Abstract
Purpose
The present investigation examined the effect of inflammation produced by intravesical zymosan on spinal dorsal horn neuronal responses to urinary bladder distension (UBD).
Methods
Extracellular single-unit recordings of neurons excited by UBD were obtained in spinalized female Sprague-Dawley rats. Neurons were classified as Type I - inhibited by heterotopic noxious conditioning stimuli (HNCS) or as Type II - not inhibited by a HNCS. In Experiment 1 - following neuronal characterization, 1% zymosan was infused into the bladder and after two hours spinal units were recharacterized. Control rats received intravesical saline or subcutaneous zymosan. In Experiment 2 – rats were pretreated with intravesical zymosan 24 hours prior to surgical preparation. Control rats only received anesthesia.
Results
137 spinal dorsal horn neurons excited by UBD were characterized. In comparison with controls, Type II neurons demonstrated increased spontaneous and UBD-evoked activity following intravesical zymosan treatment (both Experiments 1 & 2) whereas Type I neurons demonstrated either no change (Experiment 1) or decreased activity (Experiment 2) following bladder inflammation. No significant changes were noted in neuronal activity in control experiments.
Conclusions
Inflammation differentially affects subpopulations of spinal dorsal horn neurons excited by UBD that can be differentiated according to the effect of HNCS. This results in an altered pattern of spinal sensory transmission that may serve as the mechanism for the generation of visceral nociception.
Keywords: visceral, urinary bladder, cystitis, zymosan, spinal
INTRODUCTION
Stimulation of the viscera occurs continuously as part of normal physiological function, but conscious sensation of visceral events is normally minimal. However this changes under certain conditions when visceral stimuli may manifest as pain sensation. Visceral pain often occurs in the presence of inflammation of visceral structures (e.g., appendicitis). The mechanism whereby a low sensibility state is converted to a high sensibility state is still unknown although both central and peripheral mechanisms of sensitization have been proposed [1,6,9]. Leading theories include the recruitment of previously “silent” nociceptors by pathological processes such as inflammation or the sensitization of spinal dorsal horn neurons by the release of neuropeptides or glutamate. These processes are not mutually exclusive and each may have variable relevance to acute versus chronic visceral pain disorders. A combination of both peripheral and central phenomena is likely in most acute pathological disorders whereby the activation of a specific subset of afferent neurons may lead to alterations in central nervous system processing. This has been demonstrated in models of somatic inflammatory pain [19] and extrapolations to visceral models have been proposed [8,9]. In order to determine which components of the nervous system are altered by events that lead to visceral pain, a quantitative characterization of neurons responsive to UBD in the rat has been performed [12]. As characterized, these neurons demonstrate vigorous and reliable responses to UBD at graded intensities that extend into the noxious range (>40 mm Hg) and presentation of heterosegmental noxious conditioning stimuli (HNCS) produces a selective inhibition of a subgroup of these spinal nociceptive neurons. This phenomenon, termed “diffuse noxious inhibitory controls (DNIC)” by LeBars and colleagues [7] is the proposed mechanism of counterirritation whereby one noxious stimulus inhibits painful sensations from another body part. HNCS have been employed extensively in human studies to examine counterirritation effects [e.g.17]. The stratification of dorsal horn neurons according to their response to HNCS has proven to be predictive of their response to various pharmacological treatments [12,13,16] and to sensitization phenomena such as chemically-induced inflammation [14]. For this reason, neurons examined in the present study were characterized for both excitatory and inhibitory responses to segmental and heterosegmental stimuli to allow for appropriate stratification. The modulatory effect of bladder inflammation produced by the intravesical administration of the yeast cell wall component, zymosan, was assessed by examining responses to UBD in an acute fashion (before and two hours after the induction of cystitis) and after inflammation had been established (24 hours after intravesical zymosan treatment). These studies were approved by our local institutional animal use committee and adhered to the ethical guidelines established by the U.S. National Institutes of Health.
MATERIALS AND METHODS
Common methods
Female Sprague-Dawley rats (220–290g; Harlan) were anesthetized with mask halothane (2–5% induction; 1–2% maintenance). Jugular venous, arterial carotid, and tracheal cannulae were placed and the rats artificially ventilated. The upper cervical spinal cord was exposed at the level of the atlanto-occipital joint and infiltrated with 50 µl of 1% lidocaine and subsequently transected. The brain was mechanically pithed and the halothane anesthesia discontinued. Arterial blood pressure was continuously monitored and normal saline was administered as needed to prevent hypovolemia. The animals were kept warm with heating pads and overhead radiant heating and allowed to recover for four or more hours at which time they demonstrated brisk flexion reflex responses to tail and hindlimb pinch. Paralysis was then established with pancuronium bromide (0.2 mg/h i.v.) and a lumbar laminectomy was performed exposing the L6-S2 spinal cord segments. The rats were suspended by vertebral clamps and the dura mater was cut and removed and the exposed spinal cord covered with a protective layer of mineral oil.
Constant-pressure UBD (20–60 mm Hg, 20 s) was the noxious visceral test stimulus employed and was produced by inflating the urinary bladder with air via a 22 gauge angiocatheter placed via the urethra and held in place by a tight suture around the distal urethral orifice. UBD was administered as a phasic stimulus (rapid onset, rapid offset) as previously described distension [12]. Intravesical distending pressure was monitored via an in-line, low-volume pressure transducer. Multiple responses to a 60 mm Hg, 20 s UBD with intertrial interval of 4 minutes were determined and averaged as a “standard” response to UBD.
Tungsten microelectrodes were used for single-unit extracellular recordings 0–1.0 mm lateral to midline, 0.1–1.0 mm ventral to the spinal cord dorsum. To quantify neuronal responses, units were displayed on an oscilloscope for continuous monitoring, discriminated conventionally from background, converted into uniform pulses and saved by computer as peristimulus-time histograms. Spontaneous Activity was determined as the average rate of action potentials per second in the 10 second period prior to the onset of UBD. Total Activity was determined as the rate of action potentials in the 20 second period during the UBD stimulus. Evoked Activity was calculated as the difference between the Total Activity measure and the Spontaneous Activity. Responses (excitatory/inhibitory) to cutaneous inputs were determined following the presentation of multiple UBD trials using the following stimuli: brush with a cotton-tipped applicator (nonnoxious mechanical), pinch with a rat-tooth forceps at sufficient intensity to produce pain in the investigator (noxious mechanical.) The effect on Spontaneous Activity of a 5 second duration application of noxious mechanical stimulus to cervical or upper thoracic dermatomes (a heterosegmental noxious conditioning stimulus) was determined in all units. If inhibited >20%, then the neuron was defined as a Type I neuron. If excited, unaffected or inhibited < 20%, then the neuron was defined as a Type II neuron.
Descriptive statistics are reported as means ± SEM. Statistical comparisons were made using paired t-tests and/or repeated measures analysis of variance (ANOVA) with post hoc analysis performed using Tukeys HSD. Incidence data was analyzed using a χ2 analysis. p ≤ 0.05 was considered significant.
Experiment 1
In this experiment rats underwent surgical preparation and individual neurons were characterized. 0.5 ml of a 1% solution of zymosan A (Sigma-Aldrich, St. Louis, MO) in normal saline (14 rats) or 0.5 ml of normal saline (12 rats) was infused into the bladder and allowed to dwell for 30 minutes. A third group of 12 rats received 0.5 ml of the zymosan solution injected subcutaneously into the scalp instead of an intravesical treatment to serve as a systemic drug control group. The individual neurons were then recharacterized 2 hours later in order to assess changes that occur during the onset of inflammation. To allow a within-neuron assessment of the effect of the different treatments, the mean value of Evoked Activity produced by a standard 60 mm Hg, 20 s UBD (mean of multiple measures) was calculated for each individual neuron and the responses from each graded trial normalized as a percentage of this response.
Experiment 2
In this experiment 15 rats were anesthetized and eight had 0.5 ml of a 1% zymosan solution in normal saline instilled into their bladders via a transurethral catheter held in place with tape and allowed to dwell for 30 min prior to removal of the catheter and awakening from anesthesia; seven other rats were only anesthetized for 30 min. All rats received ampicillin (50 mg/kg s.c.). 24 hours after their pretreatment, rats were surgically prepared as described above and multiple dorsal horn neurons identified and characterized. The 24 hour timepoint was selected for study as it represents the peak period of inflammation due to intravesical zymosan treatment [21].
Evan’s Blue Extravasation
In order to determine whether intravesical zymosan produced an inflammatory response, plasma extravasation in the bladder was quantified two hours after zymosan treatment. Specifically, rats were anesthetized with inhaled halothane in oxygen, an intravesical catheter placed and the bladder distended with either 0.5 ml of normal saline (n=7) or a 1% zymosan solution (n=7) and allowed to dwell for 30 minutes. These rats were allowed to recover from anesthesia and then two hours later, were reanesthetized with urethane (1.5 gm/kg i.p.) along with an additional group of rats (n=7) naïve to any intravesical treatment. A jugular venous line was placed and Evan’s Blue (EB; 50 mg/kg i.v.) administered. After 15 min of circulation time, rats were overdosed with sodium pentobarbital, perfused intracardiac with 500 ml normal saline and the bladders removed, weighted and placed in 20 ml of dimethyl sulfoxide for the extraction of the EB. The amount of EB extracted was determined spectrophotometrically (620 nm) and normalized to the bladder weight. This protocol is identical to one previously published [21]
RESULTS
Experiment 1
Effects of acutely developing bladder inflammation
38 neurons were studied in these before-after (repeated measures) type of experiments. Of these, half (n=19) were inhibited by heterosegmental noxious conditioning stimuli (HNCS) and so were designated as Type I neurons. All of these neurons had cutaneous receptive fields in which both noxious and non-noxious stimuli produced excitatory responses. These receptive fields were unilaterally located and had well defined borders as noxious stimuli became inhibitory once skin outside the area of excitation was stimulated. Half of the neuronal sample (n=19) were not inhibited by HNCS and so designated as Type II neurons. Of these, ten were excited by both noxious and non-noxious cutaneous stimuli but nine were only excited by noxious stimuli. These cutaneous receptive fields were not as well defined and typically extended to include portions of the contralateral side. Baseline spontaneous activity of these groups were comparable (Type I neurons 7.6±1.4 Hz; Type II neurons 8.9±1.2 Hz). Two hours following intravesical zymosan treatment both groups had increased their levels of spontaneous activity (Type I neurons by 1.1±0.9 Hz, change not significant; Type II neurons by 8.4±2.4 Hz, p<0.05 for the change). There was a differential effect of intravesical zymosan on Type I versus Type II neurons with a robust increase in Evoked Response responses of Type II neurons but no statistically significant effect of intravesical zymosan on the Type I neurons (Figure 1 – top panel). There was no statistically significant effect of the intravesical saline or the subcutaneous zymosan injections in either Type I or Type II neurons two hours after treatment (Figure 1 – middle & bottom panel). Anecdotally, an expansion of cutaneous receptive field size was noted in multiple neurons following repeated distension and inflammation, but this phenomenon was not formally examined by the present study.
Figure 1.
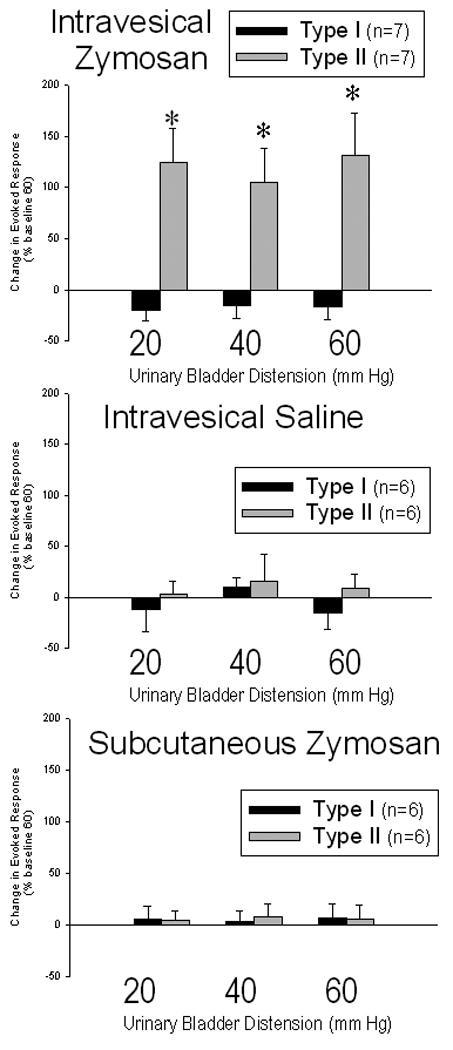
Group mean activity (± SEM) of L6-S1 dorsal horn neurons excited by urinary bladder distension (UBD) measured two hours after treatment with intravesical zymosan (top panel- inflamed bladder), intravesical saline (middle panel - procedure control) or subcutaneous zymosan (bottom panel – nonspecific action control). Activity was quantified as an Evoked Response produced by 20s of the indicated intensity of UBD and was normalized for each neuron as a percentage of the activity evoked by a 60 mm Hg, 20 s UBD measured 2 hrs previously. Neurons were stratified according to the effect of heterosegmental noxious conditioning stimuli (HNCS): Type I neurons were inhibited by HNCS and Type II neurons were not inhibited by HNCS. There was a differential effect of zymosan on Type I versus Type II neurons with a robust increase in Evoked Responses of Type II neurons but no statistically significant effect of intravesical zymosan on the Type I neurons. There was no statistically significant effect of the intravesical saline or the subcutaneous zymosan treatments in either Type I or Type II neurons. * indicates significant difference from baseline measurement, p<0.05.
Experiment 2
Effects of established bladder inflammation
A total of 99 dorsal horn neurons responsive to UBD were characterized: 54 in zymosan-pretreated rats and 45 in anesthesia pre-treated rats. Typical examples of activity of Type I and Type II neurons are presented as insets in Figure 2. There was a differential response of established bladder inflammation on the Type I versus Type II neurons. Bladder inflammation was associated with significantly higher rate of spontaneous activity in 28 Type II neurons (16.1 ± 2.1 Hz) when compared with spontaneous activity in 23 Type II neurons in rats with non-inflamed bladders (8.4 ± 1.6 Hz; difference p<0.01). This is in contrast to the lower rate of spontaneous activity of 26 Type I neurons in rats with inflamed bladders (2.6 ± 0.5 Hz) when compared with 22 Type I neurons in rats with non-inflamed bladders (11.3 ± 1.6 Hz; difference p<0.01). Evoked Responses to a 60 mm Hg, 20 UBD stimulus in this same sample of neurons followed the same pattern with greater activity in Type II neurons in rats with inflamed bladders (20.7±2.5 Hz) than in rats with non-inflamed bladders (12.0±2.8 Hz ; difference p<0.01) and lesser activity in Type I neurons in rats with inflamed bladders (11.3±1.9 Hz) than in rats with non-inflamed bladders (19.7±2.6Hz; difference p<0.05). Complete stimulus-response functions in a smaller sample of Type I and Type II neurons (Figure 2) suggest that the effects of bladder inflammation on neuronal responsiveness were present at a wide range of stimulus intensities.
Figure 2.
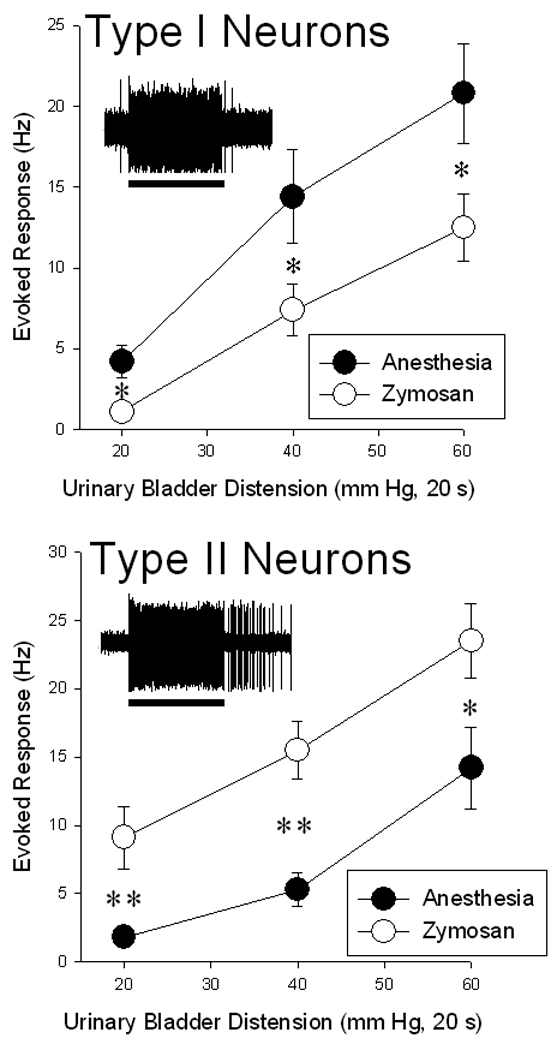
Stimulus-response functions relating group mean activity (± SEM) of L6-S1 dorsal horn neurons excited by Urinary Bladder Distension (UBD) in rats treated 24 hours prior to testing with either intravesical zymosan (open symbols – inflamed bladders) or with anesthesia alone (closed symbols – non-inflamed bladders). Neurons were stratified according to the effect of heterosegmental noxious conditioning stimuli (HNCS): Type I neurons (upper panel) were inhibited by HNCS and Type II neurons (lower panel) were not inhibited by HNCS. Insets indicate oscillographic tracings of typical extracellular recordings of individual neurons responding to a standard UBD (60 mm Hg, 20 s; indicated by bar below tracing). There was a differential effect of zymosan on Type I versus Type II neurons with a robust increase in Evoked Responses of Type II neurons and a statistically significant decrease in Evoked Responses of Type I neurons. * and ** indicate significant differences between groups with p<0.05 and p<0.01 respectively. N=18–22/group.
Evidence of inflammation
There was significantly more Evan’s Blue extravasated in the bladders of rats treated with intravesical zymosan two hours prior to measurement (786 ± 92 µg/kg) than in the bladders of rats which did not receive any intravesical treatments (254 ± 28 µg/kg; p<0.01 compared with the zymosan-treated rats). Distension of the bladder with intravesical normal saline also increased the amount of Evan’s Blue extravasated (502 ± 64 µg/kg; p<0.01 compared with rats that did not receive intravesical treatments) but not to the same extent as the intravesical zymosan treatment (p<0.05 for difference between saline-treated and zymosan-treated rats). Distension-evoked plasma extravasation has been noted by others [9] and may be due to peripheral neuropeptide release from primary afferents. In previous studies using identical methodology the amount of Evan’s Blue extravasated in the bladders of rats treated with intravesical zymosan 24 hours prior to testing was approximately 1200 µg/kg [21].
DISCUSSION
The most important finding of the present study was the demonstration of a differential effect of inflammation on two neuronal subpopulations of spinal dorsal horn neurons excited by UBD that can be stratified according to the effects of HNCS. Inflammation is a manipulation known to convert nonpainful bladder sensations into pain. A decreased vigor of responses was noted in one population (Type I neurons) and an increased vigor of responses in a different population (Type II neurons). These differences in responsiveness occurred without the presence of descending modulatory control as these neurons were studied in spinally transected rats. It is not known whether similar effects may have been observed in intact rats. Increased nociceptive reflex responses to UBD in rats has also been noted to occur in response to the same zymosan treatment [21]. Similar phenomena have been noted in studies using the visceral stimulus of colorectal distension (CRD) [14]. In the presence of colorectal inflammation produced by the mucosal application of the irritant turpentine, SUSTAINED neurons excited by CRD (a correlate to Type II neurons) became more active and ABRUPT neurons excited by CRD (a correlate to Type I neurons) became less active.
The present study used the stimulus of phasic urinary bladder distension due to its long history of use in electrophysiological studies [e.g., 8–10,11,20] and its utility in studies of reflex reponses to UBD [e.g. 2]. Although phasic distension does not mimic the slow filling that occurs during normal bladder function, the rapid onset and offset of this highly reproducible stimulus allow for the observation and quantification of time-linked phenomena. Effects of inflammation have been examined in bladder-related neuronal systems [8] with similar but less quantitative results. The neurons characterized in this study are quantitatively similar to those of previous studies in relation to ongoing spontaneous activity, cutaneous inputs and modulatory influences[12].
Female rats were employed in these studies for both technical and interpretative reasons: bladders are more easily manipulated in females and bladder pain is more prevalent in females. We have previously demonstrated that estrous cycle effects contribute to the variability of responses to urinary bladder distension [15] but chose not to formally control for these effects since the changes that occurred secondary to bladder inflammation were of sufficient magnitude to observe statistically significant effects despite the added variability.
The finding that inflammation produces both excitatory and inhibitory effects is important to understanding the spinal processing of nociceptive information. In the present study, there is evidence of a “balance” between excitatory and inhibitory influences on spinal nociceptive transmission that occurs when the sensory system is perturbed by an external stimulus such that an increase in the activity of one neuronal group was associated with a decrease in the activity of a different group. An inverse relation between the activity of two neuronal populations suggests that they may interact with each other forming a dual sensory pathway encoding for the same peripheral stimulus. A dual pathway allows both for redundancy of neuronal systems vital to the organism but also allows components of the systems to serve different functions. Central interpretation of peripheral events may therefore relate to the ratio of activities within differing pathways as much as it may relate to specific excitatory connections. Stating this mechanistically in relation to the present study, increased visceral pain perception may be a simple function of increased Type II neuronal activity ascending to supraspinal sites or alternatively may be more complexly due to the reduced Type I to Type II ratio of neuronal activities that ascend to supraspinal sites of processing. At the very least, it is clear that increased excitatory input to the spinal cord from the bladder results in a reactive inhibitory process that selectively affects Type I neurons with yet-to-be-defined secondary effects. Future experiments utilizing pharmacological manipulation of these neuronal subgroups may allow for a more precise definition of mechanism.
The present study did not attempt to localize precise laminar locations of neuronal recordings, but others such as Vizzard [22] have noted a change in the spinal distribution of neurons demonstrating excitation by UBD following bladder inflammation by using Fos-protein induction as an index of excitation. In her studies, inflammation produce a relative decrease in the number of UBD-excited neurons in the sacral parasympathetic nucleus and an absolute increase in the number of UBD-excited neurons located in the dorsal commissure area as well as the medial and lateral dorsal horn. The deep dorsal horn and dorsal commissure areas are of particular interest as they are the sites of location of many post-synaptic dorsal column neurons which are important in visceral nociception and which are induced to express increase NK1 receptors following visceral inflammation [5,18]. Vizzard [23] has also noted increases in CGRP and substance P input to the lumbosacral spinal cord following bladder inflammation. Hence, neuropeptides and receptors associated with increased neuronal excitability are both increased following bladder inflammation and so serve as possible mechanisms for the increased activity of Type II neuron. Numerous other biochemical changes occur in the spinal dorsal horn following bladder inflammation including alterations in TRPV1 channel expression [3] and neurotrophin receptors [4]. In aggregate, these changes may be responsible for the alterations in spinal neuronal activity noted in the present study.
In summary, the present study quantitatively examined the responses of neurons excited by UBD and determined the effect of inflammation on their responses. A differential effect was noted on two neuronal populations that could be differentiated by their individual responses to HNCS . Neurons which were inhibited by HNCS became less responsive to UBD whereas neurons which were not subject to this endogenous inhibitory system (not inhibited by HNCS) demonstrated an increased vigor of responses to UBD. This further demonstrates that at least two populations of spinal dorsal horn neurons exist which encode for UBD and which likely serve differing sensory functions including the perception of pain due to UBD. The mechanism of this differential response is yet to be elucidated.
ACKNOWLEDGEMENTS
Technical assistance of T. Uzzell and R. Cannon is gratefully acknowledged. Supported by DK51413 and DK078655.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Anand P, Aziz Q, Willert R, van Oudenhove L. Peripheral and central mechanisms of visceral sensitization in man. Neurogastroenterol. Motil. 2007;19:29–46. doi: 10.1111/j.1365-2982.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 2.Blatt LK, Lashinger ESR, Laping NJ, Su X. Evaluation of pressor and visceromotor reflex responses to bladder distension in urethane anesthetized rats. Neurourol. Urodyn. 2008 Nov; doi: 10.1002/nau.20650. epub. [DOI] [PubMed] [Google Scholar]
- 3.Charrua A, Reguenga C, Paule CC, Nagy I, Cruz F, Avelina A. Cystitis is associated with TRPV1b-downregulation in rat dorsal root ganglia. Neuroreport. 2008;19:1469–1472. doi: 10.1097/WNR.0b013e32830f1e73. [DOI] [PubMed] [Google Scholar]
- 4.Forrest SL, Keast JR. Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets pf pelvic afferent fibers. J.Comp. Neurol. 2008;506:989–1002. doi: 10.1002/cne.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishigooka M, Zermann D-H, Doggweiler R, Schmidt RA, Hashimoto T, Nakada T. Spinal NK1 receptor is upregulated after chronic bladder irritation. Pain. 2001;93:43–50. doi: 10.1016/S0304-3959(01)00288-3. [DOI] [PubMed] [Google Scholar]
- 6.Joshi SK, Gebhart GF. Visceral pain. Curr.Rev.Pain. 2000;4:499–506. doi: 10.1007/s11916-000-0074-7. [DOI] [PubMed] [Google Scholar]
- 7.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurons in the rat. Pain. 1979;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 8.McMahon SB. Neuronal and behavioural consequences of chemical inflammation of rat urinary bladder. Agents Actions. 1988;25:231–233. doi: 10.1007/BF01965020. [DOI] [PubMed] [Google Scholar]
- 9.McMahon SB, Dmitrieva N, Koltzenburg M. Visceral pain. Br. J. Anaesthesia. 1995;75:132–144. doi: 10.1093/bja/75.2.132. [DOI] [PubMed] [Google Scholar]
- 10.McMahon SB, Morrison JFB. Two group of spinal interneurones that respond to stimulation of the abdominal visceral of the cat. J. Physiol. (Lond) 1982;322:21–34. doi: 10.1113/jphysiol.1982.sp014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milne RJ, Foreman RD, Giesler GJ, Jr, Willis WD. Convergence of cutaneous and pelvic visceral nociceptive inputs onto primate spinothalamic neurons. Pain. 1981;11:163–183. doi: 10.1016/0304-3959(81)90003-8. [DOI] [PubMed] [Google Scholar]
- 12.Ness TJ, Castroman P. Evidence for two populations of rat spinal dorsal horn neurons excited by urinary bladder distension. Brain Res. 2001;923:147–156. doi: 10.1016/s0006-8993(01)03216-4. [DOI] [PubMed] [Google Scholar]
- 13.Ness TJ. Fedotozine and other kappa opioid agonists differentially inhibit two classes of spinal neurons excited by noxious colorectal distension. Gastroenterol. 1999;117:388–394. doi: 10.1053/gast.1999.0029900388. [DOI] [PubMed] [Google Scholar]
- 14.Ness TJ, Gebhart GF. Chemically-induced inflammation enhances reflex and spinal neuronal responses to noxious visceral stimulation in rats. Am. J. Physiol. 2001;280:G649–G657. doi: 10.1152/ajpgi.2001.280.4.G649. [DOI] [PubMed] [Google Scholar]
- 15.Ness TJ, Lewis-Sides A, Castroman P. Characterization of pressor and visceromotor reflex responses to bladder distention in rats: sources of variability and effect of analgesics. J. Urol. 2001;165:968–974. [PubMed] [Google Scholar]
- 16.Ness TJ, Randich A. Which spinal cutaneous neurons are inhibited by intravenous lidocaine in the rat? Reg. Anesth. Pain Med. 2006;31:248–253. doi: 10.1016/j.rapm.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Oono Y, Fujii K, Motohashi K, Umino M. Diffuse noxious inhibitory controls triggered by heterotopic CO2 laser conditioning stimulation decreased the SEP amplitudes induced by electrical tooth stimulation with different intensity at an equally inhibitory rate. Pain. 2008;136:256–365. doi: 10.1016/j.pain.2007.07.016. 2008. [DOI] [PubMed] [Google Scholar]
- 18.Palecek J, Paleckova V, Willis WD. Postsynaptic dorsal column neurons express NK1 receptors following colon inflammation. Neurosci. 2003;116:565–572. doi: 10.1016/s0306-4522(02)00660-7. [DOI] [PubMed] [Google Scholar]
- 19.Pace MC, Mazzariello L, Passavanti MB, Sansone P, Barbarisi M, Aurilio C. Neurobiology of pain. J.Cell Physiol. 2006;209:8–12. doi: 10.1002/jcp.20693. [DOI] [PubMed] [Google Scholar]
- 20.Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterol. 2005;129:1967–1978. doi: 10.1053/j.gastro.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Randich A, Uzzell T, Cannon R, Ness TJ. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC Urol. 2006;6:2. doi: 10.1186/1471-2490-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am.J.Physiol.Reg.Int.Comp.Physiol. 2000;278:R1027–R1039. doi: 10.1152/ajpregu.2000.278.4.R1027. [DOI] [PubMed] [Google Scholar]
- 23.Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J.Chem.Neuroanat. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]


