Abstract
Profilin-1 (Pfn1), a ubiquitously expressed actin-binding protein, is downregulated in several different types of adenocarcinoma and elicits tumor-suppressive effect on breast cancer cell lines. MDA-MB-231 (MDA-231), a breast cancer cell line that displays all the characteristics of post-epithelial-to-mesenchymal transition and does not form cell-cell adhesion, can be reverted to an epithelioid phenotype by Pfn1 overexpression. This morphological transition is caused by restoration of adherence junctions (AJ) requiring Pfn1’s interaction with actin. Pfn1 overexpression increases the expression level of R-cadherin (a type of cadherin that is endogenously expressed in the parental cell line) and restores AJ in MDA-231 cells in R-cadherin dependent manner. These findings highlight important role of Pfn1 in the regulation of epithelial cell-cell adhesion.
Keywords: Profilin-1, R-cadherin, MDA-MB-231, adherence junction, cell-cell adhesion
INTRODUCTION
Cell-cell adhesion plays critical role in embryonic development, differentiation and maintenance of tissue architecture (Gloushankova 2008). Cadherin family of transmembrane proteins, which mediates homophilic adhesion in Ca2+-dependent manner, is the major player in the makeup of adherence junctions (AJ) in cells. E-cadherin is the main form of cadherin in the AJ of epithelial cells whereas other cadherins including N-, P-, R-, and VE-cadherin form AJ in other cell types. The cytoplasmic domain of cadherin binds directly to β-catenin and p120-catenin at two distinct sites. Because of α-catenin’s ability to bind to both β-catenin and actin, it has been traditionally thought that AJ connects to the underlying actin cytoskeleton through a linkage formed by β-catenin and actin filaments with α-catenin serving as the bridging molecule. However, this view has been recently challenged by experimental evidence of mutual exclusion of α-catenin’s binding to β-catenin and actin, based on which it has been proposed that instead of acting as a stable linker, α-catenin may function as a regulator of actin dynamics near the adhesion sites (Drees et al. 2005; Yamada et al. 2005).
Disruption of cell-cell adhesion which occurs during epithelial-to-mesenchymal transformation (EMT) through downregulation of junctional components promotes cell migration and proliferation. Although EMT was originally described as a morphogenetic process that occurs during normal embryonic development, some aspects of EMT are also recapitulated during metastatic progression of epithelial-derived tumors (Thiery 2002). Loss of E-cadherin function, a hallmark of EMT, occurs through downregulation of its expression either via epigenetic mechanisms (promoter methylation, increased expression of transcriptional repressors) or inactivating mutations, and directly correlates with cancer grade (Berx et al. 1995; Birchmeier and Behrens 1994; Hennig et al. 1995; Hirohashi 1998; Yokoyama et al. 2001; Yoshiura et al. 1995). Experimental restoration of E-cadherin suppresses tumorigenic ability and invasive phenotype of E-cadherin negative cancer cells (Birchmeier and Behrens 1994). Collectively, these findings point to critical role of cell-cell adhesion in the regulation of tumor initiation and progression.
Actin polymerization de novo at the sites of cell-cell contacts is essential for formation of stable AJ (Vasioukhin et al. 2000). Actin is under dynamic control of different classes of actin-binding proteins (ABPs). Several ABPs that promote nucleation and elongation of actin filaments including N-WASP (neural Wiskott Aldrich Syndrome protein), WAVE2 (WASP-associated verprolin homology protein), Arp2/3, diaphanous and Ena (enabled) /VASP (vasodilator stimulated phosphoprotein) have been shown to be involved in the formation of AJ (Carramusa et al. 2007; Ivanov et al. 2005; Kobielak et al. 2004; Scott et al. 2006; Verma et al. 2004; Yamazaki et al. 2007). These ABPs also contain proline-rich domains to interact with profilin (Pfn), a class of G-actin binding protein (Carlsson et al. 1977) and an important regulator of actin polymerization in vivo (Ding et al. 2006; Zou et al. 2007). Therefore, it is not unlikely that at least some of these ABPs might cooperate with Pfn to regulate actin polymerization at the cell-cell adhesion sites. Very little at best is currently known about Pfn’s role in regulating epithelial cell-cell adhesion. In a recent study, we showed that silencing expression of Pfn1 (the founding and the ubiquitously expressed member of Pfn family of genes) in normal human mammary epithelial cells (HMEC) leads to dramatic delocalization of E-cadherin from cell-cell junctions with concomitant reduction in cell-cell adhesion strength and scattering in culture (Zou et al. 2007). These findings suggested for the first time that Pfn1 could play a role in the regulation of epithelial cell-cell adhesion.
Pfn1’s role has been queried in epithelial-derived cancer because i) its expression is significantly downregulated in various adenocarcinomas (breast, pancreas, hepatic and gastric) (Gronborg et al. 2006; Janke et al. 2000; Oien et al. 2003; Wu et al. 2006) and ii) it can suppresses tumorigenicity of breast cancer cells, as demonstrated previously in MDA-MB-231 (MDA-231) and CAL51 cell lines by us and others (Janke et al. 2000; Zou et al. 2007). Although MDA-231 cell line is null for expression of most of the major cadherins (E, P, N), does not form functional cell-cell adhesion and exhibit traits of post-EMT including robust vimentin and loss of cytokeratin expression (Chakrabandhu et al. 2008; Nieman et al. 1999), interestingly, we found that Pfn1 overexpression can induce MDA-231 cells to undergo morphological transformation into an epithelioid phenotype without actually re-expressing E-cadherin (Zou et al. 2007). This observation suggested that Pfn1 upregulation is capable of restoring cell-cell adhesion in breast cancer cells; however, the nature of cell-cell adhesion and the underlying mechanisms remained to be identified. This gap was addressed in the present study.
MATERIAL AND METHODS
Antibodies and reagents
Monoclonal GAPDH antibody is a product of Abd Serotec (Raleigh, NC). Polyclonal Pfn1 antibody and cytochalasin-D were purchased from Cytoskeleton Inc. (Denver, CO). Polyclonal pan-cadherin antibody was obtained from Abcam (Cambridge, MA). Monoclonal β-catenin antibody was purchased from BD Biosciences (San Diego, CA). Monoclonal p120-catenin antibody was a product of Santa Cruz (Santa Cruz, CA). All other cell culture reagents are products of Invitrogen (Carlsbad, CA).
Cell culture and transfection
Generation and culture of MDA-MB-231 (MDA-231) cell lines stably expressing GFP, GFP-Pfn1 and GFP-Pfn1-H119E have been described previously (Zou et al., 2007). Normal human mammary epithelila cells (HMEC; source: Cambrex, Walkersville, MD) were cultured in a complete growth media supplied by the manufacturer.The sequence of a single-target specific Pfn1-siRNA has been previously described (Ding et al., 2006). Smart-pool of non-targeting control and R-cadherin siRNAs were purchased from Dharmacon (Chicago, IL) and Santa Cruz (Santa Cruz, CA) respectively. All siRNA transfections were performed at a 100 nM working concentration using transfection reagent available through Dharmacon according to the manufacturer’s instruction (Chicago, IL).
RT-PCR
Total RNA was extracted from cells cultured in normal serum-containing growth medium using the RNeasy mini kit commercially available through Qiagen (Valencia, CA). RT-PCR reactions were performed using a commercial kit from Qiagen. The primer sequences for R-cadherin were 5′- GTT GGG GCA GAT GGG ACA GT -3′ (sense) and 5′- ACG TTG ATG GGC GGG ATG AC -3′ (antisense). The primer sequences for GAPDH were 5’-CGGAGTCAACGGATT TGGTCGTAT-3’ (sense) and 5’- AGGCTTCTCCATGGTGGTGAAGAC-3’ (antisense). The RT-PCR cycle conditions were 94°C (30 sec), 60°C (30 sec), and 72°C (30 sec) with a total of 40 cycles. RT-PCR details for GAPDH have been previously described (Das et al. 2009). PCR amplified products were run on a 2% agarose gel. For qRT-PCR experiments, SYBR green method was employed and fold-change in transcript level was quantified by the standard delta-delta-CT method.
Protein extraction, immunoprecipitation and immunoblotting
Total cell lysate was prepared by extracting cells with either warm 1X sample buffer or modified RIPA buffer (50 mM Tris-HCl -pH 7.5, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 0.1% SDS, 2 mM EDTA) supplemented with 50 mM NaF, 1mM sodium pervanadate, and protease inhibitors. For immunoprecipitation, 700-1000 μg cell lysate was first precleared with 25 μl of protein-G/protein A-conjugated agarose beads. Precleared lysate was incubated with either 3 μg of β-catenin or 2 μg of p120-catenin antibody overnight and then with 50 μl of the same beads for an additional 2 hours. Immunoprecipitated protein samples were washed 3 times with the lysis buffer, resuspended in 30 μl of 2X sample buffer and run on a SDS-PAGE. Immunoblotting concentrations for different antibodies were: GAPDH (1:1000), β-catenin (1:1000), p120-catenin (1:200) and R-cadherin (1:1000).
Immunostaining
For immunostaining, cells were washed with warm PBS, fixed with 4% formaldehyde for 10 minutes, permeabilized with 0.5% Triton X-100 in PBS for 10 minutes, and then blocked with 1% BSA in PBS for 1 hour at room temperature (RT). Incubation with primary antibody (β-catenin: 1:100; R-cadherin: 1: 200, pan-cadherin: 1:50) was carried out at RT for 1 hr. Cells were then washed 5 times (3 times with PBS containing 0.02% tween followed by 2 times with PBS) before incubating with the appropriate secondary antibodies was added in secondary antibody whenever the for an additional hour at RT. Stained cells were washed 5 times in a similar fashion before mounting on slides. All fluorescence images were acquired with a 60X objective using an Olympus IX-71 inverted microscope.
RESULTS
Pfn1 overexpression restores AJ in MDA-231 cells
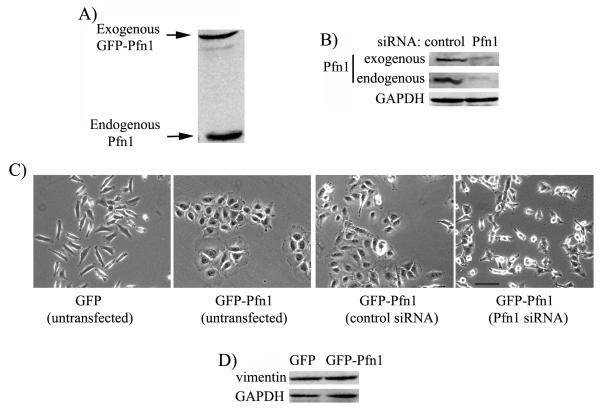
The central aim of the present study was to determine how Pfn1 upregulation can induce morphological transformation of mesenchymal breast cancer cells into an epithelioid phenotype. To address this question, we used our previously described stable transfectants of MDA-231 cells overexpressing either GFP-Pfn1 (show propensity to form clusters in culture) or GFP as control (displays mesenchymal phenotype similar to the parental cell line) (Zou et al. 2007). Pfn1 immunoblot of total lysate extracted from GFP-Pfn1 expressing cells confirms a modest (< 2-fold) level of Pfn1 overexpression in our cell system (Fig 1A). We first asked whether clustering of GFP-Pfn1 overexpressers can be reversed by downregulating Pfn1 expression. We therefore treated GFP-Pfn1 overexpressing cells with either non-targeting control- or Pfn1-siRNA. Immunoblot in Fig 1B shows that Pfn1-siRNA treatment strongly suppresses the expression of both endogenous and exogenous Pfn1 in GFP-Pfn1 expressers. Morphological examination revealed that control siRNA transfected GFP-Pfn1 expressers show clustering phenotype similar to untransfected cells as expected and this clustering effect can be completely eliminated when Pfn1 expression is downregulated (Fig 1C). This observation demonstrates that clustering of GFP-Pfn1 expressers is specifically due to an increase in the overall Pfn1 content. Given our previous observation that E-cadherin is not re-expressed in GFP-Pfn1 expressers (Zou et al. 2007) and the expression level of vimentin (a mesenchymal marker) in MDA-231 cells is unaltered by Pfn1 overexpression (Fig 1D), one can conclude that Pfn1 overexpression introduces epithelial-like morphological traits but does not cause a true mesenchymal-to-epithelial (MET) reversion of MDA-231 breast cancer cell line.
Figure 1. Pfn1-induced clustering of MDA-231 cells is reversible.
A) Relative levels of endogenous vs exogenous Pfn1 in GFP-Pfn1 expressing cells. B) Pfn1 immunoblot showing strong suppression of endogenous and exogenous Pfn1 in GFP-Pfn1 expressers 72 hours after Pfn1-siRNA treatment (GAPDH blot serves as the loading control). C) Phase-contrast micrographs of GFP-Pfn1 expressers 72 hours after treatment with either control or Pfn1-siRNA (morphology of untransfected GFP and GFP-Pfn1 expressers are shown side-by-side for comparison). D) Vimentin immunoblot of GFP and GFP-Pfn1 expressers (GAPDH blot serves as the loading control). Scale — 100 μm.
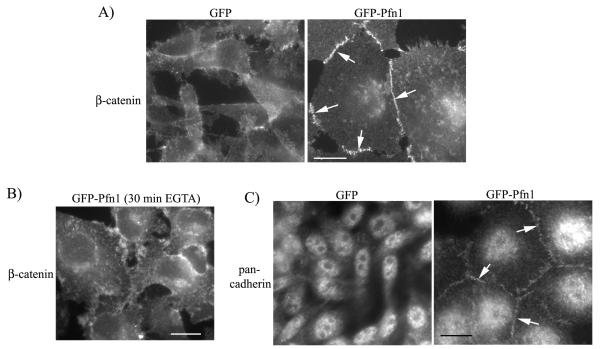
We next asked whether clustered phenotype of Pfn1 overexpressers is through restoration of AJ complex. AJ consists of two basic types: the classical cadherin-catenin complex (Ca2+-dependent) and the nectin-afadin complex (Ca2+-independent). We performed immunostaining for β—catenin (a marker for AJ) which showed strong localization at the cell-cell junctions in GFP-Pfn1 expressers, but not in control GFP-expressing cells (Fig 2A). Junctional localization of β—catenin in GFP-Pfn1 expressers becomes completely disrupted upon brief treatment of EGTA (a calcium chelator) which demonstrates that cell-cell adhesion in these cells is Ca2+ sensitive and therefore likely to involve classical cadherin-dependent AJ assembly (Fig 2B). Indeed, immunostaining data showed that cell-cell junctions of GFP-Pfn1 expressers are positive for pan-cadherin immunoreactivity (Fig 2C — note that the pan-cadherin antibody recognizes all members of cadherin family of proteins).
Figure 2. Pfn1 overexpression restores AJ in MDA-231 cells.
A) β-catenin immunostaining of GFP and GFP-Pfn1 expressers (arrows show junctional localization). B) β-catenin immunostaining of GFP-Pfn1 expressers 20 minutes after treatment with 2 mm EGTA. C) Pan-cadherin immunostaining of GFP and GFP-Pfn1 overexpressers (arrows show junctional localization). Scale bar — 20 μm.
Actin-binding of Pfn1 is required for AJ restoration in MDA-231 cells
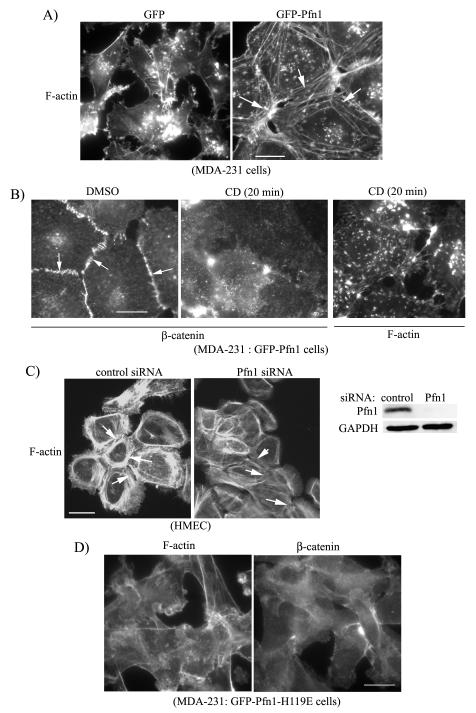
It is known that stable AJ in epithelial cells is closely associated with circumferential belt of F-actin underneath the plasma membrane. Given that Pfn1 is a key regulator of actin polymerization in cells, we speculated that Pfn1 overexpression may alter actin cytoskleletal structures in MDA-231 cells. Indeed phalloidin staining revealed circumferential cables of F-actin in GFP-Pfn1 expressers but not in control GFP-expressing cells (Fig 3A). These actin structures are critical for AJ formation since brief treatment of cytochalasin-D (CD), which completely depolymerizes those F-actin cables, also disrupts AJ in Pfn1 overexpressing cells as judged by loss of junctional localization of β-catenin (Fig 3B — diluent control DMSO treatment preserves β-catenin localization at the cell-cell junctions as expected). Consistent with our overexpression data in MDA-231 cells, in a complementary set of experiments we found that silencing Pfn1 expression causes a marked reduction in circumferential F-actin cables in normal HMEC (Fig 3C - note that we previously showed that HMEC express Pfn1 at a much higher level compared to MDA-231 cells and exhibit junctional delocalization of E-cadherin upon Pfn1 depletion (Zou et al. 2007)). Together these data demonstrate that Pfn1 plays an important role in the formation of cell-adhesion-promoting actin cytsokeletal structures. To directly test that it is the actin polymerizing action of Pfn1 that contributes to generation of circumferential F-actin cables and AJ formation in our case, we examined F-actin and β-catenin distribution in stable transfectants of MDA-231 cells overexpressing GFP-Pfn1-H119E, a mutant form of Pfn1 that is deficient in actin-binding (loss of actin-binding function of the mutant and generation of this mutant cell line have been previously described (Zou et al. 2007)). Fig 3D shows that MDA-231 cells expressing GFP-Pfn1-H119E do not display circumferential belt of F-actin and also fail to form AJ as judged by β-catenin immunostaining thus demonstrating actin-binding is critical for Pfn1-induced epithelioid conversion of MDA-231 cells.
Figure 3. Perturbing Pfn1 expression alters actin cytoskeletal structures in MDA-231 and normal HMEC.
A) Phalloidin staining shows circumferential F-actin cables in GFP-Pfn1 expressers (right panel — see arrows), but not in GFP expressers (left panel). B) β-catenin immunostaining (left and middle panels) of GFP-Pfn1 expressers of MDA-231 cells 20 min after either DMSO (vehicle control) or 500 nM CD treatment (arrows show junctional localization). The right most panel shows phalloidin staining of CD-treated GFP-Pfn1 expressers. C) Phalloidin staining of HMEC treated with control and Pfn1-siRNA (Pfn1 immunoblot next to the micrographs shows siRNA—mediated downregulation of Pfn1 expression; GAPDH blot serves as the loading control). D) Phalloidin (left panel) and β-catenin (right panel) staining of GFP-Pfn1-H119E expressers of MDA-231 cells (Scale bar — 20 μm).
Pfn1 overexpression restores AJ in MDA-231 cells in an R-cadherin dependent manner
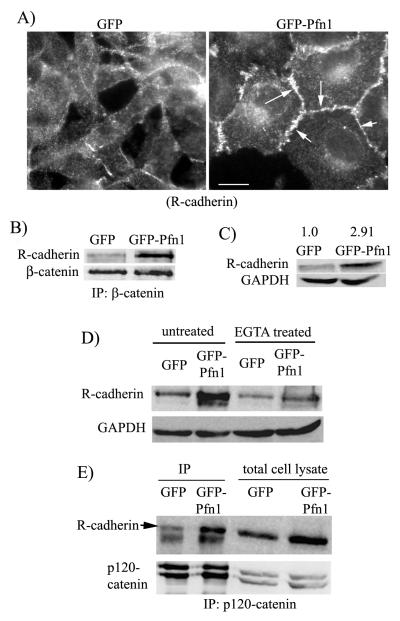
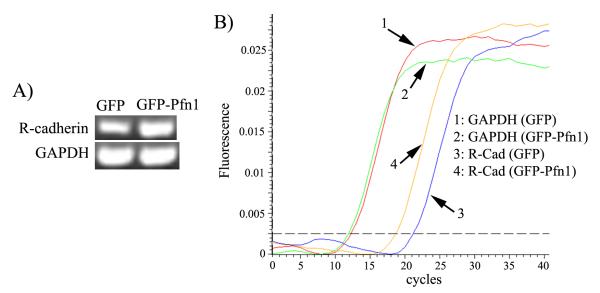
Our next goal was to determine how Pfn1 overexpression restores AJ in MDA-231 cells in an E-cadherin suppressed background. Besides E-cadherin, expressions of two other classical cadherin genes (P, N) are also silenced in MDA-231 cells and our DNA microarray analyses confirmed that GFP-Pfn1 overexpressing MDA-231 cell line does not re-express either of those two cadherins (data not shown). However, MDA-231 cell line is known to express R(retinal)-cadherin and cadherin-11. Since R-cadherin belongs to the classical cadherin family of proteins, we asked whether R-cadherin might be responsible for AJ formation in Pfn1 overexpressing MDA-231 cells. Our immunostaining experiments showed strong junctional localization of R-cadherin in GFP-Pfn1 expressers, but not in control GFP expressing cells (Fig 4A). Subsequent co-immunoprecipitation experiments revealed increased β-catenin-R-cadherin complex formation in GFP-Pfn1 expressing cells compared to GFP expressers (Fig 4B) thus suggesting incorporation of R-cadherin in functional AJ complex. Interestingly, when we compared the total protein level of R-cadherin between the two cell lines, we found a ~ 3-fold increase in R-cadherin in MDA-231 cells as a result of Pfn1 overexpression (Fig 4C). It is known that the membrane pool of E-cadherin is subjected to endosomal trafficking and degradation when cell-cell adhesions are disrupted. In our case, the excess pool (i.e equivalent to the protein differential) of R-cadherin in Pfn1 overexpressers appears to be membrane localized since the protein level R-cadherin in Pfn1 overexpressing cells is dramatically reduced and becomes nearly comparable to that in GFP expressers within a short time-frame (3 hours) after EGTA treatment (Fig 4D). Binding to p120-catenin protects cadherins from endocytosis and subsequent degradation thereby determining the cadherin level in cells. We found that the total expression level of p120-catenin is comparable between the two cell lines (Fig 4E). Although co-immunoprecipitation experiments revealed an overall increase in R-cadherin/p120-catenin complex in Pfn1 overexpressing cells (Fig 4E), given a 3-fold higher total protein content of R-cadherin in Pfn1 overexpressing cells, the intrinsic binding capability between R-cadherin and p120-catenin does not appear to be affected by Pfn1 upregulation. These findings ruled out any specific effect of Pfn1 on p120-catenin-mediated R-cadherin stabilization mechanism and prompted us to examine whether increased R-cadherin protein in Pfn1 overexpressing cells can be explained by any possible changes in the mRNA level. Interestingly, both regular RT-PCR and real-time qRT-PCR experiments showed a similar ~3-fold higher transcript level of R-cadherin in GFP-Pfn1 expressing cells (Figs 5A-B) thus demonstrating that Pfn1 overexpression increases R-cadherin expression in MDA-231 cells at the mRNA level.
Figure 4. Pfn1 overexpression causes junctional accumulation of R-cadherin in MDA-231 cells.
A) R-cadherin immunostaining of GFP and GFP-Pfn1 expressers (arrows show junctional localization; scale bar — 20 μm). B) Co-immunoprecipitation analyses of R-cadherin/β-catenin complex formation in GFP and GFP-Pfn1 expressers. C) R-cadherin immunblot of total cell lysates shows ~2.9 fold increase in R-cadherin expression in Pfn1 overexpressing cells (the numbers are based on relative densitometric analyses of R-cadherin and GAPDH (loading control) bands averaged from 4 independent experiments). D) Relative R-cadherin levels between GFP and GFP-Pfn1 expressers either untreated or following 3 hours of 2 mM EGTA treatment (GAPDH blot serves as the loading control). E) P120-catenin and R-cadherin immunoblots of total cell lysate and p120-catenin immunoprecipitates (IP) from the lysates of GFP and GFP-Pfn1 expressers.
Fig 5. Pfn1 overexpression upregulates R-cadherin expression in MDA-231 cells at the mRNA level.
A) RT-PCR data showing relative levels of R-cadherin and GAPDH mRNAs in GFP and GFP-Pfn1 expressers. B) SYBR-green fluorescence tracings of GAPDH and R-cadherin RT-PCR products in real-time qRT-PCR experiments.
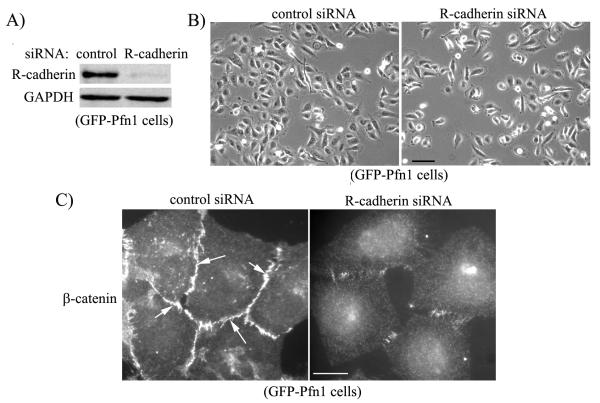
Finally, to more directly assess the role of R-cadherin in AJ formation in Pfn1 overexpressing cells, we transfected GFP-Pfn1 expressing cells with either R-cadherin-specific or control siRNA. Immunoblot in Fig 6A shows that R-cadherin expression can be dramatically downregulated by siRNA treatment within 72 hours after transfection. Phase-contrast micrographs of transfected cells show that R-cadherin depleted cells fail to form clusters and present as isolated cells in a sub-confluent culture (Fig 6B). This was further supported by immunostaining data which revealed that junctional localization of β-catenin in GFP-Pfn1 expressers is dramatically reduced when R-cadherin expression is silenced (Fig 6C). Taken together, these data clearly demonstrate that Pfn1 overexpression restores AJ in MDA-231 cells in R-cadherin dependent manner.
Fig 6. Pfn1 overexpression restores AJ in MDA-231 cells in R-cadherin-dependent manner.
A) R-cadherin immunoblot of lysates extracted from GFP-Pfn1 expressers 72 hours after either control or R-cadherin siRNA transfection (GAPDH blot serves as the loading control). B-C) Phase contrast micrographs (panel B) and β-catenin immunostaining (panel C) of GFP-Pfn1 expressers bearing either control or R-cadherin siRNA (scale bars — 100 μm in panel B, 20 μm in panel C).
DISCUSSION
Although Pfn1 has been recognized for its prominent role in actin polymerization, whether and how Pfn1 plays a role in the regulation of cell-cell adhesion has not been examined in the literature. The study described herein is the first report that characterizes Pfn1-dependent regulation of cell-cell adhesion at a molecular level. The data presented in this study revealed a novel finding that overexpression of Pfn1, which has been previously shown to elicit a strong anti-tumor effect on breast cancer cells, is also capable of restoring AJ complex in MDA-231, an E-cadherin-negative, mesenchymal breast cancer cell line. This morphological transformation did not occur through re-expression of E-cadherin suggesting that it is not a classical MET-like process. We showed that Pfn1 overexpression restores AJ and induces epithelioid phenotype in MDA-231 cell via R-cadherin, a type of classical cadherin that is endogenously expressed in the parental cell line. This is an interesting observation since 1) R-cadherin has been previously shown to be required for EMT in developing kidney (Dahl et al. 2002; Goto et al. 1998), 2) several types of tumor cells including prostate carcinoma, bladder carcinoma, and rhabodomyosarcoma show elevated levels of R-cadherin compared to their normal counterparts (Bussemakers et al. 2000; Giroldi et al. 2000; Kucharczak et al. 2008), and 3) forced expression of R-cadherin in E-cadherin expressing cells causes downregulation of AJ by promoting E-cadherin degradation (Maeda et al. 2006). Our data demonstrate that R-cadherin can actually compensate for loss of E-cadherin function in forming AJ in breast cancer cells, and is consistent with a recent report by Hazan’s group that also showed R-cadherin overexpression can induce AJ formation in MDA-231 cells (Agiostratidou et al. 2009). These findings suggest that R-cadherin may have a context dependent function. This is not unrealistic since in at least two variants of cancer (colorectal and gastric), R-cadherin expression is actually found to be downregulated (Miotto et al. 2004). We report a novel finding that Pfn1 overexpression increases R-cadherin expression in MDA-231 cells at the mRNA level. However, it is not clear at this point whether mRNA upregulation occurs at the true transcriptional level or post-transriptionally through enhanced mRNA stability. Either of these two scenarios is not unrealistic given that Pfn1 has been previously shown to affect gene transcription (Lederer et al. 2005) and a recent study has provided evidence of post-transcriptional upregulation of cadherin-11 by β-catenin and GSK3β (glycogen synthase kinase 3β) in prostate and breast cancer cells (Farina et al. 2009). These issues will need to be addressed in future studies.
We also showed that perturbation of Pfn1 expression has profound effects on actin cytoskeletal structures that are known to reinforce cadherin-catenin complex. Specifically, we showed induction of circumferential F-actin bundles (characteristic features of epithelial cells with intact AJ) by Pfn1 overexpression in MDA-231 cells and conversely, a marked reduction of those F-actin structures in normal HMEC in response to Pfn1 downregulation. Our experiments with the actin-binding deficient mutant of Pfn1 further demonstrated that actin-binding is critical for Pfn1 to facilitate formation of circumferential F-actin cables. How might Pfn1 overexpression stimulate formation of this type of F-actin structure in MDA-231 cells? Pfn1 binds to all of the major ABPs (N-WASP, WAVE2, Arp 2/3, diaphanous, Ena/VASP) which have been shown to be involved in actin polymerization at or in the proximity of AJ (Carramusa et al. 2007; Ivanov et al. 2005; Kobielak et al. 2004; Scott et al. 2006; Verma et al. 2004; Yamazaki et al. 2007). Actin polymerizing activity of those ABPs is enhanced in the presence of Pfn1. There is structural distinction in the organization of actin filaments generated by these different ABPs. While Arp2/3 complex creates a branched network of actin filaments, diaphanous and Ena/VASP proteins generate straight actin filaments. Indeed, both diaphanous and Ena/VASP proteins have been shown to participate in the formation of circumferential actin cables underneath AJ in epithelial cells (Carramusa et al. 2007; Kobielak et al. 2004; Scott et al. 2006). Therefore, it will be interesting to see whether enhanced interaction of Pfn1 with either of diaphanous or Ena/VASP proteins plays a role in AJ formation in Pfn1 overexpressing MDA-231 cells.
Finally, we will need to extend our findings to other mesenchymal breast cancer cell lines or even other types of carcinoma cells (example: hepatic, pancreatic) which also express reduced Pfn1 compared to their normal counterparts (Gronborg et al. 2006; Wu et al. 2006) to establish whether Pfn1 induced restoration of AJ is a generalized phenomenon in tumor cells. This knowledge will be valuable for further examining whether tumor-suppressive property of Pfn1 is, at least, partially linked to its ability to modulate cell-cell adhesion.
Acknowledgement
The authors wish to thank Dr. Tuhin Das and Guo Guang for technical assistance. This study was funded by a grant from the National Cancer Institute (R01-CA108607) to P.R.
REFERENCES
- Agiostratidou G, Li M, Suyama K, Badano I, Keren R, Chung S, Anzovino A, Hulit J, Qian B, Bouzahzah B. Loss of R-cadherin facilitates tumor progression and metastasis. Cancer Research. 2009 doi: 10.1158/0008-5472.CAN-08-4007. others. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. Embo J. 1995;14(24):6107–15. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198(1):11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Bussemakers MJ, Van Bokhoven A, Tomita K, Jansen CF, Schalken JA. Complex cadherin expression in human prostate cancer cells. Int J Cancer. 2000;85(3):446–50. [PubMed] [Google Scholar]
- Carlsson L, Nystrom LE, Sundkvist I, Markey F, Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. Journal of Molecular Biology. 1977;115(3):465–483. doi: 10.1016/0022-2836(77)90166-8. [DOI] [PubMed] [Google Scholar]
- Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci. 2007;120(Pt 21):3870–82. doi: 10.1242/jcs.014365. [DOI] [PubMed] [Google Scholar]
- Chakrabandhu K, Huault S, Hueber AO. Distinctive molecular signaling in triple-negative breast cancer cell death triggered by hexadecylphosphocholine (miltefosine) FEBS Lett. 2008;582(30):4176–84. doi: 10.1016/j.febslet.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Dahl U, Sjodin A, Larue L, Radice GL, Cajander S, Takeichi M, Kemler R, Semb H. Genetic dissection of cadherin function during nephrogenesis. Mol Cell Biol. 2002;22(5):1474–87. doi: 10.1128/mcb.22.5.1474-1487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Bae YH, Wells A, Roy P. Profilin-1 overexpression upregulates PTEN and suppresses AKT activation in breast cancer cells. J Cell Physiol. 2009;218(2):436–43. doi: 10.1002/jcp.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Lambrechts A, Parepally M, Roy P. Silencing profilin-1 inhibits endothelial cell proliferation, migration and cord morphogenesis. J Cell Sci. 2006;119(Pt 19):4127–37. doi: 10.1242/jcs.03178. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123(5):903–15. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina AK, Bong YS, Feltes CM, Byers SW. Post-transcriptional regulation of cadherin-11 expression by GSK-3 and beta-catenin in prostate and breast cancer cells. PLoS ONE. 2009;4(3):e4797. doi: 10.1371/journal.pone.0004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroldi LA, Shimazui T, Schalken JA, Yamasaki H, Bringuier PP. Classical cadherins in urological cancers. Morphologie. 2000;84(265):31–8. [PubMed] [Google Scholar]
- Gloushankova NA. Changes in regulation of cell-cell adhesion during tumor transformation. Biochemistry (Mosc) 2008;73(7):742–50. doi: 10.1134/s000629790807002x. [DOI] [PubMed] [Google Scholar]
- Goto S, Yaoita E, Matsunami H, Kondo D, Yamamoto T, Kawasaki K, Arakawa M, Kihara I. Involvement of R-cadherin in the early stage of glomerulogenesis. J Am Soc Nephrol. 1998;9(7):1234–41. doi: 10.1681/ASN.V971234. [DOI] [PubMed] [Google Scholar]
- Gronborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, Molina H, Jensen ON, Hruban RH, Goggins MG. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5(1):157–71. doi: 10.1074/mcp.M500178-MCP200. others. [DOI] [PubMed] [Google Scholar]
- Hennig G, Behrens J, Truss M, Frisch S, Reichmann E, Birchmeier W. Progression of carcinoma cells is associated with alterations in chromatin structure and factor binding at the E-cadherin promoter in vivo. Oncogene. 1995;11(3):475–84. [PubMed] [Google Scholar]
- Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153(2):333–9. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16(6):2636–50. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke J, Schluter K, Jandrig B, Theile M, Kolble K, Arnold W, Grinstein E, Schwartz A, Estevez-Schwarz L, Schlag PM. Suppression of tumorigenicity in breast cancer cells by the microfilament protein profilin 1. J Exp Med. 2000;191(10):1675–86. doi: 10.1084/jem.191.10.1675. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6(1):21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharczak J, Charrasse S, Comunale F, Zappulla J, Robert B, Teulon-Navarro I, Pelegrin A, Gauthier-Rouviere C. R-Cadherin expression inhibits myogenesis and induces myoblast transformation via Rac1 GTPase. Cancer Res. 2008;68(16):6559–68. doi: 10.1158/0008-5472.CAN-08-0196. [DOI] [PubMed] [Google Scholar]
- Lederer M, Jockusch BM, Rothkegel M. Profilin regulates the activity of p42POP, a novel Myb-related transcription factor. J Cell Sci. 2005;118(Pt 2):331–41. doi: 10.1242/jcs.01618. [DOI] [PubMed] [Google Scholar]
- Maeda M, Johnson E, Mandal SH, Lawson KR, Keim SA, Svoboda RA, Caplan S, Wahl JK, 3rd, Wheelock MJ, Johnson KR. Expression of inappropriate cadherins by epithelial tumor cells promotes endocytosis and degradation of E-cadherin via competition for p120(ctn) Oncogene. 2006;25(33):4595–604. doi: 10.1038/sj.onc.1209396. [DOI] [PubMed] [Google Scholar]
- Miotto E, Sabbioni S, Veronese A, Calin GA, Gullini S, Liboni A, Gramantieri L, Bolondi L, Ferrazzi E, Gafa R. Frequent aberrant methylation of the CDH4 gene promoter in human colorectal and gastric cancer. Cancer Res. 2004;64(22):8156–9. doi: 10.1158/0008-5472.CAN-04-3000. others. [DOI] [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147(3):631–44. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oien KA, Vass JK, Downie I, Fullarton G, Keith WN. Profiling, comparison and validation of gene expression in gastric carcinoma and normal stomach. Oncogene. 2003;22(27):4287–300. doi: 10.1038/sj.onc.1206615. [DOI] [PubMed] [Google Scholar]
- Scott JA, Shewan AM, den Elzen NR, Loureiro JJ, Gertler FB, Yap AS. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell. 2006;17(3):1085–95. doi: 10.1091/mbc.E05-07-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100(2):209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Verma S, Shewan AM, Scott JA, Helwani FM, den Elzen NR, Miki H, Takenawa T, Yap AS. Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J Biol Chem. 2004;279(32):34062–70. doi: 10.1074/jbc.M404814200. [DOI] [PubMed] [Google Scholar]
- Wu N, Zhang W, Yang Y, Liang YL, Wang LY, Jin JW, Cai XM, Zha XL. Profilin 1 obtained by proteomic analysis in all-trans retinoic acid-treated hepatocarcinoma cell lines is involved in inhibition of cell proliferation and migration. Proteomics. 2006;6(22):6095–106. doi: 10.1002/pmic.200500321. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123(5):889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D, Oikawa T, Takenawa T. Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion. J Cell Sci. 2007;120(Pt 1):86–100. doi: 10.1242/jcs.03311. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Kamata N, Hayashi E, Hoteiya T, Ueda N, Fujimoto R, Nagayama M. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001;37(1):65–71. doi: 10.1016/s1368-8375(00)00059-2. [DOI] [PubMed] [Google Scholar]
- Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci U S A. 1995;92(16):7416–9. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Jaramillo M, Whaley D, Wells A, Panchapakesa V, Das T, Roy P. Profilin-1 is a negative regulator of mammary carcinoma aggressiveness. Br J Cancer. 2007;97(10):1361–71. doi: 10.1038/sj.bjc.6604038. [DOI] [PMC free article] [PubMed] [Google Scholar]