Abstract
Matrix Metalloproteinases (MMPs) comprise a family of more than 20 members, each with the ability to degrade components of the extracellular matrix. The interstitial collagenases have the unique capacity to degrade the stromal collagens, types I, II and III, the body's most abundant proteins. These collagenases include MMP-1, MMP-8, MMP-13 and MMP-14. MMP-1, with a very broad expression pattern, has major roles in mediating matrix destruction in many diseases. We have described a single nucleotide polymorphism (SNP) in the MMP-1 promoter that augments transcription. This SNP is the presence or absence of an extra guanine (G) at -1607 bp, which creates the sequence 5'-GGAA-3'(2G allele), and which is an ETS binding site. Compared to the 1G allele (5'-GAA-3'), the 2G SNP is associated with enhanced transcription of MMP-1 and increased enzymatic activity.
Although murine systems are often used to model human diseases, mice have only distant homologues of human MMP-1. Therefore, we used a technique for the targeted insertion of a single copy of a gene at the HPRT locus to compare expression of the 1G and 2G alleles. We generated transgenic mice with -4372 bp of the human MMP-1 promoter containing either the 1G or 2G SNP in front of the Lac Z (E.coli ß-galactosidase) gene. We measured relative expression of the transgenes in vitro in embryonic stem (ES) cells and in fibroblasts derived from embryonic mice. Our data show modest constitutive expression of ß-galactosidase mRNA and protein from these alleles, with the 2G allele more transcriptionally active than the 1G allele. We conclude that these mice represent a model for integration of a single copy of the human MMP-1 promoter into the murine genome, and could be used to study MMP-1 gene expression in a murine system.
Keywords: mRNA, ß-galactosidase, gene expression, single nucleotide polymorphism, fibroblasts
INTRODUCTION
The family of Matrix Metalloproteinases (MMPs) is comprised of more than 20 members, each with the ability to degrade various components of the extracellular matrix (reviewed in Brinckerhoff and Matrisian, 2002; Burrage et al., 2006; Burrage and Brinckerhoff, 2007). Most MMPs are secreted in latent form and are activated proteolytically in the extracellular space Although there is some redundancy among the MMPs in terms of their substrates, the interstitial collagenases have the unique capacity to degrade the stromal collagens, types I, II and III, the body's most abundant proteins. These collagenases include MMP-1, MMP-8, MMP-13 and MMP-14, which is a membrane-bound MMP. MMP-8 is primarily a product of neutrophils, while MMP-13 is synthesized by cells in cartilage and bone, and it preferentially degrades the type II collagen found in cartilage. On the other hand, MMP-1 is expressed by most cells and can readily degrade all stromal collagens.
MMP-1, with its very broad expression pattern, has possible roles in mediating matrix destruction in many diseases, including joint degradation in arthritis, tumor invasion and metastasis in cancer, plaque rupture in atherosclerosis and bone dissolution in periodontal disease (Brinckerhoff and Matrisian, 2002). Previously, we have described a single nucleotide polymorphism (SNP) in the MMP-1 promoter that augments transcription (Rutter et al. 1998). This SNP is the presence or absence of an extra guanine (G) at -1607 bp (SNP data base rs 1799750), which creates the sequence 5'-GGAA-3'(2G allele) vs. 5'-GAA-3' (1G allele). The sequence, 5'-GGAA-3', is a consensus binding site for the Ets family of transcription factors, which are the downstream targets of several growth factors (Rutter et al., 1998). Compared to the 1G allele (5'-GAA-3'), the 2G SNP is associated with enhanced transcription of MMP-1 and increased enzymatic activity. This SNP is common in the population (Rutter et al., 1998), and the 2G allele has been linked to increased incidence or progression of several diseases, including cancer (Kanamori et al., 1999; Ye et al., 2001; Nishioka et al., 2000, 2003; Hughes et al., 2007) periodontitis (Astolfi et al., 2006; Pirhan et al., 2008) cardiovascular disorders (Ye et al., 2003; Pearce et al., 2005) and lung conditions (Mercer et al., 2009; Li et al., 2009).
Because of its prominent role in disease, there is interest in model systems that can be used to monitor MMP-1 expression. Due to their genetic uniformity and relative economy, mice are convenient and important models for the study of human diseases. Unfortunately, mice have only a pair of distant homologues of human MMP-1, mcolA and mcolB, genes with limited homology to human MMP-1. These genes are expressed primarily during development and in a tissue-specific manner (Balbin et al., 2001; Brinckerhoff and Matrisian, 2002). Murine MMP-13 shares > 90% sequence homology/identity with human MMP-13, and in contrast to human MMP-13, has a broad profile of expression in murine tissues (Balbin et al., 2001; Brinckerhoff and Matrisian, 2002). It has, therefore, served as a surrogate for a ubiquitously expressed interstitial collagenase in mouse models.
Transgenic mice expressing human MMP-1 have been described (D'Armiento et al., 1992, 1995). Expression of the transgene is associated with emphysema (D'Armiento et al., 1992), and with the development of hyperkeratosis and increased tumorigenesis (D'Armiento et al. 1995), indicating a direct link between over expression of MMP-1 in mice and tumor development and progression. Because of issues related to site of integration and variations in copy number, these standard transgenic systems are unable to quantify differences between expression of the 1G and 2G alleles in an in vivo mouse environment. Therefore, we used a technique for the targeted insertion of a single copy of a gene at the HPRT locus (Bronson et al., 1996) to compare expression of the 1G and 2G alleles. HPRT is X-linked and is not required in mice, so even males that lack HPRT are normal. Furthermore, the targeting vector mediates a highly efficient, directly selectable homologous recombination event at the HPRT locus (Bronson et al., 1996; Vivian et al., 1999; Evans et al., 2000; Guillot et al., 2000; Yang et al., 2000; Magness et al., 2000; Misra et al., 2001).
We have generated mice expressing the 1G or 2G alleles of the human MMP-1 promoter linked to the ß-galactosidase reporter. Assessing MMP-1 promoter activity of the 1G and 2G alleles via a reporter gene is an appropriate measure of collagenase activity. For most MMPs, including MMP-1, an increase in transcription is followed by an increase in mRNA, with protein synthesized and secreted within about 40 minutes (Brinckerhoff and Matrisian; Burrage et al., 2006; Burrage and Brinckerhoff, 2007). Thus, an increase in transcription is paralleled by an increase in protein. Even though the secreted collagenase protein is in latent form, in an in vivo environment, serine proteinases and other MMPs activate latent MMP-1. Therefore, gene expression correlates with protein expression, and even considering the possibility of blocking some enzyme activity with endogenous Tissue Inhibitors of Metalloproteinases (TIMPs), MMP-1 promoter activity reflects the potential for collagenolytic activity.
Thus, our study documents that we have successfully made transgenic mice with -4372 bp of the human MMP-1 promoter containing either the 1G or 2G SNP at -1607 in front of the Lac Z (E.coli ß-galactosidase) gene. The transgenes are in the HPRT (hypoxanthine-guanine phosphoribosyltransferase) locus and are transmissible from generation to generation on the X chromosome. We measured relative expression of the transgenes in vitro in embryonic stem (ES) cells and in fibroblasts derived from embryonic mice. Although our data show modest expression of ß-galactosidase mRNA and protein from these alleles, these mice represent a model for integration of a single copy of the human MMP-1 promoter into the murine genome.
RESULTS
Expression of the MMP-1 1G and 2G alleles in murine ES cells
Once we determined that the transgenes were properly inserted (Figure 1), we tested ES cells for constitutive expression of each allele (Table 1). The table shows that the human promoter is expressed in ES cells, and the 2G allele has a significantly higher level of expression than the 1G allele, indicating that the 1G and 2G alleles are regulated as expected.
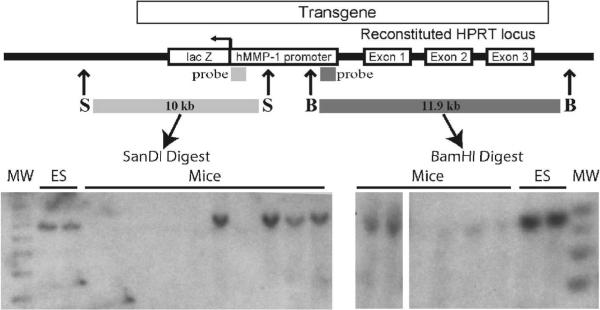
Figure 1. Representative Southern blot of MMP-1 transgenes.
Mice transgenic for the MMP-1 promoter, with the 1G or 2G allele, were generated from Embryonic Stem (ES) cells. Genomic DNA was prepared from the ES cells or from mouse tail snips, digested with SanDI or BamHI and analyzed by Southern blot with the indicated probes. With correctly targeted insertion, digestion with these restriction enzymes should give fragments of 10 kb (SanDI digest) and 11.9 kb (BamHI digest). Although DNA from the same ES cells is presented in the figure as a positive control, DNA from different mice is shown in the two restriction digests. Thus, some mice harbor the human MMP-1 transgenes. MW = molecular weight markers. Genotyping by DNA sequencing confirmed the 1G and 2G alleles (data not shown).
Table 1.
Expression of 1G/2G MMP-1/ß-galactosidase transgenes in mouse ES cells.
| MMP-1/ß-gal Transgenic Construct | Copies ß-gal / pg GAPDH | |
|---|---|---|
| 1G | 6.01 +/- 1.7 | |
| 2G | 15.25 +/- 2.9 |
Mouse ES cells that harbored 4.3 kb of the human MMP-1 promoter containing 1G or 2G at -1607 bp linked to the ß-gal reporter inserted at the HPRT locus, were grown on plastic dishes coated with 0.1% gelatin. At 80% confluence, the medium was changed and 24 hours later, RNA was harvested and subjected to real-time RT-PCR for ß-gal mRNA. Data were normalized to mouse GAPDH. Values represent the average of triplicates (+/- S.D). Rev = reverse orientation for insertion of the MMP-1 promoter relative to HPRT. P = 0.009 for 1G vs. 2G
Expression of the MMP-1 1G and 2G alleles in mouse embryonic fibroblasts (MEFs)
We next measured constitutive expression of ß-galactosidase mRNA in MEFs harboring either of the alleles. Figure 2 presents the results of two representative experiments and demonstrates that constitutive expression of the 2G allele is approximately 2 to 3-fold higher than that of the 1G allele; (P < 0.01). These levels of differential expression are in general agreement with those seen in the ES cells, confirming our results in two cell types.
Figure 2. Constitutive expression of the 1G vs. 2G alleles in mouse embryonic fibroblasts.
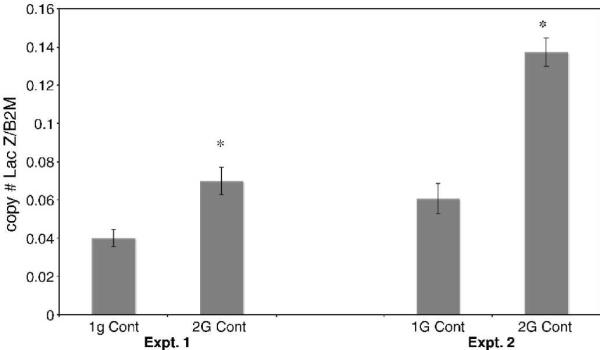
Real-time reverse transcription-polymerase chain reaction (real-time-RT-PCR) analysis of E. coli ß-galactosidase (Lac Z) transgene RNA copy number (molecules) normalized to ß2 microglobulin (ß2M) gene RNA copy number. Total RNA was purified from mouse embryonic fibroblasts (MEFs) isolated from 13.5-day old embryos which were cultured in MEF media (with serum). Data are averages for two plates of cells (60mmX15mm) analyzed in duplicate (+/- S.D.), but are representative of results from at least 6 embryos for each allele (1G/2G, SNP database rs1799750) of the human MMP-1 promoter. Experiment 1 and experiment 2 were done on cells from different embryos on different days. Experiment 1 was done on the same real time plate, experiment 2 was done on another real time plate, but the standards on each plate and their corresponding C(t) were the same. * P < 0.01 for 1G vs. 2G.
We also measured levels of ß-galactosidase protein in cells, and results were comparable to those with mRNA. Levels of protein ranged from 0.4-1.9 units ß-galactosidase/ug total protein for the 1G allele, and from 1.0-1.9 units ß-galactosidase/μg total protein for the 2G allele (data not shown). The overlap in these levels most likely reflects the facts that the assay for protein is less sensitive than mRNA detection, and that real-time PCR is a more sensitive and precise method for quantifying transcription from reporter plasmids (Ornskov et al., 2004). These experiments document that ß-galactosidase protein is expressed in cells from the transgenic mice.
Induction of the MMP-1 promoters by cytokines and growth factors
Along with MMP-1, MMP-13 is an interstitial collagenase that is increased in response to cytokines, such as IL-1ß, and growth factors, such as basic fibroblast growth factor (bFGF) (Brinckerhoff and Matrisian; Burrage et al. 2006; Burrage and Brinckerhoff, 2007; Wyatt et al., 2005; Fahmi et al., 2001). Therefore as a control in this study, we monitored increases in MMP-1 and MMP-13 mRNA in adult human fibroblasts (Figure 3). We included MMP-13 since this is the only interstitial collagenase expressed by mouse fibroblasts (Balbin et al., 2001; Brinckerhoff and Matrisian, 2002), and as expected, we found that both IL-1ß and bFGF increased MMP-1 and MMP-13. These data show that these stimuli can induce MMP-1 in our system.
Figure 3. Induction of MMP-1 and MMP-13 by cytokines and growth factors in adult human fibroblasts.
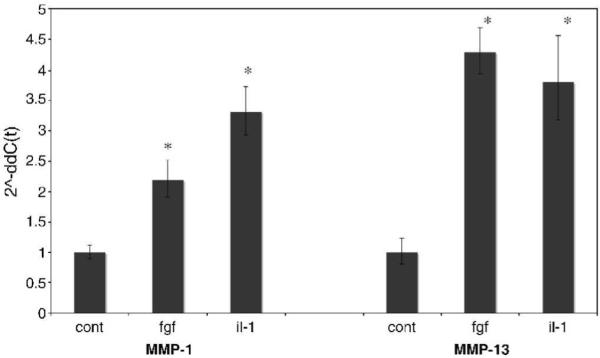
Adult human dermal fibroblasts were cultured in DMEM/10% FBS and treated for 24 hours with either recombinant mouse bFGF (12.5ng/ml) or human recombinant interleukin-1ß (IL-1) (5ng/ml). Total cell RNA was purified and analyzed by real-time reverse transcription-polymerase chain reaction. Results were normalized to human GAPDH using the 2^-ddC(t) method, therefore the bars represent fold induction. Data represents two plates of cells for each treatment analyzed in quadruplicate. * P < 0.01 for induction of MMP-1 and MMP-13.
Next we wanted to show that the 1G and 2G allele of human MMP-1 promoter could be induced appropriately in mouse fibroblasts. For this, we transiently transfected 4.3 kb of the human MMP-1 promoter, containing either the 1G or 2G allele, linked to the luciferase reporter into moue 3T3 cells. Figure 4A demonstrates that basal/constitutive expression mirrors that seen with the ß-galactosidase reporter in transgenic mice, with the 2G allele expressed at 3 - 6 fold higher levels than the 1G allele. Figure 4B shows that bFGF increased expression of both reporter constructs, but again, expression of the 2G allele was substantially higher than that of the 1G allele.
Figure 4. Transient transfection of 1G and 2G MMP-1 promoter constructs into human and mouse cells.
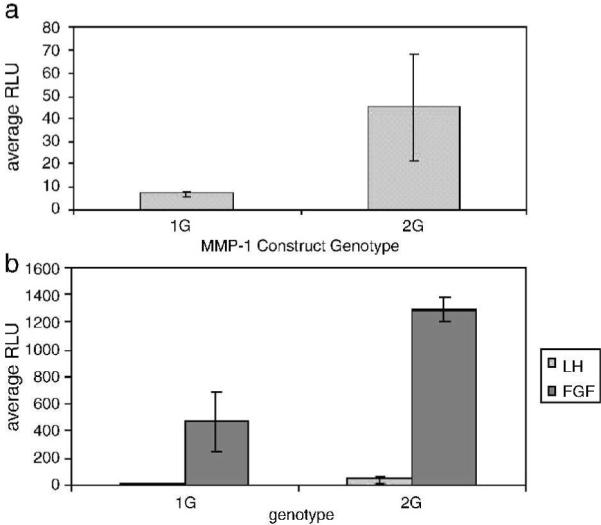
Triplicate cultures of cells were transiently co-transfected with 1 μg of MMP-1 promoter DNA containing either 1G or 2G at -1607 bp driving firefly luciferase expression (pGL3 basic) and 50 ng of a Renilla luciferase control vector. After 24 hours in serum free DMEM, luciferase levels were measured and expressed as relative light units (RLU's), and firefly luciferase levels were normalized to Renilla luciferase expression. Mouse cells were left untreated or treated with bFGF (10 ng/ml). (A) Basal expression of 4.3 kb MMP-1 promoter constructs in 3T3 mouse cells (B) bFGF-induced expression of 4.3 kb MMP-1 promoter constructs in mouse 3T3 cells.
Treatment of transfected cells with IL-1ß failed to increase luciferase expression (data not shown), a finding in keeping with our data when human fibroblasts or chondrocytic cells are transfected with the human MMP-1 promoter and treated with IL-1ß (Mengshol et al., 2001; Burrage et al., 2007a; Burrage et al., 2008). Thus, we conclude that the human MMP-1 promoter behaves similarly in human and mouse cells and the 2G allele is consistently expressed to a higher level, both constitutively and in response to bFGF. The mouse environment, therefore, seems to be appropriate for regulation of a human gene.
Lastly, we investigated whether the transgenes could be induced in MEFs treated with IL-1ß or phorbol ester. A representative experiment is shown in Figure 5, and clearly indicates a lack of induction of ß-galactosidase in MEFs harboring either the 1G or 2G allele. Treatment with bFGF also failed to induce either allele (data not shown). This finding is in direct contrast with the data in Figures 3 and 4, which show appropriate regulation of the endogenous gene in human fibroblasts and of the human MMP-1 promoters transiently transfected into murine cells. Thus, basal/constitutive expression of the 1G and 2G alleles in transgenic mice is regulated as expected, but the response to inductive stimuli is not.
Figure 5. Treatment of mouse embryonic fibroblasts (MEFs) with Interleukin-1ß (IL-1ß) or phorbol myristate acetate (PMA).

Real-time reverse transcription-polymerase chain reaction (real-time-RT-PCR) analysis of E.coli ß-galactosidase (Lac Z) transgene RNA copy number (molecules) normalized to ß2 microglobulin (ß2M) gene RNA copy number. MEFs isolated from 13.5-day old embryos were cultured in MEF media (with serum) and treated for 24 hours in the presence (or absence) of IL-1 (3.33 ng/ml) or PMA (1X10-8 M) for 24 hours, at which time total RNA was isolated and analyzed by real-time-RT-PCR. Data are averages (+/-S.D.) for two plates with duplicate samples each, but are representative of results from at least 6 embryos for each allele (1G/2G, SNP database rs1799750) of the human MMP-1 promoter.
DISCUSSION
Mice do not harbor a homologue of human MMP-1, making it impossible to carry out in vitro or in vivo experimental studies on MMP-1 in a mouse model. Consequently, there has been considerable interest in developing a transgenic mouse model for MMP-1. As noted, transgenic mice expressing human MMP-1 have been described (D'Armiento et al., 1992, 1995). The transgene was under control of a constitutive promoter, thus preventing studies on the regulation of gene expression. In addition, because of issues related to site of integration and variations in copy number, these standard transgenic systems preclude analysis of differences between expression of the 1G and 2G alleles. Another strategy is targeting expression of the transgenes to the mouse MMP-13 locus on chromosome 9. However, this may not be a viable plan since knocking out one functional copy of the mouse MMP-13 gene may severely compromise these mice. For example, haplo-insufficiency, resulting from loss of one copy of the Runx/Cbfa1 gene, has been linked to chondrodysplasia (Komori et al., 1997), indicating the importance of both copies of this gene for normal connective tissue function.
Thus, we elected to target insertion of the MMP-1 promoter linked to the ß-galactosidase reporter to the HPRT locus. This study demonstrates that (a) the human MMP-1 promoter linked to the ß-galactosidase can be inserted into the mouse genome at the HPRT locus; (b) that the chimeric construct is constitutively expressed in mouse cells at low levels; and (c) differential expression of the 1G and 2G alleles can be detected. Unfortunately, in these in vitro studies with mouse embryonic fibroblasts, the transgenes were not inducible by cytokines and growth factors.
The original paper describing the targeted insertion of a human gene at the HPRT locus used a human promoter driving expression of a single copy of murine bcl-2 (Bronson et al., 1996). Subsequent studies measured cell and tissue specific expression of human promoters linked to reporter genes, such as ß-galactosidase (Vivian et al., 1999; Evans et al., 2000; Guillot et al., 2000; Yang et al., 2000; Magness et al., 2000). One study describes the ability to discriminate between an A to G polymorphism in the promoter of the human ferrochelatase gene, demonstrating the sensitivity of this system (Magness et al., 2000). Thus, targeted human genes are appropriately expressed in mice, and gene products from a single copy are detectable.
Our results are at least partially in keeping with these previous reports. Indeed, basal/constitutive expression of the transgenes is regulated in a manner similar to the endogenous MMP-1 gene in human cells, where expression of the 2G allele is consistently higher than the 1G allele (Brinckerhoff and Matrisian, 2002; Rutter et al., 1998; Wyatt et al., 2002). Further, basal expression of MMP-1 in normal cells is often rather low and reflects a relatively low level of transcription (Brinckerhoff and Matrisian, 2002; Burrage et al., 2006; Burrage and Brinckerhoff, 2007; Wyatt et al., 2002). In contrast, the increase in expression of MMP-1 in response to inductive stimuli is often tremendous, and reflects both an increase in transcription and an increase in mRNA stability (Brinckerhoff and Matrisian, 2002; Burrage et al., 2006; Burrage and Brinckerhoff, 2007). This latter is mediated by the AUUUA sequences in the 3' UTR of MMP-1 mRNA, which is not found in the ß-galactosidase reporter.
Nonetheless, expression of the transgenes did not increase in response to growth factors and cytokines, which should have activated transcription in addition to enhancing mRNA stability. Reasons for this are not clear, but could include important response elements located within introns of the MMP-1 gene, although this does not seem likely given the fact that the MMP-1 promoter linked to luciferase responds well when expressed in murine cells (Figure 3). Further, Vincenti and colleagues (Raymond et al. 2006) transiently transfected the human MMP-1 promoter into rabbit articular chondrocytes and identified a novel IL-1ß response element in the human promoter. This element is located between -2942 bp and -2002 bp, suggesting that the promoter fragment we used does contain response region(s), but that mechanisms controlling expression in human cells are more complex than in rabbit cells.
Alternatively, perhaps there are characteristics of the chromatin at the HPRT locus that influence expression of the MMP-1 promoter. The locus has been described as “open” and accessible to transcription factors, and it is possible that repressor proteins bind to regions of the promoter, thereby squelching transcription. Indeed, deletional analysis of the MMP-1 promoter has suggested the presence of an inhibitory region in the most 5' area of the promoter, upstream of -3900 bp (Mercer et al., 2009; Li et al., 2009). The construct used to generate the transgene contained approximately 4300 bp of promoter DNA (Rutter et al., 1998), thus including the putative suppressor region, which may have dampened expression, even though the 4300 bp of promoter DNA have responded exuberantly in some cells. Finally, it is increasingly apparent that chromatin-remodeling events (Menghsol et al., 2001; Burrage et al., 2007a; Burrage et al., 2008) are important in regulating MMP-1 expression, and perhaps the locus does not permit the necessary and appropriate chromatin modifications to allow an increase in gene expression. Perhaps, too, the 4300 bp promoter used in these studies does not contain an important regulatory element that is required for induction from native chromatin, which is possibly very different from induction of transiently transfected constructs. Nonetheless, despite the absence of transcriptional induction in response to exogenous stimuli, the presence of the MMP-1 transgenes in a murine background provides a unique opportunity to monitor the basal/constitutive activity of the 1G and 2G alleles in the MMP-1 promoter in an in vivo setting. The results clearly demonstrate the increased transcription associated with the 2G allele, a result that is difficult to definitively demonstrate in the endogenous locus in human cells since there could be other linked polymorphisms influencing transcription from the endogenous 2G locus.
EXPERIMENTAL PROCEDURES
Construction of the transgenes and insertion at the HPRT locus
“pMP8” is an HPRT targeting construct designed specifically to correct the HPRT deletion in E14TG2a mouse ES cells. The construct contains 4 kb of mouse genomic DNA 5' to the deletion, 1.8 kb of human HPRT genomic DNA including the promoter and exon 1, and 7 kb of mouse HPRT genomic DNA including exons 2 and 3 (Reid et al., 1990). The pMP8SKB vector, which is a modification of pMP8, was used to target the HPRT locus of a mouse embryonic cell line (E14TG2a) lacking a functional HPRT gene (Bronson et al., 1996). The mmp1 promoter to -4372 bp with either 1G or 2G was cloned in front of the lacZ gene in pB-Gal basic (CLONTECH laboratories, Inc. Palo Alto CA 94303). The mmp-1 promoter plus the ß-galactosidase gene plus the polyadenylation signal were cloned into the targeting vector NOT 1 site in the reverse orientation relative to the HPRT replacement exons. Orientation was verified using an Mlu1 digest of the vector plus insert visualized by ethidium bromide staining on an agarose gel.
Embryonic Stem (ES) cells and generation of transgenic mice
The BK4 ES cell line was grown on mouse embryo fibroblasts using standard conditions (Nagy et al., 2003). 10 million cells were electroporated with 20 μg of linearized targeting vector. Resistant clones were selected for growth in HAT medium. Using the Gentra DNA Isolation Kit (Gentra Systems, Minneapolis, MN) DNA was isolated from targeted ES cells grown to confluency on 100mm tissue culture plates. The genomic DNA was screened for recombination by PCR using platinum Taq (Invitrogen, Carlsbad, CA) and primers to the lac z gene (B-U) (5'TATCGGCCTCAGGAAGATCGCACTC3') and the mmp1 promoter (B-L) (5'TCTAATGATTGCCTAGTCTAT3'), which gave a product of 550bp.
Homologous recombination of the HPRT locus insertion was verified by PCR using one primer outside the lesion overlap region (A-U) (5'GGAGGATCACACACTTAGAGCCAAC3') and one primer in the lac z region of the insert (A-L) (5'AATTCGCCGGATCTTTGTGAAGGAA3'), which gives a product of 5437 bp. The product was verified by sequencing the ends, and by restriction enzyme digestion with Eco RV (bands 2094 bp and 600 bp) and Kpn1 (bands 3300 bp and 1700 bp).
SNP identity was verified by PCR amplification of the genomic DNA with mmp1 promoter primers.primers: 5'TTGCCAGATGGGACAGTGTATGAG3' and 5'ACATTAAATTGTCTTGGGTACTGGT3' or primers: 5' GTCACGTCTTCACAGT3' and5'GATATCTTACTCATAAACAAT3'. The purified bands were sequenced with a nested primer (I-3) (5'AGTGTTCTTTGGTCTCTGC3'). Tail DNA from all mice was also checked for several generations for homologous recombination with primer pairs (A- U/L and B- U/L), and for the SNP (1G/2G) with the same assays as the embryonic stem cells. Chimeric founders were crossed with C57BL6 mice. Transgenic offspring from these breeding pairs were backcrossed to C57BL6 mice. Since the HPRT locus is only on the X chromosome, in the case of male founders, all male offspring were negative, so only female pups were screened for the transgene. For female founders, offspring could be either transgenic or wild type. From these screenings, hemizygous males (XtY) were crossed with heterozygous females (XtX) to obtain a colony of females that were transgenic homozygous (XtXt) and males that were hemizygous (XtY). Only homozygous females were used for mating, and these mice are XtXt. All males were XtY. Thus, every cell has one active copy of the transgene, and X inactivation does not affect expression. The genotype was verified using a PCR assay on genomic DNA isolated from tail snips using the Gentra Kit. The C57BL6 has wild type mouse HPRT. The lesion replacement has human exon 1. XtXt=no PCR band. XwtXt= PCR band. The PCR product is 238bp.Primers: 5'CGGCTGGATCTCAAATCTTATGAC3' 5'TCCGGAACTCTTATCTGACTAGGTG3'Or similarly, another area missing in the lesion, but present in the wild type.Primers: 5'TAGGCTAAAGAGTTGAACGCAAAGG3' 5'CTCGTATCTCACAGGCCAGCTATCC3': product is 889bp.
Sex determination of embryos: (sry=sex-determination region Y) male embryos will have a band with male specific primers, female embryos will not.sry-u: 5'CATGACCACCACCACCAACAA3'sry-L: 5'TCATGAGACTGCCAACCACAG3'(Rapid DNA extraction and PCR-Sexing of Mouse Embryos, Peter J. McClive and Andrew H. Sinclair, Molecular Reproduction and Development 60:225-226 (2001) orZfy-u: 5'GCCATGTTGAGATAGGTTCTA3'Zfy-L: 5'TGGTAAGTAACAGCGTGAAA3'Mouse zinc finger y-chromosome: gen bank X14382.All oligonucleotides were synthesized by IDT (Integrated DNA Technologies, Inc. Coralville, IA).
Isolation and culture of mouse embryonic fibroblasts
Mouse embryos were removed at 13-13.5 days post conception and mouse embryonic fibroblasts (MEFs) were isolated and cultured in MEF media with 10% fetal bovine serum (FBS) (Suemori and Katsuji, 1987). After 24-48 hours in culture, the media were removed and cells were treated with trypsin, and re-cultured on 60 mm tissue culture dishes in MEF media. After 72 hours, plates were trypsinized and most cell pellets were resuspended in freezing media (90% FBS, 10% DMSO) and stored at -80o C for future experiments. Alternatively, cells were divided 1:3 and allowed to grow to 80% confluence before being detached and split again. MEFs were grown and treated at 37oC, 5% CO2, in MEF media/ 10% serum and Dulbecco's Modified Eagles' Medium (DMEM) supplemented with 4mm L-glutamine, 4500mg glucose/L and 1500 mg sodium bicarb/L, 1mM sodium pyruvate. (ATCC, Manassas, VA). Treatments included human recombinant IL-1ß (BD Biosciences, Bedford MA) 10 ng/μl, dosed at 3.33ng/ml. media, recombinant mouse fibroblast growth factor basic (bFGF; R&D Systems,Inc. Minneapolis, MN) or phorbol myristate acetate (PMA; Sigma-Aldrich, St. Louis,MO) 1X10-8 M from a stock in DMSO.
Trypsin was at 0.25% trypsin/2.21mM; EDTA in Hanks Balanced Salt Solution (HBSS) without sodium bicarbonate, calcium and magnesium, (Mediatech, Inc., Hendron, VA) and Penicillin/Streptomycin (Mediatech), 10,000 IU/ml penicillin, 10,000ug/ml streptomycin. FBS was purchased from Hyclone, US Characterized Fetal Bovine Serum (Thermo Scientific), HyClone Laboratories, Inc. (Logan UT).
Human dermal fibroblasts and mouse NIH/3T3 cells
Both cell types were purchased from ATCC and cultured as described (Wyatt et al., 2002; 2005). Briefly, cells were cultured in DMEM with 10% FBS and antibiotics (Wyatt et al., 2002, 2005). They were grown to confluence and used for experiments or passaged with trypsin.
Transient transfection of MMP-1 promoter constructs and luciferase assay
NIH/3T3 murine fibroblast cells were cotransfected with 1 μg of the 4372 bp of the human MMP-1 promoter (containing either the 1G or 2G allele at -1607 bp) linked to the luciferase reporter in the pGL3 basic vector, and 50 ng of a Renilla luciferase control vector (Wyatt et al., 2005). After 24 hours in serum free DMEM, luciferase levels were measured and expressed as relative light units (RLU's), and firefly luciferase levels were normalized to Renilla luciferase expression. Cells were left untreated or treated with bFGF (10 ng/ml).
Real time RT-PCR
Opticon (Biorad)Reverse transcription: taq man rt kit, ABI (Applied Biosystems, Foster City, CA,)Oligo d(T) primer, and/or random hexamers
Real Time PCR Primers:
Lac Z(U): 5'CGGCGAGTTGCGTGACTACCT
Lac Z(L): 5'GCGCTCCACAGTTTCGGGTTT3'
MMP-13 (U): 5'CCATTTTGATGATGATGAAAC3'
MMP-13 (L): 5'GTGCAGGCGCCAGAAGAATCT3'
B2M (U): 5'GCCGAACATACTGAACTGCTAC3'
B2M (L): 5'GGCCATACTGTCATGCTTAACT
GAPDH (U): 5'ACCACAGTCCATGCCATCAC3'
GAPDH (L): 5'TCCACCACCCTGTTGCTGTA3'
GAPDH primers were from Fahmai et al. (2001).
Protein Detection
The Bio-Rad DC protein assay measured total protein (Bio-Rad Laboratories, Hercules, CA) ß-galactosidase reporter gene activity was detected with the Gal-A kit,( Sigma, Saint Louis, MO.), and expressed as units of ß-galactosidase activity/μg protein.
RNA isolation
Media were removed from cells, and cells were washed 2X with HBSS without Ca++ or Mg++. Cells were trypsinized with 2.5 mls./plate of trypsin (Trypsin EDTA,1x : 0.25% trypsin/2.21mM EDTA in HBSS without sodium bicarbonate, calcium and magnesium (Mediatech) for 3 minutes at 37oC. Trypsin was neutralized with 7 mls. media-10% serum, cells removed to 15 ml. centrifuge tubes, and pelleted at 500X G. Pelleted cells were lysed with Qiashredders (Qiagen Inc, Valencia, CA) and RNA purified using the RNEasy isolation kits (Qiagen), including on column DNase treatment. RNA was quantitated using a spectrophotometer to determine absorbance at 260nm and 280 nm. Equal amounts of RNA from each sample (4 μg) were reverse transcribed to cDNA using Taq Man reverse transcription reagents (ABI). 250ng of the cDNA was used as a template for real-time PCR using ABI sybr green master mix and using the MJ research (Bio-Rad) Opticon fluorescent detection system. Genomic DNA isolation from mouse tails: Puregene tissue core kit A, Qiagen/Gentra.Southern Probes: (Bam H1 digest)1090 bp: -4372 (Mlu1) to -3282 (Pst1) or pcr fragments 5'ACTAACGCGTCCTCACATATTTCAAATCCAT3' (U)5'CTGTGCCACTGCAGTCCAGACA3'(L)(SanD1 digest)550bp: -512(Kpn1) +63(Hind111)-512 (U) 5'TGGTGTATCGCAATAGGGTAC3'GL2R (L) 5'CTTTATGTTTTTGGCGTCTTCCA3'
Statistical analysis
Statistical significance was determined by the Student's t test.
Acknowledgements
We would like to acknowledge support from the Dartmouth Center for Molecular, Cellular, and Translational Immunological Research, COBRE P20 RR15639, and the Dartmouth Transgenic and Genetic Construct Shared Resource for their assistance in generating the mice.
Supported by NIH-AR-26599 and NIH-CA-77267 to CEB
REFERENCES
- Astolfi CM, Shinohara AL, da Silva RA, Santos MC, Line SR, de Souza AP.2006Genetic polymorphisms in the MMP-1 and MMP-3 gene may contribute to chronic periodontitis in a Brazilian population J. Clin. Periodontol 33699-703 [DOI] [PubMed] [Google Scholar]
- Balbin M, Fueyo A, Knauper V, Lopez JM, Alvarez J, Lanchez LM, Quesada V, Bordallo J, Murphy G, Lopez-Otin C.2001Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation J. Biol. Chem 27610253-10262,. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM.2002Matrix metalloproteinases: a tail of a frog that became a prince Nat. Rev. Mol. Cell. Biol 3207-214 [DOI] [PubMed] [Google Scholar]
- Bronson SK, Plaehn EG, Kluckman KD, Hagman JR, Maeda N, Smithies O.1996Single-copy transgenic mice with chosen-site integration Proc. Natl. Acad. Sci. USA 939067-9072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrage PS, Mix KS, Brinckerhoff CE.2006Matrix metalloproteinases: role in arthritis. Front Biosci 11529-543 [DOI] [PubMed] [Google Scholar]
- Burrage PS, Brinckerhoff CE.2007Molecular targets in osteoarthritis: metalloproteinases and their inhibitors Curr. Drug Targets 8293-303 [DOI] [PubMed] [Google Scholar]
- Burrage PS, Huntington JT, Sporn MB, Brinckerhoff CE.2007aRegulation of Matrix Metalloproteinase gene expression by an RXR-specific ligand Arthritis Rheum 56892-904 [DOI] [PubMed] [Google Scholar]
- Burrage PS, Schmucker AC, Ren Y, Sporn MB, Brinckerhoff CE. Retinoid X receptor and peroxisome proliferators-activated receptor gamma agonists cooperate to inhibit matrix metalloproteinase gene expression. Arthritis Res. Ther. 2008;10:R139. doi: 10.1186/ar2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Armiento J, Dalal SS, Okada Y, Berg RA, Chada K.1992Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema Cell 71955-961 [DOI] [PubMed] [Google Scholar]
- D'Armiento JM, DiColandrea T, Dalal SS, Okada Y, Huang MT, Conney AH, Chada K.1995Collagenase expression in transgenic mouse skin causes hyperkeratosis and acanthosis and increase susceptibility to tumorigenesis Mol. Cell. Biol 155732-5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans V, Hatzolpoulos A, Aird WC, Rayburn HB, Kuivenhoven JA.2000Targeting the HPRT locus in mice reveals differential regulation of Tie2 gene expression in the endothelium Physiol. Genomics 267-75 [DOI] [PubMed] [Google Scholar]
- Fahmi H, Di Battista JA, Pelletier JP, Mineau F, Ranger P, Martel-Pelletier J.2001Peroxisome proliferator--activated receptor gamma activators inhibit interleukin-1beta-induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes Arthritis Rheum 44595-607 [DOI] [PubMed] [Google Scholar]
- Guillot PV, Liu L, Kuivenhoven JA, Guan J, Rosenberg RD, Aird WC.2000Targeting of human eNOS promoter to the HPRT locus of mice leads to tissue-restricted transgene expression Physiol. Genomics 277-83 [DOI] [PubMed] [Google Scholar]
- Hughes S, Agbaje O, Bowen RL, Holliday DL, Shaw JA, Duffy S, Jones JL.2007Matrix metalloproteinase single-nucleotide polymorphisms and haplotypes predict breast cancer progression Clin. Cancer Res 136673-6680 [DOI] [PubMed] [Google Scholar]
- Kanamori Y, Matsushima M, Minaguchi T, Kobayashi K, Sagae S, Kudo R, Terakawa N, Nakamura Y.1999Correlation between expression of the matrix metalloproteinase-1 gene in ovarian cancers and an insertion/deletion polymorphism in its promoter region Cancer Res 594225-4227 [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T.1997Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts Cell 89755-764 [DOI] [PubMed] [Google Scholar]
- Li J, Ghio AJ, Cho S-H, Brinckerhoff CE, Sidney A, Simon SA, Liedtke W.2009Diesel Exhaust Particles Activate the Matrix-Metalloproteinase-1 Gene in Human Bronchial Epithelia in a β-Arrestin-Dependent Manner via Activation of RAS Environ. Health Perspect 117400-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magness ST, Tugores A, Brenner DA.2000Analysis of ferrochelatase expression during hematopoietic development of embryonic stem cells Blood 953568-3577 [PubMed] [Google Scholar]
- Mengshol JA, Vincenti MP, Brinckerhoff CE.2001IL-1 induces collagenase-3 (MMP-13) promoter activity in stably transfected chondrocytic cells: requirement for Runx-2 and activation of p38 MAPK and JNK pathways Nucleic Acids Res 294361- 4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer BA, Wallace AM, Brinckerhoff CE, D'Armiento JM.2009Identification of a cigarette smoke-responsive region in the distal MMP-1 promoter Am. J. Respir. Cell Mol. Biol 404-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra RP, Bronson SK, Xiao Q, Garrison W, Jixuan L, Zhao R, Duncan SA.2001Generation of single-copy transgenic mouse embryos directly from ES cells by tetraploid embryo complementation BMC Biotechnol 112 -20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vinstersten K, Behringer R. Manipulating the Mouse Embryo. 3rd edition. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2003. [Google Scholar]
- Nishioka Y, Kobayashi K, Sagae S, Ishioka S, Nishikawa A, Matsushima M, Kanamori Y, Minaguchi T, Nakamura Y, Tokino T, Kudo R.2000A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter in endometrial carcinomas Jpn. J. Cancer Res 90612-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y, Sagai S, Nishikawa A, Ishioka S, Kudo R.2003A relationship between Matrix metalloproteinase-1 (MMP-1) promoter polymorphism and cervical cancer progression Cancer Lett 20049-55 [DOI] [PubMed] [Google Scholar]
- Ornskov D, Nexo E, Sorensen BS.Real-time PCR is a sensitive and precise method for quantifying transcription from reporter plasmids Scan. J. Lab. Invest 200464723-728 [DOI] [PubMed] [Google Scholar]
- Pearce E, Tregouet DA, Samnegård A, Morgan AR, Cox C, Hamsten A, Eriksson P, Ye S.2005Haplotype effect of the matrix metalloproteinase-1 gene on risk of myocardial infarction Circ. Res 971070-1076 [DOI] [PubMed] [Google Scholar]
- Pirhan D, Atilla G, Emingil G, Sorsa T, Tervahartiala T, Berdeli A.2008Effect of MMP-1 promoter polymorphisms on GCF MMP-1 levels and outcome of periodontal therapy in patients with severe chronic periodontitis J. Clin. Periodontol 35862-870 [DOI] [PubMed] [Google Scholar]
- Reid LH, Sheseley EG, Kim HS, Smithies O.1991Cotransformation and gene targeting in mouse embryonic stem cells Mol Cell. Biol 112769-2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond L, Eck S, Mollmark J, Hays E, Tomek I, Kantor S, Elliott S, Vincenti M.2006Interleukin-1 beta induction of matrix metalloproteinase-1 transcription in chondrocytes requires ERK-dependent activation of CCAAT enhancer-binding protein-beta J. Cell. Physiol 207683-688 [DOI] [PubMed] [Google Scholar]
- Rutter JL, Mitchell TI, Buttice G, Meyers J, Gusella JF, Ozelius LJ, Brinckerhoff CE.1998A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription Cancer Res 585321-5325 [PubMed] [Google Scholar]
- Suemori H, Katsuji N.1987Establishment of the embryo-derived stem (ES) cell lines from mouse blastocysts: effect of the feeder layer Dev. Growth Differ 29133-139 [DOI] [PubMed] [Google Scholar]
- Vivian JL, Klein WH, Hasty P.1999Temporal, spatial and tissue-specific expression of a myogenin-lacZ transgene targeted to the HPRT locus in mice Biotechniques 27154-162 [DOI] [PubMed] [Google Scholar]
- Wyatt CA, Coon CI, Gibson JJ, Brinckerhoff CE.2002Potential for the 2G single nucleotide polymorphism in the promoter of Matrix Metalloproteinase-1 to enhance gene expression in normal stromal cells Cancer Res 627200-7202 [PubMed] [Google Scholar]
- Wyatt CA, Geoghegan JC, Brinckerhoff CE.2005Short hairpin RNA mediated inhibition of Matrix Metalloproteinase-1 in MDA-231 cells: effects on matrix destruction and tumor growth Cancer Res 6511101-11108 [DOI] [PubMed] [Google Scholar]
- Yang B, Williamson RA, Sigmund CD.2000Appropriate tissue- and cell-type expression of a single copy human angiotensinogen transgene specifically targeted upstream of the HPRT locus by homologous recombination J. Biol. Chem 2751073-1078 [DOI] [PubMed] [Google Scholar]
- Ye S, Dhillon S, Turner SJ, Bateman AC, Theaker JM, Pickering RM, Day I, Howell WM.2001Invasiveness of cutaneous malignant melanoma is influenced by matrix metalloproteinase 1 gene polymorphism Cancer Res 611296-1298 [PubMed] [Google Scholar]
- Ye S, Gale CR, Martyn CN.2003Variation in the matrix metalloproteinase-1 gene and risk of coronary heart disease Eur. Heart J 241668-1671 [DOI] [PubMed] [Google Scholar]