigen (rPA102) anthrax vaccine: a randomized, double-blinded, controlled, multicenter trial. Vaccine. 2006;24:5950–5959. doi: 10.1016/j.vaccine.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Grabenstein JD. Vaccines: countering anthrax: vaccines and immunoglobulins. Clin Infect Dis. 2008;46:129–136. doi: 10.1086/523578. [DOI] [PubMed] [Google Scholar]
- Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3–26. doi: 10.1093/epirev/mxj003. [DOI] [PubMed] [Google Scholar]
- Greidanus TG, Honl BA. Delayed-type hypersensitivity reaction to anthrax vaccine. Mil Med. 2002;167:74–75. [PubMed] [Google Scholar]
- Gu ML, Leppla SH, Klinman DM. Protection against anthrax toxin by vaccination with a DNA plasmid encoding anthrax protective antigen. Vaccine. 1999;17:340–344. doi: 10.1016/s0264-410x(98)00210-2. [DOI] [PubMed] [Google Scholar]
- Hachisuka Y, Kojima K, Sato T. Fine filaments on the outside of the exosporium of Bacillus anthracis spores. J Bacteriol. 1966;91:2382–2384. doi: 10.1128/jb.91.6.2382-2384.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn UK, Boehm R, Beyer W. DNA vaccination against anthrax in mice-combination of anti-spore and anti-toxin components. Vaccine. 2006a;24:4569–4571. doi: 10.1016/j.vaccine.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Hahn UK, Aichler M, Boehm R, Beyer W. Comparison of the immunological memory after DNA vaccination and protein vaccination against anthrax in sheep. Vaccine. 2006b;24:4595–4597. doi: 10.1016/j.vaccine.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Hambleton P, Carman JA, Melling J. Anthrax: the disease in relation to vaccines. Vaccine. 1984;2:125–132. doi: 10.1016/0264-410x(84)90003-3. [DOI] [PubMed] [Google Scholar]
- Harrison LH, Ezzell JW, Abshire TG, Kidd S, Kaufmann AF. Evaluation of serologic tests for diagnosis of anthrax after an outbreak of cutaneous anthrax in Paraguay. J Infect Dis. 1989;160:706–710. doi: 10.1093/infdis/160.4.706. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Boyer JL, Hackett NR, Wilson JM, Crystal RG. Induction of protective immunity to anthrax lethal toxin with a nonhuman primate adenovirus-based vaccine in the presence of preexisting anti-human adenovirus immunity. Infect Immun. 2005;73:6885–6891. doi: 10.1128/IAI.73.10.6885-6891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J Hyg. 1956;54:28–36. doi: 10.1017/s0022172400044272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler RW, Kelly R, McNeely TB, Fan H, Losada MC, George HA, Woods A, Cope LD, Bansal A, Cook JC, Zang G, Cohen SL, Wei X, Keller PM, Leffel E, Joyce JG, Pitt L, Schultz LD, Jansen KU, Kurtz M. A recombinant 63-kDa form of Bacillus anthracis protective antigen produced in the yeast Saccharomyces cerevisiae provides protection in rabbit and primate inhalational challenge models of anthrax infection. Vaccine. 2006;24:1501–1514. doi: 10.1016/j.vaccine.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Hermanson G, Whitlow V, Parker S, Tonsky K, Rusalov D, Ferrari M, Lalor P, Komai M, Mere R, Bell M, Brenneman K, Mateczun A, Evans T, Kaslow D, Galloway D, Hobart P. A cationic lipid-formulated plasmid DNA vaccine confers sustained antibody-mediated protection against aerosolized anthrax spores. Proc Natl Acad Sci U S A. 2004;101:13601–13606. doi: 10.1073/pnas.0405557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewetson JF, Little SF, Ivins BE, Johnson WM, Pittman PR, Brown JE, Norris SL, Nielsen CJ. An in vivo passive protection assay for the evaluation of immunity in AVA-vaccinated individuals. Vaccine. 2008;26:4262–4266. doi: 10.1016/j.vaccine.2008.05.068. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Costello C, Menich M, Grabenstein JD, Engler RJ. Using a structured medical note for determining the safety profile of anthrax vaccine for US soldiers in Korea. Vaccine. 2003;21:4399–4409. doi: 10.1016/s0264-410x(03)00435-3. [DOI] [PubMed] [Google Scholar]
- Huang J, Mikszta JA, Ferriter MS, Jiang G, Harvey NG, Dyas B, Roy CJ, Ulrich RG, Sullivan VJ. Intranasal administration of dry powder anthrax vaccine provides protection against lethal aerosol spore challenge. Hum Vaccin. 2007;3:90–93. doi: 10.4161/hv.3.3.4011. [DOI] [PubMed] [Google Scholar]
- Hunter D, Zoutman D, Whitehead J, Hutchings J, MacDonald K. Health effects of anthrax vaccination in the Canadian forces. Mil Med. 2004;169:833–838. doi: 10.7205/milmed.169.10.833. [DOI] [PubMed] [Google Scholar]
- Ivins B, Fellows P, Pitt L, Estep J, Farchaus J, Friedlander A, Gibbs P. Experimental anthrax vaccines: efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine. 1995;13:1779–1784. doi: 10.1016/0264-410x(95)00139-r. [DOI] [PubMed] [Google Scholar]
- Ivins BE, Ezzell JW, Jr, Jemski J, Hedlund KW, Ristroph JD, Leppla SH. Immunization studies with attenuated strains of Bacillus anthracis. Infect Immun. 1986;52:454–458. doi: 10.1128/iai.52.2.454-458.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins BE, Pitt ML, Fellows PF, Farchaus JW, Benner GE, Waag DM, Little SF, Anderson GW, Jr, Gibbs PH, Friedlander AM. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine. 1998;16:1141–1148. doi: 10.1016/s0264-410x(98)80112-6. [DOI] [PubMed] [Google Scholar]
- Ivins BE, Welkos SL, Knudson GB, Little SF. Immunization against anthrax with aromatic compound-dependent (Aro-) mutants of Bacillus anthracis and with recombinant strains of Bacillus subtilis that produce anthrax protective antigen. Infect Immun. 1990;58:303–308. doi: 10.1128/iai.58.2.303-308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins BE, Welkos SL, Little SF, Crumrine MH, Nelson GO. Immunization against anthrax with Bacillus anthracis protective antigen combined with adjuvants. Infect Immun. 1992;60:662–668. doi: 10.1128/iai.60.2.662-668.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Cetron M, Cohen M, Doyle T, Fischer M, Greene C, Griffith KS, Guarner J, Hadler JL, Hayslett JA, Meyer R, Petersen LR, Phillips M, Pinner R, Popovic T, Quinn CP, Reefhuis J, Reissman D, Rosenstein N, Schuchat A, Shieh WJ, Siegal L, Swerdlow DL, Tenover FC, Traeger M, Ward JW, Weisfuse I, Wiersma S, Yeskey K, Zaki S, Ashford DA, Perkins BA, Ostroff S, Hughes J, Fleming D, Koplan JP, Gerberding JL. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8:1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joellenbeck LM, Zwanziger LL, Durch JS, Strom BL. The Anthrax Vaccine: Is It Safe? Does It Work? Washington, D.C.: National Academy Press; 2002. [PubMed] [Google Scholar]
- Joyce J, Cook J, Chabot D, Hepler R, Shoop W, Xu Q, Stambaugh T, ste-Amezaga M, Wang S, Indrawati L, Bruner M, Friedlander A, Keller P, Caulfield M. Immunogenicity and protective efficacy of Bacillus anthracis poly- -D-glutamic acid capsule covalently coupled to a protein carrier using a novel triazine-based conjugation strategy. J Biol Chem. 2006;281:4831–4843. doi: 10.1074/jbc.M509432200. [DOI] [PubMed] [Google Scholar]
- Keitel WA. Recombinant protective antigen 102 (rPA102): profile of a second-generation anthrax vaccine. Expert Rev Vaccines. 2006;5:417–430. doi: 10.1586/14760584.5.4.417. [DOI] [PubMed] [Google Scholar]
- Kenney RT, Yu J, Guebre-Xabier M, Frech SA, Lambert A, Heller BA, Ellingsworth LR, Eyles JE, Williamson ED, Glenn GM. Induction of protective immunity against lethal anthrax challenge with a patch. J Infect Dis. 2004;190:774–782. doi: 10.1086/422694. [DOI] [PubMed] [Google Scholar]
- Keppie J, Smith H, Harris-Smith PW. The chemical basis of the virulence of Bacillus anthracis. III. The role of the terminal bacteraemia in death of guinea-pigs from anthrax. Br J Exp Pathol. 1955;36:315–322. [PMC free article] [PubMed] [Google Scholar]
- Klas SD, Petrie CR, Warwood SJ, Williams MS, Olds CL, Stenz JP, Cheff AM, Hinchcliffe M, Richardson C, Wimer S. A single immunization with a dry powder anthrax vaccine protects rabbits against lethal aerosol challenge. Vaccine. 2008;26:5494–5502. doi: 10.1016/j.vaccine.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Dearmon IA, Jr, Lincoln RE, Mahlandt BG, Fernelius AL. Immunological studies of anthrox. II. Levels of immunity against Bacillus anthracis obtained with protective antigen and live vaccine. J Immunol. 1962;88:15–19. [PubMed] [Google Scholar]
- Klinman DM, Xie H, Little SF, Currie D, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine. 2004;22:2881–2886. doi: 10.1016/j.vaccine.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Knockenhauer KE, Sawicka KM, Roemer EJ, Simon SR. Protective antigen composite nanofibers as a transdermal anthrax vaccine. Conf Proc IEEE Eng Med Biol Soc; 2008. pp. 1040–1043. [DOI] [PubMed] [Google Scholar]
- Kobiler D, Gozes Y, Rosenberg H, Marcus D, Reuveny S, Altboum Z. Efficiency of protection of guinea pigs against infection with Bacillus anthracis spores by passive immunization. Infect Immun. 2002;70:544–560. doi: 10.1128/IAI.70.2.544-550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Murphy WJ, Brandt S, Blazar BR, Lovchik JA, Thorkildson P, Percival A, Lyons CR. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc Natl Acad Sci U S A. 2004;101:5042–5047. doi: 10.1073/pnas.0401351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Thorkildson P, Brandt S, Welch WH, Lovchik JA, AuCoin DP, Vilai J, Lyons CR. Protective and immunochemical activities of monoclonal antibodies reactive with the Bacillus anthracis polypeptide capsule. Infect Immun. 2007;75:152–163. doi: 10.1128/IAI.01133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange JL, Lesikar SE, Rubertone MV, Brundage JF. Comprehensive systematic surveillance for adverse effects of anthrax vaccine adsorbed, US Armed Forces, 1998-2000. Vaccine. 2003;21:1620–1628. doi: 10.1016/s0264-410x(02)00723-5. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hadjipanayis AG, Welkos SL. Venezuelan Equine Encephalitis Virus-Vectored Vaccines Protect Mice against Anthrax Spore Challenge. Infect Immun. 2003;71:1491–1496. doi: 10.1128/IAI.71.3.1491-1496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SF. Anthrax vaccines: a development update. BioDrugs. 2005;19:233–245. doi: 10.2165/00063030-200519040-00003. [DOI] [PubMed] [Google Scholar]
- Little SF, Ivins BE, Fellows PF, Friedlander AM. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect Immun. 1997;65:5171–5175. doi: 10.1128/iai.65.12.5171-5175.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SF, Ivins BE, Fellows PF, Pitt ML, Norris SL, Andrews GP. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004;22:422–430. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Little SF, Ivins BE, Webster WM, Fellows PF, Pitt ML, Norris SL, Andrews GP. Duration of protection of rabbits after vaccination with Bacillus anthracis recombinant protective antigen vaccine. Vaccine. 2006;24:2530–2536. doi: 10.1016/j.vaccine.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Little SF, Knudson GB. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect Immun. 1986;52:509–512. doi: 10.1128/iai.52.2.509-512.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TH, Oscherwitz J, Schnepp B, Jacobs J, Yu F, Cease KB, Johnson PR. Genetic vaccines for anthrax based on recombinant adeno-associated virus vectors. Mol Ther. 2009;17:373–379. doi: 10.1038/mt.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxembourg A, Hannaman D, Nolan E, Ellefsen B, Nakamura G, Chau L, Tellez O, Little S, Bernard R. Potentiation of an anthrax DNA vaccine with electroporation. Vaccine. 2008;26:5216–5222. doi: 10.1016/j.vaccine.2008.03.064. [DOI] [PubMed] [Google Scholar]
- Mabry R, Rani M, Geiger R, Hubbard GB, Carrion R, Jr, Brasky K, Patterson JL, Georgiou G, Iverson BL. Passive protection against anthrax by using a high-affinity antitoxin antibody fragment lacking an Fc region. Infect Immun. 2005;73:8362–8368. doi: 10.1128/IAI.73.12.8362-8368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano N, Plikaytis BD, Martin SW, Rose C, Semenova VA, Martin SK, Freeman AE, Li H, Mulligan MJ, Parker SD, Babcock J, Keitel W, El SH, Poland GA, Jacobson RM, Keyserling HL, Soroka SD, Fox SP, Stamper JL, McNeil MM, Perkins BA, Messonnier N, Quinn CP. Effects of a reduced dose schedule and intramuscular administration of anthrax vaccine adsorbed on immunogenicity and safety at 7 months: a randomized trial. JAMA. 2008;300:1532–1543. doi: 10.1001/jama.300.13.1532. [DOI] [PubMed] [Google Scholar]
- Marcus H, Danieli R, Epstein E, Velan B, Shafferman A, Reuveny S. Contribution of immunological memory to protective immunity conferred by a Bacillus anthracis protective antigen-based vaccine. Infect Immun. 2004;72:3471–3477. doi: 10.1128/IAI.72.6.3471-3477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas GR, Friedlander AM, Glenn GM, Little S, Yu J, Alving CR. Needle-Free Skin Patch Vaccination Method for Anthrax. Infect Immun. 2004;72:1181–1183. doi: 10.1128/IAI.72.2.1181-1183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride BW, Mogg A, Telfer JL, Lever MS, Miller J, Turnbull PC, Baillie L. Protective efficacy of a recombinant protective antigen against Bacillus anthracis challenge and assessment of immunological markers. Vaccine. 1998;16:810–817. doi: 10.1016/s0264-410x(97)00268-5. [DOI] [PubMed] [Google Scholar]
- McNeil MM, Chiang IS, Wheeling JT, Zhang Y. Short-term reactogenicity and gender effect of anthrax vaccine: analysis of a 1967-1972 study and review of the 1955-2005 medical literature. Pharmacoepidemiol Drug Saf. 2007;16:259–274. doi: 10.1002/pds.1359. [DOI] [PubMed] [Google Scholar]
- Mendelson I, Gat O, oni-Grinstein R, Altboum Z, Inbar I, Kronman C, Bar-Haim E, Cohen S, Velan B, Shafferman A. Efficacious, nontoxigenic Bacillus anthracis spore vaccines based on strains expressing mutant variants of lethal toxin components. Vaccine. 2005;23:5688–5697. doi: 10.1016/j.vaccine.2004.11.077. [DOI] [PubMed] [Google Scholar]
- Midha S, Bhatnagar R. Anthrax protective antigen administered by DNA vaccination to distinct subcellular locations potentiates humoral and cellular immune responses. Eur J Immunol. 2009;39:159–177. doi: 10.1002/eji.200838058. [DOI] [PubMed] [Google Scholar]
- Mikszta JA, Dekker JP, III, Harvey NG, Dean CH, Brittingham JM, Huang J, Sullivan VJ, Dyas B, Roy CJ, Ulrich RG. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect Immun. 2006;74:6806–6810. doi: 10.1128/IAI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikszta JA, Sullivan VJ, Dean C, Waterston AM, Alarcon JB, Dekker JP, III, Brittingham JM, Huang J, Hwang CR, Ferriter M, Jiang G, Mar K, Saikh KU, Stiles BG, Roy CJ, Ulrich RG, Harvey NG. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J Infect Dis. 2005;191:278–288. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M, Duong T, Hoover T, Klaenhammer TR. Targeting mucosal dendritic cells with microbial antigens from probiotic lactic acid bacteria. Expert Rev Vaccines. 2008;7:163–174. doi: 10.1586/14760584.7.2.163. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M, Duong T, Sandwick SJ, Hoover T, Klaenhammer TR. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0900029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed N, Clagett M, Li J, Jones S, Pincus S, D′Alia G, Nardone L, Babin M, Spitalny G, Casey L. A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect Immun. 2005;73:795–802. doi: 10.1128/IAI.73.2.795-802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed N, Li J, Ferreira CS, Little SF, Friedlander AM, Spitalny GL, Casey LS. Enhancement of anthrax lethal toxin cytotoxicity: a subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect Immun. 2004;72:3276–3283. doi: 10.1128/IAI.72.6.3276-3283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar DM, Altenburn RA. Alterations in the biologocal activity of protective antigen of Bacillus anthracis toxin. Proc Soc Exp Biol Med. 1963;114:294–297. [PubMed] [Google Scholar]
- Nakouzi A, Rivera J, Rest RF, Casadevall A. Passive administration of monoclonal antibodies to anthrolysin O prolong survival in mice lethally infected with Bacillus anthracis. BMC Microbiol. 2008;8:159. doi: 10.1186/1471-2180-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. Anthrax vaccine. Model of a response to the biologic warfare threat. Infect Dis Clin North Am. 1999;13:187–208. viii. doi: 10.1016/s0891-5520(05)70050-7. [DOI] [PubMed] [Google Scholar]
- Niu MT, Ball R, Woo EJ, Burwen DR, Knippen M, Braun MM. Adverse events after anthrax vaccination reported to the Vaccine Adverse Event Reporting System (VAERS), 1990-2007. Vaccine. 2009;27:290–297. doi: 10.1016/j.vaccine.2008.10.044. [DOI] [PubMed] [Google Scholar]
- Osorio M, Wu Y, Singh S, Merkel TJ, Bhattacharyya S, Blake MS, Kopecko DJ. Anthrax protective antigen delivered by Salmonella enterica serovar Typhi Ty21a protects mice from a lethal anthrax spore challenge. Infect Immun. 2009;77:1475–1482. doi: 10.1128/IAI.00828-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DC, Franzke LH, Stehr-Green PA, Schwartz B, McNeil MM. Development of the Vaccine Analytic Unit's research agenda for investigating potential adverse events associated with anthrax vaccine adsorbed. Pharmacoepidemiol Drug Saf. 2007;16:46–54. doi: 10.1002/pds.1213. [DOI] [PubMed] [Google Scholar]
- Peachman KK, Rao M, Alving CR, Burge R, Leppla SH, Rao VB, Matyas GR. Correlation between lethal toxin-neutralizing antibody titers and protection from intranasal challenge with Bacillus anthracis Ames strain spores in mice after transcutaneous immunization with recombinant anthrax protective antigen. Infect Immun. 2006;74:794–797. doi: 10.1128/IAI.74.1.794-797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelat T, Hust M, Laffly E, Condemine F, Bottex C, Vidal D, Lefranc MP, Dübel S, Thullier P. High-affinity, human antibody-like antibody fragment (single-chain variable fragment) neutralizing the lethal factor (LF) of Bacillus anthracis by inhibiting protective antigen-LF complex formation. Antimicrob Agents Chemother. 2007;51:2758–2764. doi: 10.1128/AAC.01528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JW, Comer JE, Baze WB, Noffsinger DM, Wenglikowski A, Walberg KG, Hardcastle J, Pawlik J, Bush K, Taormina J, Moen S, Thomas J, Chatuev BM, Sower L, Chopra AK, Stanberry LR, Sawada R, Scholz WW, Sircar J. Human monoclonal antibody AVP-21D9 to protective antigen reduces dissemination of the Bacillus anthracis Ames strain from the lungs in a rabbit model. Infect Immun. 2007;75:3414–3424. doi: 10.1128/IAI.00352-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JW, Comer JE, Noffsinger DM, Wenglikowski A, Walberg KG, Chatuev BM, Chopra AK, Stanberry LR, Kang AS, Scholz WW, Sircar J. Human Monoclonal Anti-Protective Antigen Antibody Completely Protects Rabbits and Is Synergistic with Ciprofloxacin in Protecting Mice and Guinea Pigs against Inhalation Anthrax. Infect Immun. 2006;74:1016–1024. doi: 10.1128/IAI.74.2.1016-1024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect Immun. 1991;59:3472–3477. doi: 10.1128/iai.59.10.3472-3477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezard C, Weber M, Sirard JC, Berche P, Mock M. Protective immunity induced by Bacillus anthracis toxin-deficient strains. Infect Immun. 1995;63:1369–1372. doi: 10.1128/iai.63.4.1369-1372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt ML, Little S, Ivins BE, Fellows P, Boles J, Barth J, Hewetson J, Friedlander AM. In vitro correlate of immunity in an animal model of inhalational anthrax. J Appl Microbiol. 1999;87:304. doi: 10.1046/j.1365-2672.1999.00897.x. [DOI] [PubMed] [Google Scholar]
- Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, Gibbs P, Dertzbaugh M, Friedlander AM. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine. 2001;19:4768–4773. doi: 10.1016/s0264-410x(01)00234-1. [DOI] [PubMed] [Google Scholar]
- Pittman PR, Coonan KM, Gibbs PH, Scott HM, Cannon TL, McKee KT., Jr Long-term health effects of repeated exposure to multiple vaccines. Vaccine. 2004;23:525–536. doi: 10.1016/j.vaccine.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Pittman PR, Gibbs PH, Cannon TL, Friedlander AM. Anthrax vaccine: short-term safety experience in humans. Vaccine. 2001;20:972–978. doi: 10.1016/s0264-410x(01)00387-5. [DOI] [PubMed] [Google Scholar]
- Pittman PR, Kim-Ahn G, Pifat DY, Coonan K, Gibbs P, Little S, Pace-Templeton JG, Myers R, Parker GW, Friedlander AM. Anthrax vaccine: immunogenicity and safety of a dose-reduction, route-change comparison study in humans. Vaccine. 2002;20:1412–1420. doi: 10.1016/s0264-410x(01)00462-5. [DOI] [PubMed] [Google Scholar]
- Pittman PR, Leitman SF, Oro JG, Norris SL, Marano NM, Ranadive MV, Sink BS, McKee KT., Jr Protective antigen and toxin neutralization antibody patterns in anthrax vaccinees undergoing serial plasmapheresis. Clin Diagn Lab Immunol. 2005;12:713–721. doi: 10.1128/CDLI.12.6.713-721.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman PR, Mangiafico JA, Rossi CA, Cannon TL, Gibbs PH, Parker GW, Friedlander AM. Anthrax vaccine: increasing intervals between the first two doses enhances antibody response in humans. Vaccine. 2000;19:213–216. doi: 10.1016/s0264-410x(00)00174-2. [DOI] [PubMed] [Google Scholar]
- Pittman PR, Norris SL, Barrera Oro JG, Bedwell D, Cannon TL, McKee KT., Jr Patterns of antibody response in humans to the anthrax vaccine adsorbed (AVA) primary (six-dose) series. Vaccine. 2006;24:3654–3660. doi: 10.1016/j.vaccine.2006.01.054. [DOI] [PubMed] [Google Scholar]
- Price BM, Liner AL, Park S, Leppla SH, Mateczun A, Galloway DR. Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect Immun. 2001;69:4509–4515. doi: 10.1128/IAI.69.7.4509-4515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puziss M, Wright GG. Studies on immunity in anthrax. IV. Factors influencing elaboration of the protective antigen of Bacillus anthracis in chemically defined media. J Bacteriol. 1954:474–482. doi: 10.1128/jb.68.4.474-482.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DM, Leppla SH, Schneerson R, Shiloach J. Production, recovery and immunogenicity of the protective antigen from a recombinant strain of Bacillus anthracis. J Ind Microbiol Biotechnol. 2002;28:232–238. doi: 10.1038/sj/jim/7000239. [DOI] [PubMed] [Google Scholar]
- Reuveny S, White MD, Adar YY, Kafri Y, Altboum Z, Gozes Y, Kobiler D, Shafferman A, Velan B. Search for correlates of protective immunity conferred by anthrax vaccine. Infect Immun. 2001;69:2888–2893. doi: 10.1128/IAI.69.5.2888-2893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie GE, Park YM, Han JS, Yu JY, Seong WK, Oh HB. Efficacy of non-toxic deletion mutants of protective antigen from Bacillus anthracis. FEMS Immunol Med Microbiol. 2005;45:341–347. doi: 10.1016/j.femsim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Rhie GE, Roehrl MH, Mourez M, Collier RJ, Mekalanos JJ, Wang JY. A dually active anthrax vaccine that confers protection against both bacilli and toxins. Proc Natl Acad Sci U S A. 2003;100:10925–10930. doi: 10.1073/pnas.1834478100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot WJ, Panchal RG, Brittingham KC, Ruthel G, Kenny TA, Lane D, Curry B, Hoover TA, Friedlander AM, Bavari S. Anthrax lethal toxin impairs innate immune functions of alveolar macrophages and facilitates Bacillus anthracis survival. Infect Immun. 2006;74:5029–5034. doi: 10.1128/IAI.00275-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemenschneider J, Garrison A, Geisbert J, Jahrling P, Hevey M, Negley D, Schmaljohn A, Lee J, Hart MK, Vanderzanden L, Custer D, Bray M, Ruff A, Ivins B, Bassett A, Rossi C, Schmaljohn C. Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine. 2003;21:4071–4080. doi: 10.1016/s0264-410x(03)00362-1. [DOI] [PubMed] [Google Scholar]
- Sawada-Hirai R, Jiang I, Wang F, Sun SM, Nedellec R, Ruther P, Alvarez A, Millis D, Morrow PR, Kang AS. Human anti-anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J Immune Based Ther Vaccines. 2004;2:5. doi: 10.1186/1476-8518-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R, Kubler-Kielb J, Liu TY, Dai ZD, Leppla SH, Yergey A, Backlund P, Shiloach J, Majadly F, Robbins JB. Poly(gamma-D-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc Natl Acad Sci U S A. 2003;100:8945–8950. doi: 10.1073/pnas.1633512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellman BR, Mourez M, Collier RJ. Dominant-negative mutants of a toxin subunit: an approach to therapy of anthrax. Science. 2001a;292:695–697. doi: 10.1126/science.109563. [DOI] [PubMed] [Google Scholar]
- Sellman BR, Nassi S, Collier RJ. Point mutations in anthrax protective antigen that block translocation. J Biol Chem. 2001b;276:8371–8376. doi: 10.1074/jbc.M008309200. [DOI] [PubMed] [Google Scholar]
- Sever JL, Brenner AI, Gale AD, Lyle JM, Moulton LH, Ward BJ, West DJ. Safety of anthrax vaccine: an expanded review and evaluation of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) Pharmacoepidemiol Drug Saf. 2004;13:825–840. doi: 10.1002/pds.936. [DOI] [PubMed] [Google Scholar]
- Shivachandra SB, Li Q, Peachman KK, Matyas GR, Leppla SH, Alving CR, Rao M, Rao VB. Multicomponent anthrax toxin display and delivery using bacteriophage T4. Vaccine. 2007;25:1225–1235. doi: 10.1016/j.vaccine.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyakhov EN, Rubinstein E. Human live anthrax vaccine in the former USSR. Vaccine. 1994;12:727–730. doi: 10.1016/0264-410x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Singer DE, Schneerson R, Bautista CT, Rubertone MV, Robbins JB, Taylor DN. Serum IgG antibody response to the protective antigen (PA) of Bacillus anthracis induced by anthrax vaccine adsorbed (AVA) among U.S. military personnel. Vaccine. 2008;26:869–873. doi: 10.1016/j.vaccine.2007.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y, Khanna H, Chopra AP, Mehra V. A dominant negative mutant of Bacillus anthracis protective antigen inhibits anthrax toxin action in vivo. J Biol Chem. 2001;276:22090–22094. doi: 10.1074/jbc.M010222200. [DOI] [PubMed] [Google Scholar]
- Sirisanthana T, Nelson KE, Ezzell JW, Abshire TG. Serological studies of patients with cutaneous and oral-oropharyngeal anthrax from northern Thailand. Am J Trop Med Hyg. 1988;39:575–581. doi: 10.4269/ajtmh.1988.39.575. [DOI] [PubMed] [Google Scholar]
- Skoble J, Beaber JW, Gao Y, Lovchik JA, Sower LE, Liu W, Luckett W, Peterson JW, Calendar R, Portnoy DA, Lyons CR, Dubensky TW., Jr Killed But Metabolically Active Bacillus anthracis Vaccines Induce Broad and Protective Immunity Against Anthrax. Infect Immun. 2009;77:1649–63. doi: 10.1128/IAI.00530-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloat BR, Cui Z. Nasal immunization with a dual antigen anthrax vaccine induced strong mucosal and systemic immune responses against toxins and bacilli. Vaccine. 2006a;24:6405–6413. doi: 10.1016/j.vaccine.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sloat BR, Cui Z. Nasal immunization with anthrax protective antigen protein adjuvanted with polyriboinosinic-polyribocytidylic acid induced strong mucosal and systemic immunities. Pharm Res. 2006b;23:1217–1226. doi: 10.1007/s11095-006-0206-9. [DOI] [PubMed] [Google Scholar]
- Smith B, Leard CA, Smith TC, Reed RJ, Ryan MA. Anthrax vaccination in the Millennium Cohort: validation and measures of health. Am J Prev Med. 2007;32:347–353. doi: 10.1016/j.amepre.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Smith H, Keppie J, Stanley JL. The chemical basis of the virulence of Bacillus anthracis. V. The specific toxin produced by B. Anthracis in vivo. Br J Exp Pathol. 1955a;36:460–472. [PMC free article] [PubMed] [Google Scholar]
- Smith H, Keppie J, Stanley JL, Harris-Smith PW. The chemical basis of the virulence of Bacillus anthracis. IV. Secondary shock as the major factor in death of guinea-pigs from anthrax. Br J Exp Pathol. 1955b;36:323–335. [PMC free article] [PubMed] [Google Scholar]
- Staats HF, Alam SM, Scearce RM, Kirwan SM, Zhang JX, Gwinn WM, Haynes BF. In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity. Infect Immun. 2007;75:5443–5452. doi: 10.1128/IAI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steichen CT, Kearney JF, Turnbough CL., Jr Characterization of the exosporium basal layer protein BxpB of Bacillus anthracis. J Bacteriol. 2005;187:5868–5876. doi: 10.1128/JB.187.17.5868-5876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternbach G. The history of anthrax. J Emerg Med. 2003;24:463–467. doi: 10.1016/s0736-4679(03)00079-9. [DOI] [PubMed] [Google Scholar]
- Sterne M. The use of anthrax vaccines prepared from avirulent (uncapsulated) variants of Bacillus anthracis. Onderstepoort J Vet Sci Anim Ind. 1939;13:307–312. [Google Scholar]
- Stokes MG, Titball RW, Neeson BN, Galen JE, Walker NJ, Stagg AJ, Jenner DC, Thwaite JE, Nataro JP, Baillie LW, Atkins HS. Oral administration of a Salmonella enterica-based vaccine expressing Bacillus anthracis protective antigen confers protection against aerosolized B. anthracis. Infect Immun. 2007;75:1827–1834. doi: 10.1128/IAI.01242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson-Biearman B, Krenzelok EP. Delayed life-threatening reaction to anthrax vaccine. J Toxicol Clin Toxicol. 2001;39:81–84. doi: 10.1081/clt-100102885. [DOI] [PubMed] [Google Scholar]
- Sylvestre P, Couture-Tosi E, Mock M. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol Microbiol. 2002;45:169–178. doi: 10.1046/j.1365-2958.2000.03000.x. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Gursel I, Ishii KJ, Suzuki K, Gursel M, Klinman DM. Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin Immunol. 2004;16:17–22. doi: 10.1016/j.smim.2003.10.009. [DOI] [PubMed] [Google Scholar]
- The Jackson Laboratory. A/J Strain Information Page. 2008 http://jaxmice.jax.org/strain/000646.html.
- Tigertt WD. Anthrax. J Hyg (Lond) 1980;85:415–420. doi: 10.1017/s0022172400063488. William Smith Greenfield, M.D., F.R.C.P., Professor Superintendent, the Brown Animal Sanatory Institution (1878-81). Concerning the priority due to him for the production of the first vaccine against anthrax. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JN, Ulrich RG, Quesnel-Hellmann A, Mohamadzadeh M, Stiles BG. Anthrax, toxins and vaccines: a 125-year journey targeting Bacillus anthracis. Expert Rev Anti Infect Ther. 2009;7:219–236. doi: 10.1586/14787210.7.2.219. [DOI] [PubMed] [Google Scholar]
- Turnbull PC. Anthrax vaccines: past, present and future. Vaccine. 1991;9:533–539. doi: 10.1016/0264-410x(91)90237-z. [DOI] [PubMed] [Google Scholar]
- Turnbull PC. Current status of immunization against anthrax: old vaccines may be here to stay for a while. Curr Opin Infect Dis. 2000;13:113–120. doi: 10.1097/00001432-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Turnbull PC, Leppla SH, Broster MG, Quinn CP, Melling J. Antibodies to anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Med Microbiol Immunol. 1988;177:293–303. doi: 10.1007/BF00189414. [DOI] [PubMed] [Google Scholar]
- Vasudev M, Zacharisen MC. New-onset rheumatoid arthritis after anthrax vaccination. Ann Allergy Asthma Immunol. 2006;97:110–112. doi: 10.1016/S1081-1206(10)61379-8. [DOI] [PubMed] [Google Scholar]
- Wang JY, Roehrl MH. Anthrax vaccine design: strategies to achieve comprehensive protection against spore, bacillus, and toxin. Med Immunol. 2005;4:4. doi: 10.1186/1476-9433-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Fellows PF, Leighton TJ, Lucas AH. Induction of opsonic antibodies to the -D-glutamic acid capsule of Bacillus anthracis by immunization with a synthetic peptide-carrier protein conjugate. FEMS Immunol Med Microbiol. 2004;40:231–237. doi: 10.1016/S0928-8244(03)00366-3. [DOI] [PubMed] [Google Scholar]
- Wasserman GM, Grabenstein JD, Pittman PR, Rubertone MV, Gibbs PP, Wang LZ, Golder LG. Analysis of adverse events after anthrax immunization in US Army medical personnel. J Occup Environ Med. 2003;45:222–233. doi: 10.1097/01.jom.0000058345.05741.6b. [DOI] [PubMed] [Google Scholar]
- Weiss S, Kobiler D, Levy H, Marcus H, Pass A, Rothschild N, Altboum Z. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect Immun. 2006;74:394–398. doi: 10.1128/IAI.74.1.394-398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos S, Friedlander A, Weeks S, Little S, Mendelson I. In-vitro characterisation of the phagocytosis and fate of anthrax spores in macrophages and the effects of anti-PA antibody. J Med Microbiol. 2002;51:821–831. doi: 10.1099/0022-1317-51-10-821. [DOI] [PubMed] [Google Scholar]
- Welkos S, Little S, Friedlander A, Fritz D, Fellows P. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology. 2001;147:1677–1685. doi: 10.1099/00221287-147-6-1677. [DOI] [PubMed] [Google Scholar]
- Welkos SL, Friedlander AM. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb Pathog. 1988a;5:127–139. doi: 10.1016/0882-4010(88)90015-0. [DOI] [PubMed] [Google Scholar]
- Welkos SL, Friedlander AM. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis. Microb Pathog. 1988b;4:53–69. doi: 10.1016/0882-4010(88)90048-4. [DOI] [PubMed] [Google Scholar]
- Whiting GC, Rijpkema S, Adams T, Corbel MJ. Characterisation of adsorbed anthrax vaccine by two-dimensional gel electrophoresis. Vaccine. 2004;22:4245–4251. doi: 10.1016/j.vaccine.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Wild MA, Kumor K, Nolan MJ, Lockman H, Bowdish KS. A human antibody against anthrax protective antigen protects rabbits from lethal infection with aerosolized spores. Hum Antibodies. 2007;16:99–105. [PubMed] [Google Scholar]
- Wild MA, Xin H, Maruyama T, Nolan MJ, Calveley PM, Malone JD, Wallace MR, Bowdish KS. Human antibodies from immunized donors are protective against anthrax toxin in vivo. Nat Biotechnol. 2003;21:1305–1306. doi: 10.1038/nbt891. [DOI] [PubMed] [Google Scholar]
- Williamson ED, Beedham RJ, Bennett AM, Perkins SD, Miller J, Baillie LW. Presentation of protective antigen to the mouse immune system: immune sequelae. J Appl Microbiol. 1999;87:315–317. doi: 10.1046/j.1365-2672.1999.00901.x. [DOI] [PubMed] [Google Scholar]
- Williamson ED, Hodgson I, Walker NJ, Topping AW, Duchars MG, Mott JM, Estep J, Lebutt C, Flick-Smith HC, Jones HE, Li H, Quinn CP. Immunogenicity of recombinant protective antigen and efficacy against aerosol challenge with anthrax. Infect Immun. 2005;73:5978–5987. doi: 10.1128/IAI.73.9.5978-5987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimer-Mackin S, Hinchcliffe M, Petrie CR, Warwood SJ, Tino WT, Williams MS, Stenz JP, Cheff A, Richardson C. An intranasal vaccine targeting both the Bacillus anthracis toxin and bacterium provides protection against aerosol spore challenge in rabbits. Vaccine. 2006;24:3953–3963. doi: 10.1016/j.vaccine.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Wright GG, Hedberg MA, Slein JB. Studies on immunity in anthrax. III. Elaboration of protective antigen in a chemically defined, non-protein medium. J Immunol. 1954;72:263–269. [PubMed] [Google Scholar]
- Wright GG, Hedberg MA, Feinberg RJ. Studies on immunity in anthrax. II. In vitro elaboration of protective antigen by non-proteolytic mutants of Bacillus anthracis. J Exp Med. 1951a;93:523–527. doi: 10.1084/jem.93.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GG, Slein JB. Studies on immunity in anthrax. I. Variation in the serum T-agglutinin during anthrax infection in the rabbit. J Exp Med. 1951b;93:99–106. doi: 10.1084/jem.93.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Roehrl MH, Basar E, Wang JY. Selection and evaluation of the immunogenicity of protective antigen mutants as anthrax vaccine candidates. Vaccine. 2008;26:947–955. doi: 10.1016/j.vaccine.2007.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M, Xu Q, Pichichero ME. Protection against anthrax by needle-free mucosal immunization with human anthrax vaccine. Vaccine. 2007;25:3588–3594. doi: 10.1016/j.vaccine.2007.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qiu J, Zhou Y, Farhangfar F, Hester J, Lin AY, Decker WK. Plasmid-based vaccination with candidate anthrax vaccine antigens induces durable type 1 and type 2 T-helper immune responses. Vaccine. 2008;26:614–622. doi: 10.1016/j.vaccine.2007.11.072. [DOI] [PubMed] [Google Scholar]
- Zhou B, Carney C, Janda KD. Selection and characterization of human antibodies neutralizing Bacillus anthracis toxin. Bioorg Med Chem. 2008;16:1903–1913. doi: 10.1016/j.bmc.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]