Abstract
Objective
Mesenchymal stem cells (MSCs) are capable of modulating the immune system through interaction with a wide range of immune cells. This study investigates the hypothesis that interaction of MSCs with macrophages could play a significant role in their anti-inflammatory/immune modulatory effects.
Materials and Methods
MSCs were derived from bone marrow and monocytes were isolated from peripheral blood of healthy donors. We cultured human monocytes for seven days without any added cytokines to generate macrophages, and then co-cultured them for three more days with culture-expanded MSCs. We used cell surface antigen expression and intracellular cytokine expression patterns to study the immunophenotype of macrophages at the end of this co-culture period, and phagocytic assays to investigate their functional activity in vitro.
Results
Macrophages co-cultured with MSCs consistently showed high level expression of CD206, a marker of alternatively activated macrophages. Furthermore, these macrophages expressed high levels of IL-10 and low levels of IL-12, as determined by intracellular staining, typical of alternatively activated macrophages. However, macrophages co-cultured with MSCs also expressed high levels of IL-6 and low levels of TNF-α, compared to controls. Functionally, macrophages co-cultured with MSCs showed a higher level of phagocytic activity.
Conclusions
We describe a novel type of human macrophage generated in vitro after co-culture with MSCs that assume an immunophenotype defined as IL-10 high, IL-12 low, IL-6 high and TNF-α low secreting cells. These MSC-educated macrophages may be a unique and novel type of alternatively activated macrophages with potentially significant role in tissue repair.
Keywords: Mesenchymal stem cells, Alternatively activated macrophages, Immune modulation
Mesenchymal stem cells (MSCs) are fibroblast-like cells originally isolated from bone marrow (BM) more than 40 years ago [1]. Inside the BM they provide the stromal support tissue for hematopoietic stem cells, but are also capable of differentiating into mesenchymal lineages such as bone, fat and cartilage [2, 3]. Cells with similar characteristics to BM-MSCs have been found in most human tissues examined [4-6], as well as other animals [7-9]. In vitro studies using murine and human MSCs show these cells are capable of interacting with a plethora of immune cells in their different stages of activation, and modulating their responses through a variety of mechanisms [10-13]. Such unique immunological properties of MSCs combined with their other tissue regeneration properties [14, 15], have caused great enthusiasm about the potential of these cells for treatment of a wide variety of disorders [16-18]. Currently, ex vivo culture-expanded MSCs from bone marrow, or other tissues such as adipose tissues, are being actively investigated for treatment of a variety of disorders in a multitude of several phase I-III clinical trials [19].
Although the interaction of MSCs with T-lymphocytes, B-lymphocytes, natural killer cells and dendritic cells have been investigated in detail [20], there is very little known about the interaction of MSCs with cells of monocytic lineages, specifically macrophages. Due to the central role of inflammation in many conditions for which MSCs are proposed to be potentially beneficial, we hypothesize that macrophages are a major type of effector cells through which MSCs might exert their therapeutic effects. In this study, we investigated the effect of MSCs on the immunophenotype and functional characteristics of human monocyte-derived macrophages.
Materials and methods
Cell culture
We used human blood and bone marrow to derive monocytes and MSCs, respectively. All protocols were approved by the Health Sciences Institutional Review Board of University of Wisconsin-Madison School of Medicine and Public Health. Monocytes were isolated from human peripheral blood by using magnetic bead separation methods according to manufacturers’ protocol. Briefly, peripheral blood mononuclear cells were collected from blood of healthy donors by density gradient separation using Percoll (GE Healthcare Bio-sciences, Piscataway, NJ, USA). Red blood cells were lysed by incubating cells in ACK lysis buffer for 3 minutes and mononuclear cells were washed with phosphate buffered saline (PBS). To reduce platelet contamination, cell suspensions were centrifuged at 700 rpm for 15 min and cell pellets were resuspended and incubated with anti-human CD14 microbeads (Miltenyi Biotech, Auburn, CA, USA) for 15 minutes at 4° C degree. After washing to remove unbound antibody, cell separation was done using autoMACS Pro Separator (Miltenyi Biotech). Purity of isolated CD14+ cells was over 95% when checked with flow cytometry. Purified CD14+ monocytes were plated into 6 well cell culture plates at a concentration of 0.5-1 × 106 per well in IMDM media supplemented with 10% human serum blood type AB (Mediatech, Herndon, VA, USA), 1X Non-essential amino acids (NEAA-Lonza, Walkersville, MD, USA), 4mM L-Glutamine (Invitrogen, Carlsbad, CA, USA), 1mM Sodium pyruvate (Mediatech) and 4ug/ml recombinant human insulin (Invitrogen). Cells were cultured for 7 days, without adding any cytokines, to generate macrophages with one change of media 3-4 days after initiation of cultures at 37° C with 5% CO2.
Mesenchymal stem cells were isolated from filters left over after BM harvest from normal healthy donors to HLA-matched siblings. Briefly, leftover BM cells trapped in filter [21] were washed with PBS and mononuclear cells were separated using Ficoll-Hypaque 1.073 (GE Healthcare Bio-sciences) and Leucosep tube (Greiner Bio-one, Monroe, NC, USA) according to the manufacturer’ protocol. Red blood cells were lysed with 3 minute incubation in ACK lysis buffer and mononuclear cells were suspended in αMEM supplemented with 10% fetal bovine serum (FBS, US origin, uncharacterized - Hyclone, Logan, UT, USA), 1X NEAA, and 4mM L-Glutamine. Cells were cultured in 75 cm2 filter cap cell culture flask (Greiner Bio-one, Monroe, NC). Attached cells (passage 0) were harvested using TrypLE cell dissociation enzyme (Invitrogen) and then replated into new flasks as described before [22]. Passage 4 cells were characterized by flow cytometry and used for co-culture studies.
For co-culture experiments, 2 × 105 MSCs were added to each well of monocyte-derived macrophages at day seven and incubated for an additional three to four days. For transwell co-culture, 0.4 um pore size transwells (Corning, Lowell, MA, USA) were placed in 6 well plates containing macrophages plated at the bottom 7 days earlier. Then, 2 × 10 5 MSCs suspended in macrophage culture media were added on top of each transwell and cultured for an additional three days.
Flow cytometry
Antibodies used were anti-CD3 Allophycocyanin (APC), anti-CD14 APC, anti-CD31 APC, anti-CD45 Phycoerythrin (PE), anti-CD90 PerCP-Cy5.5, anti-CD105 APC, anti-CD163 PE, anti-HLA-ABC Fluorescein isothiocyanate (FITC) (eBioscience, San Diego, CA, USA), anti-CD1a FITC, anti-CD209 FITC, anti-IL10 PE, anti-IL12 PE (Miltenyi Biotech), anti-CD54 APC, anti-IL6 PE, anti-IL10 PE, unconjugated anti-IL10 (RnD systems, Minneapolis, MN, USA), anti-CD14 FITC, anti-CD29 PE, anti-CD34 FITC, anti-CD44 PE, anti-CD73 PE, anti-CD90 APC, anti-CD163 PE, anti-CD206 FITC, anti-CD206 PE, anti-HLA-DR FITC (BD Pharmingen, San Diego, CA, USA). For surface staining, cells were harvested with cell scraper; Fc receptors were blocked with Fc Receptor Blocking agent (Miltenyi Biotech) for 15 min at 4° C. Surface antibodies were added and incubated for 30 minutes at 4° C in dark and then cells were washed and fixed with 1% paraformaldehyde (PFA) in PBS. Cell surface staining was anlayzed using a FACScalibur (Becton Dickinson, Franklin Lakes, NJ, USA) within 24 hour of staining. FlowJo software version 7.2.5 (Tree Star, Ashland, OR, USA) was used to analyze the acquired data.
Intracellular cytokine staining
For detection of cytokine expression inside the macrophages, per respective protocols [23-25], cells were stimulated with LPS 1μg/mL for 24 hours (for IL-10); LPS 1μg/mL for 5 hours (for IL-6); IFN-γ 2000 IU/mL for 2 hours then 1μg/ml LPS for 24 hours (for IL-12); and 1μg/ml Ionomycin with 50nM PMA for 5 hours (for TNF-α). Brefeldin A (Invitrogen) or Monensin (eBioscience) was added to block secretion of cytokines [26], and staining was performed using Fix & Perm kit (Invitrogen). Briefly, after blocking of Fc receptor mediated non-specific binding, cells were stained with antibodies against surface markers for 15 minutes at room temperature. Cells were then fixed, washed, and permeabilized. Cytokine-specific antibodies were added and incubated for 20 minutes at room temperature, then washed and fixed with 1% PFA in PBS for flow cytometry as above.
Phagocytic assay
Macrophages were incubated with Alexa 488-conjugated E. coli (Invitrogen) for 1 hour at 37° C and 4° C, respectively. After incubation, cells were washed with cold PBS three times to reduce non-specific attachment of E. Coli and then collected by scraping. Collected cells were treated with Fc Receptor blocker for 10 minutes and then stained with CD90-PerCP-Cy5.5 and CD14-APC for 30 minutes at 4° C. After washing, cells were fixed with 1% PFA in PBS and stored for < 24 hours until analysis.
Statistical analysis
Student’ t-test was performed to compare results. A p-value of less than 0.05 was considered statistically significant. Data are presented as mean ± SD.
Results
Isolation and characterization of MSCs from human bone marrow
All MSCs derived from normal healthy BM were positive for MSC markers (CD29, CD44, CD73, CD90, CD105) and negative for hematopoietic markers (CD31, CD34, CD45, CD54) (data not shown), as previously described [22]. Specifically, MSCs expressed high levels of surface CD90 and were negative for CD14 cell surface marker, while macrophages expressed an opposite pattern. MSCs derived from BM could differentiate into bone, fat and cartilage in vitro, using appropriate growth factors (data not shown).
MSCs increase expression of CD206 on co-cultured macrophages
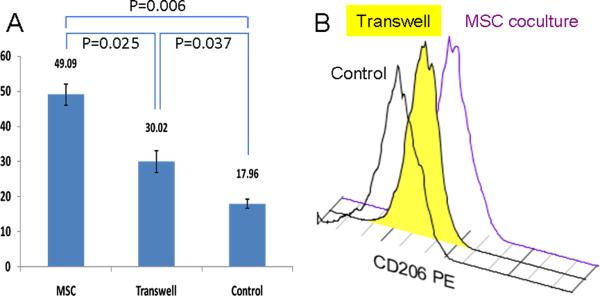
To test our hypothesis that MSCs induce anti-inflammatory phenotype in macrophages, we examined cell surface expression of CD206, a well known marker for anti-inflammatory macrophages [27]. After three to four days of co-culture with MSCs, peripheral blood monocyte derived macrophages increased expression levels of CD206 significantly, compared to macrophages cultured alone, as determined by mean fluorescence intensity (MFI) [28] (Figure-1). Using CD1a and CD209 [29] staining we did not detect presence of any dendritic cells either at the end of seven days culture of monocyte-derived macrophages or at the end of co-culture period (data not shown).To test whether secreted factors are responsible for the upregulation of CD206, we used transwell inserts during co-culture to prevent direct physical contact between MSCs and macrophages. Transwell results were compared to macrophages directly co-cultured with MSCs and macrophages cultured alone (Figure-1). Macrophages co-cultured in direct contact with MSCs showed MFI value of 49.09 ± 3.07, macrophages co-cultured with MSCs in transwell showed MFI value of 30.02 ± 3.11and control macrophage showed MFI value of 17.96 ±1.28. The p-values for all these differences are < 0.05. The intermediate CD206 expression after co-culture in the transwells suggests direct physical cell to cell interaction is partly responsible for changes in CD206 expression.
Figure-1.
Comparison of expression of CD206 cell surface marker on macrophages co-cultured in direct contact to MSCs (MSC) or within a transwell system (Transwell), and without any MSCs (Control). Results are shown comparing mean fluorescence intensity of CD206 expression.
Cytokine secretion of MSC co-cultured macrophages
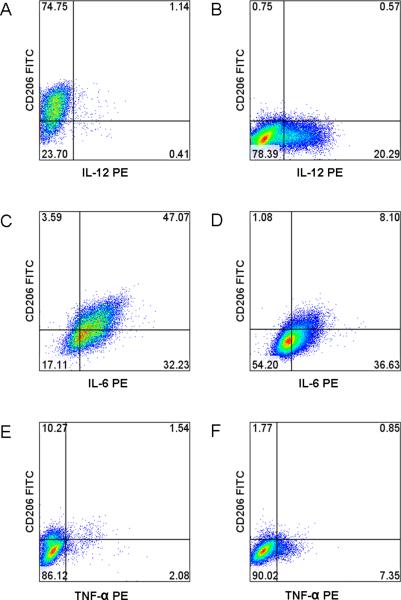
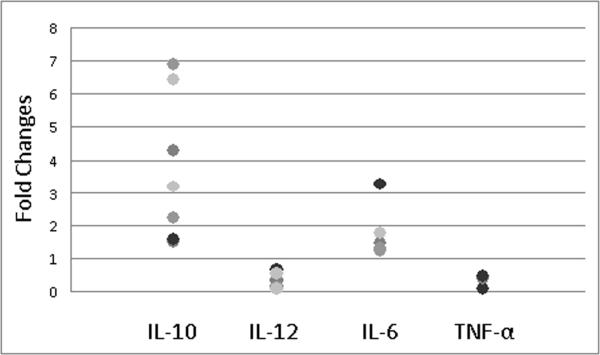
As MSC-cocultured macrophages expressed anti-inflammatory macrophage marker CD206, intracellular cytokine staining, utilizing established stimulation protocols [30, 31], was done to further characterize their immunophenotype. The results show MSC co-cultured macrophages produce more IL-10 (Figure-2) compared to control macrophages. MSC co-cultured macrophages also express lower levels to no IL-12 (Figures-3A and 3B), higher levels of IL-6 (Figures-3C and 3D) and lower levels of TNF-α (Figures-3E and 3F). Comparison of fold changes in the percentage of positive cells, with intracellular staining, for multiple repeats is summarized in Figure-4. The fold level increase in expression of IL-10 was 3.44 ± 2.21 and of IL-6 was 1.74 ± 0.76, while IL-12 and TNF-α expressions were decreased in MSC co-cultured macrophages (compared to controls) by 0.31 ± 0.20 and 0.38 ± 0.17, respectively.
Figure-2.
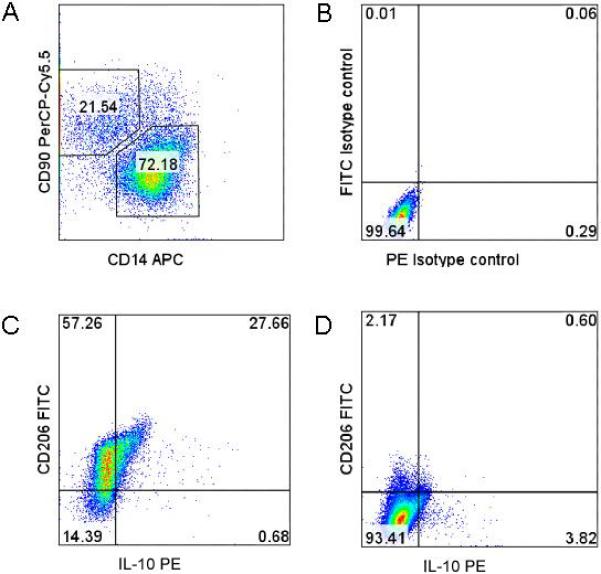
Gating strategy to separate macrophage from MSC and comparison of expression of IL-10 in MSC co-cultured macrophage versus control macrophage. A) Gating of MSC co-cultured macrophages using CD14 APC and CD90 PerCP-Cy5.5; B) Isotype control to show specificity of IL-10 staining of MSC co-cultured macrophage; C) Expression of IL10 in MSC co-cultured macrophages; D) Expression of IL-10 in control macrophages.
Figure-3.
Intracellular cytokine staining of MSC co-cultured and control macrophages. A) MSC co-cultured macrophages stained with anti-IL-12 PE; B) Control macrophage stained with anti-IL12 PE; C) MSC co-cultured macrophage stained with anti-IL6 PE; D) Control macrophage stained with anti-IL6 PE; E) MSC co-cultured macrophage stained with anti-TNF-α PE; F) Control macrophage stained with anti-TNF-α PE.
Figure-4.
Fold changes of percentages of MSC co-cultured macrophages versus control macrophage positively stained for the intracellular target cytokine.
MSC co-cultured macrophage shows increased phagocytic activity
To investigate differences in the function of MSC co-cultured macrophages and control macrophages, we measured phagocytic activity of these cells using Alexa 488 conjugated E. coli [32]. Macrophage co-cultured with MSCs and control macrophage were gated using CD14 and CD90 (Figures-5A and 5B). Nonspecific attachment of E. coli to macrophages was negligible as demonstrated by incubation at 4° C (Figure-5C). Assays performed at 37° C to allow phagocytosis showed higher activity in MSC co-cultured macrophages compared to control macrophages (Figure-5D). Fold increase of MSC co-cultured macrophages over control macrophages in fluorescence intensity was 5.80±1.81 (p value = 0.044). As nonspecific surface attachments of E. coli are identical between MSC co-cultured macrophage and control macrophages (Figure-5C), the difference observed after incubating macrophages with Alexa 488 conjugated inactivated E. coli at 37 degree C is due to phagocytosis and not nonspecific attachment.
Figure-5.
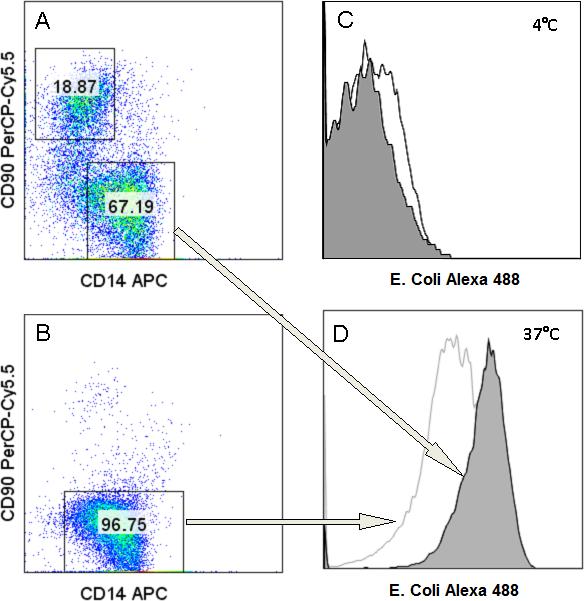
Phagocytic assay: A) Gating strategy for MSC co-cultured macrophages looking at CD14+ CD90− cells; B) Gating of control macrophages; C) MSC co-cultured macrophage in gray and control macrophage in white, Incubated at 4° C for 1 hour; D) MSC co-cultured macrophages in gray and control macrophage in white incubated at 37° C for 1 hour.
Discussion
Macrophages are widely distributed in many different tissues in the human body and are a key component of innate immunity. These cells differentiate from circulating peripheral blood monocytes after migration into tissues, either to replace long-lived tissue macrophages or in response to injurious insults [33]. Macrophages, in addition to their role in initial phases of tissue defense, play an indispensable role in later phases of tissue homeostasis and repair, such as removal of cellular debris and clearance of apoptotic cells [34]. Indeed, macrophages can be polarized by their microenvironment to mount specific functional activities relevant to different phases of inflammation [35]. Although various categories of classification have been proposed, macrophages are typically classified in two main groups: classically-activated macrophages and alternatively-activated macrophages [36]. Classically-activated macrophages and alternatively-activated macrophages are frequently referred to as M1 and M2 macrophages, respectively. Although an oversimplification, in general M1 macrophages exhibit potent anti-microbial properties reminiscent of Th1 responses, and M2 macrophages promote Th2 type of responses, secrete less pro-inflammatory cytokines and play a role in resolution of inflammation through trophic factor synthesis and high phagocytic activities [37]. Importantly, M2 macrophages now encompass several distinct classes of macrophages, M2a, M2b, and M2c that are defined by their specific patterns of cytokine production [38]. In general, these categories of M2 macrophages are characterized by low production of pro-inflammatory cytokines, such as IL-12, and high production of anti-inflammatory cytokines such as IL-10; however, M2b macrophages retain high levels of inflammatory cytokine production, such as TNF-α and IL-6 [39].
In many phase I and II clinical studies MSCs, autologous or allogeneic, have been shown to be potentially efficacious for treatment of a wide variety of clinical conditions such as acute graft versus host disease after allogeneic hematopoietic stem cell transplantation [40, 41], myocardial, infarction [42], amyotrophic lateral sclerosis [43], stroke [44], Crohn’ disease [45], diabetes mellitus [46] and refractory wounds [47]. What is shared between these seemingly diverse clinical conditions is the role of inflammation in their pathogenesis [48]. To investigate the interactions between MSCs and macrophages we used macrophages derived from peripheral blood monocytes by seven days of culture without added cytokines; these macrophages are equivalent to steady state peripheral blood monocyte derived-macrophages [49-51]. Importantly, we did not use cytokines GM-CSF and IL-4, known inducers of dendritic cell generation from monocytes, and the absence of dendritic cells in our co-culture system was verified by lack of expression of dendritic cell markers. [52]. The interactions between MSCs and dendritic cells, generated by adding GM-CSF and IL-4 to monocyte cultures, have been investigated before [53-55]. Upon addition of MSCs to these macrophages, we observed an increase in expression of cell surface marker CD206, a marker upregulated in M2 macrophages [27]. Although in many studies the level of cytokines in different types of cells, including macrophage, is typically measured by ELISA or other assays to define their immunophenotype [56], to further characterize these cells we used intracellular staining as a direct measurement of different types of cytokines. This methodology has advantages over measurement of cytokines in culture supernatants. For example, using ELISA for measurement of cytokines in co-culture experiments can not define the cellular source of those cytokines, as many of these cytokines can also being secreted by MSCs. Furthermore, in these co-culture experiments we could directly define the cytokine expression of different populations of macrophages, CD206+ versus CD206−. We used a stimulation step prior to detection of intracellular expression of each individual cytokine, as without these stimulation steps it is very difficult to detect cytokines intracellulary [30]. However, as a baseline control, we used the exact same stimulation steps in macrophages cultured without MSCs and then used the ratio of expression of each individual cytokine between these two groups to show their differences. Based on these findings we define MSC-educated macrophages (IL-10 high, IL-12 low, IL-6 high and TNF-α low) as a type of alternatively activated population of macrophages (M2M or MEM for MSC-educated macrophages) different from other subcategories of macrophages reported so far.
Our study was focused on the effects of third party human BM-derived MSCs, types of cells used in the majority of clinical trials, on human peripheral blood monocyte derived macrophages. Oritz et al. showed MSC-conditioned media inhibits the capacity of RAW-264.7 cells activated by silica or LPS to secrete TNF-α [57], and Nemeth et al. investigated the effect of mouse BM-derived MSCs in a murine model of septicemia and showed LPS stimulated macrophages produced more IL-10 when co-cultured with BM-derived MSCs [58]. Similar to our study, Nemethe et al. showed, by intracellular staining, that the number of IL-10 producing macrophages isolated from septic mice treated with MSCs was significantly higher ( about 1% versus 0.3%). The differences between human and murine MSCs [59] or macrophages [60] is very well known. Thus, the correlation of these studies, one using a murine macrophage cell line [57] and the other using murine MSCs and macrophages [58], with our study using primary allogeneic human BM-derived MSCs and peripheral blood monocyte-derived macrophages needs further investigation.
We propose the combination of IL-10 high, IL-12 Low, IL-6 High and TNF-α low cytokine expression defines a novel type of M2 macrophages potentially useful clinically for tissue repair. Although in many inflammatory conditions IL-6 is induced along with other pro-inflammatory cytokines, endogenous IL-6 from tissue macrophages [61], can also play a significant anti-inflammatory role in both local and systemic inflammatory responses [62]. Furthermore, several animal models have shown the importance of IL-6 for wound repair, such as impairment of wound healing processes in IL-6 deficient mouse [63] and IL-6 capability to restore abnormal wound repair in immuosuppressed mice [64]. MSCs made from autologous BM have shown promising results when used for treatment of refractory non-healing wounds [47]. However, a major logistical issue with use of BM-derived MSCs is the time, usually several weeks, needed to culture expand sufficient MSCs for potential application. Our study suggests collection of mononuclear cells (monocytes) through leukopheresis, followed by co-culture with a universal source of third party allogeneic MSCs, could provide sufficient autologous MSC-educated macrophages for repair of wounds in a simple and clinically feasible fashion. Interestingly, macrophages prepared from a unit of blood without manipulation have been used for treatment of decubitus ulcers through local injection [65]. The same group have also reported promising results using locally injected or applied macrophages, collected from a unit of blood of healthy donors and activated by hypo-osmotic shock, for treatment of refractory ulcers in more than 1000 patients without any side effects [66]. We propose one of potential applications of our MSC-educated macrophages cells could be for treatment of chronic non-healing wounds.
Acknowledgements
This work was supported by NIH/NHLBI HL081076 K08 grant (to PH) and also by funding from University of Wisconsin Carbone Cancer Center. The authors gratefully thank Dr. Laura Hogan for the critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure:
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow.Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 2.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental hematology. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Covas DT, Panepucci RA, Fontes AM, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Experimental hematology. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 7.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of cell science. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 8.Lennon DP, Caplan AI. Isolation of rat marrow-derived mesenchymal stem cells. Experimental hematology. 2006;34:1606–1607. doi: 10.1016/j.exphem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental hematology. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 10.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 11.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Experimental hematology. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 12.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 13.Di Ianni M, Del Papa B, De Ioanni M, et al. Mesenchymal cells recruit and regulate T regulatory cells. Experimental hematology. 2008;36:309–318. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz E, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy. 2008;10:771–774. doi: 10.1080/14653240802618085. [DOI] [PubMed] [Google Scholar]
- 15.Caplan AI. Why are MSCs therapeutic? New data: new insight. The Journal of pathology. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 17.Jones BJ, McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Experimental hematology. 2008;36:733–741. doi: 10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Experimental hematology. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: An update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 20.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007 doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 21.Sundin M, Remberger M, Lonnies H, Sundberg B, Ringden O, Le Blanc K. No increased trapping of multipotent mesenchymal stromal cells in bone marrow filters compared with other bone marrow cells. Cytotherapy. 2008;10:238–242. doi: 10.1080/14653240801965164. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Experimental hematology. 2008;36:350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellor-Pita S, Citores MJ, Castejon R, et al. Monocytes and T lymphocytes contribute to a predominance of interleukin 6 and interleukin 10 in systemic lupus erythematosus. Cytometry. 2009;76B:261–270. doi: 10.1002/cyto.b.20468. [DOI] [PubMed] [Google Scholar]
- 24.Stephens SA, Brownlie J, Charleston B, Howard CJ. Differences in cytokine synthesis by the sub-populations of dendritic cells from afferent lymph. Immunology. 2003;110:48–57. doi: 10.1046/j.1365-2567.2003.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachdeva N, Asthana D. Cytokine quantitation: technologies and applications. Front Biosci. 2007;12:4682–4695. doi: 10.2741/2418. [DOI] [PubMed] [Google Scholar]
- 27.Porcheray F, Viaud S, Rimaniol AC, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clinical and experimental immunology. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman RA. Standardization and quantitation in flow cytometry. Methods in cell biology. 2001;63:299–340. doi: 10.1016/s0091-679x(01)63018-8. [DOI] [PubMed] [Google Scholar]
- 29.Geijtenbeek TB, Torensma R, van Vliet SJ, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 30.Carter LL, Swain SL. Single cell analyses of cytokine production. Current opinion in immunology. 1997;9:177–182. doi: 10.1016/s0952-7915(97)80132-x. [DOI] [PubMed] [Google Scholar]
- 31.Schultz C. Intracytoplasmic detection of proinflammatory cytokines and chemokines in monocytes by flow cytometry. Methods in molecular biology (Clifton, NJ. 2003;215:29–39. doi: 10.1007/978-1-59259-345-3_4. [DOI] [PubMed] [Google Scholar]
- 32.Harvath L, Terle DA. Assay for phagocytosis. Methods in molecular biology (Clifton, NJ. 1999;115:281–290. doi: 10.1385/1-59259-213-9:281. [DOI] [PubMed] [Google Scholar]
- 33.Gordon S. The macrophage: past, present and future. European journal of immunology. 2007;37(Suppl 1):S9–17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 34.Pollard JW. Trophic macrophages in development and disease. Nature reviews. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 36.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annual review of immunology. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 37.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Mosser DM. The many faces of macrophage activation. Journal of leukocyte biology. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 40.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 41.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 42.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. The American journal of cardiology. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 43.Deda H, Inci MC, Kurekci AE, et al. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy. 2009;11:18–25. doi: 10.1080/14653240802549470. [DOI] [PubMed] [Google Scholar]
- 44.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Annals of neurology. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 45.Taupin P. OTI-010 Osiris Therapeutics/JCR Pharmaceuticals. Curr Opin Investig Drugs. 2006;7:473–481. [PubMed] [Google Scholar]
- 46.Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plastic and reconstructive surgery. 2008;121:860–877. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 48.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. The New England journal of medicine. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 49.Seager Danciger J, Lutz M, Hama S, et al. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. Journal of immunological methods. 2004;288:123–134. doi: 10.1016/j.jim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 51.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of clinical investigation. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. The Journal of experimental medicine. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 54.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 55.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 56.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature medicine. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 60.Schneemann M, Schoeden G. Macrophage biology and immunology: man is not a mouse. Journal of leukocyte biology. 2007;81:579. doi: 10.1189/jlb.1106702. discussion 580. [DOI] [PubMed] [Google Scholar]
- 61.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 62.Xing Z, Gauldie J, Cox G, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. The Journal of clinical investigation. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gallucci RM, Simeonova PP, Matheson JM, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. Faseb J. 2000;14:2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- 64.Gallucci RM, Sugawara T, Yucesoy B, et al. Interleukin-6 treatment augments cutaneous wound healing in immunosuppressed mice. J Interferon Cytokine Res. 2001;21:603–609. doi: 10.1089/10799900152547867. [DOI] [PubMed] [Google Scholar]
- 65.Danon D, Madjar J, Edinov E, et al. Treatment of human ulcers by application of macrophages prepared from a blood unit. Experimental gerontology. 1997;32:633–641. doi: 10.1016/s0531-5565(97)00094-6. [DOI] [PubMed] [Google Scholar]
- 66.Zuloff-Shani A, Kachel E, Frenkel O, Orenstein A, Shinar E, Danon D. Macrophage suspensions prepared from a blood unit for treatment of refractory human ulcers. Transfus Apher Sci. 2004;30:163–167. doi: 10.1016/j.transci.2003.11.007. [DOI] [PubMed] [Google Scholar]