To the Editor
Multiple myeloma is a plasma cell neoplasm that results in 14,000 deaths/year in the United States (1). While most patients initially responded to the standard treatments over the last 30 years, the development of multidrug resistance was common and left few therapeutic options for patients with refractory disease. Therefore newer therapeutic strategies needed to be identified for more effective treatment of the disease. Bortezomib was tested in refractory myeloma and displayed significant activity as a single agent.
Bortezomib is the first in class agent that functions by inhibiting the proteasome. The initial success of bortezomib has provided the rationale for the development of additional proteasome inhibitors like carfilzomib and NPI-0052 that are currently in clinical trials (2). The success of bortezomib and expanded use of proteasome inhibitors to include the treatment of newly diagnosed patients will likely result in the acquired resistance to this class of agents. Therefore we set out to determine the nature of acquired resistance to a proteasome inhibitor.
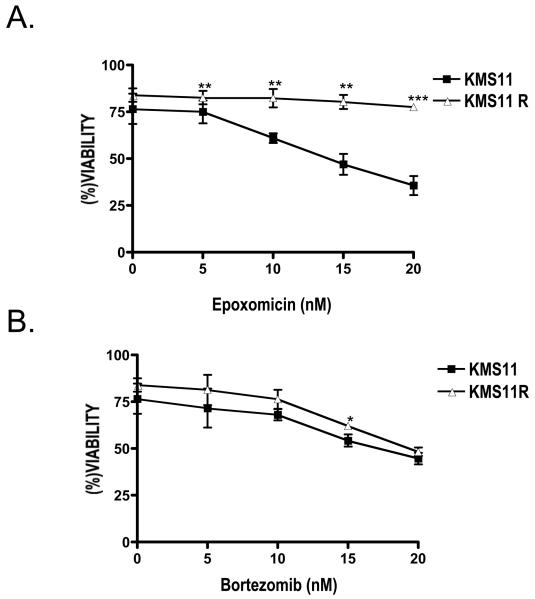
To determine factors associated with acquired resistance to proteasome inhibitors we developed KMS11R. We selected cells with the proteasome inhibitor epoxomicin for several reasons. It inhibits the same activity of the proteasome as bortezomib, namely the chymotryptic activity found in the β5 subunit (3). However epoxomicin is an irreversible inhibitor while bortezomib is reversible therefore selection would be easier and require less drug. Additionally carfilzomib, a proteasome inhibitor in clinical trials for the treatment of multiple myeloma, is derived from epoxomicin (4). KMS11R did not display overt changes phenotypically as the cells maintained expression of surface CD138 as well as FGFR3 (not shown). However the cells became resistant to epoxomicin at concentrations that are 10 times greater than the initial selecting concentration (2 nM). As seen in Figure 1A KMS11 cells display a dose-dependent response to epoxomicin while KMS11R cells are unresponsive through 20 nM at 24 h. We next asked if acquired resistance to epoxomicin resulted in cross-resistance with bortezomib. Surprisingly the KMS11R displayed no change in sensitivity to bortezomib (Figure 1B). We previously demonstrated that both epoxomicin and bortezomib activated the terminal unfolded protein response (UPR) in myeloma cell lines (5). KMS11R did not induce a terminal UPR as measured by increased ATF3 and ATF4 expression in response to epoxomicin (not shown). In contrast bortezomib induced a terminal UPR in both KMS11 and KMS11R cells (not shown).
Figure 1. Cell viability of KMS11 and KMS11R treated with epoxomicin and bortezomib.
Cells were treated with the indicated concentrations of epoxomicin (A) or bortezomib (B) for 24 h. Cell viability was determined by flow cytometry after annexin-V- FITC/ PI staining. The data are presented as the mean (± SD) of three independent experiments. *p<0.05, **p<0.01, ***P<0.001
Lysates from untreated cells or treated cells were assayed for chymotryptic activity with a fluorogenic substrate. KMS11R cells had lower activity than the parental cell line (not shown). Moreover when lysates from treated cells were assayed for chymotryptic activity, epoxomicin had a modest effect on KMS11R cells while completely inhibiting activity in the parental line. However bortezomib was able to block activity in both lines (not shown). A possible explanation for these differences in sensitivity is a mutation in the β5 subunit resulting in loss epoxomicin binding without effecting bortezomib binding. Alternatively epoxomicin has decreased access to the proteasome in cells. To discriminate between these possibilities we determined the effect of adding the proteasome inhibitors to lysates from untreated cells on chymotryptic activity. Surprisingly both lysates displayed similar dose-dependent activity in the presence of epoxomicin (not shown). Additionally we cloned and sequenced the β5 subunit (PSMB5) from both lines and found no mutations (not shown). These data suggest that the ability of epoxomicin to access the proteasome is altered in KMS11R cells.
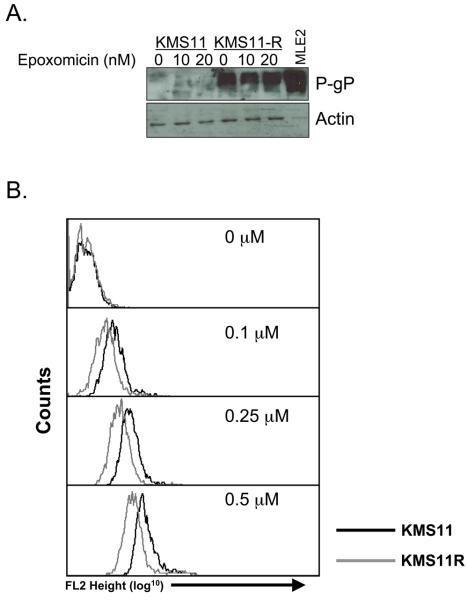
Since epoxomicin is a natural product we considered the possibility that chronic treatment of cells resulted in the induction of p-Glycoprotein (P-gP) (3). Western blot analysis revealed that KMS11R cells expressed P-gP in the absence of treatment (Figure 2A). Moreover expression is associated with acquisition of resistance, as acute treatment of cells did not induce expression of the pump. Since we are unable to directly measure epoxomicin uptake we utilized another P-gP substrate to determine if drug efflux is altered in KMS11R cells. Doxorubicin is an established substrate for P-gP and it can be easily measured in the cell since it fluoresces red. We compared the fluorescence of KMS11 and KMS11R cells treated with various concentrations of doxorubicin for 24 h. No differences in background fluorescence were observed and both cell lines could take up doxorubicin in a dose dependent fashion (Figure 2B). However the uptake observed was 3-5 fold lower in the resistant line. Additionally KMS11R cells are significantly resistant to doxorubicin-induced cell death (not shown).
Figure 2. Expression and activity of the MDR protein P Glycoprotein.
(A) Western Blot analysis of untreated KMS11 and KMS11R cells as well as cells treated with the indiated concentrations of epoxomicin for 24 h. A murine liver extract (MLE2) was used as a positive control for P-gP expression. (B) KMS11 (black histograms) and KMS11R (grey histograms) cells were treated with the indicated concentrations of doxorubicin for 24 h and the drug uptake was measured by flow cytometry. The data are representative at least three independent experiments.
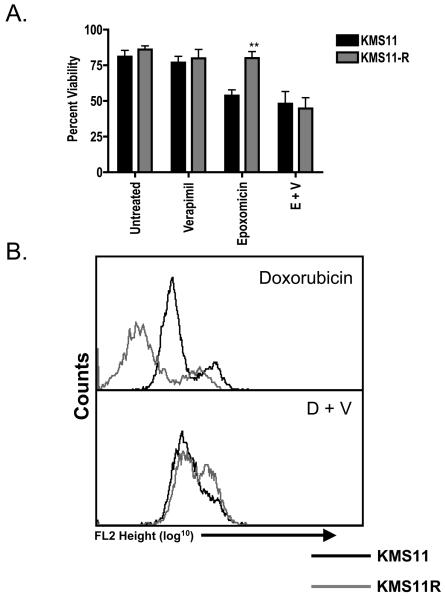
Finally we determined the role of P-gP in the acquired resistance to epoxomicin by observing the effects of inhibiting P-gP function with verapamil. Verapamil had no effect on the viability of either cell line nor did it alter the sensitivity of KMS11 cells to epoxomicin (Figure 3A) or doxorubicin (not shown). In contrast verapamil resensitized KMS11R to both drugs (Figure 3A and not shown). FACS analysis of cells treated with doxorubicin and verapamil revealed that the addition of verapamil to the KMS11R cells resulted in an increase in red fluorescence in doxorubicin-treated cells (Figure 3B).
Figure 3. Verapamil treatment of KMS11R restores sensitivity to epoxomicin.
(A). KMS11 (black histograms) and KMS11R (grey histograms) cells were treated with 20 nM epoxomicin as a single agent or in combination with 40 μM verapamil. Cell viability was determined by flow cytometry after annexin-V- FITC/PI staining. Similar results were found using a range of expoximicin concentrations from 5 nM to 20 nM (not shown). The data are presented as the mean (± SD) of three independent experiments. **p <0.01. (B). KMS11 and KMS11R cells were treated with 0.25 μM doxorubicin in the presence (lower panel) or absence (upper panel) of 40 μM verapamil for 24 h and drug uptake measured by flow cytometry.
Given the promise of bortezomib it is not surprising that other proteasome inhibitors that are either irreversible and/or inhibit the other activities of the proteasome are currently being tested in the clinic. As proteasome inhibitors become more commonly used in newly diagnosed disease it will be important to understand mechanisms of acquired resistance to this class of agents. Additionally we will need to know how acquired resistance to proteasome inhibitors alters the response to other therapeutics used to treat myeloma patients.
Acquired expression of P-gP has been observed in myeloma patients and cell lines. P-gP is rarely seen in newly diagnosed patients, however increased expression was observed in cells from approximately 75% of patients treated with vincristine, doxorubicin and dexamethasone (VAD) (6). We observed that P-gP is expressed and the P-gP inhibitor verapimil sensitizes KMS11R to epoxomicin. These data suggest that the acquired resistance observed was the result of P-gP expression. This is the first report to demonstrate the acquisition of P-gP with a proteasome inhibitor. While it is possible that the induction of P-gP could be the result of inhibition of protein turnover, this does not appear to be a likely mechanism. First epoxomicin does not acutely induce P-gP (Figure 2). More importantly we also observe increased expression of the MDR mRNA in these cells (not shown). Thus the mechanism is likely to be due to increased gene expression, possibly due to gene amplification. This remains to be determined.
Finally our data suggest that carfilzomib could be ineffective in the treatment P-gP positive myeloma. Consistent with this possibility, a recent study demonstrated that the P-gP-postive cell line, RPMI8226-Dox40, are resistant to carfilzomib however they can be sensitized by co-treatment with verapamil (7). Unfortunately such resensitization of cells to P-gP substrates has not proven to be effective clinically (6,8). Several agents that inhibit drug efflux have been tested and either proven to be too toxic or had little effect on efficacy. In addition to confirming that epoxomicin-based compounds may be subject to this type of resistance mechanism we demonstrated that they can initiate resistance in this fashion. Therefore careful consideration of the use of these compounds both as single agents and in combination therapies will be needed to assure both efficacy of this therapy as well as subsequent treatment regimens.
Acknowledgements
The work was supported by R01 CA127910.
References
- 1.Kyle RA, Vincent Rajkumar S. Treatment of multiple myeloma: an emphasis on new developments. Ann Med. 2006;38:111–115. doi: 10.1080/07853890500472078. [DOI] [PubMed] [Google Scholar]
- 2.McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–179. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Almond JB, Cohen GM. The proteasome: a novel target for cancer chemotherapy. Leukemia. 2002;16:433–443. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- 4.Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 5.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr., Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang HH, Ma MH, Vescio RA, Berenson JR. Overcoming drug resistance in multiple myeloma: the emergence of therapeutic approaches to induce apoptosis. J Clin Oncol. 2003;21:4239–4247. doi: 10.1200/JCO.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedenberg WR, Rue M, Blood EA, Dalton WS, Shustik C, Larson RA, et al. Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95): a trial of the Eastern Cooperative Oncology Group. Cancer. 2006;106:830–838. doi: 10.1002/cncr.21666. [DOI] [PubMed] [Google Scholar]