Abstract
We have previously demonstrated a neuroprotective mechanism of facial motoneuron (FMN) survival after facial nerve axotomy that is dependent on CD4+ Th2 cell interaction with peripheral antigen-presenting cells, as well as CNS resident microglia. To investigate this mechanism, we chose to study the Th2-associated chemokine, CCL11, and Th1-associated chemokine, CXCL11, in wild type and presymptomatic mSOD1 mice after facial nerve axotomy. In this report, the results indicate that CCL11 is constitutively expressed in the uninjured facial motor nucleus, but CXCL11 is not expressed at all. Facial nerve axotomy induced a shift in CCL11 expression from FMN to astrocytes, whereas CXCL11 was induced in FMN. Differences in the number of CCL11- and CXCL11-expressing cells were observed between WT and mSOD1 mice after facial nerve axotomy.
Keywords: neuroprotection, chemokine, CCL11, CXCL11, facial nerve axotomy
1. Introduction
Our lab discovered that facial motoneuron (FMN) survival is decreased in mice that lack functional adaptive immune systems (Serpe et al., 1999). Using the recombination activating gene 2-deficient mouse and adoptive transfer of CD4+, CD8+ and B lymphocytes, we demonstrated a neuroprotective role for CD4+ T cells in FMN survival after injury in the mouse (Serpe et al. 2003). Additionally, previous studies found a maximum increase in T cell infiltration of the axotomized mouse facial motor nucleus between 7–21 days after injury (Raivich et al., 1998). Furthermore, it has been demonstrated that CD4+ T cells infiltrate the facial motor nucleus after facial nerve injury (Ha et al., 2007). In support of these findings, our laboratory has shown that CNS resident microglia are necessary to reactivate CD4+ T cells centrally (Byram et al., 2004), suggesting that a mechanism must exist for peripheral CD4+ T cell recruitment to the injured facial motor nucleus from the draining cervical lymph nodes.
After activation, naïve CD4+ T cells differentiate into T helper 2 (Th2) and Th1 cells, as characterized by the production and/or secretion of the cytokines interleukin 4 (IL-4) and interferon gamma (IFN-γ), respectively (Stout et al., 1989). Additionally, we have found that facial nerve axotomy induces the development of both Th2 and Th1 cells in draining cervical lymph nodes (Xin et al., 2008) and that the Th2, but not the Th1, effector subset mediates neuroprotection of FMN after injury (Deboy et al., 2006) following its re-activation centrally (Byram et al., 2004). Armstrong et al. (2003) demonstrated increased mRNA levels for pituitary adenylyl cyclase activating polypeptide (PACAP) mRNA levels in FMN after facial nerve axotomy. Since murine microglia cultured in the presence of PACAP increase Th2-associated chemokine expression (Wainwright et al., 2008), the data collectively suggest that, following a peripheral nerve injury outside the blood-brain-barrier (BBB), microglia that are capable of antigen presentation centrally (Carson et al., 1999) interact with neuroprotective Th2 cells after their recruitment in a Th2-associated chemokine expression-dependent manner.
Chemokines, otherwise known as chemotactic cytokines, are grouped into a family of 8–14 kilodalton proteins capable of recruiting cells that express the cognate chemokine receptor (Tanabe et al., 1997). Based on the amino terminal arrangement of cysteine residues, the 48 currently identified chemokines are classified as α,β, δ, and γ. Chemokines are further classified into homeostatic and inflammatory tissue subsets, based on constitutive or inducible expression, respectively (Gardner et al., 2004). Homeostatic chemokines that contribute to T cell immunosurveillance in the lymph nodes, gut and skin have been identified and reviewed (Kunkel and Butcher, 2002), while chemokines responsible for T cell recruitment to normal CNS have not yet been identified.
Previous investigations using models of peripheral nerve injury, in which the BBB remains intact, have demonstrated inflammatory chemokine expression in FMN (Flügel et al., 2001; Harrison et al. 1998), dorsal root ganglion sensory and spinal neurons (Zhang et al., 2006), as well as glia (Babcock et al., 2003). Furthermore, Zhang et al. (2007) have shown that chemokine neutralization after peripheral nerve injury decreases leukocyte homing to the CNS (Zhang et al., 2007), confirming the hypothesis that CNS-expression of chemokines contributes to the mechanism of recruitment from peripheral to central compartments. However, few chemokines have been studied in the context of motoneuron injury or as a result of neurodegenerative disease progression.
Dysregulated expression of the T cell/monocyte-recruiting chemokine, CCL5, has been reported to be elevated in serum and cerebrospinal fluid (CSF) of patients with the degenerative motoneuron disease, amyotrophic lateral sclerosis (ALS) (Rentzos et al., 2007). Additionally, the monocyte-recruiting chemokine, CCL2, has been demonstrated to be increased in spinal cord and CSF of patients with ALS (Nagata et al., 2007; Henkel et al., 2004). Furthermore, mutant superoxide dismutase 1 (mSOD1) mice that recapitulate familial-like ALS pathology have also been shown to exhibit dysregulated CCL5 and CCL2 expression (Henkel et al., 2006; Hensley et al., 2003), validating this model for the study of chemokines in the context of ALS pathogenesis. Additionally, we have recently investigated chemokines that recruit CD4+ T cell subsets at the mRNA level in a mSOD1 mouse model after facial nerve axotomy, focusing on the Th2-associated chemokine, CCL11, and the Th1-associated chemokine, CXCL11 (Wainwright et al., 2009).
Since lymphocytes upregulate the expression of specific chemokine receptors after differentiation, polarized leukocytes can be selectively recruited to sites of chemokine expression. Naïve CD4+ T lymphocytes that develop into the Th2 subset upregulate the Th2-associated chemokine receptor, CCR3, which cells use to migrate toward the Th2-associated chemokine, CCL11 (Sallusto et al., 1997). In contrast, Th1 cells express the chemokine receptor, CXCR3 (Bonecchi et al., 1998), which cells use to migrate toward the Th1-associated chemokine, CXCL11 (Lord et al., 2005). Based on the neuroprotection of injured FMN by Th2, but not Th1 cells (Deboy et al., 2006), and the requirement for CD4+ T cell activation by microglia (Byram et al., 2004), as well as the accelerated motoneuron death in mSOD1 mice during CD4+ T cell deficiency (Beers et al., 2008), we hypothesized that Th2, but not Th1-associated chemokines are expressed in the facial motor nucleus after peripheral facial nerve axotomy in WT and mSOD1 mice.
In this report, we used immunohistochemistry to investigate Th2- and Th1-associated chemokine expression in the facial motor nucleus after facial nerve axotomy at time points reported to be associated with significant T cell infiltration (Raivich et al., 1998). Collectively, these results demonstrate that the Th2-associated chemokine, CCL11, but not the Th1-associated chemokine, CXCL11, is constitutively expressed in the WT and mSOD1 facial motor nucleus. Unexpectedly, both chemokines were observed in the facial motor nucleus after facial nerve axotomy, with differences being evident between WT and mSOD1 mice for the number of CCL11+ and percentage of CXCL11+ cells after facial nerve axotomy. A hypothesis with regard to the recruitment of neuroprotective CD4+ T cells after motoneuron injury and its relevance to the degenerative motoneuron disease, ALS, is discussed.
2. Materials and methods
2.1 Animals and surgical procedures
Seven-week old female C57BL/6 wild type (WT) and transgenic mice harboring the G93A mutant of human superoxide dismutase 1 (mSOD1) (B6. CgTg(SOD1*G93A)1Gur) were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were provided autoclaved pellets and water ad libitum. Mice were permitted 1 week to acclimate to their environment before being manipulated and used at 8 weeks of age in all experiments. All experimental manipulations were performed ~4 hr into the light cycle under aseptic conditions. All surgical procedures were completed in accordance with National Institutes of Health guidelines on the care and use of laboratory animals for research purposes. Mice were anesthetized with 3% isoflurane for all surgical procedures. Using aseptic techniques, the right facial nerve of each animal was exposed and transected at its exit from the stylomastoid foramen (Jones and LaVelle, 1985). The distal nerve stump was pushed away from the proximal nerve stump, thereby preventing reconnection of the 2 transection ends of the facial nerve. Behavioral observations were used to ensure that reconnection of the facial nerve was prevented. None of the animals in the present study showed any signs of recovering from unilateral facial paralysis after complete transection of the facial nerve.
2.2 Immunohistochemistry
WT and mSOD1 mice were uninjured (control), 7, 14 or 30 days post-axotomy (DPA) when euthanized with CO2, followed by flash freezing [in 62.5% n-Butyl Bromide (Fisher Scientific, Pittsburgh, PA) + 37.5% 2-methylbutane (Fisher Scientific) surrounded by crushed dry ice]. Frozen brains were sectioned on the Leica CM3000 cryostat (Leica, Bannockburn, IL) with a temperature of −24°C and immersed into Tissue Teck O.C.T. Compound (Sakura Finetek USA, Inc., Torrance, CA) at 8 μm intervals and thaw-mounted onto pre-cleaned SuperFrost slides (Fisher Scientific). Sections were post- fixed with 4% paraformaldehyde, blocked for endogenous biotin for 5 min. (1% H2O2 in PBS), and blocked for non-specific staining with 10% bovine serum albumin (A4503; Sigma-Aldrich; Saint Louis, MO) in PBS for 1 hr. Sections were incubated overnight with rabbit anti-CCL11 (Ebioscience; San Diego, CA) or rat anti-CXCL11 (131327; R&D Systems; Minneapolis, MN) antibodies that have previously been used with mouse tissue (Kool et al., 2008; Fujita et al., 2007; Cruise et al., 2006), in PBS at 4°C. Sections were washed extensively (PBS) and incubated with the secondary antibodies, donkey anti-rabbit HRP (Invitrogen; Carlsbad, CA) or biotinylated chicken anti-rat (Santa Cruz; Santa Cruz, CA), respectively, at room temperature for 1 hr. Sections incubated with biotinylated antibody were also incubated with an avidin-biotin HRP complex (Standard Ultra-Sensitive ABC Staining Kit; Pierce; Rockford, IL). All sections were developed by chromagenic reaction with 3, 3′-diaminobenzidine (Peroxidase Substrate Kit; Vector Laboratories; Burlingame, CA). Sections were dehydrated through ascending concentrations of ethanol:H2O (30%, 50%, 70%, 95% and 100%), cleared in Hemo-De (Fisher-Scientific; Houston, TX), mounted with Permount (Fisher-Scientific), coverslipped and viewed using an Olympus IX70 Fluoview microscope (Tokyo, Japan). As a negative control, adjacent sections were processed with primary antibody omission (2° only). Furthermore, preliminary experiments utilized a dilution series of 1:50, 1:100, 1:500, 1:1,000 and 1:10,000. The highest signal to noise ratio for anti-CCL11 and anti-CXCL11 immunoreactivity was determined by an independent blinded investigator. No signal was detected for either antibody at 1:10,000. Finally, no signal was detected for sections processed with the isotype control antibodies, rabbit IgG or rat IgG2A [(Santa Cruz) (supplementary Fig. 1)].
2.3 Co-immunofluorescence
At 14 DPA, WT mouse brains were flash frozen, post-fixed, sectioned, blocked for endogenous biotin and non-specific binding as described for immunohistochemistry. For CCL11 co-immunofluorescence, rabbit anti-CCL11 (Ebioscience) was co-incubated with biotinylated mouse anti-NeuN (A60, Millipore; Billerica, MA), mouse anti-GFAP alexafluor 488 (131-17719; Invitrogen; Carlsbad, CA) or rat anti-CD68 alexafluor 488 (FA-11; AbD Serotec; Raleigh, NC) in PBS at 4°C overnight. Sections were washed extensively and donkey anti-rabbit alexafluor 350 (for CCL11; Invitrogen) was co-incubated with streptavidin-alexafluor 488 (for NeuN; Invitrogen), PBS (for GFAP) or donkey anti-rat alexafluor 488 (for CD68; Invitrogen) for 1 hr. For CXCL11 co-immunofluorescence, rat anti-CXCL11 (R&D Systems) was co-incubated with biotinylated mouse anti-NeuN (Millipore), goat anti-GFAP (Santa Cruz) or rabbit anti-CD68 (Santa Cruz) in PBS at 4°C overnight. Sections were washed extensively and donkey anti-rat alexafluor 488 (for CXCL11; Invitrogen) was co-incubated with streptavidin-alexafluor 555 (for NeuN; Invitrogen), donkey anti-goat alexafluor 555 (for GFAP; Invitrogen) or donkey anti-rabbit alexafluor 555 (for CD68; Invitrogen) for 1 hr. Following extensive washing, sections were covered with ProLong Gold Antifade reagent (Invitrogen).
2.4 Image Analysis
Images of antibody-stained sections were captured using the IX70 Fluoview (Olympus; Center Valley, PA) microscope attached to a Retiga 2000R (QImaging; Surrey, BC) CCD camera and image capturing system using Image Pro Plus software (v.6.3; Media Cybernetics; Bethesda, MD). Light microscopic images of control and 14 DPA facial motor nucleus were captured using the 10× and 60× objective, for 100× and 600× magnification, respectively. Fluorescent images of control and 14 DPA facial motor nucleus were captured using the 60× objective, for 600× magnification.
2.5 Quantitative immunoreactivity
For each chemokine, five systematically random sampled sections of brain stem, throughout the extent of the facial motor nucleus, with a minimum of 80 μm between each section, were analyzed (n=4). Furthermore, all cell counts were performed by an independent blinded investigator with no knowledge of the experimental conditions. For CCL11 immunoreactivity, each sample was calculated as the average number of CCL11+ FMN or CCL11+ astrocytes per section. Since FMN are the only neurons in the facial motor nucleus, a morphological-based counting analysis was used that distinguished round FMN nuclei from stellate/spindle-shaped astrocytes.
For CXCL11 immunoreactivity, each sample was calculated as the percent CXCL11+ FMN using the formula (CXCL11+ FMN/thionin+ FMN × 100). For each section tested for immunoreactivity, adjacent sections were thionin-stained. This allowed a comparison of the number of immunoreactive FMN compared to the total number of FMN identified with the Nissl stain, thionin. A morphological-based counting analysis was used that distinguished large multipolar cells as FMN.
2.6 Statistical analysis
All data are presented as means ± SEM. The results from the experiments were analyzed using GB-STAT School Pak (Dynamic Microsystems, Inc.; Silver Spring, MD). Data were analyzed using the analysis of variance (ANOVA) method, followed by post hoc comparisons using the Newman-Keuls test.
3. Results
3.1 CCL11 is constitutively expressed in control FMN
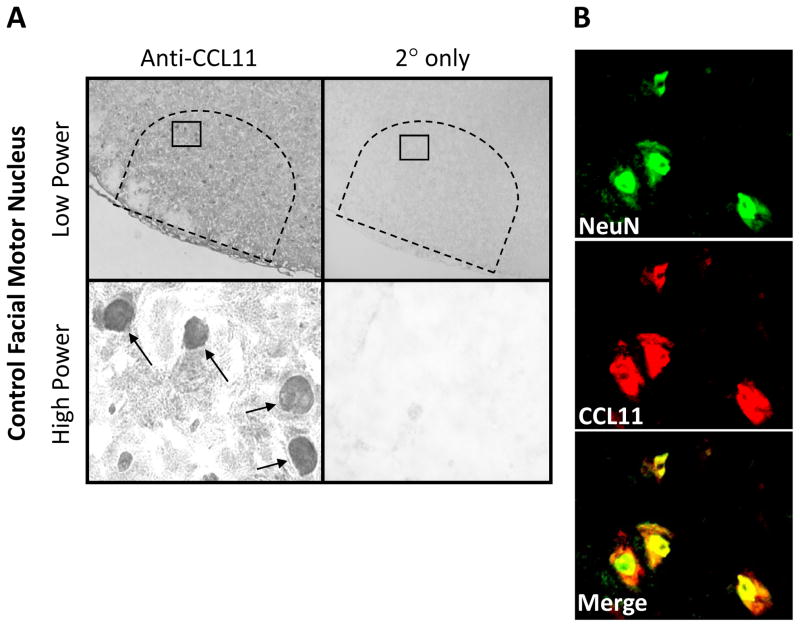
To determine Th2-associated chemokine, CCL11, localization in the control (uninjured) WT facial motor nucleus, CCL11 immunohistochemistry was performed on uninjured brainstem sections (Fig. 1). CCL11 was observed in FMN nuclei throughout the facial motor nucleus as shown at low and high power. In control experiments in which the primary antibody was omitted, CCL11 immunoreactivity was abolished. To verify that CCL11 localized to FMN nuclei, co-immunofluorescence for CCL11 and NeuN (neuronal nuclei marker) was accomplished in the control facial motor nucleus. Colocalization of CCL11 with NeuN demonstrated labeling overlap and confirmed that CCL11 localizes to the nucleus of FMN in the uninjured facial motor nucleus. Similar qualitative results were observed in mSOD1 mice (data not shown). Thus, there is expression of CCL11 in normal FMN nuclei.
Fig. 1.
CCL11 immunoreactivity in control mouse facial motor nucleus. (A) Low- (original magnification, 100×) and high-power (original magnification, 600×) photomicrographs of mouse facial motor nucleus. Sections were processed for CCL11 immunohistochemistry. Control experiments include omission of CCL11 primary antibody (2° only). Tailed arrows indicate positive CCL11 immunoreactivity. (B) High-power immunofluorescence photomicrographs of mouse facial motor nucleus immunoreactive for NeuN (green) and CCL11 (red).
3.2 CCL11 expression is induced in astrocytes after facial nerve axotomy
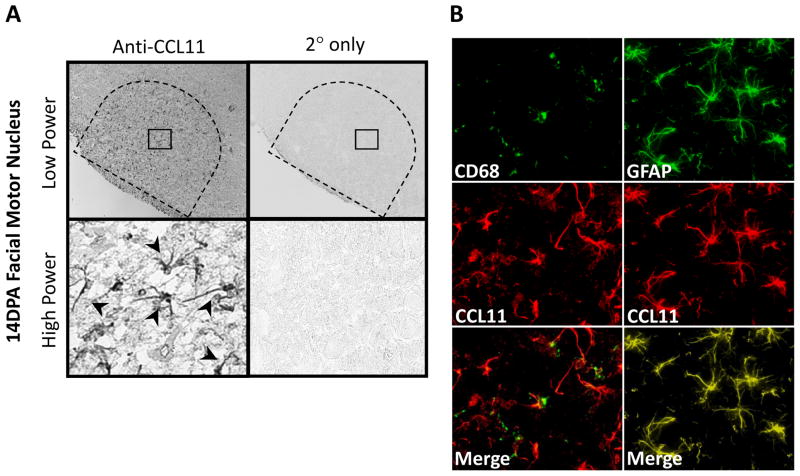
To determine CCL11 localization in the injured WT facial motor nucleus, CCL11 immunohistochemistry was performed on brainstem sections from animals 14 DPA (Fig. 2). CCL11 was observed in astrocytes throughout the facial motor nucleus as shown at low and high power. In control experiments in which the primary antibody was omitted, CCL11 immunoreactivity was abolished. To verify that CCL11 localized to astrocytes, co-immunofluorescence for CCL11 and GFAP (astrocytic marker) was accomplished in the injured facial motor nucleus. Colocalization of CCL11 with GFAP demonstrated labeling overlap and confirmed that CCL11 localizes to astrocytes in the injured facial motor nucleus. Additional immunofluorescent experiments with a microglial marker, CD68, demonstrated no overlap of signal. Similar qualitative results were observed in mSOD1 mice (data not shown). Thus, there is injury-induced expression of CCL11 in astrocytes, but not microglia.
Fig. 2.
CCL11 immunoreactivity in 14 day post-axotomy (DPA) mouse facial motor nucleus. (A) Low- (original magnification, 100×) and high-power (original magnification, 600×) photomicrographs of mouse facial motor nucleus. Sections were processed for CCL11 immunohistochemistry. Control experiments include omission of CCL11 primary antibody (2° only). Untailed arrows indicate positive CCL11 immunoreactivity. (B) High-power immunofluorescence photomicrographs of mouse facial motor nucleus immunoreactive for CD68 (green) and CCL11 (red; left column) or GFAP (green) and CCL11 (red; right column).
3.3 Quantitation of cellular immunoreactivity for CCL11 in the control and axotomized facial motor nucleus
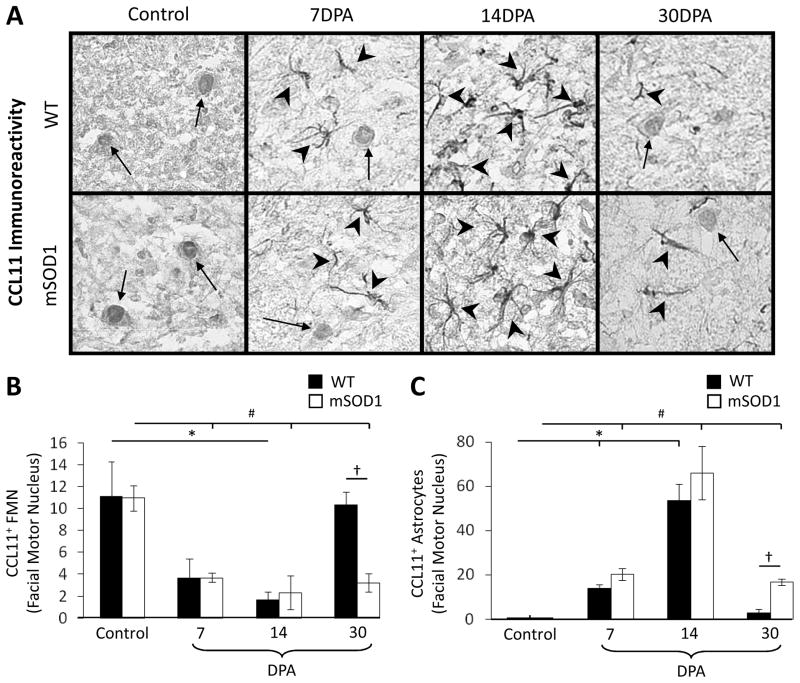
To quantify CCL11+ FMN immunoreactivity in WT and mSOD1 mouse facial motor nucleus, CCL11 immunohistochemistry was performed on brainstem sections that were control (uninjured), or 7, 14, and 30 DPA (Fig. 3A). In the WT control facial motor nucleus, the number of CCL11+ FMN was 11.1 ± 3.2 (Fig. 3B). At 7 and 14 DPA, the number of WT mouse CCL11+ FMN significantly decreased to 3.7 ± 1.7 and 1.7 ± 0.7 [F(3,11) = 5.13, p ≤ 0.05], respectively. By 30 DPA, the number of WT mouse CCL11+ FMN returned to control levels (10.3 ± 1.2). In the mSOD1 control facial motor nucleus, the number of CCL11+ FMN was 11.0 ± 1.2. At 7 and 14 DPA, the number of mSOD1 mouse CCL11+ FMN significantly decreased to 3.7 ± 0.4 and 2.3 ± 1.5, respectively [F(3,11) = 13.75, p ≤ 0.05]. By 30 DPA, the number of mSOD1 mouse CCL11+ FMN was 3.2 ± 0.8, and remained significantly decreased compared to control. No significant differences were found between WT and mSOD1 mouse at control, 7 DPA and 14 DPA time points for the number of CCL11+ FMN. At 30 DPA, the number of WT mouse CCL11+ FMN was significantly increased, when compared with mSOD1 mouse [F(1,4) = 24.01, p ≤ 0.05]. Thus, facial nerve axotomy decreases the number of CCL11+ FMN in WT and mSOD1 mouse facial motor nucleus. However, while WT CCL11+ FMN return to control values by 30 DPA, mSOD1 CCL11+ FMN remain decreased.
Fig. 3.
CCL11 immunoreactivity in control or axotomized wild-type (WT) and presymptomatic mutant superoxide dismutase 1 (mSOD1) mouse facial motor nucleus 7, 14 and 30 days post-axotomy (DPA). (A) High-power (original magnification, 600×) photomicrographs of CCL11 immunoreactivity in control and axotomized mouse facial motor nucleus. Tailed and untailed arrows indicate CCL11+ FMN and astrocytes, respectively. (B) The average number of CCL11+ FMN in the control and axotomized WT (black bars) and mSOD1 (open bars) mouse facial motor nucleus (±SEM). (C) The average number of CCL11+ astrocytes in the control and axotomized WT (black bars) and mSOD1 (open bars) mouse facial motor nucleus (±SEM). * and # denote significant differences from WT control and mSOD1 control mice at p ≤0.05, respectively. † denotes significant differences from WT 30 DPA mice at p ≤ 0.05.
To quantify CCL11+ astrocyte immunoreactivity in WT and mSOD1 mouse facial motor nucleus, CCL11 immunohistochemistry was performed on brainstem sections that were control (uninjured), or 7, 14, and 30 DPA (Fig. 3A). In the WT control facial motor nucleus, the number of CCL11+ astrocytes was 0 (Fig. 3C). At 7 and 14 DPA, the number of WT mouse CCL11+ astrocytes significantly increased to 14 ± 1.5 and 54 ± 7.3, respectively [F(3,11) = 37.31, p ≤ 0.05]. By 30 DPA, the number of WT mouse CCL11+ astrocytes returned to control levels (2.7 ± 1.9). In the mSOD1 control facial motor nucleus, the number of CCL11+ astrocytes was 0. At 7 and 14 DPA, the number of mSOD1 mouse CCL11+ astrocytes significantly increased to 20 ± 2.8 and 66 ± 12, respectively [F(3,11) = 18.65, p ≤ 0.05]. By 30 DPA, the number of mSOD1 mouse CCL11+ astrocytes was 17 ± 1.5, and remained significantly increased compared to control. No significant differences were found between WT and mSOD1 mouse at control, 7 DPA and 14 DPA time points for the number of CCL11+ astrocytes. At 30 DPA, the number of WT mouse CCL11+ astrocytes was significantly decreased, when compared with mSOD1 mouse [F(1,4) = 30.78, p ≤ 0.05]. Thus, facial nerve axotomy increases the number of CCL11+ astrocytes in WT and mSOD1 mouse facial motor nucleus. However, while WT CCL11+ astrocytes return to control values by 30 DPA, mSOD1 CCL11+ astrocytes remain increased.
3.4 CXCL11 is not expressed in control facial motor nucleus
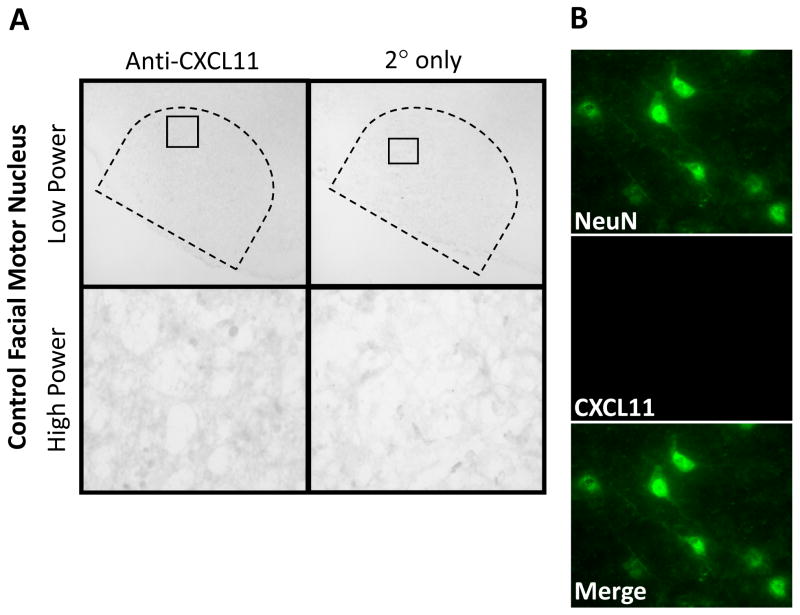
To determine Th1-associated chemokine, CXCL11, localization in the control WT facial motor nucleus, CXCL11 immunohistochemistry was performed on uninjured brainstem sections (Fig. 4). CXCL11 was not present in the facial motor nucleus as shown at low and high power. In control experiments in which the primary antibody was omitted, CXCL11 immunoreactivity was not present. To verify that CXCL11was not present, co-immunofluorescence for CXCL11 and NeuN was accomplished in the control facial motor nucleus. There was no colocalization of CXCL11 with NeuN. Similar qualitative results were observed in mSOD1 mice (data not shown). Thus, there is no expression of CXCL11 in the normal facial motor nucleus.
Fig. 4.
CXCL11 immunoreactivity in control mouse facial motor nucleus. (A) Low- (original magnification, 100×) and high-power (original magnification, 600×) photomicrographs of mouse facial motor nucleus. Sections were processed for CXCL11 immunohistochemistry. Control experiments include omission of CXCL11 primary antibody (2° only). (B) High-power immunofluorescence photomicrographs of mouse facial motor nucleus immunoreactive for NeuN (green) and CXCL11 (red).
3.5 CXCL11 expression is induced in FMN after facial nerve axotomy
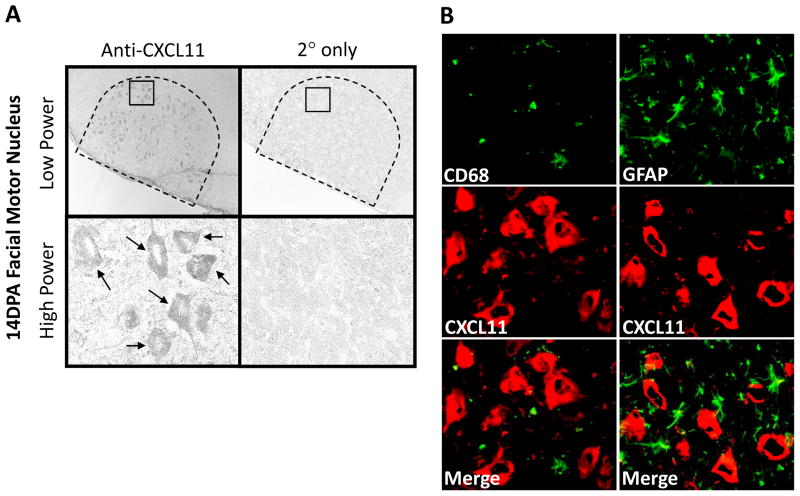
To determine CXCL11 localization in the injured WT facial motor nucleus, CXCL11 immunohistochemistry was performed on brainstem sections from animals 14 DPA (Fig. 5). CXCL11 was observed in FMN throughout the facial motor nucleus as shown at low and high power. In control experiments in which the primary antibody was omitted, CXCL11 immunoreactivity was abolished. To verify that CXCL11 localized to FMN, additional immunofluorescent experiments with a microglial marker, CD68, and an astrocytic marker, GFAP, demonstrated no overlap of signal. Similar qualitative results were observed in mSOD1 mice (data not shown). Thus, there is injury-induced expression of CXCL11 in FMN, but not microglia or astrocytes.
Fig. 5.
CXCL11 immunoreactivity in 14 day post-axotomy (DPA) mouse facial motor nucleus. (A) Low- (original magnification, 100×) and high-power (original magnification, 600×) photomicrographs of mouse facial motor nucleus. Sections were processed for CXCL11 immunohistochemistry. Control experiments include omission of CXCL11 primary antibody (2° only). Tailed arrows indicate positive CXCL11 immunoreactivity. (B) High-power immunofluorescence photomicrographs of mouse facial motor nucleus immunoreactive for CD68 (green) and CXCL11 (red; left column) or GFAP (green) and CXCL11 (red; right column).
3.6 Quantitation of cellular immunoreactivity for CXCL11 in the control and axotomized facial motor nucleus
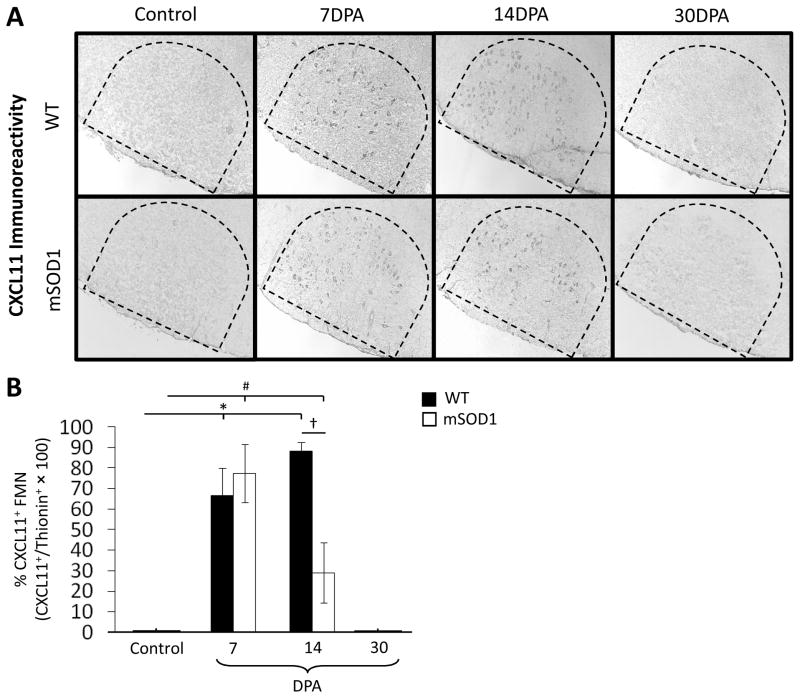
To quantify CXCL11+ FMN immunoreactivity in WT and mSOD1 mouse facial motor nucleus, CXCL11 immunohistochemistry was performed on brainstem sections that were control (uninjured), or 7, 14, and 30 DPA (Fig. 6A). In the WT control facial motor nucleus, the percent of thionin-stained FMN that were CXCL11+ was 0% (Fig. 6B). At 7 and 14 DPA, the percent of WT mouse thionin-stained FMN that were CXCL11+ significantly increased to 67% ± 14% and 88% ± 14%, respectively [F(3,11) = 21.42, p ≤ 0.05]. By 30 DPA, the percent of WT mouse thionin-stained FMN that were CXCL11+ returned to control levels (0%). In the mSOD1 control facial motor nucleus, the percent of thionin-stained FMN that were CXCL11+ was 0%. At 7 and 14 DPA, the percent of mSOD1 mouse thionin-stained FMN that were CXCL11+ significantly increased to 78% ± 4.5% and 29% ± 15%, respectively [F(3,11) = 38.11, p ≤ 0.05]. By 30 DPA, the percent of mSOD1 mouse thionin-stained FMN that were CXCL11+ returned to control levels (0%). No significant differences were found between WT and mSOD1 mouse at control, 7 DPA and 30 DPA time points for the percent of thionin-stained FMN that were CXCL11+. At 14 DPA, the percent of WT mouse thionin-stained FMN that were CXCL11+ was significantly increased, when compared with mSOD1 mouse [F(1,5) = 10.46, p ≤ 0.05]. Thus, facial nerve axotomy increases the percent of thionin-stained FMN that are CXCL11+ in WT and mSOD1 mouse facial motor nucleus. However, while the percent of WT mouse thionin-stained FMN that are CXCL11+ is maintained at increased values by 14 DPA, the percent of mSOD1 mouse thionin-stained FMN that are CXCL11+ is decreased.
Fig. 6.
CXCL11 immunoreactivity in control or axotomized wild-type (WT) and presymptomatic mutant superoxide dismutase 1 (mSOD1) mouse facial motor nucleus 7, 14 and 30 days post-axotomy (DPA). (A) Low-power (original magnification, 100×) photomicrographs of CXCL11 immunoreactivity in control and axotomized mouse facial motor nucleus. (B) The average percent CXCL11+ FMN in the control and axotomized WT and mSOD1 mouse facial motor nucleus (±SEM). * and # denote significant differences from WT control and mSOD1 control mice at p ≤ 0.05, respectively. † denotes significant differences from WT 14 DPA mice at p ≤ 0.05.
4. Discussion
We have previously established that mouse FMN survival after facial nerve axotomy depends on peripheral APCs for initial CD4+ T cell activation, as well as centrally-located microglia for CD4+ T cell reactivation (Byram et al., 2004). Additionally, we discovered that CD4+ Th2 cells develop after facial nerve axotomy (Xin et al., 2007). Furthermore, PACAP mRNA is expressed by injured mouse FMN after facial nerve axotomy (Armstrong et al., 2003) and PACAP increases Th2-associated chemokine expression in cultured murine microglia (Wainwright et al., 2008). Since CD4+ T cells are present in the facial motor nucleus after injury (Ha et al., 2007) and the Th2, but not the Th1, effector subset mediates FMN survival (Deboy et al., 2006), we hypothesized that Th2-associated chemokines would be expressed in the facial motor nucleus after facial nerve axotomy. To delineate this mechanism, we studied the expression of Th2-associated chemokine, CCL11, and Th1-associated chemokine, CXCL11, in the facial motor nucleus after facial nerve axotomy. Additionally, we compared the immunoreactivity for CCL11 and CXCL11 between WT and presymptomatic mSOD1 mice, given the previous studies that have reported dysregulated chemokine expression in mice that develop ALS-like motoneuron pathology (Henkel et al., 2006; Hensley et al., 2003).
The results of the present investigation demonstrate that the Th2-associated chemokine, CCL11, but not Th1-associated chemokine, CXCL11, is constitutively expressed in the facial motor nucleus of WT and mSOD1 mice. Specifically, CCL11 expression was observed in the nuclei of FMN. In contrast, both CCL11 and CXCL11 are expressed after facial nerve axotomy in the facial motor nucleus of WT and mSOD1 mice. Facial nerve injury resulted in a CCL11 expression pattern change from the neuron to the astrocyte at time points associated with significant T cell infiltration (Raivich et al., 1998). Interestingly, at a time point in which the glial reaction has dissipated (30 DPA; Streit, 2006), neuronal CCL11+ expression returns in WT, but not the mSOD1 facial motor nucleus. In addition, facial nerve axotomy induces the expression of CXCL11 in FMN, with the number of thionin-stained CXCL11+ FMN significantly greater in the WT vs. mSOD1 facial motor nucleus at the time of peak T cell infiltration (14 DPA; Ravich et al., 1998). The cause(s) of dysregulated CCL11 and CXCL11 expression in mSOD1 mice could be related to the enhanced neurotoxicity of mSOD1-expressing glia (Yamanaka et al., 2008; Xiao et al., 2007; Boillée et al., 2006). Determining whether the response is maladaptive, or simply a result of compensation to the combination of FMN trauma and SOD1 mutation, is a critical question. Importantly, the data support the hypothesis that at least one chemokine capable of recruiting Th2 cells (CCL11) is expressed in the facial motor nucleus after facial nerve axotomy.
CCL11 was first characterized from guinea pig samples of bronchoalveolar lavage taken after aerosol challenge with ovalbumin (Jose et al., 1994), a model that recapitulates the Th2-mediated disease, asthma. Sallusto et al. (1997) then demonstrated that CD4+ T cells that develop into the Th2 subtype, upregulate the chemokine receptor, CCR3, which is the cognate high affinity receptor for CCL11. Additionally, Th2 cells respond to CCL11 stimulation through a rise in intracellular Ca2+ and increased chemotaxis. Furthermore, while several chemokine receptors are upregulated on differentiated Th2 cells, experiments with receptor antagonists have demonstrated a critical requirement of CCR3 for Th2 cell accumulation after antigenic challenge (Mori et al., 2007). Thus, the Th2-associated chemokine, CCL11, appears to act as a critical chemokine for Th2 cell-mediated recruitment.
CCL11 has previously been demonstrated to be expressed by in vitro cultured human neurons, as well as human airway nerves in vivo (Fryer et al., 2006). In agreement, this study found CCL11 expressed by normal FMN. Unexpectedly, CCL11 localized to the nucleus of FMN. While the significance of nuclear CCL11 localization remains to be determined, the nuclear compartmentalization may be the result of a transcription factor-like function, involved with maintenance and growth under normal conditions. Possibly, CCL11 undergoes posttranslational modifications that target it toward the nucleus normally. When CCL11 was discovered, laser desorption time of flight analysis gave four different signals, which the authors postulated represented different forms of O-linked glycosylation (Jose et al., 1994) shown to contribute to nuclear localization of proteins (Juang et al., 2002). Alternatively, CCL11 may be trafficked to the nucleus from the plasma membrane as a result of chemokine receptor interaction. It has previously been demonstrated that receptor mediated internalization of chemokines results in nuclear localization (Gortz et al., 2002). It is also possible that CCL11 is alternatively spliced in FMN, giving rise to nuclear localization. It has previously been demonstrated that chemokines can be alternatively spliced in the CNS relative to PNS, giving rise to a nuclear- relative to secreted-isoform, respectively (Baird et al., 1999).
After facial nerve axotomy, the number of astrocytes in the facial motor nucleus that were immunoreactive for CCL11 increased significantly at time points consistent with significant T cell infiltration (Raivich et al., 1998; Ha et al., 2007). There is a growing literature demonstrating chemokine expression by astrocytes as a result of neuronal injury (Katayama et al., 2009; Khorooshi et al., 2008; Babcock et al., 2003). Since astrocytes are the most populous cell within the CNS, it is logical that they would serve as amplifiers of an immune response after neuronal injury. In the context of chemokine expression, astrocytes would be the most effective cells at recruiting leukocytes to the injured CNS, since their processes contribute the largest parenchymal proportion to the basement membrane of the perivascular space (Rebenko-Moll et al., 2006) and thus, are strategically positioned for secreting molecules that peripherally circulating leukocytes surveil.
In contrast to the Th2-associated chemokine, CCL11, the Th1-associated chemokine, CXCL11, was not detectable in the normal facial motor nucleus. However, CXCL11 immunoreactivity was induced in FMN after facial nerve axotomy. CXCL11 was first characterized based on cDNAs from in vitro cultured human astrocytes exposed to interferon-gamma (IFN-γ) (Cole et al., 1998). Additionally, it was shown that interleukin 1 beta (IL-1β) and IFN-γ synergize to produce a 400,000 fold increase of CXCL11 mRNA expression, when exposed to cultured astrocytes. Interestingly, we found maximal CXCL11 immunoreactivity at 14 DPA in the WT facial motor nucleus, a time point when IL-1β and IFN-γ mRNA are both present (Raivich et al., 1998).
Since Th1 cells do not mediate FMN survival (Deboy et al., 2006), the presence of the Th1-associated chemokine, CXCL11, was unexpected. However, since the BBB is not disturbed by facial nerve axotomy (Raivich et al., 1998) and FMN are not in a strategic location for the release of chemokines into the perivascular space, it is possible that the effects of CXCL11 are entirely mediated within the CNS parenchyma. Perhaps CXCL11 is released by FMN to recruit microglia, which have been demonstrated to express the cognate high affinity chemokine receptor, CXCR3 (Rappert et al., 2002). Alternatively, CXCL11 may be expressed by injured FMN to interact with the recently deorphanized high affinity chemokine receptor, CXCR7 (Burns et al., 2006), which has been shown to be expressed in rat brain stem motor nuclei using in situ hybridization for CXCR7 mRNA (Schönemeier et al., 2008). Determining where CXCL11 acts, what cell(s) it acts on and what function(s) it performs is a future direction of this study.
Our lab first considered chemokines as important mediators of CD4+ T cell recruitment based on data suggesting that CD4+ T cell interaction with microglia supports FMN survival after injury (Byram et al., 2004). Coupled with evidence indicating decreased levels of FMN survival in presymptomatic mSOD1 mice (Mariotti et al., 2002), we hypothesized that CD4+ T cells promote motoneuron survival in a mSOD1 mouse model. Recent studies have validated this hypothesis, demonstrating a positive role for CD4+ T cells during mSOD1-mediated disease progression (Beers et al., 2008), as well as a profound and progressive immunodeficiency that is linked to T cell dysfunction (Banerjee et al., 2008). While the data suggest that mSOD1 mice have a defect in immune mediated-neuroprotection, investigation of chemokine expression in the CNS of mice affected by ALS pathogenesis has been limited.
mSOD1 mice have altered levels of expression for the monocyte-recruiting chemokines, CCL2 and CCL5 (Henkel et al., 2006; Hensley et al., 2003). Furthermore, a recent report demonstrated significantly greater levels of expression for CCL2 and the neutrophil-recruiting chemokine, CXCL8, in CSF of patients with, or likely to have, ALS (ALS group), relative to a patient population of non-inflammatory neurological disease (NIND group) (Kuhle et al., 2009). The Th2-associated chemokine, CCL11, was significantly increased in serum of the ALS group, relative to the NIND group. These studies collectively support the hypothesis that mice or humans predisposed to ALS have dysregulated chemokine expression levels.
In summary, this immunohistochemical investigation has identified the presence of both Th2- and Th1-associated chemokine expression in the facial motor nucleus after facial nerve axotomy. Furthermore, the data indicate differences in chemokine-expressing cells between WT and presymptomatic mSOD1 mice after facial nerve axotomy. From the work presented in this study and evidence presented in the recent literature, we hypothesize that injury to an otherwise healthy motoneuron provokes an immune response that is geared towards neuroprotection. This model is dependent on a microenvironment within the injured facial motor nucleus that is conducive to Th2 cell recruitment. With increasing information finding an association between the presence of Th2 cytokines and increased lifespan in mSOD1 mice (Garbuzova-Davis et al., 2008), there is increased hope that the effects of this CD4+ T cell subset may provide therapeutic relief to ALS patients in the near future. Given the important role of Th2 cells after facial nerve axotomy (Deboy et al., 2006) and the profound immunodeficiency in mSOD1-mediated disease (Banerjee et al., 2008), understanding the role of injury-induced CNS expression of Th2-associated chemokines may eventually lead to more specifically targeted therapeutics in motoneuron diseases, such as ALS.
Supplementary Material
Acknowledgments
This work was supported by NIH grant NS40433 (KJJ and VMS) and the Les Turner ALS Foundation (DAW and KJJ)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Tam J, Gomariz RP, Patterson PH, Waschek JA. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–7. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–30. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JW, Nibbs RJ, Komai-Koma M, Connolly JA, Ottersbach K, Clark-Lewis I, Liew FY, Graham GJ. ESkine, a novel beta-chemokine, is differentially spliced to produce secretable and nuclear targeted isoforms. J Biol Chem. 1999;274:33496–503. doi: 10.1074/jbc.274.47.33496. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Mosley RL, Reynolds AD, Dhar A, Jackson-Lewis V, Gordon PH, Przedborski S, Gendelman HE. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS One. 2008;3:e2740. doi: 10.1371/journal.pone.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci U S A. 2008;105:15558–63. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–92. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24:4333–9. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Sutcliffe JG, Campbell IL. Microglia stimulate naïve T-cell differentiation without stimulating T-cell proliferation. J Neurosci Res. 1999;55:127–34. doi: 10.1002/(SICI)1097-4547(19990101)55:1<127::AID-JNR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–21. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruise MW, Lukens JR, Nguyen AP, Lassen MG, Waggoner SN, Hahn YS. Fas ligand is responsible for CXCR3 chemokine induction in CD4+ T cell-dependent liver damage. J Immunol. 2006;176:6235–44. doi: 10.4049/jimmunol.176.10.6235. [DOI] [PubMed] [Google Scholar]
- Deboy CA, Xin J, Byram SC, Serpe CJ, Sanders VM, Jones KJ. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4(+) T cells. Exp Neurol. 2006;201:212–24. doi: 10.1016/j.expneurol.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Flügel A, Hager G, Horvat A, Spitzer C, Singer GM, Graeber MB, Kreutzberg GW, Schwaiger FW. Neuronal MCP-1 expression in response to remote nerve injury. J Cereb Blood Flow Metab. 2001;21:69–76. doi: 10.1097/00004647-200101000-00009. [DOI] [PubMed] [Google Scholar]
- Fryer AD, Stein LH, Nie Z, Curtis DE, Evans CM, Hodgson ST, Jose PJ, Belmonte KE, Fitch E, Jacoby DB. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest. 2006;116:228–36. doi: 10.1172/JCI25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Asahina A, Komine M, Tamaki K. The direct action of 1alpha,25(OH)2-vitamin D3 on purified mouse Langerhans cells. Cell Immunol. 2007;245:70–9. doi: 10.1016/j.cellimm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Sanberg CD, Kuzmin-Nichols N, Willing AE, Gemma C, Bickford PC, Miller C, Rossi R, Sanberg PR. Human umbilical cord blood treatment in a mouse model of ALS: optimization of cell dose. PLoS ONE. 2008;3:e2494. doi: 10.1371/journal.pone.0002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner L, Patterson AM, Ashton BA, Stone MA, Middleton J. The human Duffy antigen binds selected inflammatory but not homeostatic chemokines. Biochem Biophys Res Commun. 2004;321:306–12. doi: 10.1016/j.bbrc.2004.06.146. [DOI] [PubMed] [Google Scholar]
- Gortz A, Nibbs RJ, McLean P, Jarmin D, Lambie W, Baird JW, Graham GJ. The chemokine ESkine/CCL27 displays novel modes of intracrine and paracrine function. J Immunol. 2002;169:1387–94. doi: 10.4049/jimmunol.169.3.1387. [DOI] [PubMed] [Google Scholar]
- Ha GK, Huang Z, Parikh R, Pastrana M, Petitto JM. Immunodeficiency impairs re-injury induced reversal of neuronal atrophy: relation to T cell subsets and microglia. Exp Neurol. 2007;208:92–9. doi: 10.1016/j.expneurol.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Siklós L, Appel SH. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol Cell Neurosci. 2006;31:427–37. doi: 10.1016/j.mcn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Engelhardt JI, Siklós L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221–35. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- Hensley K, Fedynyshyn J, Ferrell S, Floyd RA, Gordon B, Grammas P, Hamdheydari L, Mhatre M, Mou S, Pye QN, Stewart C, West M, West S, Williamson KS. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14:74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- Jones KJ, LaVelle A. Changes in nuclear envelope invaginations in axotomized immature and mature hamster facial motoneurons. Brain Res. 1985;353:241–9. doi: 10.1016/0165-3806(85)90212-3. [DOI] [PubMed] [Google Scholar]
- Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, Truong O, Hsuan JJ, Williams TJ. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–7. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang YT, Solomou EE, Rellahan B, Tsokos GC. Phosphorylation and O-linked glycosylation of Elf-1 leads to its translocation to the nucleus and binding to the promoter of the TCR zeta-chain. J Immunol. 2002;168:2865–71. doi: 10.4049/jimmunol.168.6.2865. [DOI] [PubMed] [Google Scholar]
- Katayama T, Tanaka H, Yoshida T, Uehara T, Minami M. Neuronal injury induces cytokine-induced neutrophil chemoattractant-1 (CINC-1) production in astrocytes. J Pharmacol Sci. 2009;109:88–93. doi: 10.1254/jphs.08298fp. [DOI] [PubMed] [Google Scholar]
- Khorooshi R, Babcock AA, Owens T. NF-kappaB-driven STAT2 and CCL2 expression in astrocytes in response to brain injury. J Immunol. 2008;181:7284–91. doi: 10.4049/jimmunol.181.10.7284. [DOI] [PubMed] [Google Scholar]
- Kool M, Soullié T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–82. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhle J, Lindberg RL, Regeniter A, Mehling M, Steck AJ, Kappos L, Czaplinski A. Increased levels of inflammatory chemokines in amyotrophic lateral sclerosis. Eur J Neurol. 2009 doi: 10.1111/j.1468-1331.2009.02560.x. (in press) [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–9. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti R, Cristino L, Bressan C, Boscolo S, Bentivoglio M. Altered reaction of facial motoneurons to axonal damage in the presymptomatic phase of a murine model of familial amyotrophic lateral sclerosis. Neuroscience. 2002;115:331–5. doi: 10.1016/s0306-4522(02)00448-7. [DOI] [PubMed] [Google Scholar]
- Mori A, Ogawa K, Someya K, Kunori Y, Nagakubo D, Yoshie O, Kitamura F, Hiroi T, Kaminuma O. Selective suppression of Th2-mediated airway eosinophil infiltration by low-molecular weight CCR3 antagonists. Int Immunol. 2007;19:913–21. doi: 10.1093/intimm/dxm049. [DOI] [PubMed] [Google Scholar]
- Nagata T, Nagano I, Shiote M, Narai H, Murakami T, Hayashi T, Shoji M, Abe K. Elevation of MCP-1 and MCP-1/VEGF ratio in cerebrospinal fluid of amyotrophic lateral sclerosis patients. Neurol Res. 2007;29:772–6. doi: 10.1179/016164107X229795. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–16. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappert A, Biber K, Nolte C, Lipp M, Schubel A, Lu B, Gerard NP, Gerard C, Boddeke HW, Kettenmann H. Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl- current and chemotaxis in murine microglia. J Immunol. 2002;168:3221–6. doi: 10.4049/jimmunol.168.7.3221. [DOI] [PubMed] [Google Scholar]
- Rebenko-Moll NM, Liu L, Cardona A, Ransohoff RM. Chemokines, mononuclear cells and the nervous system: heaven (or hell) is in the details. Curr Opin Immunol. 2006;18:683–9. doi: 10.1016/j.coi.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Rentzos M, Nikolaou C, Rombos A, Boufidou F, Zoga M, Dimitrakopoulos A, Tsoutsou A, Vassilopoulos D. RANTES levels are elevated in serum and cerebrospinal fluid in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2007;8:283–7. doi: 10.1080/17482960701419232. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–7. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- Schönemeier B, Kolodziej A, Schulz S, Jacobs S, Hoellt V, Stumm R. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol. 2008;510:207–20. doi: 10.1002/cne.21780. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RD, Bottomly K. Antigen-specific activation of effector macrophages by IFN-gamma producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989;142:760–5. [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29:506–10. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Heesen M, Berman MA, Fischer MB, Yoshizawa I, Luo Y, Dorf ME. Murine astrocytes express a functional chemokine receptor. J Neurosci. 1997;17:6522–8. doi: 10.1523/JNEUROSCI.17-17-06522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright DA, Xin J, Mesnard NA, Sanders VM, Jones KJ. Effects of facial nerve axotomy on Th2- and Th1-associated chemokine mRNA expression in the facial motor nucleus of wild-type and pre-symptomatic SOD1 mice. J Neurodegen Regen. 2009 In Press. [PMC free article] [PubMed] [Google Scholar]
- Wainwright DA, Xin J, Sanders VM, Jones KJ. Differential actions of pituitary adenylyl cyclase-activating polypeptide and interferon gamma on Th2- and Th1-associated chemokine expression in cultured murine microglia. J Neurodegen Regen. 2008;1:31–4. [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Zhao W, Beers DR, Yen AA, Xie W, Henkel JS, Appel SH. Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J Neurochem. 2007;102:2008–19. doi: 10.1111/j.1471-4159.2007.04677.x. [DOI] [PubMed] [Google Scholar]
- Xin J, Wainwright DA, Serpe CJ, Sanders VM, Jones KJ. Phenotype of CD4+ T cell subsets that develop following mouse facial nerve axotomy. Brain Behav Immun. 2008;22:528–37. doi: 10.1016/j.bbi.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–3. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–83. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.