Abstract
Amyotrophic lateral sclerosis (ALS) is an incurable progressive paralytic motor neuron disease with limited therapeutic options. Since their creation by Gurney et al. (1994), transgenic SOD1G93A mice have become the benchmark pre-clinical model for screening ALS therapies. Surprisingly, despite physiological, anatomical, ultrastructural and biochemical evidence of early motor system dysfunction it has proven difficult to detect motor performance deficits in pre-symptomatic SOD1G93A mice. As an alternative to conventional forced motor tests, we investigated the progression of motor performance deficits in freely behaving pre-symptomatic congenic B6.SOD1G93A mice. We found that motor performance deficits began several weeks prior to the onset of overt clinical symptoms (postnatal day 45). More importantly, once motor performance deficits manifested, they persisted in parallel with disease progression. In addition, two physical measures of muscle girth revealed progressive hindlimb muscle atrophy that predicted genotype in individual pre-symptomatic mice with 80% accuracy. Together, these data suggest that muscle girth is a reliable and indirect measure of hindlimb muscle denervation and an early, objective marker for disease onset in congenic B6.SOD1G93A ALS mice. Moreover, we present regression equations based on hindlimb muscle girth for predicting genotype in future studies using B6.SOD1G93A mice. These findings support new objective criteria for clinical disease onset and provide objective measures that require little expertise. These studies demonstrate a cost-effective approach for more thorough evaluation of neuroprotective strategies that seek to disrupt disease mechanisms early in the disease process. To our knowledge, these findings are the first to report early chronic motor performance and physical deficits that are coincident with the earliest known motor dysfunction in any ALS mouse model.
Keywords: amyotrophic lateral sclerosis, mutant SOD1, behavioral testing, motor neuron disease, early motor deficits, open field, discriminant analysis
Amyotrophic lateral sclerosis (ALS) is an incurable motor neuron disease characterized by the degeneration of upper and lower motor neurons (Mulder et al., 1982). Due to the progressive nature of neurodegeneration, early signs and symptoms of ALS can be subtle and go unnoticed or mistaken as other illnesses thus losing a valuable therapeutic window (Mulder et al., 1976; Garbuzova-Davis and Sanberg, 2009). Transgenic mice that carry missense mutations in the Cu2+/Zn2+ superoxide dismutase-1 gene (SOD1) remain the standard pre-clinical model for screening treatments that seek to reverse the progressive loss of motor neurons in the cortex, brainstem and spinal cord (Gurney et al., 1994; Turner and Talbot 2008). Recently, congenic SOD1G93A mice have been generated and display the most reproducible phenotype of any ALS SOD1G93A model allowing for more thorough evaluation of therapeutic interventions (Heiman-Patterson et al., 2005).
Mutant SOD1 mice show functional and physical deficits that manifest from the progressive deterioration of the motor nervous system (Gurney et al., 1994). One motivation for the present study stems from observations that traditional measures have proven inadequate for detecting chronic motor performance and physical deficits in pre-symptomatic mice regardless of the background strain used to carry the mutant SOD1 transgene. This is surprising given the overwhelming biological evidence showing early motor system dysfunction occurring well before the onset of clinical symptoms (Wong et al., 1995; Mourelatos et al., 1996; Kennel et al., 1996; Frey et al., 2000; Shefner et al., 2001; Amendola et al., 2004; Fischer et al., 2004; Kirkinezos et al., 2005; Browne et al., 2006; Durand et al., 2006; Gould et al., 2006; Hegedus et al., 2007; Niessen et al., 2007). One possibility for the delayed clinical phenotype may be over-activation of striatal cholinergic neurons (Azzouz et al., 1999). Another possibility is axon regeneration, a phenomena that has been reported following partial muscle denervation such that axonal sprouting from nearby intact motor neurons can go on to form new synaptic connections with denervated muscle fibers (Son and Thompson 1995a, 1995b; Kang and Tian et al., 2003; Love et al,. 2003; Lubischer and Thompson, 1999). Evidence for axon regeneration has been reported in mutant SOD1 hindlimb muscles (Gurney et al., 1994; Fischer et al., 2004; Schaefer et al., 2005). Although biologically relevant, this compensatory action likely renders diagnosis difficult, the consequence being that by the time regenerative mechanisms become overwhelmed and overt symptoms manifest, the toxic buildup of mutant SOD1 within cells has put in motion an irreversible set of pathogenic events. Lastly, conventional tests used most often in the past 15 years for evaluating mutant SOD1 mice are not sensitive enough to detect subtle behavioral phenotypes that occur early in the disease process.
Here, we have taken an alternative approach for describing disease onset and progression in mutant ALS mice. In addition to conventional forced exercise tests (rotarod, hanging grip, treadmill) and body weight we included tests that measured motor performance in freely behaving B6.SOD1G93A mice. We also examined shaved hindlimbs for evidence of muscle atrophy as an indirect measure of the effects of mutant SOD1G93A overexpression on hindlimb muscle denervation. This approach allowed us to demonstrate for the first time chronic motor performance and physical deficits in pre-symptomatic congenic B6.SOD1G93A mice ~45 days prior to tremor onset, to predict genotype in asymptomatic mice, and to follow the progression of changes from postnatal day 45 until the animals are moribund.
EXPERIMENTAL PROCEDURES
B6.SOD1G93A transgenic Mice
Experiments were performed in accordance with NIH guidelines for the use of experimental animals and approved by the University of Texas Institutional Animal Care and Use Committee. Animals were provided food and water ad libitum and housed on a 12:12 hr light cycle (on at 7 am). A single male B6.Cg-Tg[SOD1-G93A]1Gur/J, hereafter referred to as B6.SOD1G93A, was purchased from The Jackson Laboratory, Bar Harbor, Maine (stock# 004435). These mice over-express a point mutated form of the human SOD1 gene (single amino acid substitution of glycine to alanine at codon 93) driven by the endogenous human promoter. Offspring hemizygous for the mutant SOD1 transgene used in this study were produced by strict crossings of hemizygous male B6.SOD1G93A mice with wildtype C57BL/6 inbred females to maintain the mutant transgene on a C57BL/6 congenic background (>N10). Isolated genomic tail DNA was used to genotype animals using a standard protocol provided by The Jackson Laboratory.
The number of mice used for each test was as follows: open field, body weight and paw grip endurance measures for wildtype (WT) females n = 46, males n = 55; B6.SOD1G93A females n = 35, males n = 51. Weight measures WT females n = 51, males n = 56; B6.SOD1G93A females n = 36, males n = 55. Survival and disease onset WT females n = 58, males n = 55; B6.SOD1G93A females n = 40, males n = 53. Muscle girth measures WT females n = 17, males n = 29; B6.SOD1G93A females n = 16, males n = 25. Rotarod measures WT females n = 20, males n = 16; B6.SOD1G93A females n = 10, males n = 12.
Open field activity
Two open field 50×50×50 cm acrylic boxes each fitted with 3 LED light bar sets made of infrared emitters (IR) and detectors were used to monitor motor activity. For motion detection in the X,Y plane two IR/detector light bar sets were arranged such that IR light beams generated a 5.0 cm2 grid-like pattern 2 cm above the floor of the box. To detect behavior in the Z-dimension a third IR/ detector light bar set was placed 6.5 cm above the floor such that the head of the mouse broke the beams when they reared on their hindlimbs. The IR beams for the Z-dimension were 2.5 cm apart. Hardware consisted of a power source (SG-506) a 48 channel IR controller (ENV-520) and activity monitor software (Med Associates, St. Albans, VT). On testing days, animals were transported to a 25 degree Celsius, soundproofed and dimly lit (~85 lumens / m3 non-fluorescent bulb) room starting at 10 am. Testing followed after a 10 min acclimation period. Open field measures included average velocity of locomotion in the X, Y planes, rearing counts (vertical beam breaks), ambulatory distance, and resting time. Dependent variables were automatically scored by computer using Med Associates software.
Rotarod
Performance testing was conducted on a Series 8 rotarod (IITC Life Sciences, Woodland Hills, CA) that housed 5 partitioned 5 cm diameter aluminum cylinders. Because of their smooth surface the cylinders were covered with a paper-thin textured plastic. A ramped accelerating program was used such that during the first 120 s the cylinder accelerated from 5-18 rpm, then held at 18 rpm for the final 60 s and longest latency to fall over two trials was used for analysis.
Paw grip endurance test (PaGE)
Using a 1 cm2 stainless steel mesh grid, mice were suspended upside down for 180 s and longest latency to fall over 2 trials was kept for analysis. On the 1st trial, mice fell into a bath of 25 degree Celsius tap water as a motivational tool for non-performers.
Muscle measurements
For muscle girth measures, animals were lightly anesthetized via nose-cone administration of isoflurane gas anesthesia. Animals were placed at a slight angle belly-side up on a water-heated stainless steel platform to give easy access to the hindlimbs. Using small animal clippers, the entire left rear hindlimb was shaved smooth from the ankle to the upper thigh allowing the accurate measure of calf and thigh muscle girth. Under well lighted conditions, with the leg in a relaxed position and without pulling or pushing on the leg three measures of muscle girth were taken: 1) calf (dorsal/ventral) - from the middle of the tibia to the belly of the calf, 2) calf (medial/lateral) - from the medial to the lateral edge of the calf muscle and 3) thigh - a measure that spanned the thickness of the thigh medial to lateral as close as possible just above the femur-pelvic joint. In general, animals were anesthetized for no longer than 3-4 min. Open field, grip test, muscle girth and body weight measures were taken every 10th day beginning at postnatal day (P) 45 whereas rotarod performance began at P55.
Clinical symptom onset and survival
Clinical symptom onset was determined by the presence of fine motor tremors in one or both hindlimbs when suspended by the tail and upon the consensus of three blinded observers who monitored mice daily beginning at P65. When muscle weakness became pronounced, pulverized food was placed at the bottom of the cage along with angled spout water bottles that allowed continuous water access. For ethical reasons, mice were considered moribund and sacrificed when they failed to right themselves within 20 s of being placed on their side (Boillee et al., 2006).
Statistical analysis
Apart from 2 animals that were wounded by littermates, all data from all animals were analyzed. Kaplan-Meier analysis was performed to determine disease onset and survival times. To find significant differences between wildtype and B6.SOD1G93A mice that emerged across time (P45-P125), a repeated-measures ANOVA was performed with genotype (wildtype and B6.SOD1G93A) as a between-subject variable and the within-subjects variables were time and either open field, muscle girth, weight, paw grip endurance or rotarod measures. To determine the age at which significant group differences emerged we performed simple-effects tests on all measures for male and female mice every 10 days between P45-P95. To protect against the increased likelihood of a type-I error, all repeated measures analyses were Bonferroni corrected for multiple comparisons. To determine the diagnostic accuracy of each behavioral test and physical measure, a receiver-operated-characteristics (ROC) curve using 95% confidence intervals was calculated for each dependent variable. To determine the variable or combination of variables that best predicted genotype before the onset of overt clinical symptoms a stepwise discriminant analysis of all variables measured was performed. Stepwise selection was based on minimizing Wilks' lambda, leave-one-out classification and F value entry = 3.84, F value removal = 2.71. For all analyses a p≤0.05 significance level was used and data were analyzed with SPSS 11.5 software.
RESULTS
Delayed tremor symptom onset in female B6.SOD1G93A mice
In B6.SOD1G93A mice, the first clinical symptoms of motor neuron disease presented as fine tremors in one or both hindlimbs when suspended by the tail (Chiu et al., 1995). Kaplan-Meier analysis revealed that tremor onset occurred significantly sooner in male (91 ± 2 days) compared to female (100 ± 2 days) B6.SOD1G93A mice, (p≤0.01) (Table 1). Evidence of tremor was undetected at any age in control mice. Similar to previously published results (Heiman-Patterson et al., 2005), Kaplan-Meier analysis revealed no significant differences in survival times between male (153 ± 2 d) and female (161 ± 2 d) B6.SOD1G93A mice (Figure 1). These results showed that while disease symptom onset was delayed in female B6.SOD1G93A mice, disease progression was similar between male and female B6.SOD1G93A mice.
Table 1.
Kaplan-Meier tremor onset and survival analysis in B6.SOD1G93A mice.
| Tremor Onset (days) ± SEM | Survival (days) ± SEM | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Mean | 91±2 d | 100±2 d | 153±2 d | 161±2 d |
| 95% confidence interval | 87-94 d | 95-104 d | 149-158 d | 158-164 d |
| Log Rank Significance | p=.003 | p=.082 | ||
Figure 1. Delayed clinical symptom onset in female B6.SOD1G93A mice.
Kaplan-Meier curves to compare tremor onset and survival time (moribund animals unable to right themselves within 20 s) between male and female B6.SOD1G93A mice. Clinical symptom onset (tremor in at least 1 hindlimb upon tail suspension) was delayed ~10 days in females (n=39) compared to male mice (n=49). No significant difference in survival time between male and female B6.SOD1G93A mice was observed.
Early chronic motor performance deficits in pre-symptomatic B6.SOD1G93A mice
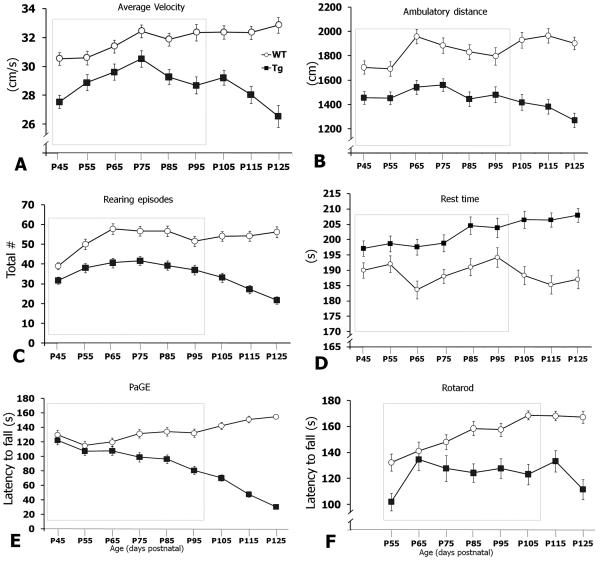
Conventional tests like the rotarod, paw grip endurance (PaGE), hanging wire, loaded grid and treadmill gait analyses have proven insensitive for detecting functional deficits that are coincident with the early pathophysiological and histopathological motor defects in mutant SOD1 mice. We hypothesized that before tremor onset, B6.SOD1G93A mice would exhibit motor performance deficits when allowed to roam freely versus forced motor behavior tests. To investigate the likelihood that freely behaving B6.SOD1G93A transgenic mice displayed motor-related deficits during the pre-symptomatic phase, animals were placed in an open field apparatus for 5 min every 10th day beginning at postnatal day 45 to postnatal day 125 (P45-P125). In general, performance of B6.SOD1G93A transgenic mice on all open-field measures was below that of control mice (Figure 2). Compared to control mice, B6.SOD1G93A mice showed significant deficits across time (P45-P125) on all motor performance evaluations including average locomotion velocity, ambulatory distance, rearing episodes, resting time, PaGE and rotarod (Figure 2). Analysis of males and females separately also showed significant deficits across time (P45-P125) on all motor tests (data not shown).
Figure 2. Progression of motor deficits in B6.SOD1G93A mice.
Line graphs showing time course of disease progression from motor tests. Animals were monitored every 10 d from P45-P125 except for rotarod (P55-P125). For simplicity, all motor performance graphs for wildtype (WT, open circles) and B6.SOD1G93A (Tg, black squares) were collapsed across sex. (A-D) Open field measures. (E) Paw grip endurance (PaGE) test and (F) Rotarod. The dashed box indicates the time points for post hoc statistical analysis of male and female mice (see Table 2).
To further demonstrate the specific time point when motor deficits were present, Bonferroni-corrected simple-effects tests were performed on control and B6.SOD1G93A male and female mice at each time point from P45 to the age of tremor symptom onset at ~P95. The simple-effects tests allowed us to determine the age when motor performance deficits manifested and whether these deficits remained present up to the onset of tremor symptoms.
Beginning at P45, male B6.SOD1G93A mice showed significant motor deficits in average locomotion velocity, ambulatory distance, rearing episodes and resting time (Table 2). Not only did these motor deficits manifest 45 d prior to the onset of tremor symptoms, they persisted at each subsequent time point up to and beyond the onset of overt clinical symptoms (Table 2). Moreover, all four open-field performance measures in males detected significant group differences at 20 d and 60 d earlier than the conventional PaGE (P65) or rotarod (P105) tests, respectively (Table 2).
Table 2.
B6.SOD1G93A mice show early chronic motor performance deficits.
| Males | ||||||
|---|---|---|---|---|---|---|
| Age | P45 | P55 | P65 | P75 | P85 | P95 |
| Ave. Velocity (cm/s) | ** | ** | * | * | ** | ** |
| Ambulatory distance (cm) | ** | ** | ** | ** | ** | ** |
| Rearing episodes (total #) | ** | ** | ** | ** | ** | ** |
| Resting time (s) | * | * | ** | ** | ** | * |
| Paw grip endurance (s) | * | ** | ** | ** | ||
| Rota rod (s) | n.d. | |||||
| Calf girth dorsal/ventral (cm) | * | ** | ** | ** | ** | |
| Calf girth medial/lateral (cm) | ** | ** | ** | ** | ||
| Thigh girth medial/lateral (cm) | * | ** | ** | ** | ||
| Weight (g) | ** | ** | ** | ** | ||
| Females | ||||||
| Age | P45 | P55 | P65 | P75 | P85 | P95 |
| Ave. Velocity (cm/s) | ** | ** | ** | ** | ** | |
| Ambulatory distance (cm) | * | |||||
| Rearing episodes (total #) | * | ** | ** | ** | ** | |
| Resting time (s) | ||||||
| Paw grip endurance (s) | * | ** | ||||
| Rotarod (s) | n.d. | * | * | ** | ** | |
| Calf girth dorsal/ventral (cm) | ** | ** | ** | ** | ** | |
| Calf girth medial/lateral (cm) | * | ** | ** | ** | ||
| Thigh girth medial/lateral (cm) | ** | ** | ** | |||
| Weight (g) | ** | ** | ** | ** | ||
Table depicting significant simple-effects comparisons on control and B6.SOD1G93A male and female mice. Physical measures of muscle girth and body weight (shaded), motor performance measures (not shaded). All measures were Bonferroni-corrected for repeated measures.
A single star denotes significance level at p ≤ 0.05
a double star denotes significance level at p ≤ 0.01.
Rotarod was not determined (n.d.) at P45 and it showed a significant effect in males from P105 on (not shown).
Female B6.SOD1G93A mice showed an early transient deficit in average velocity until P65 when mice remained significantly slower up to and beyond the onset of hindlimb tremors at P100 (Table 2). More importantly, similar to males, female B6.SOD1G93A mice showed a persistent deficit in rearing episodes beginning 45 d prior to the onset of overt clinical symptoms (Table 2).
Overall, these data showed that motor performance deficits manifested several weeks prior to the onset of overt clinical symptoms in pre-symptomatic, freely behaving B6.SOD1G93A mice. Secondly, open-field measures of average locomotion velocity, ambulatory distance, rearing episodes and resting behavior were more sensitive to the effects of mutant SOD1G93A overexpression than PaGE or rotarod. Interestingly, female B6.SOD1G93A mice showed transient or no deficits in ambulatory distance and rest behaviors, respectively. Significant chronic deficits in average velocity and PaGE were also delayed compared to male B6.SOD1G93A mice (Table 2).
Early chronic deficits in hindlimb muscle girth in pre-symptomatic B6.SOD1G93A mice
Several studies have shown loss of functional motor units and muscle denervation in gastrocnemius muscle from P40-P50 mutant SOD1G93A mice (Kennel, et al., 1996; Frey et al., 2000; Hegedus et al., 2007). To determine the extent of hindlimb muscle atrophy in living animals and whether these changes could be used as an indirect measure of muscle denervation, we took repeated measures of hindlimb muscle girth from calf muscles in the dorsal/ventral and in the medial/lateral planes (i.e. calf (d/v) and calf (m/l)) and one measure of thigh muscle girth in the medial/lateral plane with Vernier calipers every 10 d beginning at P45. In general, all three measures of muscle girth were consistently larger in both male and female wildtype mice (Figure 3). Statistical analysis averaged across time (P45-P125) revealed that compared to control mice all three hindlimb muscle measures were significantly smaller in B6.SOD1G93A mice (Figure 3). To further demonstrate the specific time point when muscle atrophy appeared we conducted Bonferroni-corrected simple-effects tests. Both male and female B6.SOD1G93A mice showed atrophy in calf (d/v) muscles at P55 (Figure 3 and Table 2). Male and female B6.SOD1G93A mice also showed group differences in body weight beginning at P65 (Figure 3 and Table 2). Together, changes in muscle girth were a sensitive indirect measure of hindlimb muscle denervation in pre-symptomatic B6.SOD1G93A mice.
Figure 3. Progression of muscle size and body weight deficits in B6.SOD1G93A mice.
Line graphs showing time course of disease progression from physical measures. Animals were monitored every 10 d from P45-P125. For clarity, all muscle girth graphs for each group (wildtype and B6.SOD1G93A) were collapsed across sex (A-C). The dashed box indicates the time points for post hoc statistical analysis of male and female mice (shaded region in Table 2).
Hindlimb muscle girth predicts genotype in pre-symptomatic mice
While open field and muscle girth showed early and chronic deficits we wanted to know the likelihood that any of the measures used in this study could actually predict genotype. To determine whether genotype could be predicted in individual pre-symptomatic animals a stepwise discriminate analysis of all dependent variables in this study was performed at each 10 d time point from P45 to P125. Interestingly, only two variables, calf (d/v) and thigh muscle girth, were able to predict genotype in pre-symptomatic animals with 80% or greater accuracy (Figure 4). Further analysis revealed that by P55 (45 d prior to tremor symptom onset) genotype was predicted in 82% of female mice based on muscle girth (29 out of 33 subjects) (X2(2) = 15.4, p < 0.001), whereas genotype was predicted in 83% of males by P65 (45 out of 54 subjects) (X2(2) = 36.4, p < 0.001) (Figure 4). Furthermore, this analysis revealed that at P55, female mice with calf (d/v) muscles smaller than 0.80 cm in girth were 10 times more likely to carry the SOD1G93A transgene. Males, on the other hand, were 4.9 times more likely to carry the SOD1G93A transgene if at P65 calf (d/v) muscle girth was less than 0.88 cm. Taken together, physical measures of muscle girth reliably predicted genotype in individual pre-symptomatic B6SOD1G93A mice (~ 40 d prior to tremor onset). Importantly, these data also supported earlier data that muscle girth was a reliable indirect marker of muscle denervation.
Figure 4. Muscle girth predicts genotype in pre-symptomatic SOD1 G93A mice.
Line graph showing at each time point the percentage of animals correctly classified as wildtype or transgenic based on calf (d/v) and thigh muscle girth. Notice that 27out of 35 females can be correctly g by P55 whereas 43 out of 54 males can be correctly classified at P65.
Diagnostic accuracy of physical and motor measures
Since tests revealed different time courses in disease progression (e.g. deficits in rearing episodes occurred much earlier than deficits in rotarod performance), receiver-operating-characteristic (ROC) curves were used to provide a measure of the accuracy that each test can indeed correctly indentify phenotypic differences between groups. ROC curves have been used in recent studies to evaluate the diagnostic accuracy of dependent measures including body weight, rotarod and PaGE in SOD1G93A mice (Weydt, et al., 2003; Miana-Mena, et al., 2005). The ROC curve was constructed by plotting the true positive rate ((true positives ÷ (true positives + false negatives)) as a function of the true negative rate ((true negatives ÷ (true negatives + false positives)). An ideal test has a diagnostic accuracy of 1 (100%) in which case each level of the dependent variable (e.g. each muscle girth value from smallest to largest) can serve as a criterion that can accurately detect all true positives while excluding false positives and simultaneously detecting all true negatives while excluding false negatives. A poor test has the same diagnostic accuracy as a coin toss (50%). Therefore, we determined the diagnostic accuracy of all tests at each time point from P45 to P125 in male and female mice. Tests were considered diagnostically accurate when test accuracy was ≥ 0.80 for three consecutive time points. In both male and female mice, calf (d/v) muscle girth was diagnostically accurate from P55 and increased over time to greater than 95% accuracy by P75 (Figure 5A; 5B). Similar results were observed for calf (m/l) and thigh muscle from P65 on (Figure 5A; 5B). The next best accurate tests were body weight followed by rotarod and PaGE, reaching 80% diagnostic accuracy at ~P115 in males and females, well after tremor symptom onset (Figure 5C; 5D). Interestingly, few open field measures ever reached 80% diagnostic accuracy.
Figure 5. Phenotypic diagnostic accuracy in muscle girth but not motor performance measures.
Receiver-operated-characteristics (ROC) curves to determine test accuracy were generated for each time point from P45 to P125 for all physical measures (A and B) and motor performance tests (C and D). Notice how rapidly the phenotypic diagnostic accuracy for calf (d/v) muscle girth measures reaches 80% in pre-symptomatic males and females (A and B) and is quickly matched by calf (m/l) and thigh at P65. While motor tasks are not as diagnostically accurate as muscle girth measures (C and D), in general, they are more accurate for males versus female mice. Shaded region indicates high diagnostic accuracy.
Taken together, these results provided further evidence for asynchronous progression of motor system dysfunction in B6.SOD1G93A ALS mice. Moreover, these data supported our findings that muscle girth was an accurate predictor of genotype and a reliable indirect measure of hindlimb muscle denervation in pre-symptomatic B6.SOD1G93A mice.
DISCUSSION
The evaluation of treatment efficacy in mouse models of ALS has traditionally relied on forced exercise tasks (e.g. rotarod, paw grip endurance, treadmill, gait analysis) (Gurney et al., 1994; Kong et al., 1998; Weydt et al., 2003; Fischer et al., 2004; Wang et al., 2008). However, this battery of tests has proven insensitive at detecting motor deficits prior to the onset of overt clinical symptoms despite ultrastructural, histological, electrophysiological and metabolic evidence that have demonstrated early motor system defects in pre-symptomatic mutant SOD1 mice (Wong et al., 1995; Mourelatos et al., 1996; Kennel et al., 1996; Frey et al., 2000; Shefner et al., 2001; Amendola et al., 2004; Fischer et al., 2004; Kirkinezos et al., 2005; Browne et al., 2006; Durand et al., 2006; Gould et al., 2006; Hegedus et al., 2007; Niessen et al., 2007). One possibility for the delayed clinical phenotype may be that axon regeneration (Chiu et al., 1995; Kennel et al., 1996; Frey et al., 2000; Schaefer et al., 2005; Pun et al., 2006), or over-activation of cholinergic neurons in the basal ganglia can compensate for early deficits in muscle function (Azzouz et al., 1999). With age, however, mutant SOD1 and the toxic sequelae that accompany its accumulation may overwhelm these compensatory mechanisms leading to the rapid degeneration of motor neurons. Another likely possibility for the inability to detect chronic pre-symptomatic behavioral deficits may be the heterogeneous genetic background of mouse strains used to maintain the mutant SOD1G93A transgene.
To date, there have been no reports of motor performance deficits in ALS mouse models that 1) were coincident with the earliest known age when muscles are known to be compromised and 2) followed disease progression once pre-symptomatic deficits manifest. Therefore, we sought to identify chronic motor performance and physical deficits in pre-symptomatic mutant congenic B6.SOD1G93A transgenic mice. This study demonstrated the earliest known chronic motor performance and physical deficits in pre-symptomatic congenic B6.SOD1G93A mice from P45 (~45 d prior to tremor onset) until mice were considered moribund.
Delayed clinical symptom onset in female congenic B6.SODG93A mice
Lifespan in mutant SOD1G93A mice has been found to be gender and genetic background dependent (Veldink et al., 2003; Heiman-Patterson et al., 2005). Our results replicated previous studies using the same congenic B6.SOD1G93A line (Wooley et al., 2005; Haenggeli et al., 2007) and showed the average survival time for males and females was ~158 d with no significant gender differences.
Sex differences in disease onset have been reported in both low and high copy number mutant SOD1G93A mice (mixed B6/SJL background) with female onset occurring later than males (hind paw extension reflex) (Veldink et al., 2003). Whereas others have reported no sex differences in tremor onset in congenic B6.SOD1G93A mice (Wooley et al., 2005; Heiman-Patterson et al., 2005) we found that tremor onset was delayed ~10 d in female B6.SOD1G93A mice. In addition, the study by Wooley at al. (2005) reported tremor onset at 143 ± 10 d whereas we found that tremor onset occurred much earlier in both males (91 ± 2 d) and females (100 ± 2 d). The simplest explanation for these discrepancies may be observer variability. Despite the fact that tremor onset in this study was defined as the consensus from the same 3 trained/blinded individuals, the subjective nature of the examination should be cause for concern when attempting to reproduce results between different observers or laboratories. Further compounding the problem may be the use of this subjective measure when evaluating the efficacy of nutraceutical, drug, growth-factor, gene transfer, cell replacement and stem cell interventions (Koh et al., 2006; Nagano et al., 2005; Azzouz et al., 2004; Zhao et al., 2007; Lunn et al., 2009). However, in light of our results that showed early detection of chronic motor performance and physical deficits in pre-symptomatic mice, we suggest new objective criteria for determining clinical onset and the evaluation of therapeutic interventions in congenic ALS mouse models.
Chronic motor performance deficits in pre-symptomatic B6.SOD1G93A mice
Early therapeutic interventions are vital for disrupting the progression of neurodegenerative disorders. Sensitive behavioral assays for the early detection of disease states not only facilitates treatment efficacy but helps further our understanding of the mechanisms that initiate the disease process.
An in-house study by the commercial vendor (The Jackson Laboratory) using computerized treadmill gait analysis found modified gait patterns at ~P55 and later at ~P70 (Wooley et al., 2005). However, in the former study these gait deficits were not investigated after P70 even though they reported the onset of clinical symptoms at P142 ± 10 d. Nonetheless, the gait deficits described by the commercial vendor could not be reproduced in a later study (Guillot et al., 2008). Others reported that treadmill performance declined from ~P70 in B6/SJL SOD1G93A mice but the authors stated that these effects may be due to muscle fatigue caused by forced exercise (Kirkinezos et al., 2003). Another study reported transient reflex deficits between the first and second postnatal weeks (Amendola et al., 2004).
This study is the first to demonstrate sustained functional motor deficits in the pre-symptomatic disease phase of any mutant ALS rodent model. Freely behaving male B6.SOD1G93A mice showed significant motor performance deficits beginning at P45 (~45 d before clinical tremor onset). These motor deficits were also coincident with the earliest reports of hindlimb muscle denervation (Wong et al., 1995; Kennel et al. 1996; Frey et al., 2000; Fischer et al., 2004; Hegedus et al., 2007). Moreover, after their early detection, these functional deficits were chronic and advanced in parallel with disease progression up to and beyond clinical disease onset until death. A similarity between our study and Smittkamp et al. (2008) was that ambulatory distance was less affected in freely behaving female mice. Although they reported only on female mice, this similarity supports the use of open field measures, as shown in this study, to monitor motor performance in ALS mouse models.
Body weight
A recent study by Smittkamp et al. (2008) suggested that orolingual motor deficits may affect feeding behavior and consequently the ability of mutant SOD1 mice to gain weight. Several reports suggested that a 10% decline in peak body weight can be used as an objective measure of disease onset in mutant ALS mice (Wong et al., 1995; Liu et al., 2005; Boillee et al., 2006; Lobsiger et al., 2009). However, there has been a varied range of weight differences reported for mutant SOD1 mice. Some reported persistent body weight differences at ~P75 (Chiu et al., 1995) or near tremor symptom onset at ~P100 (Weydt et al., 2003) or in late-stage disease at ~P125 (Fischer et al., 2004; Tankersley et al., 2007), whereas others reported that male and female mutant SOD1 mice weighed significant less from P14 to death (Miana-Mena et al., 2005). But the last study presented several inconsistencies that warrant caution: background strain was not mentioned; their methods stated that only heterozygous mutant SOD1G93A mice were used but they reported on homozygous animals and finally the analyses of the repeated measures design for weight were not corrected for multiple comparisons.
Our results supported previous findings (Smittkamp et al., 2008; Azari et al., 2003) that weight gain in both male and female congenic B6.SOD1G93A mice reached a plateau from ~P55-P125. Although both sexes weighed significantly less than controls from P65-death, this was due to the continued weight gain of control mice rather than weight loss in transgenic mice. We did eventually see a rapid decrease in body weight typical of other mutant SOD1 lines during disease end-stage when B6.SOD1G93A mice were almost paralyzed and near death (~P135-P155). These results argue that weight loss is not a sensitive measure of disease onset or progression in congenic B6.SOD1G93A mice.
Rotarod performance
Our results showed that while the ability of B6.SOD1G93A mice to maintain balance on a rotarod apparatus was less than controls, group differences in males did not appear until P105 (data not shown) whereas in females chronic deficits appeared at P75. Others reported similar results for overall deficits in rotarod performance but significant group differences in females showed up at P113 (Smittkamp et al., 2008) or P95 (Miana-Mena et al., 2005). These discrepancies may reflect different ramping protocols, the make, model and diameter of the rotating cylinders and the genetic background of mutant SOD1G93A mice in each study. While rotarod performance may be a good tool for evaluating therapeutic efficacy on balance and strength late in the disease process, our results suggest that it is not a sensitive measure of disease onset in ALS mice.
Paw grip endurance
We found chronic muscle limb weakness in female and male B6.SOD1G93A mice beginning at P85 and P65 respectively. Others reported persistent muscle limb weakness beginning at ~P80 in females (Smittkamp et al., 2008) at ~P80 (males and females combined) (Fischer et al., 2004) and ~P100 in males and females (Miana-Mena et al., 2005). Taken together, our results suggest that rotarod and PaGE tests are unable to detect motor performance deficits in the pre-symptomatic phase (before tremor onset) in this model.
Progressive hindlimb muscle atrophy predicts genotype in pre-symptomatic B6.SOD1G93A
An early hallmark of ALS is hindlimb muscle denervation (Dantes et al., 1991; Gurney et al., 1994; Felice et al., 1997). A leading theory, proposed by Fischer et al. (2004), suggests that ALS is a distal axonopathy such that lumbar spinal motor neuron degeneration occurs in a retrograde fashion (Fischer et al., 2004; Siklós et al., 1996; Nguyen et al., 2009). During the pre-symptomatic phase, motor neuron degeneration begins with dying back of motor nerve terminals and pre-terminal axons and is followed by a symptomatic phase characterized by rapid cell death. As motor neurons become progressively disconnected they no longer stimulate their postsynaptic muscle targets resulting in paralysis and muscle wasting.
Studies in ALS mouse models have shown anatomically that white muscle fibers (fast-twitch, fatiguable, glycolytic, type-IIb muscle fibers) are the most vulnerable fiber population and become denervated at ~P45 (Fischer et al., 2004, Frey et al., 2000; Pun et al., 2006) or earlier at P25 (Gould et al., 2006). These findings are supported by electrophysiological reports showing loss of functional motor units at the same age (Kennel et al., 1996; Hegedus et al., 2007), ultrastuctural changes in cellular organelles (Mourelatos et al., 1996; Kong et al., 1997) and metabolic changes (Browne et al., 2006). Our results support all of these studies and strongly suggest that hindlimb muscle girth serves as a diagnostically accurate indirect marker for hindlimb muscle denervation in pre-symptomatic B6.SOD1G93A mice. Secondly, in similar fashion as open field motor performance, muscle girth provides an early objective marker of pre-symptomatic disease onset. Equally important, muscle atrophy is chronic and parallels disease progression.
Test accuracy and genotype prediction
Similar to previous studies (Weydt et al., 2003; Miana-Mena et al., 2005) we found that our battery of tests showed different time courses in disease progression of congenic B6.SOD1G93A mice. For example, the most sensitive test in terms of early detection was the open field. Several measures (average velocity, ambulatory distance, rest time and rearing episodes) were able to detect group differences at each time point from P45-P125, followed by less sensitive PaGE and rotarod measures. However, after P55-P65, physical measures of muscle girth, and to a lesser degree body weight, detected significant group differences in pre-symptomatic mice.
The questions then became how well each test can detect phenotypic differences between wildtype and transgenic mice on an individual basis and second, whether the test can predict genotype pre-symptomatically. The majority of open field measures were successful in detecting early motor deficits yet these measures failed to achieve 80% diagnostic accuracy (c.f. male open field measures in Table 2 and Figure 5C). This discrepancy highlights a common misconception about the meaning of a statistically “significant difference”. While analyses can show a significant difference between group means this does not imply that group distributions are mutually exclusive. For this reason receiver operating characteristic (ROC) curves were used to evaluate the diagnostic accuracy of each dependent measure. ROC curves serve as a measure of the degree of overlap between the distribution of dependent measures from wildtype and SOD1.B6G93A mice. As these distributions become increasingly separated diagnostic accuracy increases. ROC curves determine the ability of a test to discriminate groups by plotting the true positive rate as a function of the false positive rate at varying cutoff points of a classifying measure.
For example, in the case of muscle girth higher cutoff values are more likely to correctly identify every transgenic subject as transgenic, but are also more likely to misidentify wildtype subjects as transgenic. The greater the proportion of correctly identified transgenics to incorrectly identified transgenics the greater the area underneath the ROC curve and the greater the diagnostic accuracy of that particular measure. Discriminant analysis also attempts to classify subjects based on individual scores but goes further than the ROC curve analysis in that it determines the single optimal threshold value (e.g. muscle girth of 0.80 mm) that generates the most accurate discrimination between groups. Thus, by comparing individual scores with that threshold value a prediction of genotype can be made. For example, female mice with calf (d/v) muscle girth ≥ 0.80 mm would be predicted as wildtype while below 0.80 mm would be predicted as transgenic. Next, a ratio of the predicted genotype to the known genotype then provides a level of the prediction.
Besides muscle girth being highly accurate at detecting phenotype (diagnostically accurate), we found using stepwise discriminant analysis that calf (d/v) together with thigh girth predicted genotype well before tremor symptom onset in 80% of females at P55 and 80% of males at P65. More importantly however, the discriminant analysis provided a regression equation (D) that can be used to predict genotype of unknown subjects based on their individual muscle girth measures. These equations were: Female mice D = −23.368 + (35.289 * calf (d/v) muscle girth) + (−8.088 * thigh muscle girth). Male mice D = −19.550 + (28.881 * calf (d/v) muscle girth) + (−8.611 * thigh muscle girth). Positive D values predict wildtype genotype and negative D values predict B6.SOD1G93A genotype. While in practice genotype can be determined at birth by PCR, both the ROC and discriminant analyses strongly supported our findings that muscle girth is a valid early detector of pre-symptomatic phenotype and like open field measures can reliably monitor disease progression in pre-symptomatic B6.SOD1G93A ALS mouse model.
Broader implications
There is a general need to detect reliable phenotypic changes in other models of neurodegenerative diseases such as Huntington's and Parkinson's disease models. For example, a common symptom in Huntington's disease is loss of muscle mass (Sanberg et al. 1981). Bates and colleagues (1996) generated murine models of Huntington's disease that showed muscle atrophy in extracted hindlimb muscles (Mangiarini et al., 1996; Sathasivam et al., 1999) and deficits in open field (Bolivar et al., 2004). Therefore, the use of muscle girth and open field measures as described here may complement the evaluation of disease progression and treatment efficacy in pre-clinical studies with other models of motor disease.
CONCLUSION
While ~18 different murine ALS models have contributed greatly to our current understanding of the disease mechanisms they also serve as platforms for screening candidate therapies (Turner and Talbot, 2008). An important hurdle limiting the development of neuroprotective strategies in ALS models has been the inability to detect persistent behavioral and physical changes early in the disease. This has been exacerbated by the lack of objective, non-invasive, non-technical, cost-effective and high-throughput tests able to detect early and persistent motor and physical deficits. The findings of this study highlight each of these criteria. For example, the reported motor performance measures take 5 min/subject every 10th day, require no complex expertise, are done in freely behaving animals, provide objective data and are inexpensive (e.g., $4500 open field automated apparatus, or $100 for quality Vernier calipers and a light source). More importantly they detect chronic motor deficits in pre-symptomatic ALS mice. These findings are expected to contribute directly to more effective model evaluations of neuroprotective strategies that seek to disrupt disease mechanisms in the ALS disease process.
Acknowledgments
Supported by NIH grant T32 MH065728. The authors thank Dr. Jason Shumake for his generous help. We also greatly appreciate the contributions of Sonya Sui, Samir Khatani, Anvinh Nguyen, Huma Vaid, Erin Keith, Justin Rhee, Kimi Vu, Shivam Patel, Brandon Martinez, Tameka Jones-Watlington and Gene Pershwitz.
List of Abbreviations
- ALS
amyotrophic lateral sclerosis
- SOD1G93A
superoxide dismutase-1 with glycine to alanine switch at codon 93
- d/v
dorsal/ventral plane
- m/l
medial/lateral plane
- RM ANOVA
repeated measures analysis of variance
- ROC
receiver operating characteristic
- P
post-natal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amendola J, Verrier B, Roubertoux P, Durand J. Altered sensorimotor development in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2004;20:2822–2826. doi: 10.1111/j.1460-9568.2004.03745.x. [DOI] [PubMed] [Google Scholar]
- Azari MF, Lopes EC, Stubna C, Turner BJ, Zang D, Nicola NA, Kurek JB, Cheema SS. Behavioural and anatomical effects of systemically administered leukemia inhibitory factor in the SOD1(G93A G1H) mouse model of familial amyotrophic lateral sclerosis. Brain Res. 2003;982:92–97. doi: 10.1016/s0006-8993(03)02989-5. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Krezel W, Dolle P, Vodouhe C, Warter JM, Poindron P, Borg J. Compensatory mechanism of motor defect in SOD1 transgenic mice by overactivation of striatal cholinergic neurons. Neuroreport. 1999;10:1013–1018. doi: 10.1097/00001756-199904060-00022. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Browne SE, Yang L, DiMauro JP, Fuller SW, Licata SC, Beal MF. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Neurobiol Dis. 2006;22:599–610. doi: 10.1016/j.nbd.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Chiu AY, Zhai P, Dal Canto MC, Peters TM, Kwon YW, Prattis SM, Gurney ME. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995;6:349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- Dantes M, McComas A. The extent and time course of motoneuron involvement in amyotrophic lateral sclerosis. Muscle Nerve. 1991;14:416–421. doi: 10.1002/mus.880140506. [DOI] [PubMed] [Google Scholar]
- Durand J, Amendola J, Bories C, Lamotte d'Incamps B. Early abnormalities in transgenic mouse models of amyotrophic lateral sclerosis. J Physiol Paris. 2006;99:211–220. doi: 10.1016/j.jphysparis.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Felice KJ. A longitudinal study comparing thenar motor unit number estimates to other quantitative tests in patients with amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:179–185. doi: 10.1002/(sici)1097-4598(199702)20:2<179::aid-mus7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Sanberg PR. Feasibility of cell therapy for amyotrophic lateral sclerosis. Exp Neurol. 2009;216:3–6. doi: 10.1016/j.expneurol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, Milligan CE, Oppenheim RW. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, Asress SA, Richardson JR, Glass JD, Miller GW. Treadmill gait analysis does not detect motor deficits in animal models of Parkinson's disease or amyotrophic lateral sclerosis. J Mot Behav. 2008;40:568–577. doi: 10.3200/JMBR.40.6.568-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Haenggeli C, Julien JP, Mosley RL, Perez N, Dhar A, Gendelman HE, Rothstein JD. Therapeutic immunization with a glatiramer acetate derivative does not alter survival in G93A and G37R SOD1 mouse models of familial ALS. Neurobiol Dis. 2007;26:146–152. doi: 10.1016/j.nbd.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28:154–164. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Heiman-Patterson TD, Deitch JS, Blankenhorn EP, Erwin KL, Perreault MJ, Alexander BK, Byers N, Toman I, Alexander GM. Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J Neurol Sci. 2005;236:1–7. doi: 10.1016/j.jns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Kang H, Tian L, Thompson W. Terminal Schwann cells guide the reinnervation of muscle after nerve injury. J Neurocytol. 2003;32:975–985. doi: 10.1023/B:NEUR.0000020636.27222.2d. [DOI] [PubMed] [Google Scholar]
- Kennel PF, Finiels F, Revah F, Mallet J. Neuromuscular function impairment is not caused by motor neurone loss in FALS mice: an electromyographic study. Neuroreport. 1996;7:1427–1431. doi: 10.1097/00001756-199605310-00021. [DOI] [PubMed] [Google Scholar]
- Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2003;53:804–807. doi: 10.1002/ana.10597. [DOI] [PubMed] [Google Scholar]
- Kirkinezos IG, Bacman SR, Hernandez D, Oca-Cossio J, Arias LJ, Perez-Pinzon MA, Bradley WG, Moraes CT. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci. 2005;25:164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SH, Lee SM, Kim HY, Lee KY, Lee YJ, Kim HT, Kim J, Kim MH, Hwang MS, Song C, Yang KW, Lee KW, Kim SH, Kim OH. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci Lett. 2006;395:103–107. doi: 10.1016/j.neulet.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shinobu LA, Ward CM, Young D, Cleveland DW. Elevation of the Hsp70 chaperone does not effect toxicity in mouse models of familial amyotrophic lateral sclerosis. J Neurochem. 2005;93:875–882. doi: 10.1111/j.1471-4159.2005.03054.x. [DOI] [PubMed] [Google Scholar]
- Lobsiger CS, Boillee S, McAlonis-Downes M, Khan AM, Feltri ML, Yamanaka K, Cleveland DW. Schwann cells expressing dismutase active mutant SOD1 unexpectedly slow disease progression in ALS mice. Proc Natl Acad Sci U S A. 2009;106:4465–4470. doi: 10.1073/pnas.0813339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love FM, Son YJ, Thompson WJ. Activity alters muscle reinnervation and terminal sprouting by reducing the number of Schwann cell pathways that grow to link synaptic sites. J Neurobiol. 2003;54:566–576. doi: 10.1002/neu.10191. [DOI] [PubMed] [Google Scholar]
- Lubischer JL, Bebinger DM. Regulation of terminal Schwann cell number at the adult neuromuscular junction. J Neurosci. 1999;19:RC46. doi: 10.1523/JNEUROSCI.19-24-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JS, Hefferan MP, Marsala M, Feldman EL. Stem cells: comprehensive treatments for amyotrophic lateral sclerosis in conjunction with growth factor delivery. Growth Factors. 2009;27:133–140. doi: 10.1080/08977190902814855. [DOI] [PubMed] [Google Scholar]
- Miana-Mena FJ, Munoz MJ, Yague G, Mendez M, Moreno M, Ciriza J, Zaragoza P, Osta R. Optimal methods to characterize the G93A mouse model of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:55–62. doi: 10.1080/14660820510026162. [DOI] [PubMed] [Google Scholar]
- Mourelatos Z, Gonatas NK, Stieber A, Gurney ME, Dal Canto MC. The Golgi apparatus of spinal cord motor neurons in transgenic mice expressing mutant Cu,Zn superoxide dismutase becomes fragmented in early, preclinical stages of the disease. Proc Natl Acad Sci U S A. 1996;93:5472–5477. doi: 10.1073/pnas.93.11.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder DW, Howard FM., Jr. Patient resistance and prognosis in amyotrophic lateral sclerosis. Mayo Clin Proc. 1976;51:537–541. [PubMed] [Google Scholar]
- Mulder DW. Clinical limits of amyotrophic lateral sclerosis. Adv Neurol. 1982;36:15–22. [PubMed] [Google Scholar]
- Nagano I, Ilieva H, Shiote M, Murakami T, Yokoyama M, Shoji M, Abe K. Therapeutic benefit of intrathecal injection of insulin-like growth factor-1 in a mouse model of Amyotrophic Lateral Sclerosis. J Neurol Sci. 2005;235:61–68. doi: 10.1016/j.jns.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Garcia-Chacon LE, Barrett JN, Barrett EF, David G. The Psi(m) depolarization that accompanies mitochondrial Ca2+ uptake is greater in mutant SOD1 than in wild-type mouse motor terminals. Proc Natl Acad Sci U S A. 2009;106:2007–2011. doi: 10.1073/pnas.0810934106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen HG, Debska-Vielhaber G, Sander K, Angenstein F, Ludolph AC, Hilfert L, Willker W, Leibfritz D, Heinze HJ, Kunz WS, Vielhaber S. Metabolic progression markers of neurodegeneration in the transgenic G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:1669–1677. doi: 10.1111/j.1460-9568.2007.05415.x. [DOI] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Sanes JR, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol. 2005;490:209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Brown RH, Jr., Cole D, Chaturvedi P, Schoenfeld D, Pastuszak K, Matthews R, Upton-Rice M, Cudkowicz ME. Effect of neurophilin ligands on motor units in mice with SOD1 ALS mutations. Neurology. 2001;57:1857–1861. doi: 10.1212/wnl.57.10.1857. [DOI] [PubMed] [Google Scholar]
- Siklós L, Engelhardt J, Harati Y, Smith RG, Joo F, Appel SH. Ultrastructural evidence for altered calcium in motor nerve terminals in amyotropic lateral sclerosis. Ann Neurol. 1996;39:203–216. doi: 10.1002/ana.410390210. [DOI] [PubMed] [Google Scholar]
- Smittkamp SE, Brown JW, Stanford JA. Time-course and characterization of orolingual motor deficits in B6SJL-Tg(SOD1-G93A)1Gur/J mice. Neuroscience. 2008;151:613–621. doi: 10.1016/j.neuroscience.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron. 1995;14:133–141. doi: 10.1016/0896-6273(95)90247-3. [DOI] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Haenggeli C, Rothstein JD. Respiratory impairment in a mouse model of amyotrophic lateral sclerosis. J Appl Physiol. 2007;102:926–932. doi: 10.1152/japplphysiol.00193.2006. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 2008;85:94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Veldink JH, Bar PR, Joosten EA, Otten M, Wokke JH, van den Berg LH. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 2003;13:737–743. doi: 10.1016/s0960-8966(03)00104-4. [DOI] [PubMed] [Google Scholar]
- Wang L, Sharma K, Deng HX, Siddique T, Grisotti G, Liu E, Roos RP. Restricted expression of mutant SOD1 in spinal motor neurons and interneurons induces motor neuron pathology. Neurobiol Dis. 2008;29:400–408. doi: 10.1016/j.nbd.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Weydt P, Hong SY, Kliot M, Moller T. Assessing disease onset and progression in the SOD1 mouse model of ALS. Neuroreport. 2003;14:1051–1054. doi: 10.1097/01.wnr.0000073685.00308.89. [DOI] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CP, Zhang C, Zhou SN, Xie YM, Wang YH, Huang H, Shang YC, Li WY, Zhou C, Yu MJ, Feng SW. Human mesenchymal stromal cells ameliorate the phenotype of SOD1-G93A ALS mice. Cytotherapy. 2007;9:414–426. doi: 10.1080/14653240701376413. [DOI] [PubMed] [Google Scholar]