Abstract
OBJECTIVES
This study evaluates the efficacy of combining proton irradiation with gemcitabine and the role the inhibitor of apoptosis proteins (IAP) survivin & XIAP play in the radiosensitive vs. radioresistant status of pancreatic cancer.
METHODS
The radioresistant (PANC-1) and radiosensitive (MIA PaCa-2) pancreatic carcinoma cells response to combined gemcitabine and proton irradiation was compared. Cells were treated with 0.1 - 500 μ-M gemcitabine and 0 - 15 Gy proton irradiation after which Trypan blue and flow cytometry were utilized to determine changes in the cell cycle and apoptosis. Expression levels of survivin were measured using Western blotting. Combination therapy with 24 h gemcitabine followed by 10-Gy proton irradiation proved most effective.
RESULTS
Gemcitabine and proton irradiation, resulted in increased survivin levels, with little apoptosis. However, combination therapy resulted in robust apoptotic induction with a concomitant survivin & XIAP reduction in the MIA PaCa-2 cells with little effect in the PANC-1 cells. siRNA studies confirmed a role for XIAP in the radioresistance of PANC-1 cells.
CONCLUSIONS
Our data demonstrate that combining gemcitabine and proton irradiation enhances apoptosis in human pancreatic cancer cells when XIAP levels decrease. Therefore, XIAP may play an important role in human pancreatic cancer proton radioresistance.
Keywords: Gemcitabine, Proton Irradiation, Survivin, XIAP, polyploidy, Inhibitor of Apoptosis
INTRODUCTION
Pancreatic cancer is the fourth most common cause of cancer death in men and women in the United States, with 5-year survival for all stages of disease less than 5% 1. Pancreatic cancer has no clear early warning signs or symptoms and is usually silent until the disease is well advanced. Patients have a median survival of 4-8 months after diagnosis due in part to the advanced stage the disease has already attained by the time it is discovered and treatment has begun. Risk factors include age with diagnosis occurring in people ages 65-79, smoking, sex, and possibly diets high in fat 2. Currently, if diagnosed early, surgical resection remains the only viable cure. However, only 20% of pancreatic cancer patients meet these criteria 3. It is therefore necessary to discover new therapies or therapeutic combinations in order to significantly impact this deadly disease. The anti-metabolite agent gemcitabine is currently being employed to treat pancreatic cancer 4. While gemcitabine has shown significant benefit in clinical applications, its ability to more than modestly impact pancreatic cancer is limited. It has been speculated that combinatory treatments using gemcitabine and other chemotherapeutics or radiotherapeutics could improve survival rates 5, 6. Proton radiotherapy has been investigated for a number of cancer types including cancers of the prostate, head & neck and brain 7-9. Protocols are also currently in progress or development for treating a variety of additional cancer types including: carcinoma of the nasopharynx, paranasal sinus carcinoma, non-small-cell lung carcinoma, hepatocellular carcinoma and pancreatic cancer 10. Pancreatic cancers though inherently resistant to photon radiation may be safely treated using protons. Proton therapy allows dose escalation to improve local tumor control in anatomic sites and histologies where local control is suboptimal with photons 9. This improved dose localization reduces normaltissue doses resulting in lower acute and late toxicity.
Survivin, a member of the inhibitor of apoptosis protein (IAP) family has previously been shown to be a prognostic marker for pancreatic cancer patients 11-13 and has also been implicated in cancer cell radio- and chemotherapy resistance 14. Many recent reports have demonstrated that inhibiting survivin expression by antisense oligonucleotides 15, dominant negative mutation 16, 17, and ribozyme 18 can reduce cancer cell radio- and chemoresistance and may be important to resensitize these tumors to therapy.
The goal of this study was to examine the combined affect of gemcitabine and proton irradiation on the pancreatic cell lines PANC-1 (photon radioresistant) and MIA PaCa-2 (photon radiosensitive) and to determine whether the same survivin involvement in proton radiation resistance would be observed16, 19, 20.
MATERIALS AND METHODS
Cell Cultures
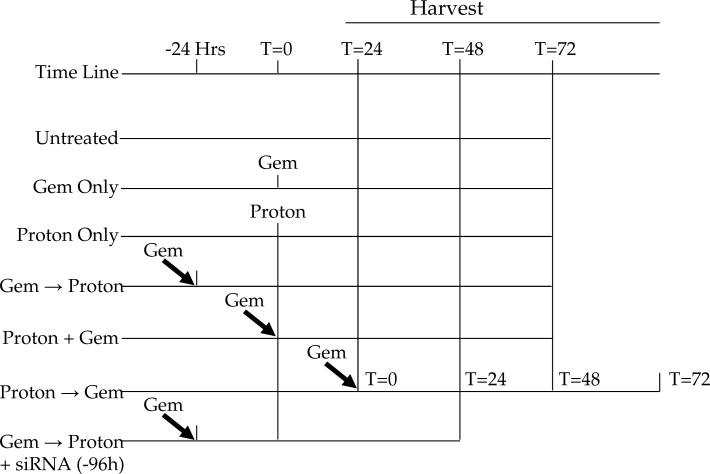
Pancreatic carcinoma (Panc-1 & MIA Paca-2) cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM supplemented with 100 units of penicillin, 100 μg/ml streptomycin, 300 μg of L-glutamine and 10% heat inactivated FBS (ATCC). MIA PaCa-2 media also included 2.5% horse serum (ATCC). Cells were grown at 37 °C in a humidified atmosphere of 95% air, 5% CO2. Gemcitabine or Gemzar™ (Eli Lilly and Company, Indianapolis, Indiana) was dissolved in water and added to cells for the duration of 24 hours prior, simultaneously or 24 hours after radiation exposure. Post treatment, the cells were returned to the incubator for an additional 24, 48, or 72 h. All radiation procedures were accomplished in the Loma Linda University Radiobiology Proton Treatment Facility, now the James M. Slater, MD, Proton Treatment and Research Center. Cells were exposed in vitro to 250 MeV protons with doses ranging from 0 to 15 Gy at four different dose rates: a low dose rate of 2.5 Gy/h, an intermediate dose rate of 5 Gy/h and two high dose rates of 10 and 15 Gy/h. Cells are treated as shown in Figure 1.
Figure 1.
Treatment schematic. Gemcitabine and protons were given at time = 0. Combination treatment of gemcitabine followed by proton radiation was treated with gemcitabine given at -24 hrs and then followed by proton irradiation at time = 0 (Gem → Proton). Simultaneous treatment was accomplished with both modalities being given at time = 0 (Proton + Gem). Proton irradiation was administered 24 hrs before gemcitabine treatment at time = 24 (Proton → Gem). All cells were harvested 24, 48, and 72 hrs after its final treatment was administered.
Apoptosis and Cell Cycle Analysis
Subconfluent cultures of the various cell lines were incubated with vehicle (water), gemcitabine (0 to 500 μM) or exposed to proton irradiation (0 to 15 Gy/h) for 0, 24, 48, and 72 hours at 37°C or combinations of gemcitabine and proton irradiation described above. Cells were harvested, prepared, and analyzed for DNA content as described previously 21. DNA content was analyzed using a Becton Dickinson FACScan flow cytometer (Becton Dickinson, San Jose, CA). The distribution of cells in the different phases of the cell cycle was analyzed from DNA histograms using BD CellQuest software (Becton Dickinson and Company, San Jose, CA) and FlowJo software (Tree Star, Ashland, OR).
Western Blot Analysis
Cells were solubilized, proteins (20-40 μg) separated using 12 or 15% Bis-Tris polyacrylamide gels, proteins transferred onto nitrocellulose membranes (Bio-Rad) and probed using the following antibodies: rabbit polyclonal anti-survivin (Novus, Littleton, CO) and GAPDH (Cell Signaling Technologies, Beverly, MA), and polyclonal β-actin (Abcam, Cambridge, MA). Secondary antibodies (IR-Dye-conjugated) were goat anti-rabbit immunoglobulin (LICOR, Lincoln, Nebraska). Immunoreactive bands were detected using the Odyssey imaging system (LICOR) and quantified using ImageQuant software. Protein quantifications presented in this report were normalized with respect to GAPDH or β-actin as indicated.
siRNA Knockdown
siRNA oligos were obtained for Survivin and XIAP knockdown (Santa Cruz Biotechnology, Santa Cruz, CA). In addition, a scramble siRNA was purchased for control. Amaxa Nucleofection technology was employed for transfection of PANC-1 cells. PANC-1 cells were cultured as described above and passaged 3 days before transfection. Nucleofection Kit R was used. PANC-1 cells were trypsinized, counted, and aliquoted into 1×106 cells per tube. Cells were spun down and resuspended in 100 uL of nucleofection solution. To this 1.5 ug of siRNA was added, the suspension was transferred to a nucleofection cuvette, and the suggested program was applied.. Immediately after program completion, 500 uL of fresh media was added and the cells were aliquoted equally into 6-well plates for further culture and treatment. Cells were cultured for 3 days after transfection to allow for gene knockdown. After this time, the appropriate treatments were applied.
Statistical Analysis
Statistical analysis was performed using a two-way analysis of variance (ANOVA) with the aid of JMP statistical software (Cary, NC). A paired t test was used for group analysis.
RESULTS
Gemcitabine-induced survivin protein is associated with growth inhibition and cytotoxicity in pancreatic cancer cells
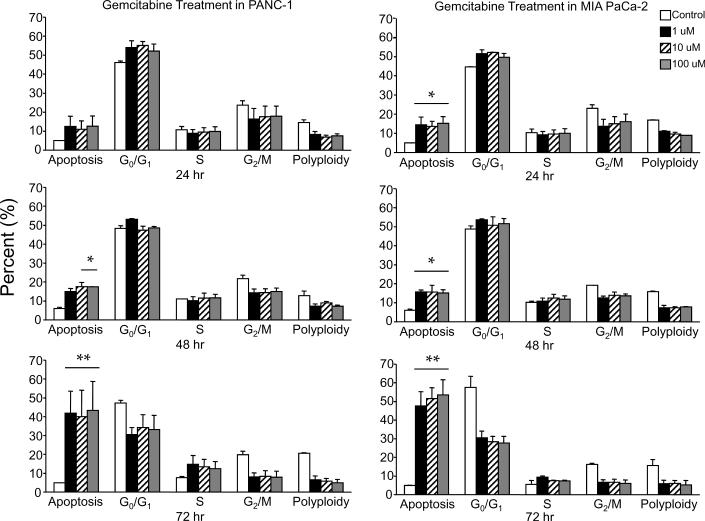
Treatment of PANC-1 or MIA PaCa-2 cells with various concentrations (100 μ1M, 10 μM and 1.0 μM) of gemcitabine resulted in a modest G0/G1 phase cell cycle arrest at 24 h, followed by the progressive appearance of apoptosis over the 48-72 h time interval (Figure 2A). Dose escalation of gemcitabine was insignificant, as 1 μM was as effective as 100 μM in inducing cell cycle arrest as well as apoptotic cell death in both cell lines. Between cell lines, the more radiosensitive MIA PaCa-2 cells were also more sensitive to gemcitabine than the radioresistant PANC-1 cells. Both cell lines in their non-treated resting state exhibited between a 10 and 20% polyploid fraction (cells containing greater then 4N DNA). Interestingly, after cells were gemcitabine treated, this polyploid fraction disappeared in both cell lines (Figure 2A).
Figure 2.
Gemcitabine treatment of PANC-1 and MIA PaCa-2 cell lines. (A) Cells were treated using 0 μM,1 μM, 10 μM, and 100 μM gemcitabine after which they were harvested and analyzed for DNA content by propidium iodide staining and flow cytometry at 24 hr, 48 hr, and 72 hr. Percentages of apoptotic cells with hypodiploid (sub-G1) DNA content as well as those in G0/G1, S, G2/M and polyploid are indicated per each condition tested. Data are the mean ± SD of three independent experiments (*p<0.01, **p<0.001). (B) Detergent-solubilized extracts of pancreatic cells treated with gemcitabine were analyzed at the indicated time intervals for reactivity with antibodies for survivin and GAPDH (loading control), by Western blotting. Molecular-weight (Mr) markers in kilodaltons are shown on the left.
Treatment of both PANC-1 and MIA PaCa-2 cell lines for 24 h with gemcitabine resulted in a dose-dependent reduction in survivin levels by Western blot analysis (Figure 2B). Further gemcitabine incubation of 48 h and 72 h resulted in survivin protein levels being enhanced or unchanged at doses of 1 and 10 μM in both cell lines, a result that is most likely due to druginduced stress 22. As a dose of 10 μM gemcitabine induced a time dependent G0/G1 arrest, enhanced cytotoxicity and 24 h reduction in survivin, this dose was chosen for all further experiments with MIA PaCa-2 cells. However, PANC-1 cells were treated with 100 μM gemcitabine due to their resistance to gemcitabine-induced cell death.
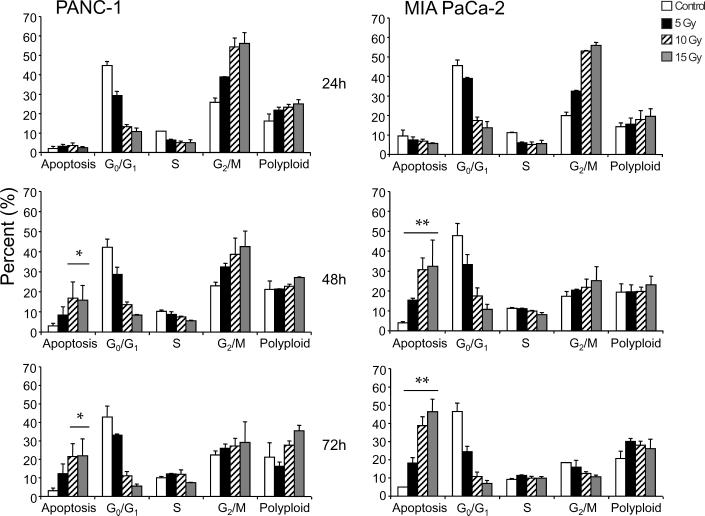
Treatment of PANC-1 or MIA PaCa-2 cells with various concentrations (0, 2.5, 5, 10, and 15 Gy) of proton irradiation resulted in significant cell cycle arrest in both the radiosensitive MIA PaCa-2 as well as the radioresistant PANC-1 pancreatic cell lines in a dose-dependent manner (Figure 3A). Unlike the results of gemcitabine in these two cell lines, only the radiation sensitive MIA PaCa-2 cells were induced to undergo notable levels of apoptosis. MIA PaCa-2 cells experienced a time and dose-dependent apoptosis with the G2/M arrested cells being the most sensitive as it is from this population of cells that the highest level of time-dependent death is recorded. Photon radioresistant PANC-1 cells were also resistant to proton radiation (Figure 3A) even though these cells also experienced a dose-dependent cell cycle arrest. In both cell lines, proton radiation induced a dose-dependent increase in polyploid cells from the 10% observed in the untreated controls to almost 30% in those treated with 15 Gy (Figure 3A).
Figure 3.
Proton irradiation of PANC-1 and MIA PaCa-2 cell lines. (A) Cells were treated using 0, 5, 10 or 15 Gy of proton radiation after which they were harvested and analyzed for DNA content by propidium iodide staining and flow cytometry at 24 hr, 48 hr, and 72 hr. Percentages of apoptotic cells with hypodiploid (sub-G1) DNA content as well as those in G0/G1, S, G2/M and polyploid are indicated per each condition tested. Data are the mean ± SD of three independent experiments (*p<0.01, **p<0.001). (B) Detergent-solubilized extracts of pancreatic cells treated with proton radiation were analyzed at the indicated time intervals for reactivity with antibodies for survivin and β-actin (loading control), by Western blotting. Molecular-weight (Mr) markers in kilodaltons are shown on the left.
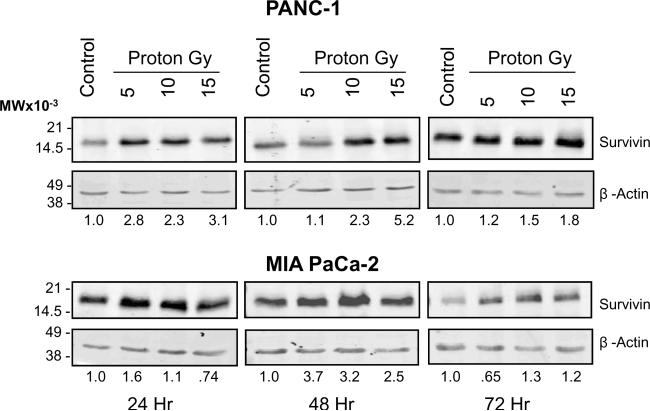
Treatment of both MIA PaCa-2 and PANC-1 cells lines with proton radiation resulted in a dose-dependent increase in survivin protein as defined by Western blot analysis (Figure 3B). This increase in survivin protein concomitant with the observed G2/M arrest is expected as survivin has been previously shown to be expressed during cell division in a cell cycle-dependent manner 22.
Sequential treatment of pancreatic cancer cells with gemcitabine and proton irradiation enhances the effect of single agent treatment in only MIA PaCa-2 cells
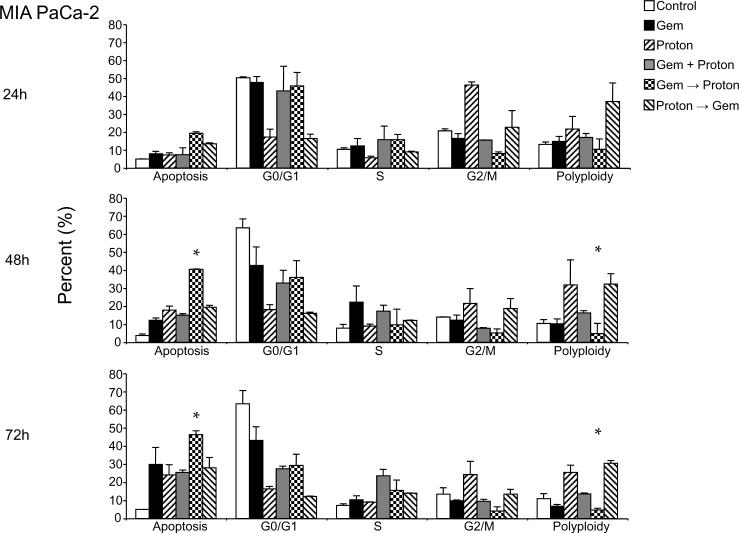
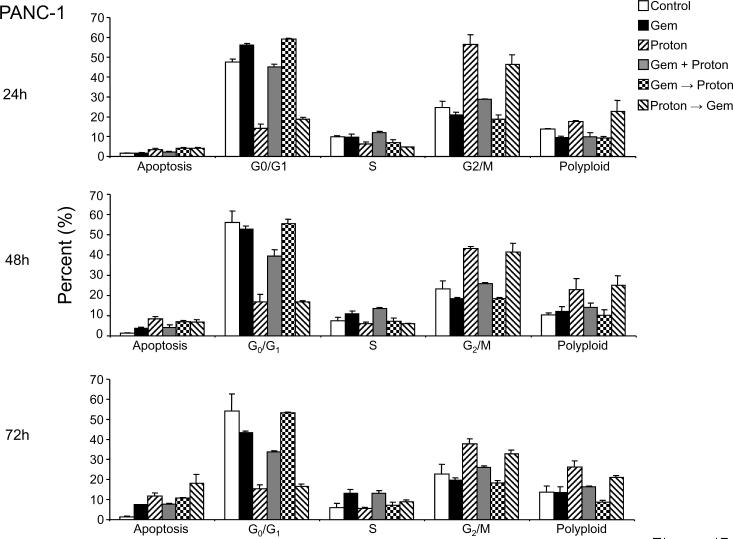
Treatment of MIA PaCa-2 cells with 10 μM gemcitabine (Figure 2A) and 10 Gy proton radiation (Figure 3A) resulted in modest levels of apoptosis, cell cycle arrest and survivin modulation in both cell lines with the most prominent killing effect in MIA PaCa-2 cells. We therefore combined the two modalities as shown in Figure 1.
Treatment of MIA PaCa-2 cells (Figure 4A) with 10 μM gemcitabine or 10 Gy proton irradiation resulted in cell cycle arrest at G0/G1 and G2/M respectively. For sequential treatments that include gemcitabine as the first modality in the treatment regimen, G0/G1 arrest was also the prominent phenotypic cell cycle change and likewise a G2/M arrest resulted from sequential treatments that used proton irradiation as the first modality in the treatment regime. Cell cycle arrest was followed by the progressive appearance of apoptosis over the 72 h time interval. However, sequential treatments where gemcitabine lead proton irradiation resulted in an enhanced apoptosis by 48 h, a trend that increased further by 72 h. An interesting observation first made with the single agent treatment experiments (Figure 2 & 3) is that gemcitabine treatment does not result in significant numbers of cells having greater than 4N DNA (polyploidy) while proton irradiation results in a progressive accumulation of polyploid cells. This is also observed in the sequential treatments where proton irradiation leads gemcitabine treatment. However, where gemcitabine and proton are given concurrently, little enhancement of this polyploid fraction is recorded and where gemcitabine leads the proton irradiation, an unremarkable number of polyploid cells are recorded (Figure 4A).
Figure 4.
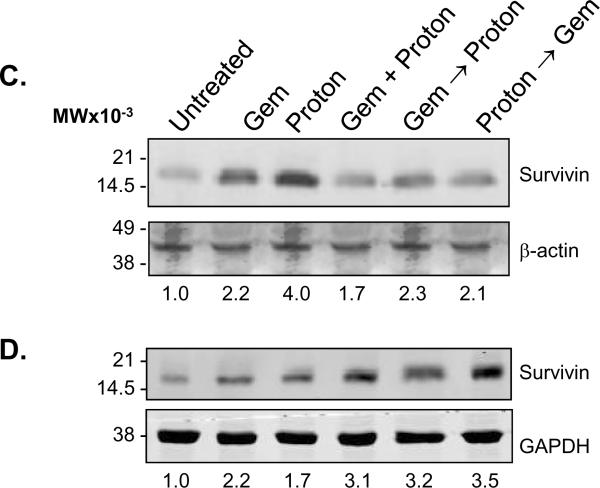
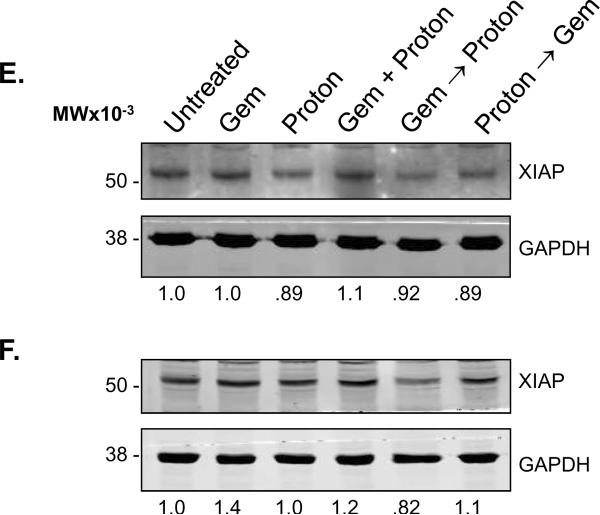
Combination Gemcitabine and Proton Radiation in PANC-1 and MIA PaCa-2 cell lines. (A) PANC-1 and (B) MIA PaCa-2 cells were treated using 10 Gy of proton radiation and 10 μM gemcitabine after which they were harvested and analyzed for DNA content by propidium iodide staining and flow cytometry at 24 hr, 48 hr, and 72 hr. Percentages of apoptotic cells with hypodiploid (sub-G1) DNA content as well as those in G0/G1, S, G2/M and polyploid are indicated per each condition tested. Data are the mean ± SD of three independent experiments (*p<0.01). Detergent-solubilized extracts of (C) PANC-1 and (D) MIA PaCa-2 cells treated with 10 Gy of proton radiation and 10 μM gemcitabine were analyzed at 48h for reactivity with antibodies for survivin and μ-actin or GAPDH (loading control), by Western blotting. (E) PANC-1 and (F) MIA PaCa-2 membranes were stripped and reprobed with antibodies for XIAP after which densitometric fold changes for each were indicated below. Molecular-weight (Mr) markers in kilodaltons are shown on the left.
Like MIA PaCa-2 cells, treatment of PANC-1 cells (Figure 4B) with 100 μM gemcitabine or 10 Gy proton irradiation alone or those combinations that lead with gemcitabine or proton irradiation also resulted in cell cycle arrest in G0/G1 and G2/M respectively. However, unlike MIA PaCa-2 cells, sequential treatments did not result in the progressive appearance of apoptotic cells, even though 10 fold higher concentration of gemcitabine was used. In fact, after 72 h of treatment, no significant changes are observed from those recorded after only 24 h of treatment. Polyploidy does however, match what was observed in the MIA PaCa-2 cells (Figure 4A).
Modulation of survivin protein expression by combining gemcitabine and proton irradiation in pancreatic cancer cell lines
To determine the potential relevance of targeting survivin for tumor cell apoptosis in sequential gemcitabine and proton irradiation treatments, survivin levels were analyzed by Western blotting in PANC-1 and MIA PaCa-2 cells treated with gemcitabine or proton irradiation alone or with the sequential combinations described previously at 48 h post treatment (Figure 1). Treatment of PANC-1 cells with gemcitabine or protons resulted in a 2 and 4 fold increase in survivin expression respectively (Figure 4C). In contrast, treatment of MIA PaCa-2 cells only showed a 2 to 3 fold increase in those cells treated with protons. Gemcitabine treatment for 48 h resulted in a down regulation of survivin protein (Figure 4D). Both cell lines exhibited very little change in survivin protein expression from that of the control in the sequential combination treatments (Figure 4C, D). XIAP has been known to interact more directly with the apoptotic pathway machinery than survivin 23. Both cell lines also exhibited very little change in XIAP protein expression compared to control, with the noticeable exception of gemcitabine → proton treatment (Figure 4E, F). This sequential treatment showed a marked decrease in XIAP protein expression, which may be responsible for the MIA PaCa-2 cells being more susceptible to the combination of gemcitabine and proton irradiation then the PANC-1 cells.
siRNA knockdown of XIAP further potentiates cell death after gemcitabine and proton combination therapy
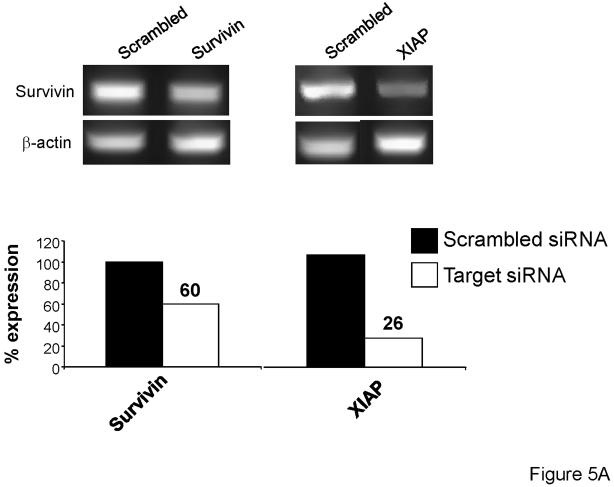
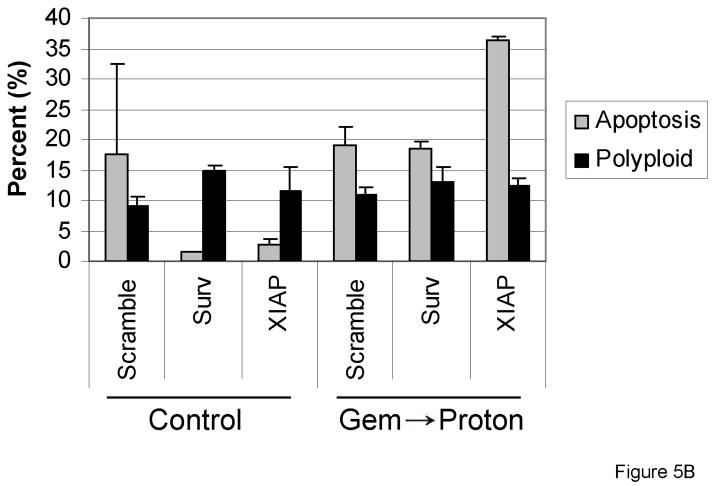
To further investigate the role survivin and XIAP may play in proton radiation resistance of the PANC-1 cells, siRNA knockdown experiments were completed. Three days after transfection with the siRNAs (described in Materials and Methods), cells were analyzed for the presence of Survivin and XIAP knockdown. PCR analysis indicated that survivin and XIAP knockdown was successful (Figure 5A), with approximately 75% knockdown of XIAP and 40% knockdown of Survivin. Furthermore, after 72h IAP knockdown, cells were treated with either gemcitabine, proton radiation, or 24h gemcitabine followed by proton radiation (Figure 1). Forty-eight hours after treatment, cells were harvested for propidium iodide flow cytometric analysis. As hypothesized, the addition of the XIAP siRNA to the PANC-1 cells resulted in a marked increase in gemcitabine/proton-induced apoptosis compared to that recorded in the cells having survivin knockdown or those of the control (Figure 5B).
Figure 5.
Knockdown of the inhibitor of apoptosis proteins survivin and XIAP, using siRNA, increases drug and radiation killing of PANC-1 pancreatic cancer cells. (A) Knockdown of survivin and XIAP expression. PANC-1 cells were transfected with either Scrambled siRNA or siRNA designed to knockdown survivin or XIAP. (B) PANC-1 cells were first transfected with siRNA against either survivin or XIAP for 12 h after which they were treated using 10 Gy of proton radiation and 10 μM gemcitabine. Cells were harvested and analyzed for DNA content by propidium iodide staining and flow cytometry at 48 hr. Percentages of apoptotic cells with hypodiploid (sub-G1) DNA content as well as the polyploid are indicated per each condition tested. Data are the mean ± SD of two independent experiments.
DISCUSSION
There has been little success in developing effective systemic therapies for the treatment of patients with locally advanced or metastatic pancreatic cancer. Chemotherapy was first combined with radiotherapy in the treatment of pancreatic cancer when clinicians at the Mayo Clinic in 1969 added 5-Fluorouracil (5-FU) to external beam radiotherapy. The result was an improved mean survival of 10.4 months for the combination therapy compared to 6.3 months for those patients receiving radiotherapy alone 24, 25. As a result, this combination has been considered standard therapy for locally advanced pancreatic cancer 25 and though multiagent regimens which include 5-FU have sought to improve upon this combination, randomized phase III trials have failed to confirm survival advantage over that with 5-FU alone 26. More recently, the nucleoside analog gemcitabine provided encouraging results in both antitumor effects and its impact on parameters of clinical benefit for patients with pancreatic cancer such as, decreased pain severity, decreased requirement for opioid analgesics, increased appetite and weight gain 26. In direct comparison on locally advanced pancreatic cancer and metastatic pancreatic cancer, gemcitabine treatment resulted in a 5.56 month overall survival compared to a 4.41 month overall survival using 5-FU. In combination with conventional radiotherapy gemcitabine extended overall survival to 11.3 months compared to 5-FU extending it by 10.4 months 25, 26. As a result, gemcitabine has become widely accepted for unresectable pancreatic cancer.
As pancreatic tumors are well advanced before detection, with survival reduced due to high rates of distant metastases, the continued use of conventional radiation-based therapies has been brought into question. As tumor loads increase, superfluous radiation delivered to surrounding normal tissue leads to increasing treatment morbidity. As a result, better control of dose distribution and localization are necessary. Proton radiotherapy allows for both. Where local control is suboptimal with conventional photon radiotherapy, proton radiotherapy provides improved physical dose distribution, and improved localization to anatomic sites and histologies. The resulting improvements to both dose distribution and localization will ultimately lead to dose escalation for anatomical sites where local control with conventional radiation dose has been suboptimal such as in advanced pancreatic disease 10, 27.
The aim of the current work was to define the involvement of survivin following chemotherapy and radiation therapy and to determine if proton irradiation followed classical radiation treatment observations. Our data shows that proton irradiation alone exhibited similar results as has been reported in photon radiation treatment. However, sequential treatment using gemcitabine before proton irradiation induced significant apoptotic cell death. While survivin seems to be minimally involved in the mechanism of radioresistance, our work provides evidence that XIAP down regulation may be involved in the sensitization of MIA PaCa-2 cells and the concomitant radioresistance of PANC-1 cells. It has been demonstrated that RNAi-mediated knockdown of XIAP as well as small molecule inhibitors of XIAP sensitize pancreatic cancer cells to apoptosis via activation of caspases 2, 3, 8 and 9, and loss of mitochondrial membrane polarization 28. Furthermore, XIAP small molecule inhibitors have been shown to synergize the effects of radiation and gemcitabine alone 29.
An important finding of these studies was the treatment of PANC-1 and MIA PaCa-2 cells with proton irradiation caused a significant number of the cells to become polyploid. Polyploidy is a state in which cells possess more than two sets of homologous chromosomes. It is commonly believed that polyploid cells arise after cellular stress, ageing, and in various diseases, perhaps because polyploidy confers a metabolic benefit 30-32. Polyploid cells have been shown to be genetically unstable and can be the intermediates where aneuploid cells become cancerous 32. In our hands, treatment of the pancreatic cancer cells lines with proton irradiation alone or before being combined with gemcitabine resulted in a significant enhanced polyploid fraction of cells (Figure 4). The cells treated with gemcitabine alone or with gemcitabine followed by proton irradiation prohibited this polyploidy. These findings suggest that proton irradiation-resistant pancreatic cells may gain enhanced genetic instability and ultimately a more aggressive tumor phenotype. However, administering gemcitabine as a pretreatment to proton irradiation may reduce this genetic instability and ultimately allow the proton irradiation to result in a more effective killing of the tumor. Furthermore, as polyploidy is a state of having more than two sets of chromosomes, survivin is a chromosomal passenger protein, and its deregulation in cancer promotes tetraploidy or aneuploidy, we strongly believe that by better understanding the role of gemcitabine and proton irradiation biology in regard to survivin expression modulation will provide useful data for the combining of therapies for the killing of radioresistant pancreatic cancer.
XIAP, a unique and best-characterized member of the inhibitor of apoptosis (IAP) family, has been identified as a central regulator of caspase-dependent apoptosis. Whether the activation of apoptosis is initiated by events that perturb the mitochondria (via caspase-9) or progress directly from cell surface receptors (via caspase-8), the ability of XIAP to inhibit the downstream executioner caspases-3 and -7 makes it a potent and broad inhibitor of cell death33 and important target for therapy. XIAP reduction has been reported in cells treated with protons and hypoxia in three kinds of cancers: lung, hepatoma and leukemia34. However, pancreatic cancers were not investigated. A broadened search to include photon and UV radiation revealed that much work has been accomplished investigating radiation-induced downregulation of XIAP and the mechanisms whereby this happens. A recent study describes UVB-induced sensitization coinciding with XIAP degradation which then allows for functional caspase 3-induced apoptosis35. Furthermore, the loss of XIAP was shown to be the result of UVB-enhanced Ikappa B alpha degradation, resulting in NF-kappaB-dependent transcriptional repression of XIAP35. Future studies will explore XIAP's involvement in the sequential chemo- and radiosensitization of pancreatic cancer as well as survivin's role in XIAP stabilization and the possibility of shifting the survival phenotype to apoptosis by interfering with this union.
ACKNOWLEDGMENTS
This work would have been impossible if not for a generous grant from the Hirshberg Foundation for Pancreatic Cancer Research and the friendship, inspiration and mentoring of Agi Hirshberg. Proton irradiation was accomplished at the Loma Linda University Radiobiology Proton Treatment Facility, now the James M. Slater, MD, Proton Treatment and Research Center. The authors would like to personally thank Dr. James Slater, Dr. Daila Gridley, Steven Rightnar and Celso Perez for all their help. We are also indebted to Dr. Jonathan Neidigh for supplying us with the gemcitabine. The authors would also like to thank Dr. Stephen Pandol and the Pancreatic Research Group he leads at the VA Greater Los Angeles Healthcare System (VAGLAHS) and UCLA.
Financial Support: Hirshberg Foundation for Pancreatic Cancer Research (NRW) NCMHD Project EXPORT Program 5P20MD001632/Project 3 (NRW) Start-up package from Loma Linda University's Center for Molecular Biology and Gene Therapy, now the Center for Health Disparities Research and Molecular Medicine (NRW).
REFERENCES
- 1.Kleeff J, Michalski C, Friess H, Buchler MW. Pancreatic cancer: from bench to 5-year survival. Pancreas. 2006;33(2):111–8. doi: 10.1097/01.mpa.0000229010.62538.f2. [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246(2):173–80. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller MW, Friess H, Koninger J, Martin D, Wente MN, Hinz U, Ceyhan GO, Blaha P, Kleeff J, Buchler MW. Factors influencing survival after bypass procedures in patients with advanced pancreatic adenocarcinomas. Am J Surg. 2008;195(2):221–8. doi: 10.1016/j.amjsurg.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Ueno H, Kiyosawa K, Kaniwa N. Pharmacogenomics of gemcitabine: can genetic studies lead to tailor-made therapy? Br J Cancer. 2007;97(2):145–51. doi: 10.1038/sj.bjc.6603860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reni M, Cereda S, Galli L. PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) for patients with advanced pancreatic cancer: the ghost regimen. Cancer Lett. 2007;256(1):25–8. doi: 10.1016/j.canlet.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Oettle H, Neuhaus P. Adjuvant therapy in pancreatic cancer: a critical appraisal. Drugs. 2007;67(16):2293–310. doi: 10.2165/00003495-200767160-00001. [DOI] [PubMed] [Google Scholar]
- 7.Olsen DR, Bruland OS, Frykholm G, Norderhaug IN. Proton therapy - a systematic review of clinical effectiveness. Radiother Oncol. 2007;83(2):123–32. doi: 10.1016/j.radonc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Slater JD, Yonemoto LT, Mantik DW, Bush DA, Preston W, Grove RI, Miller DW, Slater JM. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys. 2005;62(2):494–500. doi: 10.1016/j.ijrobp.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 9.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25(8):953–64. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 10.DeLaney TF. Clinical proton radiation therapy research at the Francis H. Burr Proton Therapy Center. Technol Cancer Res Treat. 2007;6(4 Suppl):61–6. doi: 10.1177/15330346070060S410. [DOI] [PubMed] [Google Scholar]
- 11.Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86(6):886–92. doi: 10.1038/sj.bjc.6600133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kami K, Doi R, Koizumi M, Toyoda E, Mori T, Ito D, Fujimoto K, Wada M, Miyatake S, Imamura M. Survivin expression is a prognostic marker in pancreatic cancer patients. Surgery. 2004;136(2):443–8. doi: 10.1016/j.surg.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92(2):271–8. doi: 10.1002/1097-0142(20010715)92:2<271::aid-cncr1319>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Li F. Survivin study: what is the next wave? J Cell Physiol. 2003;197(1):8–29. doi: 10.1002/jcp.10327. [DOI] [PubMed] [Google Scholar]
- 15.Kim KW, Mutter RW, Willey CD, Subhawong TK, Shinohara ET, Albert JM, Ling G, Cao C, Gi YJ, Lu B. Inhibition of survivin and aurora B kinase sensitizes mesothelioma cells by enhancing mitotic arrests. Int J Radiat Oncol Biol Phys. 2007;67(5):1519–25. doi: 10.1016/j.ijrobp.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Asanuma K, Kobayashi D, Furuya D, Tsuji N, Yagihashi A, Watanabe N. A role for survivin in radioresistance of pancreatic cancer cells. Jpn J Cancer Res. 2002;93(9):1057–62. doi: 10.1111/j.1349-7006.2002.tb02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesri M, Wall NR, Li J, Kim RW, Altieri DC. Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest. 2001;108(7):981–90. doi: 10.1172/JCI12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pennati M, Binda M, Colella G, Folini M, Citti L, Villa R, Daidone MG, Zaffaroni N. Radiosensitization of human melanoma cells by ribozyme-mediated inhibition of survivin expression. J Invest Dermatol. 2003;120(4):648–54. doi: 10.1046/j.1523-1747.2003.12082.x. [DOI] [PubMed] [Google Scholar]
- 19.Kami K, Doi R, Koizumi M, Toyoda E, Mori T, Ito D, Kawaguchi Y, Fujimoto K, Wada M, Miyatake S, Imamura M. Downregulation of survivin by siRNA diminishes radioresistance of pancreatic cancer cells. Surgery. 2005;138(2):299–305. doi: 10.1016/j.surg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Lee JU, Hosotani R, Wada M, Doi R, Kosiba T, Fujimoto K, Miyamoto Y, Tsuji S, Nakajima S, Nishimura Y, Imamura M. Role of Bcl-2 family proteins (Bax, Bcl-2 and Bcl-X) on cellular susceptibility to radiation in pancreatic cancer cells. Eur J Cancer. 1999;35(9):1374–80. doi: 10.1016/s0959-8049(99)00134-3. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Ackermann EJ, Bennett CF, Rothermel AL, Plescia J, Tognin S, Villa A, Marchisio PC, Altieri DC. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat Cell Biol. 1999;1(8):461–6. doi: 10.1038/70242. [DOI] [PubMed] [Google Scholar]
- 22.O'Connor DS, Wall NR, Porter AC, Altieri DC. A p34(cdc2) survival checkpoint in cancer. Cancer Cell. 2002;2(1):43–54. doi: 10.1016/s1535-6108(02)00084-3. [DOI] [PubMed] [Google Scholar]
- 23.Dohi T, Xia F, Altieri DC. Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection. Mol Cell. 2007;27(1):17–28. doi: 10.1016/j.molcel.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moertel CG, Childs DS, Jr., Reitemeier RJ, Colby MY, Jr., Holbrook MA. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2(7626):865–7. doi: 10.1016/s0140-6736(69)92326-5. [DOI] [PubMed] [Google Scholar]
- 25.Park JK, Ryu JK, Lee JK, Yoon WJ, Lee SH, Kim YT, Yoon YB. Gemcitabine chemotherapy versus 5-fluorouracil-based concurrent chemoradiotherapy in locally advanced unresectable pancreatic cancer. Pancreas. 2006;33(4):397–402. doi: 10.1097/01.mpa.0000236725.26672.be. [DOI] [PubMed] [Google Scholar]
- 26.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 27.Trofimov A, Nguyen PL, Coen JJ, Doppke KP, Schneider RJ, Adams JA, Bortfeld TR, Zietman AL, Delaney TF, Shipley WU. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69(2):444–53. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giagkousiklidis S, Vellanki SH, Debatin KM, Fulda S. Sensitization of pancreatic carcinoma cells for gamma-irradiation-induced apoptosis by XIAP inhibition. Oncogene. 2007;26(49):7006–16. doi: 10.1038/sj.onc.1210502. [DOI] [PubMed] [Google Scholar]
- 29.Karikari CA, Roy I, Tryggestad E, Feldmann G, Pinilla C, Welsh K, Reed JC, Armour EP, Wong J, Herman J, Rakheja D, Maitra A. Targeting the apoptotic machinery in pancreatic cancers using small-molecule antagonists of the X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2007;6(3):957–66. doi: 10.1158/1535-7163.MCT-06-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comai L. The advantages and disadvantages of being polyploid. Nat Rev Genet. 2005;6(11):836–46. doi: 10.1038/nrg1711. [DOI] [PubMed] [Google Scholar]
- 31.Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5(1):45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- 32.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17(2):157–62. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Mufti AR, Burstein E, Duckett CS. XIAP: cell death regulation meets copper homeostasis. Arch Biochem Biophys. 2007;463(2):168–74. doi: 10.1016/j.abb.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KB, Kim KR, Huh TL, Lee YM. Proton induces apoptosis of hypoxic tumor cells by the p53-dependent and p38/JNK MAPK signaling pathways. Int J Oncol. 2008;33(6):1247–56. [PubMed] [Google Scholar]
- 35.Thayaparasingham B, Kunz A, Peters N, Kulms D. Sensitization of melanoma cells to TRAIL by UVB-induced and NF-kappaB-mediated downregulation of xIAP. Oncogene. 2009;28(3):345–62. doi: 10.1038/onc.2008.397. [DOI] [PubMed] [Google Scholar]