Abstract
Objective
To assess the abilities of cisplatin, paclitaxel and flexible heteroarotinoid (Flex-Het) compound (SHetA2) to sensitize ovarian cancer cells to induction of the extrinsic apoptosis pathway by death receptor ligands, tumor necrosis factor α (TNFα) and TNF-related apoptosis-inducing ligand (TRAIL).
Study Design
The effects of various combinations of TNFα, TRAIL, cisplatin, paclitaxel and SHetA2 on viability and apoptosis in two established ovarian cancer cell lines, A2780 and SKOV3, and normal human primary endometrial cultures were measured with a cytotoxicity assay, flow cytometric analysis of annexin-V and propidium iodide staining and Western blot analysis of caspase 8 and 3 activation.
Results
Ovarian cancer and normal cells were resistant to TNFα and TRAIL. Cisplatin and paclitaxel did not increase sensitivity to these agents in either cell type. In contrast, combination of SHetA2 with TNFα or TRAIL induced a synergistic induction of apoptosis in cancer cells that involved activation of the extrinsic pathway caspase 8 and executioner caspase 3. The TRAIL combination was more potent than the TNFα combination. SHetA2 did not harm the viability of normal cells as a single agent or in combination with the death receptor ligands.
Conclusions
SHetA2, but not cisplatin or paclitaxel, can overcome resistance of ovarian cancer cells to TNFα and TRAIL without increasing sensitivity of normal cells to these death receptor ligands.
Keywords: Ovarian Cancer, Tumor Necrosis Factor alpha (TNFα), TNF-related apoptosis inducing ligand (TRAIL), Flexible Heteroarotinoid (Flex-Het), SHetA2, apoptosis
Introduction
Epithelial ovarian cancer is the most lethal of the gynecologic malignancies in developed nations with an estimated 15,000 disease related deaths in the United States annually. Due to the lack of disease-specific symptoms and adequate screening modalities, more than two-thirds of patients present with advanced-stage disease [1]. Platinum agents have long been recognized as the most active cytotoxic drugs in ovarian carcinomas. Addition of taxane to platinum-based chemotherapy demonstrated superior overall survival, with overall survival exceeding 48 months in optimally debulked, advanced stage patients, and now stands as the standard of care for adjuvant therapy of ovarian cancer [2]. Although most of these patients initially achieve complete responses to platinum-based chemotherapy regimens, they ultimately recur and succumb to progressive disease [3]. New cytotoxic agents with single-agent activity in recurrent ovarian cancer have been developed, however a recent international cooperative trial revealed that response rates or survival were not improved by combination of these agents with platinum and/or taxane in advanced epithelial ovarian carcinomas [3]. One cause of resistance to conventional chemotherapy is mutation in p53 and other molecules that mediate or regulate induction of the intrinsic apoptosis pathway by chemotherapy-induced DNA damage or cell stress [4, 5]. Targeting the intrinsic apoptosis pathway for anti-cancer drug development is also hindered by toxic side effects caused when it is induced in non-cancer cells.
A more recent approach toward developing new agents for ovarian cancer therapy has targeted induction of the extrinsic apoptosis pathway, which has shown promising differential effects in cancer versus normal cells. In contrast to the intrinsic pathway that involves mitochondrial release of proteins leading to activation of the inducer caspase 9, the extrinsic pathway is induced by activation of transmembrane death receptors leading to activation of caspase 8 [6]. Death Receptor Ligands (DRLs) and antibodies that activate death receptors have been evaluated for treatment of various cancers. Clinical trials of tumor necrosis factor α (TNFα), which activates the TNF-R1 death receptor, failed due to resistance and unacceptable toxicity, however multiple ongoing NCI-sponsored clinical trials are evaluating various methods for tumor-selective delivery of TNFα to reduce the toxicity [7-9]. TNF related apoptosis inducing ligand (TRAIL), which also activates the DR4 (TRAIL-R1) and DR5 (TRAIL-R2) death receptors, is a more promising biologic because it preferentially targets malignant cells [10, 11]. TRAIL (co-developed by Genentech and Amgen) is now in clinical trials [12]. Phase I and II clinical trials of humanized agonistic monoclonal antibodies against DR4 (mapatumumab: HGS-ERT1 developed by Human Genome Sciences) and DR5 (lexatumumab: developed by Human Genome Sciences, AMG655: developed by Amgen, and apomab: developed by Genentech) are demonstrating good tolerability and some promising clinical responses [13]. High levels of DR4 and DR5 expression have been observed in metastatic ovarian cancer effusions associated with poor response to chemotherapy and shorter survival suggesting that targeting the extrinsic apoptosis pathway is a viable alternative to overcome chemo-resistance [14].
The search for agents capable of increasing sensitivity to death receptor ligands without added toxicity could have a profound therapeutic benefit in the treatment of ovarian carcinoma. In preclinical studies, a large number of compounds have been shown to synergize with TNFα and TRAIL through a variety of mechanisms [13]. Flexible Heteroarotinoids (Flex-Hets) are a promising class of new compounds that regulate growth, differentiation, apoptosis and angiogenesis in cancer cells and tumors with minimal toxicity [15-20]. As a single agent, the Flex-Het lead compound, named SHetA2, differentially induces the intrinsic apoptosis pathway in malignant cells without impacting normal cells by directly targeting mitochondria and the Bcl2 family of proteins leading to activation of caspase 9 [19, 20]. The hypothesis of this study was that SHetA2 also differentially alters the sensitivity of cancer cells over normal cells to induction of the extrinsic apoptosis pathway by death receptor ligand activation leading to activation of caspase 8. The chemotherapeutic agents, cisplatin and paclitaxel, were also evaluated for comparison of SHetA2 with current standard of care therapeutics.
Materials and mehtods
Reagents and cell cultures
Cisplatin and Paclitaxel (LKT laboratories, St. Paul, MN) were dissolved in dimethyl sulfoxide (DMSO). Human recombinant TNFα (R&D systems, Minneapolis, MN) and TRAIL (Peprotech, Rocky Hill, NJ) were purchased as liquid stocks. SHetA2 was synthesized by Dr K. Darrel Berlin as described previously, dissolved in DMSO and manipulated under reduced lighting conditions [21]. A2780 (gift from Michael Birrer, National Cancer Institute, Bethesda, MD) and SK-OV-3 (ATCC) ovarian cancer cells were cultured in RPMI 1640 supplemented with 10% FBS, 1mmol/L HEPES buffer, 1mmol/L sodium pyruvate, and 1X antimycotic/antibiotic (Invitrogen Inc). Normal endometrial cells (EndomD1) were cultured from menstrual blood as previously described under an Institutional Review Board (IRB) approved protocol (16, 22).
Cytotoxicity Assays
Cells were plated into 96-well tissue culture dishes at a concentration of 10,000 cells/well. After a 24 hour incubation, cells were exposed to various concentrations of cisplatin (10-100μM), paclitaxel (5-20μM), SHetA2 (5-10μM) or no drug for 4 hours per the synergy data on TRAIL and cisplatin published by Kim [23]. SHetA2 was also evaluated at 0 and 6 hour pretreatment times. Cultures were then exposed to additional treatment with TNFα or TRAIL at concentrations of 5-20 ng/mL or no death receptor ligand for 24 hours. Cell Viability was measured with MTS reagent (Promega, Madison, WI) and expressed as fold of the untreated control.
Apoptosis Assays
Twenty-four hours after plating cells in 6-well tissue culture dishes, the cultures were treated with a range of SHetA2 concentrations alone or in combination with 20 or 30 ng/ml TRAIL for 24 hours. Apoptosis was detected using Annexin V FITC/PI from the Vybrant Apoptosis assay kit #3 (Invitrogen, Carlsbad, CA) and evaluated by flow cytometry using a Becton Dicknison FACS Caliber automated bench- top flow cytometer at an excitation wavelength of 488 and observation wavelengths of 530 and 575nm.
Western blots
Cells were plated at concentration of 2000 cells/10 cm tissue culture dish and allowed to adhere overnight. Media was replenished prior to treatment with varying concentrations of SHetA2 with or without 20ng/ml TNFα or TRAIL. Whole cell extracts prepared from cultures using M-PER (Pierce, Rockford, IL). Onehundred μg of protein were electrophoresed into wells of 10% SDS-Polyacrylamide gels and electrotransfered to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked in 5% non-fat dry milk in Tris-buffered Saline/Tween 20 (10nM, pH 8.0, 150mM NaCl, and 0.01% Tween 20) and then incubated caspase 8 or caspase 3 (Cell Signaling Technology, Danvers, MA) antibodies overnight at 4 °C. Membranes were washed with Tris-buffered saline/Tween 20 and then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour. After washes with Tris-buffered saline/Tween 20, bands were visualized by chemiluminiscence using luminol reagent (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were stripped with Blotfresh western blot stripping reagent (SignaGen, Ijamsville, MD), blocked with 5% nonfat dry milk in Tris-buffered Saline/Tween 20 for 1hour, and probed for loading control, B-actin (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical Analysis
Graphpad Prism software was used to plot and statistically analyse the data from the cytotoxocity assays. The differences in cell survival of cultures treated with SHetA2 in the absence and presence of TNFα or TRAIL was evaluated for statistical significance using Prism to run a two-way ANOVA. The differences in percent apoptosis were determined with Excel using two-tailed t-tests. Significance was established P<0.05.
Results
Effects of TNFα, TRAIL, cisplatin, paclitaxel and SHetA2 on cell survival
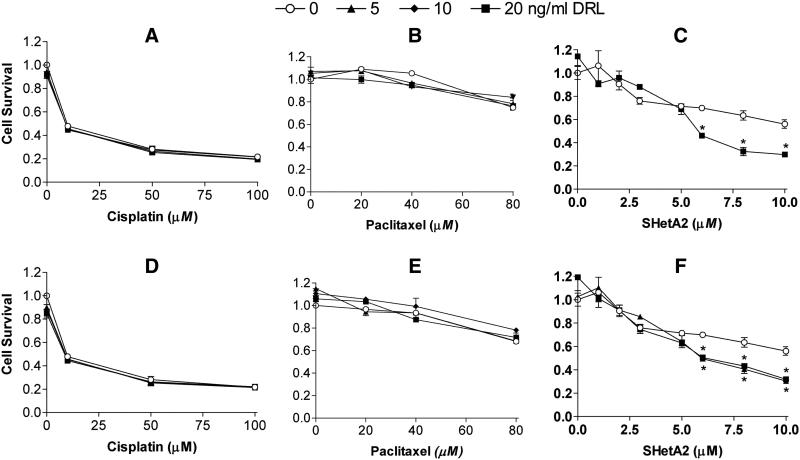
Cytotoxicity assays demonstrated that the A2780 ovarian cancer cell line was resistant to TNFα (Fig. 1A-C) and TRAIL (Fig. 1D-F) over a range of concentrations (all of the points at the zero concentration on the x-axis are not significantly different as determined by a two-tailed t-test, p>0.05). To determine if cisplatin, paclitaxel or SHetA2 could sensitize these cells, cultures were incubated with these agents for 4 hours prior to addition of TNFα or TRAIL. This pre-treatment time was chosen based on a report that maximal sensitization of cells to TRAIL by cisplatin pre-treatment was achieved at 4 hours (23). The candidate sensitizing agents were present during the subsequent 24 hr exposure to TNFα or TRAIL. Cisplatin induced a dose-responsive decrease in cell survival that was not enhanced by addition of TNFα (Fig. 1A) or TRAIL (Fig. 1D). Paclitaxel exerted a moderate effect on cell viability only at the highest concentration tested, and also did not increase cell susceptibility to TNFα (Fig. 1B) or TRAIL (Fig. 1E). A two-way ANOVA analysis demonstrated no significant effects of the combined treatments (p-values > 0.05).
Fig. 1. TNFα and TRAIL-resistant A2780 ovarian cancer cells are sensitized by SHetA2, but not by cisplatin and paclitaxel.
Cultures were pretreated with the indicated concentrations of cisplatin (A and D), paclitaxel (B and D) for 4 hours or SHetA2 (C and F) for 0 hours followed by 24 hour incubation with the addition of the indicated concentrations of DRL’s TNFα (A through C, upper panel) and TRAIL (D through F, lower panel). Cell survival was measured using a tetrazolium dye-based cytotoxicity assay and determined by dividing the average survival of cells in the treated cultures by the average survival of cultures treated with solvent only. All results were performed in triplicate. The results presented are representative of two independent experiments for cisplatin and taxol, and 4 independent experiments for SHetA2. *Two-way ANOVA comparing survival of cultures treated with SHetA2 only in comparison to the combined SHetA2 + TNFα or TRAIL indicated significant differences at SHetA2 concentrations >5 μM: Panel C) 6 μM SHetA2 ± 20 ng/ml TNFα p<0.01; 8 μM SHetA2 ± 20 ng/ml TNFα p<0.001; 10 μM SHetA2 ± 20 ng/ml TNFα p<0.01); and Panel F) 6 μM SHetA2 ± 20 ng/ml TRAIL p<0.05; 8 μM SHetA2 ± 20 ng/ml TRAIL p<0.01; 10 μM SHetA2 ± 20 ng/ml TRAIL p<0.05.
In contrast, SHetA2 induced a dose-responsive reduction in cell survival that was significantly enhanced by addition of TNFα or TRAIL only at concentrations of SHetA2 greater than 5 μM as determined by a two-way ANOVA (Fig. 1C). These data were generated with simultaneous treatment of SHetA2 and TNFα or TRAIL with no pretreatment. Additional experiments administering pretreatment for 4 and 6 hours also demonstrated significant sensitization to TNFα and TRAIL at SHetA2 concentrations greater than 5 μM, but the pretreatment did not enhance the effect (data not shown). Similar results were observed for SHetA2 sensitization of another ovarian cancer cell line (SK-OV-3) to TNFα and TRAIL (data not shown) and confirmed by the apoptosis analysis described below.
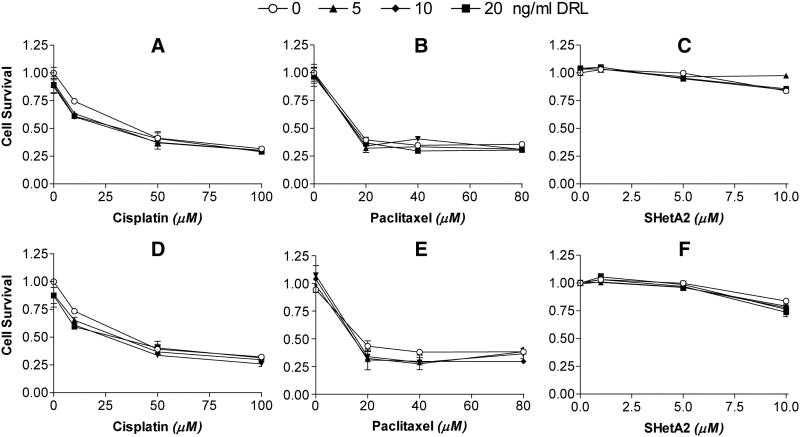
To evaluate the potential toxic effects of the SHetA2 and TNFα or TRAIL combination, primary cultures of normal endometrial cells were evaluated. These cells were chosen based on our ability to generate sufficiently large numbers of cells for repeated measurements of multiple drug combinations. Human ovarian surface epithelium (HOSE) cells were not utilized because of our inability to generate sufficient cell numbers of these slow growing cultures required for our experiments, and because our previous research demonstrated similar resistance patterns to SHetA2 as the endometrial cells used in this study (22). Also, the potential loss of the ovarian surface epithelial lining in a woman with ovarian cancer is less concerning than the potential loss of other epithelial linings. In this study, normal endometrial cells were resistant to TNFα and TRAIL (Fig. 2A-C and 2D-F), sensitive to cisplatin (Fig. 2A and D), sensitive to paclitaxel (Fig. 2B and E), and resistant to SHetA2 (Fig. 2C and F). None of these agents sensitized normal endometrial cells to TNFα (Fig. 2A through C) or TRAIL (Fig. 2 D through F).
Fig. 2. Normal endometrial cells are resistant to TNFα, TRAIL and SHetA2, but not cisplatin and paclitaxel.
Cultures were pretreated with the indicated concentrations of cisplatin (A and D), paclitaxel (B and D) or SHetA2 (C and F) for 4 hours followed by addition of the DRL’s TNFα (A through C, upper panel) or TRAIL (D through F, lower panel) at the concentrations depicted at the top of the graph. Cultures were then incubated with TNFα and TRAIL for 24 hours. Cell survival was measured using a tetrazolium dye based cytotoxicity assays and determined by dividing the average survival of cells in the treated cultures by the average survival of cultures treated with solvent only. All results were performed in triplicate. The results presented are representative of two independent experiments
Involvement of apoptosis in SHetA2 sensitization of cells to TNFα and TRAIL
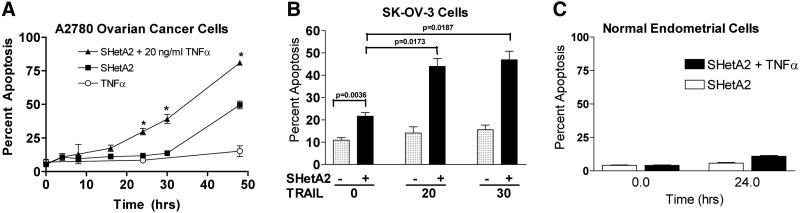
To determine if these effects on cell survival are due to induction of apoptosis, flow cytometric analysis of Annexin-V/PI staining was used to detect apoptosis in cancer and normal cultures after exposure to the various treatments. As reported previously, SHetA2 induced apoptosis in both A2780 (Fig. 3A) and SK-OV-3 (Fig. 3B) cell lines, but not in normal endometrial cells (Fig. 3B). Combined treatment of SHetA2 with TNFα induced a greater induction of apoptosis in the A2780 cancer cell line (Fig. 3A) and the SK-OV-3 cell line (data not shown), but not in the normal endometrial cells (Fig. 3C). Combined treatment of SHetA2 with TRAIL also increased the percent cells undergoing apoptosis in comparison to SHetA2 as a single agent in the SK-OV-3 (Fig. 3B) and A2780 (data not shown) cell lines. Since TNFα or TRAIL did not induce significant apoptosis as single agents (two-tailed t-tests p>0.05), the increased apoptosis observed when added to SHetA2 indicated synergy. At 24 hours, the combination of SHetA2 with 20 ng/ml TRAIL induced a greater level of apoptosis (Fig 3B, 44%) in comparison to the combination of SHetA2 with 20 ng/ml TNFα (Fig 3A. 30%).
Fig. 3. Sensitization to TRAIL and TNFα by SHetA2 is due to apoptosis.
A2780 (A) and SK-OV-3 (B) ovarian cancer cell cultures or primary normal endometrial cultures (C) were treated with indicated doses of SHetA2 in the presence or absence of the indicated concentrations of TNFα or TRAIL for the time indicated in A and C, and for 24 hours in B. The percent of cells undergoing apoptosis at the end of the treatment period was evaluated by flow cytometric analysis of Annexin-V/ PI staining of the cells. Two tailed t-tests were performed on percent apoptosis in cultures treated with SHetA2 only in comparison to SHetA2 combined with TNFα or TRAIL: A) 24 hr p value <0.0001, 30 hr p=0.0002, 48 hr p=0.0005; B) p values are depicted within the figure, and C) p>0.05 for all comparisons.
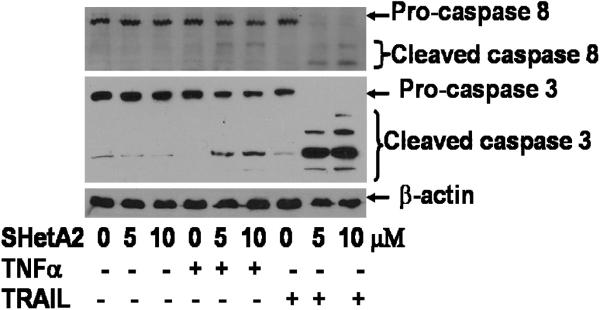
As an indicator of induction of the extrinsic apoptosis pathway, cleavage of pro-caspase 8 into the active form of caspase 8 was measured by Western blot of protein extracts from treated SK-OV-3 ovarian cancer cultures (Fig. 4). Caspase 8 cleavage was not induced by SHetA2, TNFα or TRAIL as single agents, but was induced when SHetA2 was combined with TNFα or TRAIL. Consistent with the greater enhancement of TRAIL over TNFα by SHetA2 in the apoptosis assays, caspase 8 cleavage was much more pronounced when SHetA2 was combined with TRAIL in comparison to when it was combined with TNFα. The pattern of cleavage of down-stream executioner caspase 3 was consistent with that observed for caspase 8. Similar results were observed for the A2780 cell line (data not shown)
Fig. 4.
Extrinisic initiator caspase 8 and executor caspase 3 are only activated when SHetA2 is combined with TNFα or TRAIL. SK-OV3 ovarian cancer cells were treated with indicated doses of SHetA2 with or without 20ng/ml of TRAIL or TNFα. Cell extracts were subjected SDS-PAGE and subsequent western blot analysis using antibodies against indicated proteins.
The flexible heteroarotinoid (Flex-Het) drug, SHetA2, sensitizes ovarian cancer cells, but not normal cells, to induction of extrinsic apoptosis by TNFα and TRIAL.
Discussion
The results of this paper demonstrate that resistance of ovarian cancer cells to death receptor ligands, TNFα and TRAIL, can be alleviated by a novel non-toxic drug SHetA2, but not by the current standard of care cytotoxic drugs cisplatin and paclitaxel. Consistent with our hypothesis, induction of the extrinsic pathway, as measured by cleavage of caspase 8 and downstream caspase 3, only occurred when SHetA2 was combined with TNFα and TRAIL. The combination with TRAIL was much more potent in reducing cell survival and activation of caspases 8 and 3 in comparison to TNFα, which is consistent with the different molecular mechanisms of action of these two death receptor ligands either up-stream or at the level of caspase 8 activation [24].
Lack of toxicity of these combination therapies on normal cells offers promise that the overall therapeutic ratio, and not just the general toxicities, of death receptor ligands can be improved by sensitization with a non-toxic agent like SHetA2. Differential induction of the intrinsic apoptosis pathway by SHetA2 on sets of cancer over normal cells has been noted previously in matching sets of cancer and normal cells from the same organ site (ovary, endometrium and kidney) and found to be caused by differential effects on mitochondria and the Bcl-2 family of proteins [19, 20, 25]. Lack of toxicity on normal cells in vitro is consistent with lack of overall toxicity, liver toxicity, skin irritation and teratogenicity in multiple animal models [15, 19, 26]. This lack of in vivo toxicity in combination with chemoprevention activity and in vivo inhibition of xenograft tumor growth prompted the National Cancer Institute (NCI) to award a Rapid Access to Preventive Intervention Development (RAPID) grant for preclinical development of SHetA2 as a chemoprevention agent. The results of this project indicate that SHetA2 also has potential for clinical application as sensitizer for death receptor ligands or their activating antibodies.
The results of others demonstrating additive or synergistic induction of apoptosis by combination of cisplatin, paclitaxel or other chemotherapeutic agents with TRAIL in vitro and in vivo may be due to different doses and treatment schedules or types of cancer cells used in those studies, which included small cell lung cancer, esophageal, mesothelioma, prostate and breast [37-31]. The major finding, however, is that when ovarian cancer cell lines are treated under similar conditions with these agents, SHetA2 reversed ovarian cancer cell resistance to death receptor ligands, while the standard chemotherapeutic agents did not.
In summary, the synergistic induction of the extrinsic apoptosis pathway by the combination of SHetA2 with TRAIL and to a lesser extent TNFα provides evidence that this drug could provide a means to sensitize ovarian cancers to biologics targeted at DR4, DR5 and TNF-R1 currently in clinical trials. The lack of toxicity and sensitization of SHetA2 in primary cultures of normal cells suggests that this drug will improve the overall therapeutic ratio without increasing the overall toxicity. The potential for translation of these results to clinical trials is increased as the preclinical testing of SHetA2 is almost completed and Phase I trials are being planned.
Acknowledgements
Supported by National Cancer Institute Grant #CA106713. We thank Jim Henthorn, Director of the University of Oklahoma Health Sciences Flow and Imaging lab for training and advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- [1].Young RC, Brady MF, Nieberg RK, Long HJ, Mayer AR, Lentz SS, et al. Adjuvant treatment for early ovarian cancer: a randomized phase III trial of intraperitoneal 32P or intravenous cyclophosphamide and cisplatin - a gynecologic oncology group study. J Clin Oncol. 2003;21(23):4360–55. doi: 10.1200/JCO.2003.02.154. [DOI] [PubMed] [Google Scholar]
- [2].Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2003;21(17):3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- [3].Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27(9):1355–8. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, et al. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807–10. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- [5].Fulda S, Debatin K-M. Targeting apoptosis pathways in cancer therapy. Current Cancer Drug Targets. 2004 Nov;4(7):569–76. doi: 10.2174/1568009043332763. [DOI] [PubMed] [Google Scholar]
- [6].Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006 Aug 7;25(34):4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- [7].Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncology. 2003 Sep;4(9):565–73. doi: 10.1016/s1470-2045(03)01196-3. [DOI] [PubMed] [Google Scholar]
- [8].Lans DLB Titia E., Libutti Steven K., Gnant Michael F.X., Liewehr David J., Venzon David J., Turner Ewa M., Richard Alexander H. Role of Tumor Necrosis Factor on Toxicity and Cytokine Production after Isolated Hepatic Perfusion1. Clinical Cancer Research. 2001 April;7:784–90. [PubMed] [Google Scholar]
- [9].Hao Wu JT, Su-Chang Lin. Smac Mimetics and TNFa: A dangerous Liaison? Cell. 2007 November 16;131:655–8. doi: 10.1016/j.cell.2007.10.042. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takeda K, Stagg J, Yagita H, Okumura K, Smyth MJ. Targeting death-inducing receptors in cancer therapy. Oncogene. 2007;26:3745–57. doi: 10.1038/sj.onc.1210374. [DOI] [PubMed] [Google Scholar]
- [12].Oldenhuis CNAM, Stegehuis JH, Walenkamp AME, de Jong S, de Vries EGE. Targeting TRAIL death receptors. Current Opinion in Pharmacology. 2008 Aug;8(4):433–9. doi: 10.1016/j.coph.2008.06.011. [DOI] [PubMed] [Google Scholar]
- [13].Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–98. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- [14].Dong HP, Kleinberg L, Silins I, Florenes VA, Trope CG, Risberg B, et al. Death receptor expression is associated with poor response to chemotherapy and shorter survival in metastatic ovarian cancer. Cancer. 2008;112:84–93. doi: 10.1002/cncr.23140. [DOI] [PubMed] [Google Scholar]
- [15].Benbrook DM, Kamelle SA, Guruswamy SB, Lightfoot SA, Hannafon B, Rutledge TL, et al. Flexible heteroarotinoids (Flex-Hets) exhibit improved therapeutic ratios as anti-cancer agents over retinoic acid receptor antagonists. Inv New Drugs. 2005;23:417–28. doi: 10.1007/s10637-005-2901-5. [DOI] [PubMed] [Google Scholar]
- [16].Benbrook DM, Lightfoot S, Ranger-Moore J, Liu T, Chengedza S, Berry WL, et al. Gene expression analysis of biological systems driving an organotypic model of endometrial carcinogenesis and chemoprevention. Gene Regulation and Systems Biology. 2008;2:21–42. doi: 10.4137/grsb.s344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guruswamy S, Lightfoot S, Gold M, Hassan R, Berlin KD, Ivey RT, et al. Effects of retinoids on cancerous phenotype and apoptosis in organotypic culture of ovarian carcinoma. J Nat Cancer Inst. 2001;93:516–25. doi: 10.1093/jnci/93.7.516. [DOI] [PubMed] [Google Scholar]
- [18].Myers T, Chengedza S, Lightfoot S, Pan Y, Dedmond D, Cole L, et al. Flexible Heteroarotinoid (Flex-Het) SHetA2 inhibits angiogenesis in vitro and in vivo. Investigational New Drugs. 2008 September 18; doi: 10.1007/s10637-008-9175-7. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu T, Masamha P, Chengedza S, Berlin KD, Lightfoot S, He F, et al. Development of Flexible-Heteroarotinoids (Flex-Hets) for Kidney Cancer. Molecular Cancer Therapeutics. 2009;8(5):1227–38. doi: 10.1158/1535-7163.MCT-08-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu T-Z, Hannafon B, Gill L, Kelly B, Benbrook DM. Flex-Hets differentially induce apoptosis in cancer over normal cells by directly targeting mitochondria. Mol Cancer Ther. 2007;6:1814–22. doi: 10.1158/1535-7163.MCT-06-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu S, Brown CW, Berlin KD, Dhar A, Guruswamy SB, Brown D, et al. Synthesis of flexible sulfur-containing heteroarotinoids that induce apoptosis and reactive oxygen species with discrimination between malignant and benign cells. J Med Chem. 2004;47:999–1007. doi: 10.1021/jm030346v. [DOI] [PubMed] [Google Scholar]
- [22].Kamelle SA, Sienko A, Benbrook DM. Retinoids and steroids regulate menstrual phase histologic features in human endometrial organotypic cultures. Fertil Steril. 2002;78(3):596–602. doi: 10.1016/s0015-0282(02)03302-2. 22. [DOI] [PubMed] [Google Scholar]
- [23].Kim YH, Lee YJ. Time sequence of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and cisplatin treatment is responsible for a complex pattern of synergistic cytotoxicity. J Cell Biochem. 2006;98:1284–1295. doi: 10.1002/jcb.20844. [DOI] [PubMed] [Google Scholar]
- [24].Diessenbacher P, Hupe M, Sprick MR, Kerstan A, Geserick P, Haas TL, et al. NF-kappaB inhibition reveals differential mechanisms of TNF versus TRAIL-induced apoptosis upstream or at the level of caspase-8 activation independent of cIAP2. Journal of Investigative Dermatology. 2008 May;128(5):1134–47. doi: 10.1038/sj.jid.5701141. [DOI] [PubMed] [Google Scholar]
- [26].Le TC, Berlin KD, Benson SD, Eastman MA, Bell-Eunice G, Nelson AC, et al. Heteroarotinoids with Anti-Cancer Activity Against Ovarian Cancer Cells. Open Med Chem J. 2007;1:11–23. doi: 10.2174/1874104500701010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7135–40. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shamimi-Noori S, Yeow WS, Ziauddin MF, Xin H, Tran TLN, XIe J, et al. Cisplatin enhances the antitumor effect of tumor necrosis factor-related apoptosis-inducing ligand gene therapy via recruitment of the mitochondria-dependent death signaling pathway. Cancer Gene Therapy. 2008;15:356–70. doi: 10.1038/sj.cgt.7701120. [DOI] [PubMed] [Google Scholar]
- [29].Shanhar S, Chen X, Srivastava RK. Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate. 2005;62(2):165–86. doi: 10.1002/pros.20126. [DOI] [PubMed] [Google Scholar]
- [30].Singh TR, Shankar S, Chen X, Asim M, Srivastava RK. Synergistic interactions of chemotherapeutic drugs and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand on apoptosis and on regression of breast carcinoma in vivo. Cancer Res. 2003;63(17):5390–400. [PubMed] [Google Scholar]
- [31].Kim M, Liao J, Dowling ML, Voong KR, Parker SE, Wang S, et al. TRAIL inactivates the mitotic checkpoint and potentiates death induced by microtubule-targeting agents in human cancer cells. Cancer Research. 2008 May 1;68(9):3440–9. doi: 10.1158/0008-5472.CAN-08-0014. [DOI] [PubMed] [Google Scholar]
- [32].Kim M, Liao JD, Voong KR, Parker SE, Wang S, El-Deiry WS, et al. TRAIL inactivates the mitotic checkpoint and potentiates death induced by microtubule-targeting agents in human cancer cells. Cancer Res. 2008;68(9):3440–9. doi: 10.1158/0008-5472.CAN-08-0014. M.L. [DOI] [PubMed] [Google Scholar]