Abstract
Respiratory-related complications are the leading cause of death in spinal cord injury (SCI) patients. Few effective SCI treatments are available after therapeutic interventions are performed in the period shortly after injury (e.g. spine stabilization and prevention of further spinal damage). In this review we explore the capacity to harness endogenous spinal plasticity induced by intermittent hypoxia to optimize function of surviving (spared) neural pathways associated with breathing. Two primary questions are addressed: 1) does intermittent hypoxia induce plasticity in spinal synaptic pathways to respiratory motor neurons following experimental SCI? and 2) can this plasticity improve respiratory function? In normal rats, intermittent hypoxia induces serotonin-dependent plasticity in spinal pathways to respiratory motor neurons. Early experiments suggest that intermittent hypoxia also enhances respiratory motor output in experimental models of cervical SCI, (cervical hemisection) and that the capacity to induce functional recovery is greater with longer durations post-injury. Available evidence suggests that intermittent hypoxia-induced spinal plasticity has considerable therapeutic potential to treat respiratory insufficiency following chronic cervical spinal injury.
Keywords: Respiratory plasticity, Cervical Spinal cord injury, Intermittent Hypoxia, Phrenic long term facilitation
1. Introduction
Cervical spinal cord injuries (SCI) are frequent in humans, compromising both respiratory and somatic motor function. In fact, respiratory failure is the most common cause of mortality following SCI (Frankel et al, 1998). In most cases, SCI is incomplete, enabling spontaneous partial recovery of respiratory and somatic motor function due to spontaneous (but limited) plasticity in spared spinal synaptic pathways (Goshgarian, 2003). However, after immediate therapeutic interventions during the acute phases of spinal injury (eg. stabilization of the spine to prevent further spinal damage), few effective SCI treatments are available (Winslow and Rozovsky, 2003). Since spontaneous functional recovery is seldom complete, there is considerable need for new therapeutic strategies to further enhance respiratory function in patients with chronic SCI. Despite considerable focus on the goal of regenerating damaged spinal pathways following SCI, only limited progress has been made. Enhancing endogenous mechanisms of plasticity in spared neural pathways may be a more achievable goal to promote functional recovery (Blight, 2004).
In recent years, we discovered that repetitive exposure to low oxygen (intermittent hypoxia) triggers plasticity in spinal pathways to respiratory motor neurons (Fuller et al., 2000; Mitchell et al., 2001; Feldman et al., 2003; Mahamed and Mitchell, 2008; MacFarlane and Mitchell, 2008a). The fundamental goal of this paper is to review available data concerning the potential of intermittent hypoxia-induced spinal plasticity to enhance respiratory function following cervical spinal cord injury. Certain protocols of intermittent hypoxia enhance respiratory motor function in rodent models of cervical spinal injury, leading us to suggest that limited protocols of intermittent hypoxia have unique potential as a therapeutic approach in the treatment of patients with chronic cervical SCI.
2. Intermittent Hypoxia induces spinal respiratory plasticity
Respiratory plasticity in uninjured rats can be induced by multiple intermittent hypoxia protocols. Acute intermittent hypoxia (AIH)-induced long-term facilitation (LTF) is one of the most thoroughly studied models of respiratory plasticity to date (for review, see: Mitchell et al., 2001; Mahamed and Mitchell, 2008; MacFarlane and Mitchell, 2008a). On the other hand, there are a variety of chronic or repetitive intermittent hypoxia protocols that induce unique forms of spinal respiratory plasticity or metaplasticity (Ling et al., 2001; Reeves and Gozal, 2006; Pawar et al., 2008; Wilkerson and Mitchell, 2008). Both AIH and chronic intermittent hypoxia (CIH) induce spinal respiratory plasticity in intact and spinally injured animals (Bach and Mitchell, 1996; Golder and Mitchell, 2005; Ling et al., 2001; Fuller et al., 2003), suggesting that intermittent hypoxia augments spared synaptic pathways to respiratory motor neurons, particularly after spinal injury.
2.1. Long-term facilitation (LTF) following acute intermittent hypoxia (AIH)
LTF is an intermediate form of plasticity, lasting at least 2 hours. LTF is expressed as an augmentation of respiratory motor output post-AIH (3 to 10 hypoxic episodes), and is most frequently studied in phrenic (spinal) and hypoglossal (brainstem) nerves (Mitchell et al., 2001; Baker-Herman and Mitchell, 2002). LTF is predominantly expressed as an increased nerve burst amplitude, with small and inconsistent effects on burst frequency in anesthetized and vagotomized animals (Mitchell et al., 2001; Baker-Herman and Mitchell, 2008). Ventilatory LTF has been demonstrated in unanesthetized rats (McGuire et al., 2002, 2004), and can be evoked in normal humans during sleep (Pierchala et al., 2008). The magnitude of LTF in phrenic and hypoglossal motor output is affected by age, sex, genetics and previous experiences (Mitchell et al., 2001; Fuller et al., 2000; Behan et al., 2003; Bavis et al., 2005), suggesting that these factors must be considered before successful therapeutic applications of intermittent hypoxia will be possible in patients with cervical spinal injuries.
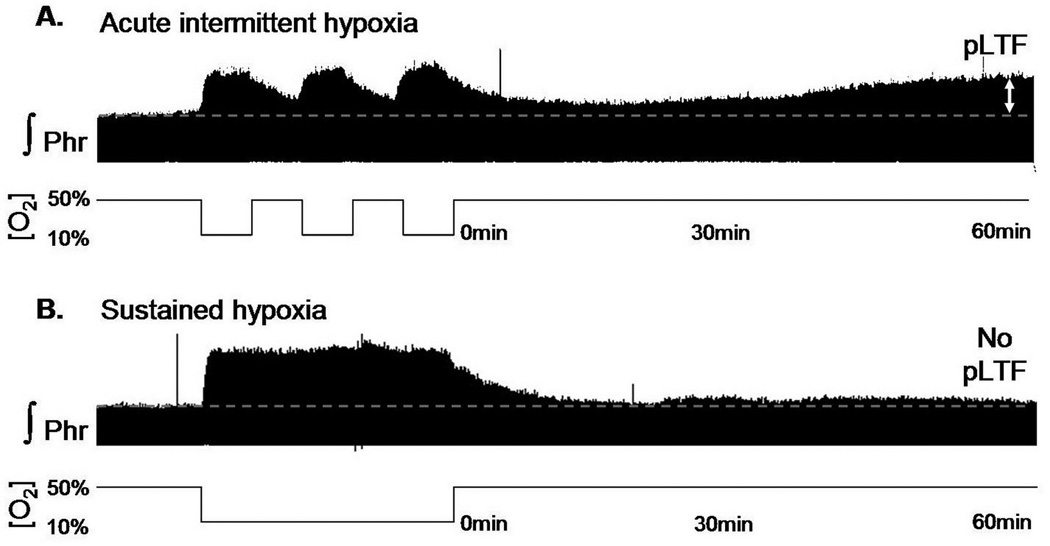
Phrenic LTF (pLTF) is pattern-sensitive since it is not induced by exposure to the same cumulative duration of sustained hypoxia (Baker and Mitchell, 2000; Figure 1). pLTF is relatively insensitive to the severity or duration of hypoxia between 28 and 70 mmHg (Fuller et al., 2000; Mahamed and Mitchell, 2008). Although pLTF is most frequently studied following AIH (3 episodes, 5 min duration, 5 min intervals; Mitchell et al., 2001), it is also induced by episodic stimulation of carotid chemoafferent neurons without hypoxia (Millhorn et al., 1980a; Hayashi et al., 1993) or by repetitive, 25 second apneas (Mahamed and Mitchell, 2008). Since episodic hypercapnia does not elicit pLTF (Bach and Mitchell, 1998; Baker et al., 2001), the relevant stimulus to pLTF appears to be swings in oxygenation or the associated (episodic) activation of carotid chemoafferent neurons. Because the intermittent pattern is an essential feature of the pLTF stimulus, intermittent versus sustained hypoxia has considerably greater potential in the development of novel therapeutic approaches for SCI. The precise details of the intermittent hypoxic protocol (e.g. duration, severity, isocapnic versus hypercapnic) appear to be relatively unimportant, with the exception of the normoxic interval between successive hypoxic episodes. The limits on the inter-episode interval are not precisely defined, but it must be greater than zero (Baker and Mitchell, 2000) and less than 30 minutes (Bach and Mitchell, 1998). Further studies defining the optimal interval are warranted.
Figure 1.
Phrenic long term facilitation : a pattern-sensitive respiratory plasticity.
A. 3×5 min acute intermittent hypoxia (10% O2, bottom line) induces a long lasting (up to 60 min) increase of the phrenic nerve activity named phrenic long term facilitation (pLTF, double white arrow). Note the continuous increase of the activity due to a molecular cascade which leads to new protein synthesis (see the review for further details). B. Sustained hypoxia of similar cumulative duration does not induce pLTF. pLTF requires intermittent hypoxia/reoxygenation to be induced.
2.2. Cellular Mechanisms of AIH-induced pLTF
Considerable progress has been made in revealing cellular/synaptic mechanisms of AIH-induced pLTF in intact rats (Mitchell et al., 2001; Feldman et al., 2003; Baker-Herman et al., 2004; Mahamed and Mitchell, 2007; MacFarlane and Mitchell, 2008a; Figure 2). pLTF is serotonin-dependent (Bach and Mitchell, 1996, Millhorn et al., 1980b; Mahamed and Mitchell, 2008) and requires spinal serotonin receptor activation (particularly 5-HT2 receptors) for its induction, but not maintenance (Baker-Herman and Mitchell, 2002; Fuller et al., 2001a; MacFarlane and Mitchell, 2008b). Episodic spinal 5-HT receptor activation is sufficient to induce pLTF in vitro neonatal preparations (Lovett-Barr et al., 2006), and in vivo anesthetized rats (MacFarlane and Mitchell, 2007). Thus, intermittent (selective) 5-HT receptor activation may represent a viable approach to facilitate respiratory motor output following cervical spinal cord injury.
Figure 2.
Phrenic long term facilitaion following acute intermittent hypoxia: schematic reperesentation of its cellular mechanisms and changes in relevant proteins following repetitive exposure to acute intermittent hypoxia. A. Acute intermittent hypoxia increases phrenic motor output, an effect referred to as pLTF (Figure 1). AIH induces serotonin release in the vicinity of phrenic motor neurons, thereby activating 5HT-2 receptors and, via the PKC pathway, induces increased BDNF expression. BDNF subsequently activates its high affinity receptor, TrkB, possibly increasing the phosphorylation state and traffiking of glutamate receptors, thereby accounting for pLTF. This pathway is modulated by inhibitory constraints, including protein pohosphatases, which are in turn regulated (inhibited) by reactive oxygen species (ROS). B. Dailly acute intermittent hypoxia or thrice weekly AIH for 10 weeks enhances pLTF. In association, these treatments increase serotoninergic terminal density near phrenic motor neurons and, presumably, serotonin release in response to subsequent AIH. Subsequent aspects of this pathway are enhanced by repetitive acute intermittent hypoxia, including increased 5HT-2 receptor expression, increased BDNF synthesis and increased expression of TrkB. Figure adapted from Mitchell and Johnson, 2003.
Episodic serotonin receptor activation initiates new protein synthesis (Baker-Herman and Mitchell, 2002), particularly new synthesis of brain-derived neurotrophic factor (BDNF). New BDNF synthesis is both necessary and sufficient for LTF (Baker-Herman et al., 2004). Once released, BDNF activates its high affinity receptor, TrkB (Baker-Herman et al., 2004), leading to pLTF. AIH and episodic spinal serotonin receptor activation both increase the formation of reactive oxygen species (ROS), and these ROS are necessary for pLTF (MacFarlane and Mitchell, 2008a). Greater ROS formation following intermittent (versus sustained) hypoxia appears to account for LTF pattern-sensitivity, largely by inhibiting okadaic acid-sensitive serine/threonine protein phosphatases that normally constrain pLTF (Wilkerson et al., 2008; MacFarlane and Mitchell, 2008a).
2.3. Chronic intermittent hypoxia (CIH) induces novel forms of respiratory plasticity
Chronic intermittent hypoxia (CIH; eg. 5 min of 11% O2 alternating with 5 min 21% O2; 12 hours per night; 7 nights) also elicits respiratory plasticity (Mitchell et al., 2001; Peng et al., 2001). CIH differs from AIH largely in the number of hypoxic episodes (eg. 504 episodes over 7 days in the example given above). CIH pre-treatment enhances AIH-induced LTF in anaesthetized (Ling et al., 2001; Zabka et al., 2003; Peng and Prabhakar, 2003) and unanaesthetized rats (McGuire et al., 2003), and these effects can be long-lasting. In intact, unanesthetized rats, CIH effects continue for more than 3, but less than 7 days post-treatment (McGuire et al., 2003). Similarly, CIH-enhanced hypoxic phrenic responses last more than one week in animals exposed to neonatal hyperoxia (Fuller et al., 2001b). CIH pre-treatment confers the ability to express AIH-induced LTF in animals not normally able to express LTF (McGuire et al., 2003; Zabka et al., 2003; Wilkerson and Mitchell, 2009).
CIH pre-treatment also decreases the apneic threshold, increases the phrenic response during hypoxia and increases central neural integration of carotid chemoafferent neuron activation (Ling et al., 2001). On the other hand, CIH pre-treatment does not change resting ventilation or metabolic rate in unanesthetized rats (McGuire et al., 2003). Thus, apart from its effects on AIH-induced pLTF, the capacity to elicit respiratory plasticity is unclear, and may depend on the state of the animal when measurements are made. Whereas CIH elicits whole-animal physiological changes (see previous paragraph), these potentially beneficial compensatory responses are often accompanied by deleterious side effects, such as impaired cognitive function (see below).
2.4. Cellular Mechanisms of CIH-induced spinal plasticity
Relatively little is known concerning cellular or synaptic mechanisms of CIH induced respiratory plasticity. Enhanced pLTF following CIH still requires serotonin receptor activation, although there is evidence that novel serotonin receptors play a greater role following CIH (Ling et al., 2001, 1994; McGuire et al, 2004; Figure 2). For example, whereas pre-treatment with a 5-HT2 receptor antagonist, ketanserin, completely blocks AIH-induced pLTF in normal rats (Kinkead and Mitchell, 1999), enhanced pLTF following CIH is only partially blocked by ketanserin at the same dose (Ling et al., 2001; McGuire et al., 2004). On the other hand, CIH-enhanced pLTF is blocked by the less selective serotonin receptor antagonist, methysergide (Ling et al., 2001; McGuire et al., 2004). Thus, novel serotonin receptor subtypes play a more prominent role following CIH (eg. 5-HT7 receptors; McGuire et al., 2004).
Enhanced pLTF in CIH pre-treated rats may arise in part from the induction of sensory LTF in carotid chemo-afferent neurons (Peng and Prabhakar, 2003). However, we believe that CIH enhances pLTF through additional actions within the spinal cord since: 1) repetitive intermittent hypoxia increases key proteins necessary for pLTF within putative phrenic motor neurons (Satriotomo et al., 2007); and 2) CIH enhances short-latency evoked responses from the ventro-lateral funiculus to phrenic motor neurons, although this effect may be unique to spinally injured rats (Fuller et al., 2003). Further investigations concerning mechanisms of CIH induced plasticity and meta-plasticity are necessary.
Severe CIH protocols similar to those described above are limited in their potential for therapeutic application since they are associated with pathology, including hypertension (Fletcher et al., 1992), hippocampal cell loss (Gozal et al., 2001) and learning deficits (Row et al., 2002, 2003). Recently, we investigated the possibility that less severe protocols of repetitive, acute intermittent hypoxia induce (presumably beneficial) respiratory plasticity, but without the attendant pathology.
2.5. Daily acute intermittent hypoxia (dAIH)
is one alternative to CIH since it exposes the rat to fewer hypoxic episodes (70 versus 504 total episodes over 7 days; Wilkerson and Mitchell, 2009; Table 1). dAIH consisting of 10 hypoxic episodes (5 min; 10.5% O2) interspersed with normoxic intervals (21% O2; 5 min) per day for 7 days appears to elicit the beneficial effects of CIH, but without negative side-effects, such as hypertension or hippocampal cell death (McGuire et al., 2002; Ling et al., 2001; Wilkerson and Mitchell, 2009). For example, dAIH pre-treatment (like CIH) enhances the magnitude of AIH-induced LTF, and can change an ineffective AIH protocol into a protocol capable of eliciting LTF in some rat strains (Wilkerson et Mitchell, 2009). dAIH also increases expression of key proteins within the phrenic motor nucleus (eg. BDNF; Satriotomo et al., 2007). In uninjured rats, dAIH increased BDNF immunoreactivity within motor neurons at C4 by 45% (p< 0.05), but had no effect on TrkB immunoreactivity (p>0.05) near the phrenic motor nucleus (Lovett-barr et al., 2008). Further investigations are necessary to determine the cellular impact of dAIH in uninjured rats.
Table 1.
Summary of the hypoxic protocols used by our laboratory and their different effects on respiration following C2HS.
| AIH | CIH | dAIH | |
|---|---|---|---|
| Number of hypoxia |
3 to 5 | 504 | 70 |
| Duration | Acute 3×5 min hypoxic exposure | 72×5 min hypoxic episodes a day during 7 days | 10×5 min hypoxic episodes a day during 7 days |
| Interval between hypoxic episodes |
5 min normoxic intervals | 5 min normoxic intervals | 5 min normoxic intervals |
| CO2 regulation | Isocapnic/Poikilocapnic | poikilocapnic | Poikilocapnic |
| Animal condition | Anaesthetized/ Unaesthetized | Unanaesthetized | Unanaesthetized |
| Delay after injury | 7 days to 2 months | 7 days | 7 days |
| Known beneficial effects |
Increased pLTF, Strenghtening of CPP | Enhanced activity on side of injury during baseline hypercapnia and hypoxia conditions, enhanee short latency evoked responses |
Enhanced activity on side of injury, restores breathing capacity (increases minute ventilation and tidal volume during chemosensitivity drive), restores LTF below injury |
| Known deleterious effects |
None | Hippocampal cell loss, learning deficits (in mices and rats) |
No hippocampal cell loss or learning deficits |
Differences in pLTF magnitude after different intermittent hypoxia protocols (AIH, CIH and dAIH; Table 1) suggest at least some differences in the underlying mechanisms. These differences may confer unique advantages when animals are confronted with the need for complex respiratory responses to physiological and pathological conditions, such as chronic spinal injury (Mitchell and Johnson, 2003).
3. Cervical spinal injuries: effects on the respiratory system
Cervical spinal injury impairs respiratory and somatic motor function, but also induces cellular and molecular changes that have lead some to describe the injured spinal cord as a “new spinal cord” (Edgerton et al., 2004). Injury triggers a cascade of molecular and cellular changes that begin within minutes, and continue for days to weeks. SCI can disrupt regional blood flow and trigger inflammatory processes (e.g., microglial activation, T-cell infiltration) that play a role in healing (see Schwab and Bartholdi, 1996 ; Popovitch and Jones, 2003 for reviews). There are gene expression changes following contusions in the mid-cervical spinal cord; for example, in one study, dynamic changes were observed in post-injury gene expression, and these change correlated with functional outcome (tissue healing, scar formation, tissue reoxygenation). Most gene expression changes in this study were associated with inflammation and the enzymatic, matrix (Velardo et al., 2004). Following C2HS, increased protein expression of trophic factors and their receptors is observed ( BDNF is increased 82%; TrkB is increased 25% from controls; both p<0.05; Lovett-Barr et al., 2008). Spinal 5HT2A receptor density also increases in the vicinity of the phrenic motor nucleus (Fuller et al., 2005). Increased 5HT2A receptor expression is thought to strengthen crossed-spinal synaptic pathways to phrenic motor neurons following C2-cervical hemisection (C2HS; Fuller et al., 2005; Goshgarian, 2003). Collectively, these reports paint the picture that spinal injury alters the expression of molecules that play important roles in spontaneous and induced spinal plasticity, including growth factors, cytokines, metabotropic receptors, transcription factors, etc. (Vinit et al., 2005; 2009, in press). Thus, it is important to bear in mind that the cellular/molecular environment of an injured spinal cord differs from the uninjured spinal cord, and that mechanisms of induced plasticity may differ in interesting ways from un-injured animals.
3.1. Spontaneous respiratory recovery in rats following C2HS
One well-described experimental model of respiratory impairment and spontaneous functional recovery following C2HS is the crossed-phrenic phenomenon (Goshgarian, 2003; Fuller et al., 2003; Vinit et al., 2006; 2007; 2008 ; see Vinit and Kastner for review in this issue). Following C2HS, bulbo-spinal projections from brainstem respiratory neurons are disrupted ipsilateral to injury. Some functional recovery can occur via spinal synaptic pathways to phrenic motor neurons that cross the spinal mid-line, but other mechanisms may contribute, including plasticity in phrenic dendrites that cross the spinal mid-line (Lindsay et al., 1991; Prakash et al., 2000). In some respects, defined experimental injuries such as C2HS are similar to human SCI since the injury is incomplete, leaving multiple pathways intact that form the substrate for functional recovery via rewiring, sprouting and increased synaptic strength. Defined injuries are quite different from spontaneous human injuries in other respects since they are defined cuts versus contusion injuries.
C2HS disrupts bulbospinal synaptic inputs to phrenic motoneurons resulting in an electrically silent ipsilateral phrenic nerve and diaphragm (Golder et al., 2001; Nantwi and Goshgarian, 1998; Vinit et al., 2006; 2007). Consequently, the capacity to maintain and increase breathing is reduced in comparison to an uninjured (sham) rat (Golder et al., 2003; Fuller et al., 2006). By 2 months post-C2HS, weak phrenic motor recovery occurs spontaneously below the injury due to strengthening of the crossed spinal synaptic pathways (Golder et al., 2001; Nantwi and Goshgarian, 1998; For review, see Goshgarian, 2003). Bulbospinal respiratory descending pathways are “rewired” (Vinit et al., 2008) and slight diaphragmagtic activity can be recorded under some experimental conditions (Vinit et al., 2006). Apart from the spinal plasticity following C2HS, additional recovery may arise from plasticity in the diaphragm neuromuscular junction (Prakash et al., 1995; Mantilla et al., 2007). Functional recovery of breathing capacity is progressive, increasing with post-lesion time; on the other hand, spontaneous increases in ipsilateral phrenic nerve activity have only small effects on the capacity to generate inspiratory tidal volume in unaesthetized rats (Fuller et al., 2006). Spared ventral medial pathways following C2HS are not sufficient to preserve ipsilateral phrenic nerve activity immediately following injury (Vinit et al., 2007), but contribute to the delayed recovery of the ipsilateral phrenic nerve activity (Fuller et al., 2009 ; Vinit et al., 2008). Thus, although there is some spontaneous plasticity and functional recovery in ventilatory capacity (Table 1) following C2HS, it is not sufficient to completely restore respiratory function. There would be considerable benefit to patients if therapies could be developed that stimulate additional plasticity and functional recovery, possibly by enhancing the same fundamental mechanisms that underlie spontaneous recovery, or by inducing new mechanisms that achieve the same functional outcome (ie. increased breathing capacity).
3.2. Intermittent hypoxia enhances functional recovery following cervical injury
Several potential therapeutic strategies induce limited improvement of respiratory function in rodent models of C2HS. For example, purified olfactory ensheathing cells transplantation around the injury site can induce slight ipsilateral phrenic and diaphragm recovery (Polentes et al., 2004); at least some of this recovery may result from alterations in the glial scar (see Ruff et al., 2008 for review). Channel rhodopsin activation induced by light stimulation of transfected phrenic motoneurons also restores diaphragm activity following a C2HS (Alilain et al., 2008). However, these approaches are invasive and the recovery is incomplete versus uninjured rats. A novel, non-invasive approach with considerable potential to induce respiratory functional recovery following a C2HS is treatment with repetitive intermittent hypoxia. This may constitute a novel noninvasive treatment for human application, but that has not been clearly established yet in clinical trials.
Different protocols of intermittent hypoxia have been tested to date, each with unique strengths and weaknesses. Typically, intermittent hypoxia protocols used to date consist of repetitive 5 minute episodes of moderate hypoxia (eg. 10.5% inspired oxygen), separated by 5 minute intervals of normoxia; details of the various protocols used to date are compared in Table 1. Protocols tested in rats with C2HS include: acute intermittent hypoxia (3 to 5 episodes, 1 day; Golder and Mitchell, 2005; Nakamura, Golder and Mitchell, Unpublished observations), chronic intermittent hypoxia (504 episodes during 7 consecutive nights; Fuller et al., 2003), and daily acute intermittent hypoxia (70 episodes during 7 consecutive days; Lovett-Barr et al., 2008).
3.2.1. Acute intermittent hypoxia
strengthens spinal synaptic pathways to phrenic motor neurons below a C2HS (Golder and Mitchell, 2005). However, the capacity of AIH to induce crossed-spinal synaptic pathways to phrenic motor neurons below the injury is highly dependent on time post-injury and the rat strain studied. For example, following C2HS, AIH induces ipsilateral phrenic long-term facilitation (>60 min post AIH) at 8 weeks post-injury, but not at two weeks post-injury in Sprague Dawley and Lewis rats (Golder and Mitchell, 2005). However, at 4 weeks post-injury in these same studies (Golder and Mitchell, 2005), the AIH effect was observed in Lewis, but not Sprague Dawley rats. The recovery of AIH-induced phrenic long-term facilitation correlated with injury-induced loss and recovery of serotonergic innervation within the phrenic motor nucleus (Golder and Mitchell, 2005). Thus, intermittent hypoxia may be more effective at restoring respiratory function in patients with chronic (versus acute) spinal injury, once descending serotonergic innervation has had sufficient time to recover below the injury. The differential strain effects suggest that there is considerable heterogeneity in the time-course of these effects, and that the timing for optimal effects of intermittent hypoxia treatments may vary considerably among individuals, particularly in genetically diverse populations such as humans. AIH-induced facilitation of phrenic motor output below a cervical spinal injury provides “proof-of-principle” that intermittent hypoxia has therapeutic potential; however, the expected magnitude and duration of induced functional recovery is limited with a single exposure to a limited number of hypoxic episodes.
AIH may also affect motor neurons contralateral to injury and brainstem respiratory neurons. For example, C2HS increases phrenic motor output and prevents expression of AIH induced pLTF on the side contralateral to injury (Doperalski and Fuller, 2006); however, this finding is somewhat at variance with limited data presented in an earlier study from our laboratory (Golder and Mitchell, 2005). The burst frequency increase following AIH is attenuated in C2HS rats versus controls (Doperalski and Fuller, 2006), suggesting that the injury affects mechanisms of respiratory rhythm generation, either through direct effects on rhythm generating neurons or on afferent pathways to those neurons. Collectively, these findings emphasize the importance of considering effects beyond the immediate impact of spinal injury since many aspects of the respiratory control system may also be (directly or indirectly) affected.
3.2.2. Pharmacological simulation of AIH also improves respiratory function following C2HS
Since AIH enhances phrenic motor output by stimulating new BDNF synthesis and subsequent TrkB activation (see above), we tested the hypothesis that trans-activation of TrkB receptors independently from BDNF synthesis or BDNF-dependent TrkB activation would also improve respiratory function following C2HS (Golder et al., 2008). In these experiments, the adenosine 2A receptor agonist, CGS 21680, was injected either systemically, or into the cervical intrathecal space. In brief, CGS 21680 greatly enhanced phrenic motor output below a C2HS for at least 120 minutes post-injection, and restored ventilatory function in unanesthetized rats studied in a whole body plethysmograph (Golder et al., 2008). In uninjured rats, this form of facilitation arises from new TrkB synthesis, and intracellular signaling from an immature TrkB isoform without ligand binding (ie, it is BDNF independent; Golder et al., 2008). Other studies have shown that theophylline, an adenosine A1 and A2 receptor antagonist, restores diaphragm activity following a C2HS (Nantwi and Goshgarian, 2002, see also Nantwi et al. in this special issue). Since theophylline is an antagonist versus agonist, these effects most likely arise from a mechanism distinct from TrkB transactivation. Further experiments concerning the actions of adenosinergic drugs on functional recovery following spinal injury are warranted. Regardless of their mechanism of action, these experiments using adenosinergic drugs point out that even if intermittent hypoxia does not prove to be a suitable therapeutic approach to human patients, a thorough understanding of basic mechanisms whereby intermittent hypoxia improves respiratory motor function may inspire novel pharmacological approaches with unique advantages and disadvantages in the treatment of spinal injury.
3.2.3. Chronic intermittent hypoxia (CIH)
also induces respiratory motor recovery following C2HS, and this effect is robust when treatment begins even 7 days post-injury. CIH (7 days) beginning 1 week post-hemisection increased spontaneous phrenic motor output ipsilateral to injury, and increases short-latency ipsilateral phrenic responses evoked by stimulation of the contralateral ventro-lateral funiculus (Fuller et al., 2003). On the other hand, CIH had no effect on phrenic motor output in uninjured control rats, nor were there significant CIH effects on contralateral (intact) phrenic nerve activity in C2HS rats (Fuller et al., 2003). Differences in CIH treatment effects between injured and uninjured rats could be due to a variety of factors, including descending pathways that inhibit the expression of plasticity and differences in the chemical milieu of the injured spinal cord (eg. increased 5-HT2A receptor expression; Fuller et al., 2005). Although CIH effects in rats with C2HS were observed many hours after the final episode of intermittent hypoxia (Fuller et al., 2003), no information is available concerning the duration of CIH effects, nor is information available concerning strain differences or relative effects at different times post-injury.
The mechanism of CIH-induced ipsilateral phrenic nerve recovery in C2HS rats is not yet clear. Serotonin receptor activation is necessary for CIH-induced respiratory plasticity in uninjured animals (Ling et al., 2001; McGuire et al., 2004). Although serotonin concentration does not appear to be altered in C2HS or CIH-pretreated rats (Fuller et al., 2005), 5- HT 2 a receptor expression is increased below C2HS (Fuller et al., 2005). CIH treatment 2 weeks post-injury has no further effect on 5- HT 2 a receptor expression below the injury (Fuller et al., 2005), but the C2HS induced receptor upregulation may be critical to enable CIH-induced plasticity and functional recovery at this early time-point when serotonergic innervation is still low (Golder et al., 2008). Contralateral to hemisection, 5- HT 2 a receptor expression is increased only in C2HS rats with CIH treatment (Fuller et al., 2005). Taken together, these data suggest that 5- HT 2 a receptors may play an important role in the expression of CIH -induced plasticity 2 weeks post-C2HS. Further studies are necessary to determine the mechanisms of CIH induced respiratory recovery.
Although CIH treatment enhances the ipsilateral phrenic nerve activity after C2HS (Fuller et al., 2003), certain protocols of CIH also exert detrimental side effects (Reeves and Gozal, 2006; Pawar et al., 2008; see previous section), limiting its potential as a therapeutic approach in the treatment of spinal injury patients. An alternative approach is to reduce the number of hypoxic episodes, preserving the capacity for plasticity without detrimental side-effects.
3.2.4.Daily acute intermittent hypoxia
(dAIH; 10, 5 minute episodes of moderate hypoxia per day for 7 days) elicits respiratory plasticity and meta-plasticity in uninjured rats without obvious side-effects such as systemic hypertension (Wilkerson and Mitchell, 2009). Preliminary findings also indicate that dAIH elicits beneficial effects in rats with cervical spinal injury. For example, dAIH (1 week post-C2HS) restores the capacity to increase minute ventilation and tidal volume during maximal chemoreceptor stimulation (measured by whole-body plethysmography in unanesthetized rats; Barr et al., 2007b); in fact, dAIH-treatment restores >80% of the lost breathing capacity caused by C2HS. dAIH also increased spontaneous phrenic motor output and short-latency evoked phrenic responses elicited by from ventrolateral funiculus stimulation ipsilateral to hemisection (Barr et al., 2007a). Thus, dAIH appears to elicit functional recovery at least in part via spinal plasticity. In addition to its effects on the capacity to increase breathing and phrenic motor output during chemoreceptor stimulation, dAIH also restores the ability to express AIH-induced pLTF contralateral to injury (Vinit and Mitchell, 2009) although hemisected rats normally are unable to do at 2 weeks post-injury (Golder and Mitchell, 2005). Taken together, these reports suggest that repetitive acute intermittent hypoxia has considerable potential as a treatment for cervical spinal injury since it increases spontaneous phrenic motor output and enhances the capacity for respiratory plasticity without evidence of unintended side effects such as systemic hypertension (Wilkerson and Mitchell, 2009).
Cellular mechanisms of dAIH-induced functional recovery are not yet clear, although dAIH differentially affects BDNF and TrkB protein expression in rats with C2HS. Immunohistochemical analysis was performed on rats following dAIH, or an equivalent duration of normoxia, beginning one week post-injury in sham and C2HS rats. In uninjured rats, dAIH increased BDNF immunoreactivity by 45% (consistent with ELISA results, Lovett-Barr et al., 2008), but had no significant effect on TrkB immunoreactivity. C2HS without dAIH also increased BDNF (82%) and TrkB (25%) immunoreactivity relative to sham animals. However, when dAIH was applied to rats with C2HS, dAIH had no further effect on BDNF immunoreactivity, and returned TrkB immunoreactivity to uninjured levels 2 weeks post-hemisection (Lovett-Barr et al., 2008). Thus, key molecules in AIH-induced spinal respiratory plasticity are affected by dAIH, but differential responses in injured and uninjured rats make interpretations concerning mechanisms of functional recovery following cervical injury unclear. Further investigations are warranted.
4. Factors that may undermine therapeutic efficacy of intermittent hypoxia
Although repetitive acute intermittent hypoxia has considerable potential as a therapeutic approach in the treatment of respiratory insufficiency following spinal injury, there are a number of factors that may undermine its efficacy.
4.1. Genetic influences
Since AIH effects vary among rat strains, genetic or epigenetic influences may be important in the underlying response (Golder and Mitchell, 2005). The capacity for AIH-induced pLTF varies among rat strains (Bavis et al., 2003), and even sub-strains (Fuller et al., 2000, 2001), suggesting genetic variations in the capacity for respiratory plasticity. Additional support for genetic factors comes from a study of athymic nude versus Sprague Dawley rats following SCI; in this study, 80 genes were found to differ between the rat strains following spinal contusion. The functionally organized gene profiles were temporally distinct between strains, and correlated with repair indices (Velardo et al., 2004). Because genetic (or epigenetic) differences appear to influence the capacity for functional recovery following SCI, genetically diverse populations, such as humans, may vary widely in their capacity for induced functional recovery following repetitive AIH.
4.2. Differential recovery of serotonergic innervation following SCI
The scarce data available suggest that repetitive intermittent hypoxia may be most effective in chronic spinal injury, after serotonergic function has been (at least partially) restored below the injury (Golder and Mitchell, 2005; Zhou et al., 2001). However, the details of spinal injuries vary widely in human patients, causing considerable variation in the extent of descending serotonergic projections below the site of injury. Furthermore, the extent of functional recovery correlates with the serotonergic innervation below the injury (Schmidt and Jordan, 2000 for review). Although early interpretations were that serotonergic innervation was simply reflecting the extent of injury, later studies suggest that serotonergic neurons actually play a causal role in functional recovery (Hashimoto and Fukuda, 1991). Thus, differences in post-injury recovery due to differential serotonergic re-innervation below the injury because of variations in specific tracts affected by injury, genetic differences in the capacity for recovery of serotonergic function below an injury or time may influence the ability to harness respiratory plasticity with intermittent hypoxia. Variations in serotonergic innervation below spinal injury due to time, genetics and details of the spinal injury may place constraints on the therapeutic efficacy of repetitive intermittent hypoxia in some patients.
4.3. Immune/Inflammatory responses
Another relatively unexplored factor that may undermine the capacity of repetitive intermittent hypoxia to induce plasticity and promote functional recovery in SCI patients is infection and inflammation. Currently, little is known concerning inflammatory/immune responses and their impact on the control of breathing. However, recent findings suggest that the immune system has a profound impact on neural function in other regions of the nervous system, including hippocampal synaptic transmission and plasticity (DiFilippo et al., 2008). In human SCI patients, inflammation is a major factor in the acute phases of spinal injury (Hagg and Oudega, 2006). Additional injuries or disease processes also frequently accompany the spinal injury per se (eg. peripheral nerve inflammation, injured organs, bacterial infections). Bacterial infections are also frequent in chronic SCI patients, often leading to severe health complications, particularly respiratory infections such as pneumonia (DeVivo et al., 1999; Winslow and Rosovsky, 2003 ; Burns, 2007). These other injuries/infections could explain some of the variation which can observed in spontaneous functional recovery among human patients with apparently similar spinal injuries.
For example, in rats, peripheral inflammation induced by capsaisin injection into the paw diminishes the capacity for spinal instrumental learning following thoracic spinal injury. However, if the animal is trained prior to inflammation, the capacity for spinal instrumental learning is preserved (Hook et al., 2008), demonstrating that peripheral inflammation inhibits the ability of the spinal cord to express new plasticity without reversing the expression of previously learned behaviors. Other studies that mimic inflammation during bacterial infection via lipopolysaccharide (LPS) injections further demonstrate that inflammation impairs spinal learning in rodents. For example, LPS disrupts the acquisition of spinal learning similar to capsaisin (Young et al., 2007). Of particular relevance to the present review, we recently demonstrated that acute, systemic LPS injections impair the expression of AIH-induced phrenic long-term facilitation (Vinit and Mitchell, 2008). Thus, immune/inflammatory responses must be considered when developing therapeutic approaches to SCI that rely on mechanisms of spontaneous or induced respiratory plasticity.
5. Repetitive intermittent hypoxia: a possible treatment in human SCI patients
In this review, we summarize evidence concerning the potential of intermittent hypoxia to induce functional recovery of breathing capacity following cervical spinal injury in rodent models. Most studies to date have been performed using the C2HS model in rats. In important respects, rats and humans share a common organization of the respiratory control system, suggesting that rats are useful as a model to investigate basic mechanisms of spinal plasticity following injury and/or for developing novel therapeutic strategies in humans (Kastner and Gauthier, 2008; Lane et al., 2008). Although lesion anatomy is highly variable in human SCI patients, functional outcomes observed both clinically (humans) and experimentally (rats) are relatively comparable depending on the outcome measures employed (Lane et al., 2008).
Repetitive intermittent hypoxia could be easily applied in human SCI patients since it is a non- invasive, safe, and easy-to-use strategy. Since few SCI patients (12 of 107 cases) that require ventilatory assistance are successfully weaned from the ventilator (Oo et al., 1999), non-invasive strategies to improve weaning success would be highly beneficial (Rossignol et al., 2007 for review of clinical trials in SCI). Some human studies have shown increased chemoreflexes following intermittent hypoxia (Sheel and MacNutt, 2008), suggesting that intermittent hypoxia may be a useful tool in the treatment of SCI patients. Since we also have evidence that repetitive intermittent hypoxia increases growth factor expression in non-respiratory motor neurons, this treatment may also be an effective strategy to treat non-respiratory motor impairments. Preliminary attempts to utilize repetitive intermittent hypoxia to improve somatic motor function in paraplegic patients have shown promising results in this regard (Rymer et al., 2007). Collectively, available evidence suggests that further investigation of rintermittent hypoxia to treat respiratory and somatic motor deficits following SCI are warranted.
Acknowledgements
This work was supported by an NIH MERIT award HL69064. Dr Stéphane Vinit is supported by a Craig H Neilsen foundation Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. Light-induced rescue of breathing after spinal cord injury. J. Neurosci. 2008;28(46):11862–11870. doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypercapnia-induced long-term depression of respiratory activity requires alpha2-adrenergic receptors. J. Appl. Physiol. 1998;84:2099–2105. doi: 10.1152/jappl.1998.84.6.2099. [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir. Physiol. 1996;104(2–3):251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J. Physiol. 2000;529:215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TL, Fuller DD, Zabka AG, Mitchell GS. Respiratory plasticity: differential actions of continuous and episodic hypoxia and hypercapnia. Respir. Physiol. 2001;129(1–2):25–35. doi: 10.1016/s0034-5687(01)00280-8. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir. Physiol. Neurobiol. 2008;162(1):8–17. doi: 10.1016/j.resp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat. Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J. Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MLR, Wilkerson JER, Hoffman M, Sibigtroth CM, Mitchell GS. Daily acute intermittent hypoxia increases ipsilateral phrenic nerve in rats with chronic cervical spinal hemisection. Society for Neuroscience Abstract. 2007a 600.23/BB19. [Google Scholar]
- Barr MRL, Sibigtroth CM, Mitchell GS. Daily acute intermittent hypoxia improves respiratory function in rats with chronic cervical spinal hemisection. FASEB J. 2007b;21 918.18. [Google Scholar]
- Bavis RW. Developmental plasticity of the hypoxic ventilatory response after perinatal hyperoxia and hypoxia. Respir. Physiol. Neurobiol. 2005;149(1–3):287–299. doi: 10.1016/j.resp.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Vidruk EH, Bisgard GE, Mitchell GS. Level and duration of developmental hyperoxia influence impairment of hypoxic phrenic responses in rats. J. Appl. Physiol. 2003;95(4):1550–1559. doi: 10.1152/japplphysiol.01043.2002. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir. Physiol. Neurobiol. 2003;136(2–3):249–263. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Blight AR. Just one word: plasticity. Nat. Neurosci. 2004;7:206–208. doi: 10.1038/nn0304-206. [DOI] [PubMed] [Google Scholar]
- Burns SP. Acute respiratory infections in persons with spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2007;18(2):203–216. doi: 10.1016/j.pmr.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch. Phys. Med. Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Doperlaski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp. Neurol. 2006;200:74–81. doi: 10.1016/j.expneurol.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu. Rev. Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Lesske J, Qian W, Miller CC, Unger T. Repetitive episodic hypoxia causes diurnal elevations of systemic blood pressure in rats. Hypertension. 1992;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- Frankel HL, Coll JR, Charlifue SW, Whiteneck GG, Gardner BP, Jamous MA, Krishnan KR, Nuseibeh I, Savic G, Sett P. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord. 1998;36:266–274. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Sandhu M, Doperalski NJ, Lane M, White TE, Bishop MD, Reier P. Graded unilateral spinal cord injury and respiratory motor recovery. Resp. Physiol. Neurobiol. 2009;165(2–3):245–253. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J. Appl. Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J. Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J. Neurosci. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J. Appl. Physiol. 2001a;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Wang ZY, Ling L, Olson EB, Bisgard GE, Mitchell GS. Induced recovery of hypoxic phrenic responses in adult rats exposed to hyperoxia for the first month of life. J. Physiol. 2001b;536:917–926. doi: 10.1111/j.1469-7793.2001.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir. Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J. Neurosci. 2008;28(9):2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J. Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases ontralateral respiratory motor output. J. Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J. Neurosci. 2001;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J. Appl. Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J. Neurosci. 2001;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma. 2006;23(3–4):264–280. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Fukuda N. Contribution of serotonin neurons to the functional recovery after spinal cord injury in rats. Brain Res. 1991;539(2):263–270. doi: 10.1016/0006-8993(91)91630-j. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am. J. Physiol. 1993;265(4 Pt 2):R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Hook MA, Huie JR, Grau JW. Peripheral inflammation undermines the plasticity of the isolated spinal cord. Behav. Neurosci. 2008;122(1):233–249. doi: 10.1037/0735-7044.122.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner A, Gauthier P. Are rodents an appropriate pre-clinical model for treating spinal cord injury ? Examples from the respiratory system. Exp. Neurol. 2008;213:249–256. doi: 10.1016/j.expneurol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am. J. Physiol. 1999;277(3 Pt 2):R658–R666. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury : translational perspectives. Trends neurosci. 2008;31(10):538–547. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AD, Greer JJ, Feldman JL. Phrenic motoneuron morphology in the neonatal rat. J. Comp. Neurol. 1991;308(2):169–179. doi: 10.1002/cne.903080204. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J. Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Bach KB, Mitchell GS. Serotonin reveals ineffective spinal pathways to contralateral phrenic motoneurons in spinally hemisected rats. Exp. Brain Res. 1994;101:35–43. doi: 10.1007/BF00243214. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Mitchell GS, Satriotomo I, Johnson SM. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neuroscience. 2006;142(3):885–892. doi: 10.1016/j.neuroscience.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Windelborn JA, Sibigtroth CM, Mitchell GS. Daily acute intermittent hypoxia differentially affects BDNF and TrkB expression in uninjured and spinally injured rats. Society for Neuroscience abstract. 2008 352.2/Y31. [Google Scholar]
- Macfarlane PM, Mitchell GS. Serotonin-induced phrenic long-term facilitation requires reactive oxygen species signaling via the NADPH oxidase complex. Neuroscience. 2008a;152(1):189–197. doi: 10.1016/j.neuroscience.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Simulated apnoeas induce serotonin-dependent respiratory long-term facilitation in rats. J. Physiol. 2008;586:2171–2181. doi: 10.1113/jphysiol.2007.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp.Physiol. 2007;92(1):27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience. 2007;146(1):178–189. doi: 10.1016/j.neuroscience.2007.01.048. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am. J. Physiol. Regul. Integr. Zomp. Physiol. 2004;286:R334–R341. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J. Appl. Physiol. 2003;95:1499–1508. doi: 10.1152/japplphysiol.00044.2003. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Effect of hypoxic episode number and severity on ventilatory long-term facilitation. J. Appl. Physiol. 2002;93:2155–2161. doi: 10.1152/japplphysiol.00405.2002. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir. Physiol. 1980a;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir. Physiol. 1980b;42:171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J. Appl. Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J. Appl. Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Effects of chronic systemic theophylline injections on recovery of hemidiaphragmatic function after cervical spinal cord injury in adult rats. Brain Res. 1998;789:126–129. doi: 10.1016/s0006-8993(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Actions of specific adenosine receptor A1 and A2 agonists and antagonists in recovery of phrenic motor output following upper cervical spinal cord injury in adult rats. Clin. Exp. Pharmacol. Physiol. 2002;29(10):915–923. doi: 10.1046/j.1440-1681.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- Oo T, Watt JW, Soni BM, Sett PK. Delayed diaphragm recovery in 12 patients after high cervical spinal cord injury.A retrospective review of the diaphragm status of 107 patients ventilated after acute spinal cord injury. Spinal Cord. 1999;37:117–122. doi: 10.1038/sj.sc.3100775. [DOI] [PubMed] [Google Scholar]
- Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J. Appl. Physiol. 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Kline DD, Dick TE, Prabhakar NR. Chronic intermittent hypoxia enhances carotid body chemoreceptor response to low oxygen. Adv. Exp. Med. Biol. 2001;499:33–38. doi: 10.1007/978-1-4615-1375-9_5. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J. Appl. Physiol. 2003;94(6):2342–2349. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir. Physiol. Neurobiol. 2008;160(3):259–266. doi: 10.1016/j.resp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Polentes J, Stamegna JC, Nieto-Sampedro M, Gauthier P. Phrenic rehabilitation and diaphragm recovery after cervical injury and transplantation of olfactory ensheathing cells. Neurobiol Dis. 2004;16(3):638–653. doi: 10.1016/j.nbd.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Jones TB. Manipulating neuroinflammatory reactions in the injured spinal cord: back to basics. Trends Pharmacol.Sci. 2003;24(1):13–17. doi: 10.1016/s0165-6147(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J. Appl. Physiol. 2000;89(2):563–572. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Zhan WZ, Miyata H, Sieck GC. Adaptations of diaphragm neuromuscular junction following inactivity. Acta Anat. (Basel) 1995;154(2):147–161. doi: 10.1159/000147762. [DOI] [PubMed] [Google Scholar]
- Reeves SR, Gozal D. Respiratory and metabolic responses to early postnatal chronic intermittent hypoxia and sustained hypoxia in the developing rat. Pediatr. Res. 2006;60(6):680–686. doi: 10.1203/01.pdr.0000246073.95911.18. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Schwab ME, Schartz M, Fehlings MG. Spinal cord injury : time to move? J.Neurosci. 2007;27(44):11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am. J. Respir. Crit. Care. 2003;167:1548–1553. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr. Res. 2002;52:449–453. doi: 10.1203/00006450-200209000-00024. [DOI] [PubMed] [Google Scholar]
- Ruff RL, McKerracher L, Selzer ME. Repair and neurorehabilitation strategies for spinal cord injury. Ann. N. Y. Acad. Sci. 2008;1142:1–20. doi: 10.1196/annals.1444.004. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Dale EA, Mitchell GS. Thrice weekly intermittent hypoxia increases expression of key proteins necessary for phrenic long-term facilitation: a possible mechanism of respiratory metaplasticity? FASEB Journal. 2007;21 918.15. [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res. Bull. 2000;53(5):689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76(2):319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Sheel AW, MacNutt MJ. Control of ventilation in human following intermittent hypoxia. Appl. Physiol. Nutr. Metab. 2008;33:573–581. doi: 10.1139/H08-008. [DOI] [PubMed] [Google Scholar]
- Rymer Z, Hornby G, Mitchell GS, Schmit B, Trumbower RD. Effects of intermittent hypoxia on motor function in persons with incomplete SCI. Society for Neuroscience Abstract. 2007 82.18/LL2. [Google Scholar]
- Velardo MJ, Burger C, Williams PR, Baker HV, Lopez MC, Mareci TH, White TE, Muzyczka N, Reier PJ. Patterns of gene expression reveal a temporally orchestrated wound healing response in the injured spinal cord. J. Neurosci. 2004;24(39):8562–8576. doi: 10.1523/JNEUROSCI.3316-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Boulenguez P, Efthimiadi L, Stamegna JC, Gauthier P, Kastner A. Axotomized bulbospinal neurons express c-jun after cervical spinal cord injury. Neuroreport. 2005;16:1535–1539. doi: 10.1097/01.wnr.0000179075.32035.0f. [DOI] [PubMed] [Google Scholar]
- Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J. Neurotrauma. 2006;23:1137–1146. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- Vinit S, Stamegna JC, Boulenguez P, Gauthier P, Kastner A. Restorative respiratory pathways after partial cervical spinal cord injury: role of ipsilateral phrenic afferents. Eur. J. Neurosci. 2007;25:3551–3560. doi: 10.1111/j.1460-9568.2007.05619.x. [DOI] [PubMed] [Google Scholar]
- Vinit S, Darlot F, Stamegna JC, Sanchez P, Gauthier P, Kastner A. Long-term respiratory pathways reorganization after partial cervical spinal cord injury. Eur. J. Neurosci. 2008;27:897–908. doi: 10.1111/j.1460-9568.2008.06072.x. [DOI] [PubMed] [Google Scholar]
- Vinit S, Mitchell GS. Lipopolysaccharide reduces phrenic long-term facilitation following acute intermittent hypoxia in rats. Society for Neurosciences Abstracts. 2008 433.18/F4. [Google Scholar]
- Vinit S, Mitchell GS. dAIH restores phrenic long-term facilitation contralateral to cervical spinal injury. FASEB J. 2009 23:784.5. [Google Scholar]
- Vinit S, Darlot F, Stamegna JC, Gauthier P, Kastner A. Effect of cervical spinal cord hemisection on the expression of axon growth markers. Neurosci. Letters. 2009 doi: 10.1016/j.neulet.2009.06.058. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J. Neurosci. 2008;28:2949–2958. doi: 10.1523/JNEUROSCI.5539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp. Neurol. 2009;217(1):116–123. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am. J. Phys. Med. Rehabil. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Young EE, Baumbauer KM, Elliot A, Joynes RL. Lipopolysaccharide induces a spinal learning deficit thqt is blocked by IL-1 receptor antagonism. Brain behav. Immun. 2007;21:748–757. doi: 10.1016/j.bbi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Olson EB, Jr, Behan M. Selected contribution: chronic intermittent hypoxia enhances respiratory long-term facilitation in geriatric female rats. J. Appl. Physiol. 2003;95:2614–2623. doi: 10.1152/japplphysiol.00476.2003. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J. Appl. Physiol. 2001;91(6):2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]