Abstract
Drug-associated cues and stress increase craving and lead to greater risk of relapse in abstinent drug addicts. This risk may be increased when these factors occur simultaneously. The current study examined whether the presentation of three different levels of intermittent footshock would trigger reinstatement or potentiate reinstatement of cocaine-seeking caused by conditioned cues. Male, Long Evans rats underwent daily i.v. cocaine self-administration, followed by extinction of lever responding in the absence of previously cocaine-paired cues. Reinstatement of cocaine-seeking was measured during presentation of cocaine-paired cues, following pretreatment with three levels of intermittent footshock (0.25, 0.5, and 0.75 mA), or after the combination of footshock and cues. Footshock at the 0.5 and 0.75 mA levels led to significant reinstatement when presented alone, and also potentiated the reinstatement triggered by the presentation of conditioned cues. These results demonstrate that while stress and drug-paired cues reinstate drug-seeking when presented in isolation, their interaction leads to potentiated reinstatement. Dual targeting of stress and cues is thus a critical consideration for treatment intervention in abstinent drug users.
Keywords: cocaine, cues, footshock, reinstatement, relapse, stress
1. Introduction
Relapse to drug-seeking and drug-taking in addicts is a major challenge to the successful treatment of cocaine addiction. A number of factors can trigger relapse, particularly exposure to drug-associated cues and contexts (such as drug paraphernalia or drug-associated environments), or stressful life events that occur during periods of abstinence. Clinical laboratory studies have shown that cocaine-associated stimuli increase craving in abstinent users [5], as does exposure to stress provoking stimuli [28]. The triggering of relapse in abstinent users has been modeled in animals using the self-administration and reinstatement paradigm. Animals trained to operantly respond for cocaine will reinstate responding on the previously drug-paired lever after exposure to cocaine-associated cues [22] or stress [24]. Over the last decade, the use of the reinstatement model has shown unique, yet overlapping neural circuitries of these varied forms of reinstatement of cocaine-seeking, as well as with other drugs of abuse [11, 14, 26], suggesting these factors could interact to promote reinstatement of drug-seeking.
Almost all previous animal model studies of the triggering events that initiate reinstatement of drug-seeking have examined these factors separately. However, the issue of the interaction of drug-related cues and stress is critical, as most human relapse situations invariably involve concurrent stress and conditioned environmental factors. We recently demonstrated that pharmacological stress induced by the noradrenergic alpha-2 receptor antagonist, yohimbine, not only produced reinstatement of cocaine-seeking, but also potentiated conditioned-cue induced reinstatement [10]. Several studies have confirmed the ability of footshock stress to trigger reinstatement of drug-seeking behavior [2, 4, 25]. In rats with a history of ethanol self-administration, footshock stress and conditioned ethanol cues interacted to augment the resumption of ethanol-seeking following extinction [18]. However, a previous study in rats with a history of cocaine self-administration reported that footshock stress only produced reinstatement in animals not extinguished to cocaine-associated cues [27], which is not the case for yohimbine-induced reinstatement [10]. This study also reported an inability of footshock to trigger reinstatement when presented without cues, which is in conflict with other data [2, 8, 9], but does support the interaction of cues and footshock to promote reinstatement.
In order to systematically determine the potentiative effects of footshock stress on conditioned cue-induced reinstatement, the current study examined the ability of four different levels of intermittent footshock (0 – 0.75 mA) alone or in the presence of previously cocaine-paired cues, to promote cocaine-seeking behavior at the time of reinstatement. We predicted that reinstatement of cocaine-seeking would occur to either stress or cues alone, and that footshock would produce an intensity-dependent enhancement of conditioned cue-induced reinstatement.
2. Experimental procedures
2.1. Subjects
Male, Long-Evans rats (initial weight 275–300 g; Charles River, Wilmington, MA, USA) were individually housed in a temperature- and humidity controlled vivarium on a reverse 12 h light–dark cycle (lights on 6 PM – 6 AM). Animals received water and standard rat chow (Harlan, Indianapolis, IN, USA) ad libitum, with the exception of 2–3 days of food restriction during the initial acquisition of cocaine self-administration. Housing and care of the rats were carried out in accordance with the guidelines of the Institute of Laboratory Animal Resources on Life Sciences, National Research Council.
2.2. Surgery
Rats were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively, IP), and equithesin (0.5 ml/kg with a solution of 9.72 mg/ml pentobarbital sodium, 42.5 mg/ml chloral hydrate, and 21.3 mg/ml magnesium sulfate heptahydrate dissolved in a 44% propylene glycol, 10% ethanol solution, IP). Surgical procedures were conducted using aseptic techniques. Catheters were constructed using previously described methods [12] and consisted of external guide cannulae with screw-type connectors (Plastics One Inc., Roanoke, VA, USA), Silastic tubing (10 cm; i.d. = 0.64 mm; o.d. = 1.19 mm; Dow Corning, Midland, MI, USA), prolite polypropylene monofilament mesh (2 cm diameter, Atrium Medical Corporation, Hudson, NH, USA), and cranioplastic cement. The end of the catheter was inserted into the right jugular vein, secured with suture, and exited on the rat’s back, posterior to the shoulder blades. To maintain patency, catheters were flushed once daily for 4 days after surgery with 0.1 ml each of an antibiotic solution of cefazolin (10.0 mg/ml; Schein Pharmaceuticals, Florham Park, NJ, USA) dissolved in heparinized saline (70 U/ml; Elkins-Sinn, Cherry Hill, NJ, USA) and heparinized saline alone (70 U/ml). For the duration of the experiment, each subject received 0.1 ml of heparinized saline (10 U/ml) immediately prior to self-administration and the cefazolin + 70 U/ml heparinized saline regimen following each session. To verify catheter patency, rats occasionally received a 0.12 ml infusion of methohexital sodium (10.0 mg/ml IV; Eli Lilly and Co., Indianapolis, IN, USA), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously.
2.3. Cocaine self-administration
Rats self-administered cocaine (cocaine hydrochloride dissolved in 0.9% sterile saline; cocaine provided by the National Institute on Drug Abuse, Research Triangle Park, NC, USA) during daily 2 h sessions according to an FR 1 schedule of reinforcement. At the start of each session, the catheter was connected to a swivel (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One Inc., Roanoke, VA, USA). Self-administration occurred in standard operant conditioning chambers (30×20×20 cm) linked to a computerized data collection program (MED-PC, Med Associates, Inc., St. Albans, VT, USA). The chambers were equipped with two levers, a stimulus light above each lever, a speaker linked to a tone generator (ENV-223HAM, Med Associates), and a house light on the back wall of the chamber. The house light signaled the initiation of the session and remained illuminated throughout the entire session. Lever presses on the active lever resulted in a 2 s infusion (0.2 mg/50 μl) and a 5 s presentation of a stimulus complex, consisting of activation of the white stimulus light above the active lever and the tone generator (78 dB, 4.5 kHz). Following each infusion, responding on the active lever had no consequences during a 20-s time-out period. Inactive lever presses had no consequences, but were recorded. Daily cocaine self-administration continued until each rat had obtained the self-administration criterion of 10 sessions with at least 10 infusions per session.
2.4. Extinction and reinstatement of cocaine-seeking
Following chronic cocaine self-administration and before the first reinstatement test, rats underwent daily 2 h extinction sessions during which active lever presses had no programmed consequences. Once extinction criterion was reached (a minimum of seven extinction sessions with ≤ 15 active lever responses per session for the last two consecutive days), each rat underwent seven separate reinstatement tests. Animals were extinguished to ≤ 15 active lever responses over 2 sessions between each reinstatement test. Footshock-induced reinstatement involved overnight housing (13 h) in the testing chamber prior to session initiation, as previously utilized in footshock-induced reinstatement studies [20]. During the last 15 minutes of those 13 h, animals then underwent 15 min of intermittent footshock (footshock duration 0.5 s, variable interval average = 40 s, intensity = 0, 0.25, 0.5, and 0.75 mA) delivered via a grid floor scrambler (ENV-414, Med Associates) within the testing chamber. We selected this range of footshock, since it includes a level (0.25 mA) below the range normally reported, as well as two levels (0.5 and 0.75 mA) that have been found to induce reinstatement of cocaine-seeking in previous studies [8, 23, 31]. After footshock delivery and a one min delay, the house light came on and both levers were extended to initiate the reinstatement test. During four of the reinstatement tests, presses on the active lever resulted in presentation of conditioned cues (0 mA test = cues alone condition, 0.25–0.75 mA test = cues + footshock condition). During the other three trials, presses on the active lever resulted in no programmed consequences (footshock alone condition). Testing was counterbalanced for condition (cue, footshock, cue+footshock). Prior studies have successfully utilized similar repeated reinstatement testing designs [10, 16] without any evidence of carryover effects between trials.
2.5. Data analysis
All data were analyzed using a two-way repeated measures analysis of variance (ANOVA) with footshock and cue as the main factors. Post-hoc pairwise comparisons were made using the Student-Newman-Keuls test. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Cocaine self-administration and extinction
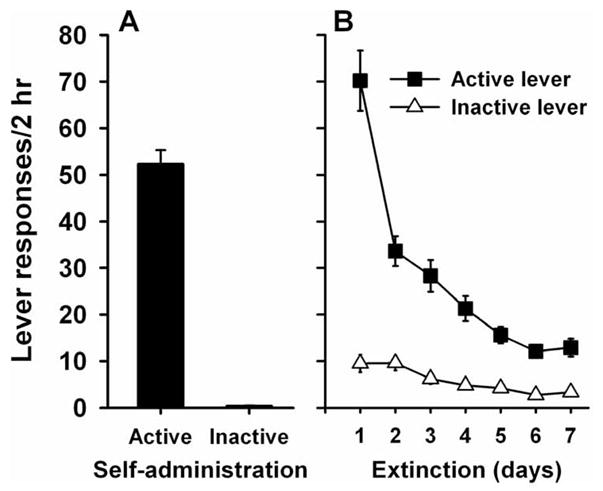
Rats readily acquired cocaine self-administration and demonstrated stable patterns of daily active lever responding and cocaine intake throughout the maintenance phase of the experiment (last 2 days of self-administration, mean active lever responses = 52.2±3.1, mean intake = 20.5±0=1.0 mg/kg, Figure 1A). Furthermore, all animals decreased responding during extinction sessions in the absence of cocaine infusions and cue presentations (Figure 1B). Active lever responding reached the established criterion (<15 active lever responses over two days) for a mean of 8.7±0.5 days before subsequent reinstatement testing. Mean lever presses on the day before the first reinstatement test = 7.9±0.7.
Fig. 1.
Mean number of responses on the active (black bars and squares) and inactive (white bars and triangles) levers on the last two days of cocaine self-administration (panel A) and the last seven days of extinction responding (panel B).
3.2. Reinstatement testing
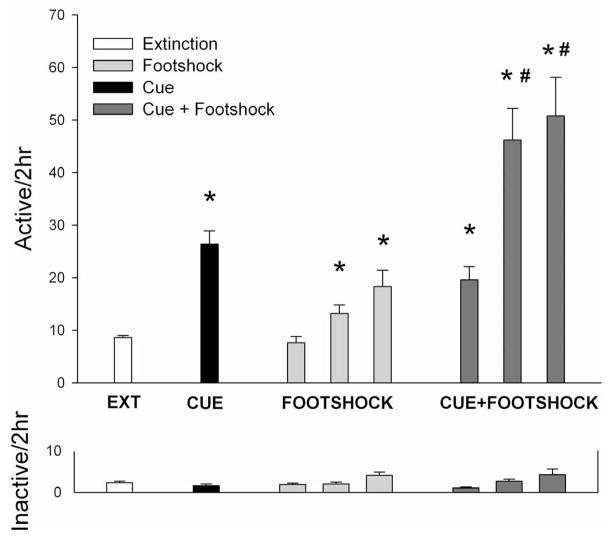
Data analysis revealed significant main effects of cue (F(1,189) = 76.25, p < 0.001) and footshock (F(3,189) = 13.016, p < 0.001), as well as the cue × footshock interaction (F(3,189) = 3.718, p < 0.05). Post-hoc analyses reveal that the presentation of each reinstatement factor led to significant reinstatement of responding on the previously cocaine-paired lever during testing. Presentation of conditioned cues significantly (p < 0.05) increased responding over extinction levels (mean active lever responses for cues = 26.4±2.5, extinction = 8.6±0.4), as did footshock at the 0.5 and 0.75 mA levels (mean active lever responses for 0.5 mA = 13.2 ± 1.6, 0.75 mA = 18.3 ± 3.1, p < 0.05). The lowest level of footshock did not significantly reinstate responding (mean active lever responses for 0.25 mA = 7.6 ± 1.2, p > 0.05). Footshock administration at the 0.5 and 0.75 mA levels prior to a conditioned cue reinstatement test led to significantly (p < 0.05) potentiated reinstatement responding beyond that of extinction levels, or that caused by footshock or cues alone (mean active lever responses for cues = 26.4±2.5; 0.5 mA + cues = 46.2 ± 6.0; 0.75 mA + cues = 50.8 ± 7.3). No order effects of reinstatement tests were found and no significant effects for inactive lever responding (which were uniformly low in magnitude) were seen between tests.
4. Discussion
The present data show the clear ability of intermittent footshock stress to enhance conditioned cue-induced reinstatement of cocaine-seeking in an animal model of relapse. Using varied levels of footshock, we demonstrated an intensity dependent facilitation (akin to a dose-response curve) for footshock, whereby the lowest mA footshock (0.25 mA) neither caused reinstatement nor potentiated conditioned cue reinstatement, while medium and high levels of footshock (0.5 and 0.75 mA) supported both types of reinstatement. These results concur with previous studies showing the additive effects for footshock+cue in the reinstatement of ethanol-seeking [18], and the potentiative effects of pharmacological stress on cue reactivity on cocaine-seeking in rats [10].
Previous studies using footshock to trigger reinstatement of drug-seeking have used variable procedures, including different footshock amplitude, length of exposure to the reinstatement chamber prior to testing, duration of the footshock session, and different strains of rats [7, 32]. As such, the degrees to which footshock has been reported to elicit reinstatement when presented in the absence of conditioned cues is varied as well. While a number of studies have shown footshock-induced reinstatement of drug-seeking [19], the magnitude of reinstatement often depends on the exact parameters used and the drug being examined. Footshock-induced reinstatement of heroin-seeking is generally quite robust, while reinstatement of cocaine-seeking caused by footshock is more moderate [23]. Previous reports have shown “responder vs. nonresponder” subpopulations for footshock-induced reinstatement for nicotine-seeking [32], and a previous report [27] suggests that footshock is ineffective at causing reinstatement of cocaine-seeking behavior in the absence of conditioned cues. The reasons for this discrepancy may be the level of footshock (1.02 mA in the Shelton and Beardsley study [27] vs. 0.25–0.75 mA in the current study), the pattern of exposure to the shock in the self-administration/reinstatement testing chamber, and/or the use of different rat strains. In order to parametrically examine the amplitude parameters necessary for reinstatement, the current study performed a within-subjects comparison of the efficacy of different levels of footshock to reinstate cocaine-seeking. While we saw clear amplitude dependent effects on reinstatement, rats generally have a “preferred” level of footshock best capable at producing reinstatement, i.e. a level of footshock that causes more robust reinstatement than others. Indeed, some rats only increased responding from extinction levels following a single footshock intensity (22% of the population). Further, although we saw significant reinstatement across the entire population, some animals did not increase responding to any level of footshock (11% of the population). These results help explain the varied results across other reports in the literature that generally report the use a single set level of footshock across all animals within a study. Alternatively, some studies assess the “best” level of footshock and adjust accordingly for each rat prior to the reinstatement test [31]. Since individual sensitivity to stressful stimuli is well-documented in other paradigms [6, 30], it is not surprising that individual variability to the effects of stress on drug-seeking are seen in relapse paradigms.
Previous studies have examined the neural circuitry underlying footshock stress-induced reinstatement and demonstrated a role for a number of critical brain nuclei [2, 8, 9, 20]. Among these structures is the bed nucleus of the stria terminalis (BNST), an area that must be functionally intact for footshock stress-induced, but not cocaine-primed reinstatement [20, 21]. While no prior studies have examined the neural substrates of footshock stress+cue reinstatement, we have recently demonstrated (Buffalari and See, unpublished observations) that yohimbine stress potentiation of conditioned cue-induced reinstatement relies on the BNST. Footshock stress potentiation of conditioned cue-induced reinstatement may be due to critical processing of stress and cue information at the level of the BNST. Alternatively, the central amygdala is important for the expression of both conditioned cue-induced [17] and footshock-induced [7] reinstatement. Footshock-induced has been shown to rely on corticotropin-releasing hormone projections from the central amygdala to the BNST [7] and noradrenergic activation of both of these areas. Activation of the BNST and/or CeA (via glutamatergic, noradrenergic and/or corticotropin-releasing hormone projections) may lead to convergent processing via corticostriatal circuits that are necessary for the expression of drug-seeking behavior triggered by all forms of relapse factors [13]. Interestingly, we see only modest potentiation of conditioned cue-induced reinstatement with intracerebroventricular corticotropin-releasing hormone administration (Buffalari and See, unpublished observations). Future experiments will need to target the exact neural substrates of stress-cue interactions on motivated drug-seeking during relapse.
The current results expand on a growing literature suggesting that drug addicted individuals may be more susceptible to drug relapse caused by exposure to drug-related cues or environments when under states of heightened stress and anxiety [29]. Further, individual variability may play a role in susceptibility to certain types of relapse factors, in particular to stress. Clinical reports suggest that cocaine addicted individuals report varied levels of craving to stressful stimuli [28], and different levels of withdrawal symptoms which may be relevant to treatment outcome [15, 1]. Whereas earlier studies, both clinical and preclinical, have focused on the effects of stress or cues in isolation, addicts are most likely to encounter multiple triggers for relapse during abstinence, and be more at risk during periods of greater stress or other maladaptive states, such as anxiety or depression [3]. Thus, animal models aimed at understanding the mechanisms underlying relapse need to consider the interactions that occur between factors that promote relapse to drug-seeking and drug-taking behaviors, and individual sensitivity to such relapse factors. Enhanced understanding of the relationship between stress and cues will inform the development of more effective treatment strategies and pharmacological interventions for the prevention of relapse in abstinent users.
Fig. 2.
Mean number of responses on the active (top panel) and inactive (bottom panel) levers on the last day of extinction responding (EXT), and conditioned-cue reinstatement tests (CUE), footshock stress reinstatement tests (FOOTSHOCK), and footshock plus conditioned-cue reinstatement test (FOOTSHOCK + CUE). Significant differences: *p<0.05 from EXT responding, #p<0.05 from CUE responding.
Acknowledgments
This research was supported by National Institute on Drug Abuse grants DA16511 and DA21690 (RES), 5F32 DA025411-02 (DMB), and NIH grant C06 RR015455. The authors thank Alisha Henderson, Sarah Wade Boatwright, and Bernard Smalls for technical assistance and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmadi J, Kampman K, Dackis C. Outcome predictors in cocaine dependence treatment trials. Am J Addict. 2006;15:434–439. doi: 10.1080/10550490600998476. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- 3.Brady KT, Verduin ML, Tolliver BK. Treatment of patients comorbid for addiction and other psychiatric disorders. Curr Psychiatry Rep. 2007;9:374–380. doi: 10.1007/s11920-007-0048-0. [DOI] [PubMed] [Google Scholar]
- 4.Buczek Y, Le AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- 5.Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 6.DeTurck KH, Vogel WH. Factors influencing plasma catecholamine levels in rats during immobilization. Pharmacol Biochem Behav. 1980;13:129–131. doi: 10.1016/0091-3057(80)90132-x. [DOI] [PubMed] [Google Scholar]
- 7.Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- 8.Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- 9.Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Feltenstein MW, See SE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 14.Kalivas PW, Toda S, Bowers MS, Baker DA, Ghasemzadeh MB. The temporal sequence of changes in gene expression by drugs of abuse. Methods Mol Med. 2003;79:3–11. doi: 10.1385/1-59259-358-5:03. [DOI] [PubMed] [Google Scholar]
- 15.Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, Cnaan A, Poole S, Muller E, Acosta T, Luce D, O’Brien C. Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001;63:69–78. doi: 10.1016/s0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 16.Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- 17.Kruzich PJ. See, R.E. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 20.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- 23.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 24.Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- 26.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Shelton KB, Beardsley PM. Interaction of extinguished cocaine-conditioned stimuli and footshock on reinstatement in rats. International Journal of Comparative Psychology. 2005;18:154–166. [Google Scholar]
- 28.Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- 29.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 30.Taylor J, Weyers P, Harris N, Vogel WH. The plasma catecholamine stress response is characteristic for a given animal over a one-year period. Physiol Behav. 1989;46:853–856. doi: 10.1016/0031-9384(89)90048-6. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]