Abstract
The regulatory role of programmed death 1 (PD-1) was investigated in the development of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis. Typical EAE could be induced by immunization without pertussis toxin (PTX) in PD-1-null but not in wild-type (WT) mice. However, both strains developed a similar EAE phenotype when immunized with PTX or by adoptive transfer of pathogenic T cells. In WT mice that did not develop EAE after immunization without PTX, the frequency of CD4+FoxP3+ Treg cells was boosted in the periphery but not in the thymus. This increase in Treg frequency was abrogated by PD-1 deficiency or inclusion of PTX. In addition, PD-1 expression was critical to in vitro conversion of naïve myelin-specific CD4 T cells into Treg cells and was directly related to Treg suppressive activity. Finally, PD-1 was markedly down-modulated in the periphery of WT mice after administration of PTX. Therefore, down-modulation of PD-1 in Treg cells may abrogate Treg-mediated immune suppression, permitting the activation of myelin-reactive T cells and induction of EAE.
Keywords: multiple sclerosis, experimental autoimmune encephalomyelitis, programmed death 1, regulatory T cells, pertussis toxin
Multiple sclerosis (MS) is a debilitating neurological disease with unknown etiology that has a strong autoimmune component. The inflammatory phase of MS likely involves activation and migration of myelin-reactive T cells across the blood–brain barrier (BBB), resulting in damage to myelin and axons in the central nervous system (CNS). However, myelin-reactive T cells are found normally in the periphery of healthy controls, without any apparent harm (Hemmer et al., 2002). Similarly, transgenic mice that predominantly express myelin antigen-specific T-cell receptors (TCRs) in their T cells usually do not spontaneously develop experimental autoimmune encephalomyelitis (EAE), an animal model of MS, unless established on the SJL background (Waldner et al., 2000), kept in nonsterile environment (Goverman et al., 1993; Bettelli et al., 2006), or challenged with immunization or microbial agents. Thus, myelin-reactive T cells are not always encephalitogenic and require extraneous factors to trigger the disease. In humans, it is believed that MS may be triggered by stressful events such as microbial infections (van Noort et al., 2000). However, concrete evidence is still lacking, and it is not clear exactly how these triggering factors activate myelin-reactive T cells and initiate CNS-directed immune attack in vivo.
One of the main factors limiting the encephalitogenicity of myelin-reactive T cells is immune tolerance. For example, many of the peptide sequences in myelin proteins are constitutively expressed in the thymus of rodents, the notable exception being SJL/J mice, in which the dominant disease-inducing peptide PLP-139–151 happens to differ from the thymic variants and to escape central tolerance induction (Kuchroo et al., 1994). As a result, PLP-139–151-specific T cells are intrinsically encephalitogenic, and pertussis toxin (PTX) is not required for PLP-139–151 peptide in complete Freund’s adjuvant (CFA) to induce EAE in this mouse strain. In other mouse strains, microbial agents such as PTX are necessary to break immune tolerance and promote EAE induction.
Programmed death 1 (PD-1) has been demonstrated to play a crucial role in the development and maintenance of peripheral tolerance (Keir et al., 2007). PD-1 gene-deficient (PD-1KO) mice develop spontaneous autoimmune disorders similar to lupus-like glomerulonephritis, arthritis, or dilated cardiomyopathy as early as 5 weeks of age. In MS patients, a PD-1 polymorphism was determined to be associated with disease progression, possibly through a partial defect in PD-1-mediated inhibition of T-cell activation (Kroner et al., 2005). In the EAE model, it was shown that genetic disruption of PD-L1, an identified PD-1 ligand that can also bind to B7-1, converted an EAE-resistant mouse strain into a fully permissive one (Latchman et al., 2004), suggesting that PD-L1 may be critically involved in the process of EAE induction. However, a more precise role played by PD-1 in the development of MS and EAE has not been elucidated.
In this study, we demonstrate that the expression levels of PD-1 represent a critical checkpoint for the activation and infiltration of myelin-reactive T cells into the central nervous system (CNS) and that PTX may enhance EAE susceptibility by modulating inhibitory effects of Treg mediated through PD-1. Our results have significant implications in the context of MS pathogenesis.
MATERIALS AND METHODS
Mice
Mice used in the experiments were age-matched (7–11 weeks old) females rested for at least 7 days prior to treatment or immunization. PD-1-null (PD-1KO) mice, which were back-crossed with C57BL/6 (B6) mice for 11 generations, were from Dr. Honjo at Kyoto University (Kyoto, Japan). B6 mice (used as WT controls), green fluorescence protein (GFP; under β-actin promoter) mice, and 2D2 (mMOG-35–55 TCR transgenic) mice were from the Jackson Laboratory (Bar Harbor, ME). FoxP3-GFP “knock-in” mice on the B6 background were provided by Drs. Ziegler and Rudensky (University of Washington, Seattle, WA). Animals were housed and cared for according to institutional guidelines in the animal resource facility at the Veterans Affairs Medical Center (Portland, OR).
Reagents
Mouse (m)MOG-35–55 peptide was purchase from Polypeptides (San Diego, CA). Incomplete Freund’s adjuvant (IFA) was purchased from Sigma (St. Louis, MO). Mycobacterium tuberculosis H37RA (MTB) was purchased from Difco (Detroit, MI). Complete Freund’s adjuvant (CFA) was prepared by mixing 20 mg MTB with 10 ml IFA. PTX was purchased from List Biological Laboratories (Campbell, CA). All antibodies (Abs) were purchased from eBioscience (San Diego, CA). MicroBeads for MACS cell separation were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). DNase I and liberase were purchased from Roche (Indianapolis, IN).
Induction of EAE
Mice were inoculated s.c. in the flanks with 0.2 ml of an emulsion containing 200 µg mMOG-35–55 peptide and CFA containing 200 µg heat-killed MTB. When indicated, mice were injected i.v. with 75 and 200 ng PTX on days 0 and 2, respectively. Passive EAE was established according to a protocol published previously (Wang et al., 2006). Briefly, 8 days after immunization without PTX, spleens and draining lymph nodes (LN) were removed, and single-cell suspensions were produced. The mixed-cell suspension was cultured with 20 µg/ml of MOG peptide at 8 × 106 cells/ml in stimulation medium (RPMI 1640 medium supplemented with 0.1 mM nonessential amino acids, 2 mM L-glutamine, 0.1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol, and 2% FBS) for 48 hr. The cells were washed twice with RPMI and transferred i.v. into naïve recipients (20 × 106 cells/mouse in RPMI). The mice were assessed daily for clinical signs of EAE: 0 5 normal, 1 = limp tail or mild hindlimb weakness, 2 = moderate hindlimb weakness or mild ataxia, 3 = moderately severe hindlimb weakness, 4 = severe hindlimb weakness or mild forelimb weakness or moderate ataxia, 5 = paraplegia with no more than moderate forelimb weakness, and 6 = paraplegia with severe forelimb weakness or severe ataxia, moribund condition, or dead.
Pathology
Intact spinal columns were removed from experimental mice at the end of each experiment. The spinal cords were dissected after fixation in 4% paraformadehyde, dehydrated, and embedded in paraffin before sectioning. To examine the tissue for demyelination, 7-µm sections from the lumbar region of spinal cord were stained with Luxol fast blue plus periodic acid Schiff (LFB-PAS) and recorded with a digital camera.
Flow Cytometry
At the end of each experiment, the spleens and LN were harvested and minced through a 200-mesh screen to obtain a single-cell suspension. Intracellular staining for FoxP3 was performed following the protocol recommended by eBioscience. Both membrane and intracellular PD-1 were stained. Data were collected on a FACSCalibur flow cytometer and analyzed in FlowJo software (Tree Star, Ashland, OR). Data represent 50,000–100,000 events, unless otherwise noted.
Mixed Lymphocyte Proliferation and T-Cell Suppression Assay
Treg-cell suppression of lymphocyte proliferation was assessed by anti-CD3 mAb activation of CD4+ indicator cells mixed at different ratios with Treg cells plus APC (Wang et al., 2006). The T-cell suppression assay was a modified mixed-lymphocyte proliferation assay including Treg cells. WT, PD-1KO, or FoxP3-GFP “knock-in” mice were immunized 2 weeks before the suppression experiments. CD4+FoxP3+ and CD4+CD25− cells from WT and PD-1KO mice as well as CD4+GFP+PD-1+ and CD4+GFP+PD-1− cells from FoxP3-GFP mice were sorted with a FACSorter. APCs were irradiated, T-cell-depleted (by CD90 microbeads on AutoMACS) splenocytes from naïve WT mice. Assays were set up according to a standard protocol (Polanczyk et al., 2007). I50 was determined as the ratio of indicator/suppressor cells that produced 50% inhibition of proliferation responses to anti-CD3/C28 mAb. Suppressive index (SI) = 100–I50.
In Vitro Conversion Assay
Naïve mMOG-35–55-specific CD4 T cells (CD4+CD25−CD62L+) and dendritic cells (DCs; CD11c+) were isolated by FACS from liberase-digested spleens of 2D2 (mMOG-35–55-specific TCR-transgenic) mice. Briefly, whole spleens were injected with HBSS containing 50 µg/ml DNase I and 250 mg/ml liberase and incubated at 37°C for 10 min before being minced and washed through a 200-mesh screen with RPMI. Isolated CD4 T cells or DCs were then incubated with neutralizing anti-PD-1 antibody (α-PD-1, NA/LE) or PBS for 30 min at 4°C and washed twice with ice-cold RPMI. For conversion, 50,000 CD4 T cells and 30,000 DCs were cultured in 200 µl RPMI media containing 2% FBS, 250 ng/ml mMOG-35–55 peptide, and 50 U/ml recombinant human interleukin-2 (IL-2) for 2 days at 37°C. Transforming growth factor-β (TGFβ; 2 ng/ml) was added as indicated. The incubation groups included the following combinations: 1) CD4 T cells (preincubated with PBS), DCs (preincubated with PBS), and mMOG-35–55 peptide; 2) CD4 T cells (preincubated with PBS), DCs (preincubated with PBS), mMOG-35–55 peptide, and TGFβ; 3) CD4 T cells (preincubated with α-PD-1), DCs (preincubated with PBS), mMOG-35–55 peptide, and TGFβ; and 4) CD4 T cells (preincubated with PBS), DCs (preincubated with α-PD-1), mMOG-35–55 peptide, and TGFβ. At the end of incubation, cells were washed with staining buffer and stained for CD4, FoxP3, and IgG.
RESULTS
PD-1 Deficiency Overrode the Necessity of PTX in EAE Induction
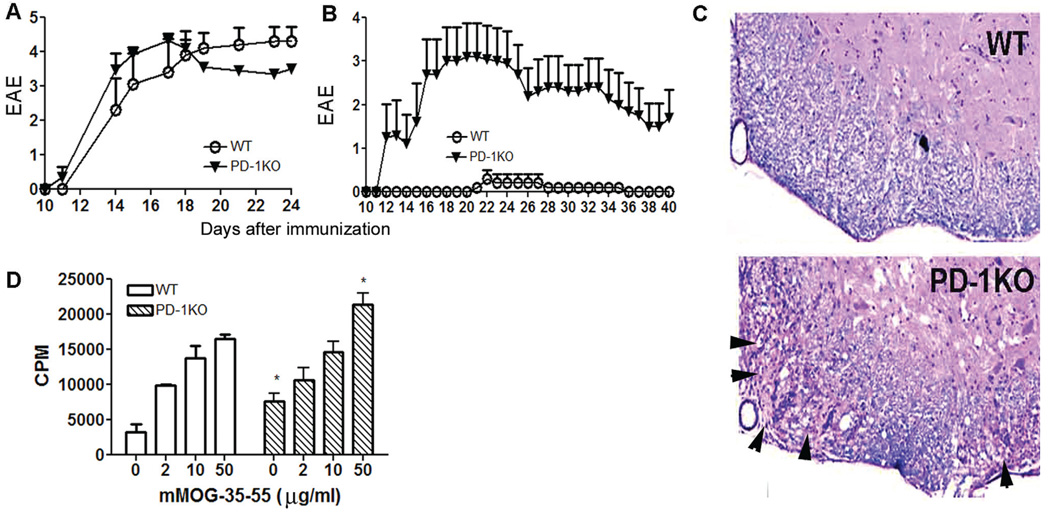
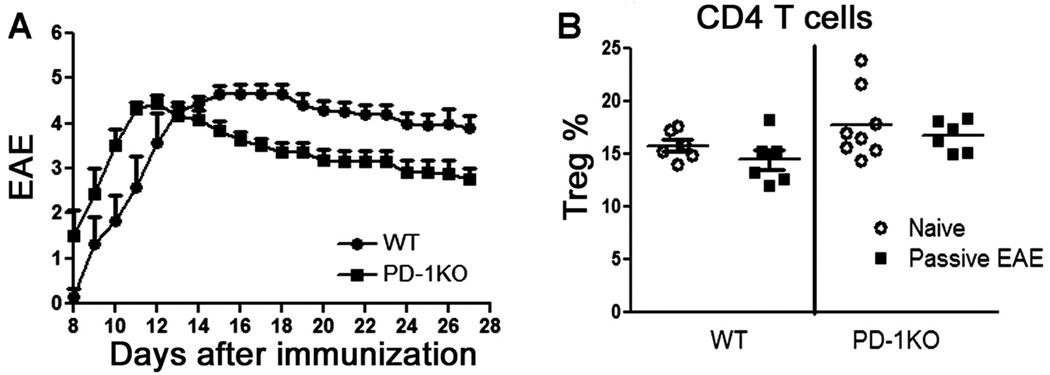
To test whether deficiency of PD-1 would affect EAE induction and development, we immunized PD-1KO and WT mice with mMOG-35–55 peptide/CFA in the presence or absence of PTX. Interestingly, although the scores of clinical EAE induced by immunization in the presence of PTX were similar for both strains (Fig. 1A), immunization in the absence of PTX resulted in distinct clinical outcomes: although no clinical or histological sign of EAE were discernible in WT mice, typical EAE developed in PD-1KO mice (Fig. 1B,C). Thus, PD-1 deficiency rendered hyperreactivity to PD-1KO mice and overrode the necessity of PTX in EAE induction. However, the severities of clinical EAE in PD-1KO mice immunized in the presence and absence of PTX and WT mice immunized with PTX were similar. When stimulated in vitro with mMOG-35–55 peptide, splenocytes obtained from WT mice 40 days after immunization in the absence of PTX proliferated significantly, although at a somewhat lower level than from PD-1KO mice (Fig. 1D), indicating the existence of mMOG-35–55-reactive T cells in both mice as expected. The differential susceptibility to EAE induction in WT and PD-1KO mice with and without PTX implies a critical role for PD-1 in EAE initiation.
Fig. 1.
The deficiency of PD-1 alters immune tolerance to mMOG-35–55 peptide in the absence of PTX. A: Immunization with mMOG-35–55 peptide/CFA in the presence of PTX induced EAE with similar severity in WT and PD-1KO mice; n = 6–10. B: Immunization with mMOG-35–55 peptide/CFA in the absence of PTX induced EAE in PD-1KO mice but not in WT mice. **P < 0.01 as assessed by the Mann-Whitney test (n = 5). C: Immunization in the absence of PTX caused demyelination in PD-1KO but not in WT mice. D: Splenocytes from WT and PD-1KO mice proliferated in vitro to mMOG-35–55 peptide. Splenocytes and LN cells were obtained ex vivo from mice used in A at the end of the experiment. *P < 0.05. PTX was injected into mice on days 0 and 2.
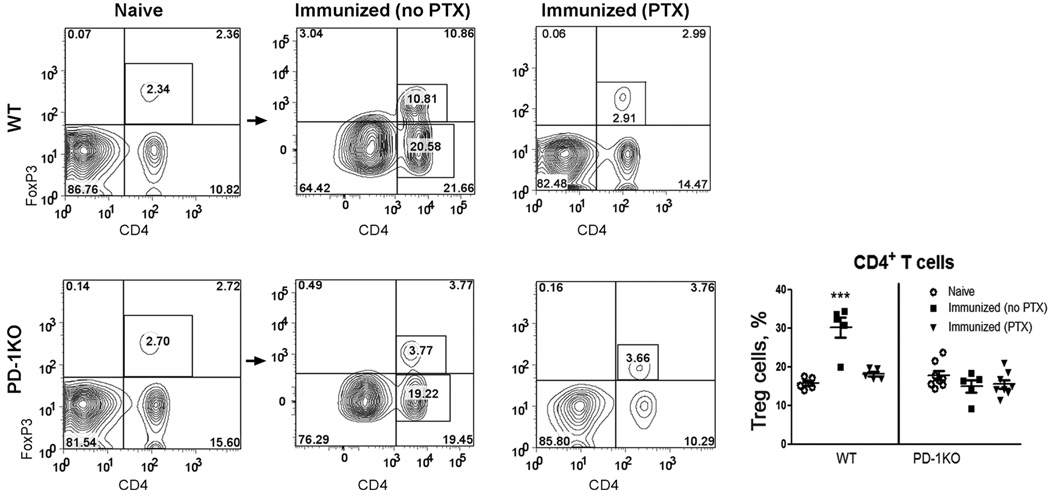
Immunization in the Absence of PTX Up-Regulated Treg in a PD-1-Dependent and PTX-Sensitive Manner
CD4+FoxP3+ Treg cells act to suppress the activation of autoreactive T cells and thereby maintain immune homeostasis and tolerance to self-antigens (Sakaguchi et al., 2008). We investigated whether these cells played an important role in causing differential susceptibility of PD-1KO and WT mice to EAE induction. In the absence of PTX, immunization with mMOG-35–55 peptide/CFA significantly boosted the frequency of CD4+FoxP3+ Treg cells in the periphery of WT mice (Fig. 2). These increased Treg cells seemed to be generated in the periphery, because there was no increase in Treg-cell frequency in the thymus (data not shown). In PD-1KO mice, on the contrary, immunization in the absence of PTX did not induce any significant up-regulation of Treg cells. Similarly, challenging the mice with PTX in the immunizing regime prevented up-regulation of Treg with mMOG-35–55/CFA. PTX did not alter the frequency of Treg cells in PD-1KO mice, indicating that PTX may depend on the presence of PD-1 to influence Treg cells. Our result indicated that both PD-1 and PTX may regulate sensitivity to EAE induction by up-regulation of Treg cells in the periphery but not in thymus.
Fig. 2.
The percentage of CD4+FoxP3+ Treg cells was markedly increased by immunization with mMOG-35–55 peptide/CFA in the absence of PTX in WT but not in PD-1KO or PTX-administered WT mice. Splenocytes were obtained from naïve or mMOG-35–55 peptide/CFA (in the presence or absence of PTX)-immunized WT or PD-1KO mice used in Figure 1B. The cells were stained with Abs for CD4, FoxP3, and rat IgG2a (or isotope control), and data were analyzed with FlowJo software. There was virtually no staining by the isotope control (not shown). The percentages of Treg cells in CD4 T cells were calculated as percentage of CD4+FoxP3+ cells/(% of CD4+FoxP3+ cells + percentage CD4+FoxP3−) × 100%. ***P < 0.001 as measured by one-way ANOVA, followed by Newman-Kuels test; n = 5–8.
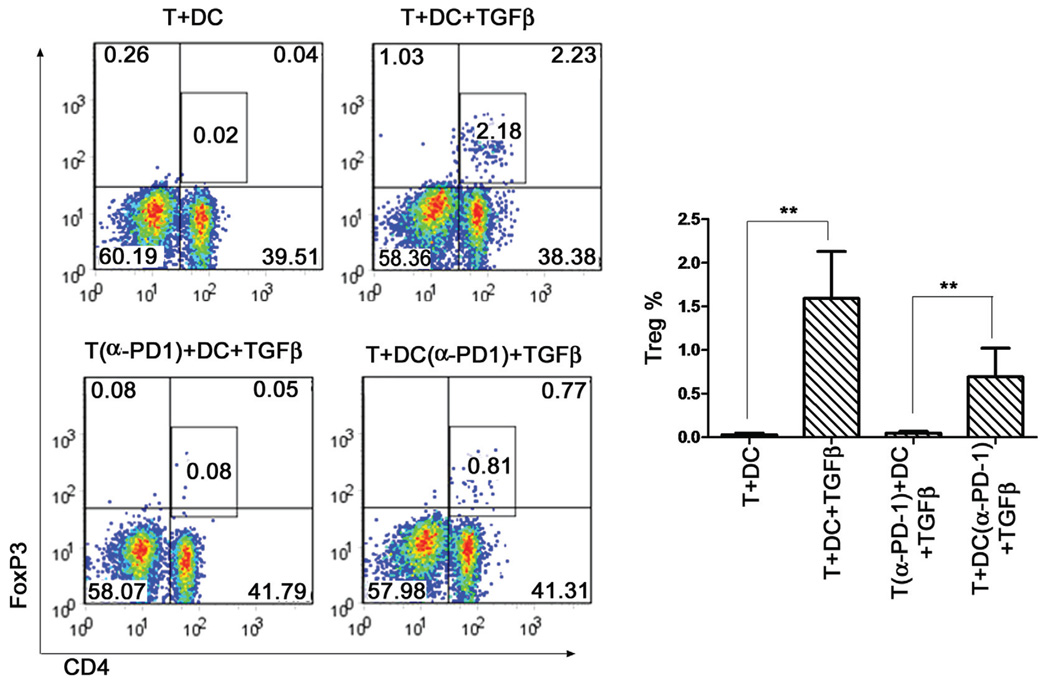
DC and TGFβ-Induced Treg Conversion Was Dependent on PD-1
It has been reported that PD-L1 signaling regulates the generation of adaptive Tregs in the periphery (Habicht et al., 2007; Wang et al., 2008). We therefore set up an in vitro conversion assay to test whether naive mMOG-35–55-specific CD4 T cells could be induced to become Treg cells and whether PD-1 was involved. As shown in Figure 3, DCs plus TGFβ induced a potent conversion from mMOG-35–55 peptide-specific naïve CD4+ T cells to Treg cells in the presence of mMOG-35–55 peptide (0.05% ± 0.05% to 1.6% ± 0.0.6% FoxP3+ cells; P < 0.01). Preincubation of naïve CD4 T cells with α-PD-1 mAb strongly reduced this conversion compared with preincubation of DC with the mAb (0.08% ± 0.03% vs. 0.75% ± 0.25% FoxP3+ cells; P < 0.01; Fig. 3). Thus, the newly generated Treg cells in mMOG-35–55 peptide/CFA-immunized WT mice without PTX are most likely adaptive Treg cells, whose generation depends on the presence of PD-1.
Fig. 3.
Neutralizing PD-1 in naïve mMOG-35–55 peptide-specific CD4 T cells, but not DCs, abolished the generation of adaptive Tregs in vitro. Naïve CD4 T cells (CD4+CD25−CD62L+) or DCs (CD11c+) were isolated by FACS and preincubated with anti-PD-1 (α-PD-1, NA/LE) or PBS. In total 50,000 naïve CD4 T cells and 30,000 DC (CD11c+) were cultured for 2 days with mMOG-35–55 peptide and IL-2, with and without TGFβ. The incubation groups included the following combinations: 1) CD4 T cells (preincubated with PBS), DCs (preincubated with PBS), and mMOG-35–55 peptide; 2) CD4 T cells (preincubated with PBS), DCs (preincubated with PBS), mMOG-35–55 peptide, and TGFβ; 3) CD4 T cells (preincubated with α-PD-1), DCs (preincubated with PBS), mMOG-35–55 peptide, and TGFβ; and 4) CD4 T cells (preincubated with PBS), DCs (preincubated with α-PD-1), mMOG-35–55 peptide, and TGFβ. At the end of incubation, cells were stained for CD4, FoxP3, and IgG. **P < 0.01 as measured by Student’s t-test; n = 4. The experiment was repeated twice.
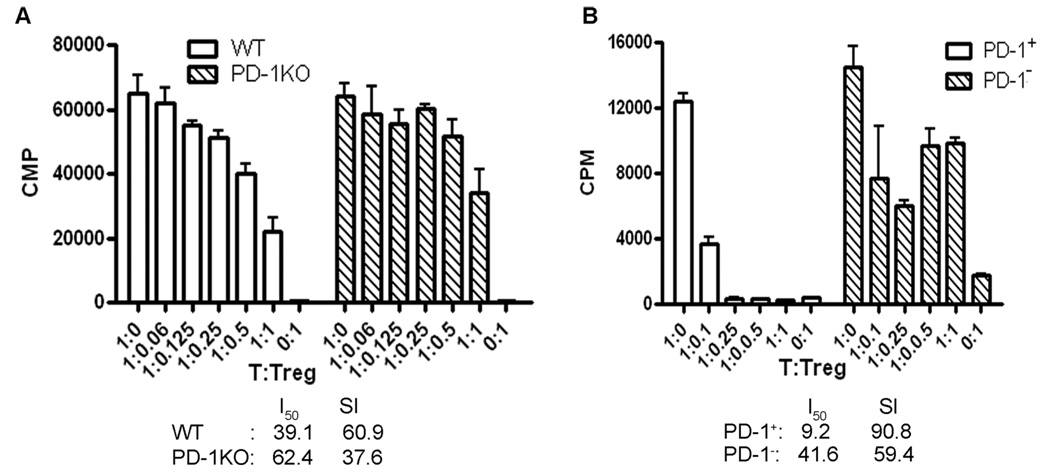
PD-1 Was Important for Treg Suppression
We next studied whether the expression level of PD-1 could be linked to the suppressive activity of CD4+FoxP3+ Treg cells. As shown in Figure 4A, Treg cells obtained from PD-1 KO mice were much less suppressive than those from WT mice. The suppressive indices (SIs) of PD-1KO and WT Treg cells were 37.6 and 60.9, respectively (higher SI indicates more suppression). On the other hand, FACS-sorted CD4+GFP+PD-1+ and CD4+GFP+PD-1− Treg cells from the spleens of FoxP3-GFP “knock-in” mice also showed different suppressive activities: PD-1+ Treg (SI = 90.8) were far more suppressive than PD-1− Treg (SI = 59.4) cells (Fig. 4B). Thus, down-regulation of PD-1 expression not only reduced the frequency but also affected the function of splenic CD4+FoxP3+Treg cells.
Fig. 4.
PD-1 expression was related to the suppressive activity of CD4+FoxP3+ Treg cells. A: PD-1 deficiency impaired the suppressive function of Treg cells from PD-1KO mice. B: CD4+PD-1+GFP+ Treg cells had greater suppression than CD4+PD-1−GFP+ Treg cells from FoxP3-GFP “knock-in” mice. CD4+FoxP3+ (in A) or CD4+PD-1+GFP+ and CD4+PD-1−GFP+ (in B) cells were sorted from splenocytes of immunized PD-1KO and WT B6 mice (in A) or FoxP3-GFP “knock-in” mice (in B). The suppressive activities of CD4+FoxP3+ cells from PD-1KO vs. WT mice or CD4+PD-1+GFP+ vs. CD4+PD-1−GFP+ from FoxP3-GFP “knock-in” mice were compared ex vivo by using the T-cell suppression assay. CD4+FoxP3− (in A) or CD4+GFP− (in B) cells from the same batch of mice were used as responder cells and T-cell-depleted splenocytes from naïve WT mice were used as APCs. The experiment was repeated twice with five mice per group in each experiment.
PD-1 Had No Effect on Passive EAE Induced by Activated T Cells
Our result showing that immunization with and without PTX induced similarly severe EAE in PD-1KO mice indicated that PD-1 may have little effect in restraining the encephalitogenicity of myelin-reactive T cells once they are activated. To reinforce this finding, we restimulated splenocytes and lymph node cells from mMOG-35–55 peptide/CFA-immunized GFP mice in vitro with mMOG-35–55 and transferred the activated cells back into naïve PD-1KO or WT recipients. As expected, passively transferred T cells caused EAE with similar severity in both strains (Fig. 5A). No differences were observed in the numbers of CD4+FoxP3+ (GFP−) recipient-derived cells in the two strains of mice (Fig. 5B). Moreover, donor Treg cells (GFP+) were not detected in recipient mice.
Fig. 5.
WT and PD-1KO recipient mice that received passively transferred mMOG-35–55 peptide-reactive T cells developed EAE with similar severities (A) and had similar numbers of CD4+FoxP3+ Treg cells (B). Spleen and LN cells were obtained from 15 female GFP mice immunized with mMOG-35–55 peptide/CFA for 8 days, restimulated in vitro for 2 days, and transferred at 20 × 106 blast cells/mouse by i.v. injection into WT or PD-1KO recipients (n = 6 for WT mice; n = 8 for PD-1KO mice). Recipients were euthanized 27 days after transfer to detect the frequency of CD4+FoxP3+ cells in both CD4+ T cells and whole-lymphocyte populations. The numbers of Treg cells in naïve WT and PD-1KO mice were also analyzed and included in the figure as baseline controls.
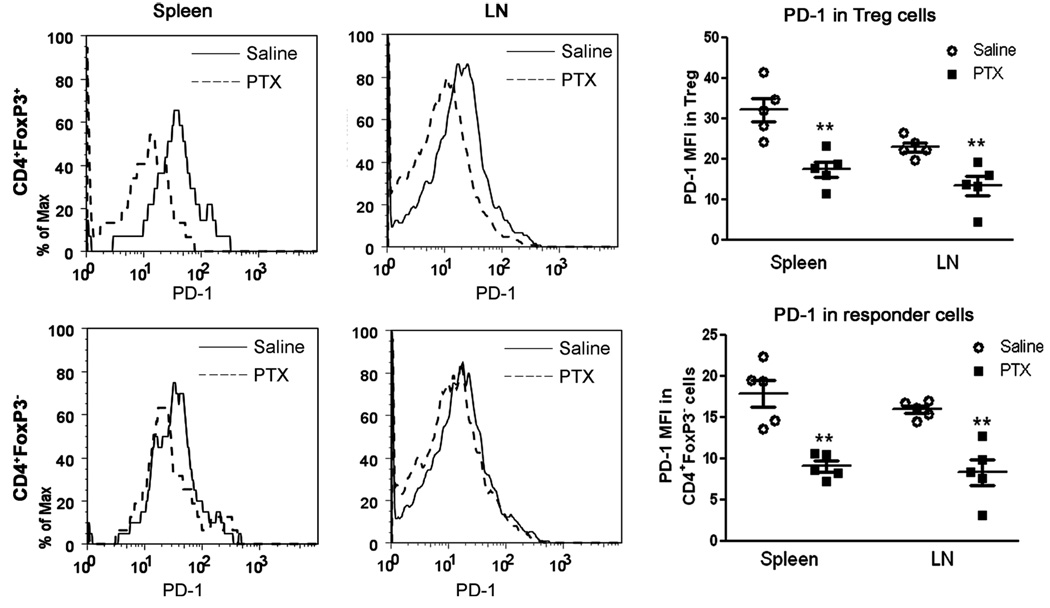
PTX Treatment Down-Regulated PD-1 Expression in Treg Cells
We next investigated whether PTX treatment can affect the expression level of PD-1 in WT mice developing EAE. Administration of PTX sharply reduced the expression of PD-1 in Treg cells in the spleen and LN harvested from WT mice euthanized 6 days after immunization with mMOG-35–55 peptide/CFA (Fig. 6) but not in naïve WT mice (data not shown). Thus, PTX treatment down-regulated PD-1 expression in Treg cells, but only in immunized mice. Insofar as PD-1 expression in CD4+FoxP3− cells was much (~50%) lower than that in CD4+FoxP3+ Treg cells, it is unlikely that its fluctuation would contribute significantly to EAE regulation, although it was further reduced by PTX (Fig. 6). Interestingly, PTX treatment (200 µg/ml) in vitro did not affect anti-CD3-stimulated up-regulation of PD-1 in Treg cells in cultured splenocytes (data not shown).
Fig. 6.
Administration of PTX reduced PD-1 expression in FoxP3+ and FoxP3− CD4 T cells. Six days after immunization with mMOG-35–55 peptide/CFA in the presence or absence of PTX, WT mice were euthanized, and the PD-1 expression levels in CD4+FoxP3+ and CD4+FoxP3− cells in both splenocytes and LN cells were analyzed. **P < 0.01 as measured by Student’s t-test (n = 5). The experiment was repeated twice.
DISCUSSION
PD-1 has been recognized as a crucial player in the development and maintenance of peripheral tolerance and immune homeostasis (Keir et al., 2007). However, little is known about the role of PD-1 in regulating onset and development of MS and EAE. In this study, we showed that EAE could be initiated in PD-1KO mice without facilitation by PTX, whereas administration of PTX prominently down-regulated the expression level of PD-1 in CD4+FoxP3+ Treg cells in the periphery of WT mice. In addition, we showed that PD-1 expression level was critical to maintaining the frequency and suppressive activity of Treg cells. It seems that down-regulation of PD-1 expression reduced the frequency and suppressive activities of Treg cells and thus permitted myelin-reactive T cells to launch an immune attack. Our in vitro data showed that PD-1 is critical for naïve CD4 T cells to convert to antigen-specific Treg cells. We can conclude, based on these results, that the PD-1 expression level is a critical checkpoint for the peripheral myelin-reactive T cells to initiate EAE and that PTX may enhance EAE induction by down-regulating PD-1 expression.
In the context of MS pathogenesis, this finding may have significant implications. It is assumed that infectious events, which are presented to the immune system as “danger signals,” may help to break immune tolerance and trigger CNS-directed attack from myelin-reactive T cells regularly circulating in the periphery (van Noort et al., 2000). Although several possible mechanisms were proposed, including molecular mimicry, bystander activation, epitope spreading, superantigenic activation of T cells, and activation of αB-crystallin-reactive T cells (van Noort et al., 2000; Wang et al., 2006), their existence in human disease has not been supported by solid evidence. We propose, based on our data from this study, that PD-1 may serve as an important link between microbial infection and onset of MS, insofar as PD-1 expression is essential for keeping myelin-reactive T cells in check and PTX can prominently down-regulate PD-1 expression. Our hypothesis is strengthened by the association of a PD-1 polymorphism with MS progression (Kroner et al., 2005). To draw a more concrete conclusion, however, we would have to measure the PD-1 expression in patients with different types of microbial infections and to determine whether PD-1 down-regulation or dysfunction activates myelin-reactive T cells in MS patients.
This study also revealed a novel mechanism for the disease-enhancing action of PTX. Classically, it is believed that PTX exacerbates EAE by increasing the permeability of the BBB (Linthicum et al., 1982; Bruckener et al., 2003). Recently, PTX was shown to enhance rolling and adhesion of activated T cells on pial vessels by inducing P-selectin expression in a TLR4-dependent manner (Racke et al., 2005; Kerfoot et al., 2006). PTX also decreased the frequency and function of Treg cells (Cassan et al., 2006; Chen et al., 2006) and the production of IL-6 and IL-10 in mast cells (Mielcarek et al., 2001). Finally, PTX increased both Th1 and Th2 responses (Ryan et al., 1998; Shive et al., 2000; Hofstetter et al., 2002) and inhibited chemokine-induced lymphocyte migration (Cyster and Goodnow, 1995; Alt et al., 2002). Our results identified PD-1 as a novel target of PTX, which regulated CD4+FoxP3+ Treg cells through regulation of PD-1. It remains to be determined how PTX-induced down-regulation of PD-1 is related to other known PTX actions, such as activation of TLR4.
PTX may not be the only microbial agent that regulates PD-1 on immune cells. It was shown previously that PD-1 expression on murine B cells was markedly reduced by signals associated with immune system danger, including lipopolysaccharide, CpG oligodeoxynucleotides, and several proinflammatory cytokines (Zhong et al., 2004; Okazaki and Honjo, 2007). On the other hand, a variety of microorganisms that cause chronic infections were shown to up-regulate PD-1 expression on CD8+ cells in individuals infected with chronic lymphocytic choriomeningitis, human immunodeficiency, and hepatitis C viruses (Barber et al., 2006; Day et al., 2006; Petrovas et al., 2006; Trautmann et al., 2006; Urbani et al., 2006; Okazaki and Honjo, 2007). To our knowledge, it has not been previously reported that a microbial agent reduced PD-1 expression on CD4+ T cells, and especially Treg cells. Whether PTX can change the PD-1 expression on other cell types and whether other microbial agents can regulate PD-1 expression on Treg cells are interesting topics currently being investigated.
The sources of increased number of Treg cells upon immunization challenge without PTX in WT were also investigated. Because thymic FoxP3+ cells were not changed, the Treg cells were likely generated in the periphery. Our in vitro conversion study clearly showed that TGFβ-activated DCs could induce a potent transition from naïve mMOG-35–55-specific CD4 T cells to Treg cells. This process was dependent on the presence of PD-1 in CD4 T cells, but not in DCs. Presumably, it is possible that immunization excluding PTX might facilitate the peripheral conversion by inducing production of TGFβ. As a result, EAE could not be initiated because of newly generated adaptive Treg cells. In PD-1KO or PTX-challenged WT mice, however, the conversion was interrupted by PD-1 deficiency or down-regulation. Consequently, EAE could be initiated.
Overall, we ahve shown that PD-1 is critically involved in EAE initiation by regulation of adoptive Treg-cell generation and activity. PTX may enhance EAE susceptibility by down-regulating PD-1. Our findings may advance the understanding of pathogenesis and immunoregulation in MS and may facilitate the development of novel therapeutic strategies for MS by targeting PD-1.
ACKNOWLEDGMENTS
The authors thank Paul Bui and William Beckman for providing excellent breeding service and animal care, Dr. Honjo at Kyoto University for providing PD-1KO mice, Drs. Rudensky and Ziegler for providing FoxP3-GFP mice, and Eva Niehaus for assistance in manuscript preparation.
Contract grant sponsor: NIH; Contract grant number: NS45445; Contract grant number: NS49210; Contract grant sponsor: National Multiple Sclerosis Society; Contract grant number: RG3405; Contract grant number: PP1295; Contract grant sponsor: The Nancy Davis Center Without Walls; Contract grant sponsor: Biomedical Laboratory R&D Service, Department of Veterans Affairs.
Footnotes
The authors declare that they have no competing financial interests.
REFERENCES
- Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32:2133–2144. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Baeten D, Jager A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckener KE, el BA, Galla HJ, Schmidt MA. Permeabilization in a cerebral endothelial barrier model by pertussis toxin involves the PKC effector pathway and is abolished by elevated levels of cAMP. J Cell Sci. 2003;116:1837–1846. doi: 10.1242/jcs.00378. [DOI] [PubMed] [Google Scholar]
- Cassan C, Piaggio E, Zappulla JP, Mars LT, Couturier N, Bucciarelli F, Desbois S, Bauer J, Gonzalez-Dunia D, Liblau RS. Pertussis toxin reduces the number of splenic Foxp3+ regulatory T cells. J Immunol. 2006;177:1552–1560. doi: 10.4049/jimmunol.177.3.1552. [DOI] [PubMed] [Google Scholar]
- Chen X, Winkler-Pickett RT, Carbonetti NH, Ortaldo JR, Oppenheim JJ, Howard OM. Pertussis toxin as an adjuvant suppresses the number and function of CD4+CD25+ T regulatory cells. Eur J Immunol. 2006;36:671–680. doi: 10.1002/eji.200535353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG, Goodnow CC. Pertussis toxin inhibits migration of B and T lymphocytes into splenic white pulp cords. J Exp Med. 1995;182:581–586. doi: 10.1084/jem.182.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, Sayegh MH, Guleria I. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179:5211–5219. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- Hemmer B, Cepok S, Nessler S, Sommer N. Pathogenesis of multiple sclerosis: an update on immunology. Curr Opin Neurol. 2002;15:227–231. doi: 10.1097/00019052-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Shive CL, Forsthuber TG. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund’s adjuvant: induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. J Immunol. 2002;169:117–125. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Kerfoot SM, Norman MU, Lapointe BM, Bonder CS, Zbytnuik L, Kubes P. Reevaluation of P-selectin and alpha 4 integrin as targets for the treatment of experimental autoimmune encephalomyelitis. J Immunol. 2006;176:6225–6234. doi: 10.4049/jimmunol.176.10.6225. [DOI] [PubMed] [Google Scholar]
- Kroner A, Mehling M, Hemmer B, Rieckmann P, Toyka KV, Maurer M, Wiendl H. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol. 2005;58:50–57. doi: 10.1002/ana.20514. [DOI] [PubMed] [Google Scholar]
- Kuchroo VK, Collins M, al Sabbagh A, Sobel RA, Whitters MJ, Zamvil SS, Dorf ME, Hafler DA, Seidman JG, Weiner HL. T cell receptor (TCR) usage determines disease susceptibility in experimental autoimmune encephalomyelitis: studies with TCR V beta 8.2 transgenic mice. J Exp Med. 1994;179:1659–1664. doi: 10.1084/jem.179.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum DS, Munoz JJ, Blaskett A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- Mielcarek N, Hornquist EH, Johansson BR, Locht C, Abraham SN, Holmgren J. Interaction of Bordetella pertussis with mast cells, modulation of cytokine secretion by pertussis toxin. Cell Microbiol. 2001;3:181–188. doi: 10.1046/j.1462-5822.2001.00106.x. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) Int Immunol. 2007;19:337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- Racke MK, Hu W, Lovett-Racke AE. PTX cruiser: driving autoimmunity via TLR4. Trends Immunol. 2005;26:289–291. doi: 10.1016/j.it.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Ryan M, McCarthy L, Rappuoli R, Mahon BP, Mills KH. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int Immunol. 1998;10:651–662. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Shive CL, Hofstetter H, Arredondo L, Shaw C, Forsthuber TG. The enhanced antigen-specific production of cytokines induced by pertussis toxin is due to clonal expansion of T cells and not to altered effector functions of long-term memory cells. Eur J Immunol. 2000;30:2422–2431. doi: 10.1002/1521-4141(2000)30:8<2422::AID-IMMU2422>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Up-regulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noort JM, Bajramovic JJ, Plomp AC, van Stipdonk MJ. Mistaken self, a novel model that links microbial infections with myelin-directed autoimmunity in multiple sclerosis. J Neuroimmunol. 2000;105:46–57. doi: 10.1016/s0165-5728(00)00181-8. [DOI] [PubMed] [Google Scholar]
- Waldner H, Whitters MJ, Sobel RA, Collins M, Kuchroo VK. Fulminant spontaneous autoimmunity of the central nervous system in mice transgenic for the myelin proteolipid protein-specific T cell receptor. Proc Natl Acad Sci USA. 2000;97:3412–3417. doi: 10.1073/pnas.97.7.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chou YK, Rich CM, Link JM, Afentoulis ME, van Noort JM, Wawrousek EF, Offner H, Vandenbark AA. AlphaB-crystallin-reactive T cells from knockout mice are not encephalitogenic. J Neuroimmunol. 2006;176:51–62. doi: 10.1016/j.jneuroim.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wang L, Pino-Lagos K, de VV, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Bai C, Gao W, Strom TB, Rothstein TL. Suppression of expression and function of negative immune regulator PD-1 by certain pattern recognition and cytokine receptor signals associated with immune system danger. Int Immunol. 2004;16:1181–1188. doi: 10.1093/intimm/dxh121. [DOI] [PubMed] [Google Scholar]