Abstract
Urothelial carcinoma of the renal pelvis is a deadly disease with an unclear tumorigenic mechanism. We conducted gene expression profiling on a set of human tumors of this type, and identified a PI3K/AKT activation expression signature in 76.9% (n=13) of our samples. Sequence analysis found both activating mutations of PIK3CA (13.6%, n = 22) and loss of heterozygosity at the PTEN locus (25%, n = 8). In contrast, none of the other subtypes of kidney neoplasms (e.g., clear cell renal cell carcinoma) harbored PIK3CA mutations (n = 87; P < 0.001). Immunohistochemical analysis of urothelial carcinoma samples found loss of PTEN protein expression (36.4%, n = 11) and elevation of phospho-mTOR (63.6%, n = 11). To confirm the role of the PI3K/AKT pathway in urothelial carcinoma, we generated mice containing biallelic inactivation of Pten in the urogenital epithelia. These mice developed typical renal pelvic urothelial carcinomas, with an incidence of 57.1% in mice older than one year. Laser capture microdissection followed by PCR confirmed the deletion of Pten exons 4 and 5 in the animal tumor cells. Immunohistochemical analyses demonstrated increased phospho-mTOR and phospho-S6K levels in the animal tumors. Renal lymph node metastases were found in 15.8% of the animals with urothelial carcinoma. In conclusion, we identified and confirmed an important role for the PI3K/AKT pathway in the development of urothelial carcinoma and suggested that inhibitors of this pathway (e.g. mTOR inhibitor) may serve as effective therapeutic agents.
Keywords: Phosphatidylinositol-3-kinase, PTEN, urothelial carcinoma, renal pelvis, animal model
INTRODUCTION
Urothelial carcinomas of renal pelvis (also known as transitional cell carcinoma) account for 7% of all kidney tumors and about 5% of all urothelial tumors (1, 2). The standard treatment for this rare malignancy is open radical nephroureterectomy. However, endoscopic management is also reasonable in selected patients (3). The known prognostic factors include pathological T stage, tumor grade, lymphovascular invasion, and tumor necrosis (1, 4-6). The proposed etiologic factors include smoking, exposure to occupational carcinogens, increased coffee consumption, and cyclophosphamide treatment (2). While urothelial carcinoma is very common in families with Balkan nephropathy (7), the underlying molecular mechanism of tumorigenesis remains unclear. Interestingly, urothelial carcinoma is also a component tumor of Lynch syndrome (hereditary nonpolyposis colon cancer (HNPCC) syndrome), especially in the Muir-Torre variant (8, 9).
Phosphatidylinositol-3-kinase (PI3K) functions as a lipid kinase that catalyzes the formation of the second messenger, phosphatidylinositol-3,4,5-trisphosphate (PIP3), from phosphatidylinositol-4,5-bisphosphate (PIP2). PI3K-mediated production of PIP3 triggers a signaling cascade which results in the activation of the serine/threonine kinase AKT and some of its downstream targets, including mTOR (mammalian target of rapamycin). Activated mTOR itself phosphorylates and activates downstream targets, including p70 S6 kinase (S6K) (10, 11). Functionally, the PI3K/AKT pathway can regulate numerous biological activities, including cellular growth, survival, and proliferation (12). Activating mutations in the PI3K p100 catalytic subunit α (PIK3CA) occur in more than 30% of solid tumors (13-15). The majority of such mutations in PIK3CA occur in exons 20, 9, 1, and 7, in order of frequency (13, 15, 16).
The PTEN tumor suppressor functions primarily as a lipid phosphatase in the cytoplasm that converts PIP3 back to PIP2. By depleting cellular levels of PIP3, PTEN acts as a brake on AKT activation. PTEN is commonly inactivated by mutation and loss of heterozygosity (LOH) in human cancers (17-20). Recently, nuclear PTEN has also been shown to play a fundamental role in the maintenance of chromosome stability, as well as a role in eliciting G1 cell cycle arrest through its nuclear phosphatase activity (21, 22).
In our gene expression study evaluating different histological subtypes of renal cell carcinoma (RCC), we found that renal pelvic urothelial carcinoma had a gene expression signature distinct from those of the clear cell, papillary, chromophobe RCC/oncocytoma, and Wilms’ subtypes (23). We therefore, hypothesized that the essential signaling pathway for the initiation of renal pelvic urothelial carcinoma could be identified by application of high-throughput screening technology and further confirmed by tissue-specific knock-out animal models.
METHODS
Patient tissue samples
Institutional Review Board approval was obtained from each participating institution providing human clinical samples. Frozen or formalin-fixed, paraffin-embedded tissue samples of 24 primary tumors with a diagnosis of urothelial carcinoma in the renal pelvis were collected from participating institutions in the United States (Spectrum Health Hospital, Grand Rapids, Michigan; Cooperative Human Tissue Network) and from the French Kidney Tumor Consortium. Of the 24 cases, 22 had sufficient tissue for DNA extraction, 13 had sufficient tissue for total RNA extraction, 11 had sufficient formalin-fixed, paraffin-embedded tissue for immunohistochemical (IHC) staining, and 8 had matched tumor/normal tissue appropriate for LOH analysis.
The DNA from another 87 kidney tumors of various pathological types (32 clear cell, 15 chromophobe, and 15 papillary RCC, 10 oncocytoma, 6 Wilms’ tumor, and 9 unknown kidney tumors) was also extracted for PIK3CA mutation screening. These kidney samples were obtained from the Cooperative Human Tissue Network with an approval from the Van Andel Research Institute Institutional Review Board.
Gene expression analysis
Gene expression profiles from 13 renal pelvic urothelial carcinoma samples were produced using the Affymetrix HG-U133 Plus 2.0 GeneChip platform, as described previously (24). Additional gene expression profiles derived from non-diseased kidney and from other subtypes of RCC were generated by our group and can be obtained from the Gene Expression Omnibus (GDS1344). Gene expression values were preprocessed using the RMA method as implemented in the BioConductor affy package for the R environment using updated probe set mappings (25). The gene expression data was filtered using an interquartile range filter (IQR > 0.5) to identify the most variable genes (n = 7634). Euclidian distance and complete linkage hierarchical clustering were used for unsupervised tumor sample evaluation. Pathway analysis was performed using a parametric gene set enrichment analysis as implemented in the BioConductor PGSEA package (26). Briefly, sets of genes that were over- or down-expressed by activation of MYC, RAS, E2F, SRC, AKT, synergistic HGF/VEGF, inactivation of VHL, or induction of hypoxia were obtained from the literature (27-31). HGF and VEGF signatures were generated using data from the Gene Expression Omnibus (GDS406 and GDS495, respectively). In all cases, cells after 24 h of treatment were compared with control cells. For consistent presentation, all “up” and “down” gene lists reflect the gene expression changes in treated/mutant cells versus the nearest approximation of wild-type cells; for example, MYC-transfected cells were compared with mock-transfected cells.
Immunohistochemistry and semi-quantitative analyses
Rabbit monoclonal antibody (Ab) against PTEN (Cat No. ab32199, Abcom, Cambridge, MA) was used for PTEN staining of mouse tissues and the human tissues using the Discovery XT System (Ventana Medical Systems, Inc., Tucson, Arizona), which automatically prepared the IHC stained slides, according to the manufacturer’s instructions. Mouse anti-PTEN mAb (1:50, 26H9, Cell Signaling Technology, Inc., Boston, MA) was also used for manual staining of some human tissues. Rabbit anti-phosphorylated mTOR (1:20, Ser2448, Cat No. 2976, Cell Signaling Technology, Inc.) and rabbit anti-phosphorylated S6-ribosomal protein (1:50, ser240/244 No.2215, Cell Signaling Technology, Inc.) were applied using the automatic staining system. Rabbit anti-phosphorylated AKT mAb (1:5, gift from Katie Crosby, Cell Signaling Technology) was used for the manual staining of some human tissues. For manual staining, epitope retrieval was performed by heating the sections at 95 °C in 10 mM citrate buffer, pH 6.0, for 25 min. The sections were incubated with primary antibodies at 4 °C overnight, and visualized using a VECTASTAIN Elite ABC kit (POD; Vector Laboratories, Burlingame, CA), 3,3′-diaminobenzidine (DAB), and hematoxylin counterstaining. For semi-quantitative analyses of the IHC staining of PTEN and phospho-mTOR, a scoring system was applied (32). Briefly, the IHC reaction was scored by multiplying the percentage of positive tumor cells (PP: 0 = no positive tumor cell; 1 < 10%; 2 = 10-50%; 3 = 51-80%; 4 > 80% positive tumor cells) by their prevalent degree of staining (SI: 0 = negative; 1 = weak; 2 = moderate; 3 = strong staining). Immunoreactive scores (IRS = PP × SI) range from 0 to 12. The average value from the scores of two independent observers was used as the final value. For phospho-mTOR staining, the interstitial tissues in all 11 cases were weakly stained with scores lower than 6. Therefore, we regard a score higher than 6 as indicating significant elevation of phospho-mTOR in the tumors.
PIK3CA mutation screening
Exons 1, 7, 9, and 20 of PIK3CA were amplified from genomic DNA with primers complementary to surrounding intronic sequences (see Supplementary Table 2). PCR was carried out with 25 ng of genomic DNA in a reaction volume of 50 μl. Products were purified on Millipore MultiScreen HTS PCR plates, cycle-sequenced with BigDye v3.1 for 45 cycles at an annealing temperature of 55°C, and run on an ABI 3700 Genetic Analyzer.
LOH analysis of the PTEN region
Eight matched samples of renal pelvic urothelial carcinoma tumor tissue and normal tissue were studied using 12 highly polymorphic microsatellite markers from human chromosome 10: D10S1652, D10S537, D10S1686, W1218, D10S1739, W213, D10S1753, D10S564, D10S583, D10S185, D10S192, and D10S597. These markers are located from 10q21.2 to 10q25.1, flanking the PTEN gene (10q23.3). PCR was performed in a 7.5-μl reaction volume containing 0.17 μM each of fluorescence-labeled forward and unlabeled reverse primer, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 4 mM MgCl2, 0.3 U AmpliTaq Gold polymerase (Applied Biosystems, Foster City, CA), 0.25 mM dNTPs, and 15 ng of genomic DNA. Amplification was performed in a thermal cycler (ABI). Assessment of LOH was performed using Genescan v. 3.7 and Genotyper v. 3.7 software (ABI). LOH was defined by the LOH index = (T2/T1)/(N2/N1), where T was the tumor sample, N was the matched normal sample, and 1 and 2 were the intensities of smaller and larger alleles, respectively. LOH was confirmed if the ratio was < 0.6 or > 1.3.
Transgenic mice, genotyping, and histological analyses
Ksp-cadherin is a tissue-specific member of the cadherin family that is expressed exclusively in the epithelial cells of the kidney and the developing genitourinary tract (33). Ksp-Cre transgenic mice expressing Cre recombinase under the control of the Ksp-cadherin promoter have been established and used for organ-specific knock-out studies (34). Pten-flox mice, carrying a pair of loxP sites that flank Pten exons 4 and 5 (35), were obtained from Tak Mak. We generated mice homozygous for the Pten-flox allele that also contained the Ksp-Cre transgene (Ksp-Cre/Ptenflox/flox). Genotyping was performed by PCR analysis of tail DNA. The primers used for Cre recombinase genotyping were as follows: 5′-CGATGCAACGAGTGATGAGGTTC-3′ (CreF) and 5′-GCACGTTCACCGGCATCAAC-3′ (CreR). Pten-flox genotyping was carried out using the following primers: 5′-CATCACACTAAGGTCTGTGG-3′ (Pten-11698-F) and 5′-GTGAACTCCCACCAATGAAC-3′ (Pten-11836-R).
After euthanization of the mice, the studied organs and tissues were isolated and fixed in 10% neutral buffered formalin for routine hematoxylin/eosin (H&E) staining or IHC staining. All mouse experiments were approved prior to initiation by the Van Andel Institute Institutional Animal Care and Use Committee.
LCM and PCR amplification
Laser capture microdissection (LCM) was performed on the urothelial carcinoma tissues as previously reported (34). Briefly, sections (8 μm) were cut from the paraffin blocks and stained with H&E. LCM was then performed using a PixCell IIe LCM system (Arcturus Engineering, Inc., Mountain View, CA) following the manufacturer’s protocols. Captured cells attached to the polymer film surface on the CapShur LCM caps (Arcturus Engineering, Inc.) were incubated with 150 μl of digestion buffer from PicoPure DNA extraction kit (Arcturus Engineering, Inc.) at 65 °C for 24 h, followed by boiling for 10 min to inactivate the proteinase K.
Primers were designed to amplify the region flanked by loxP sites in the Pten-flox allele. A third primer was also used as indicated in Figure 5C. Sequences were as follows: 5′- ATTGTATGTGATCATCTGTC -3′ (P1) ; 5′- TCACCAGGCAGTAAAAGACAAGTC -3′ (P2) ; and 5′- AACAGAACATCTGAACACTTCATCG -3′ (P3). PCR was performed using Platinum PCR SuperMix High Fidelity (Invitrogen) and 300 nM of each primer. Four DNA samples were analyzed: 1) total urothelial carcinoma tumor tissue from a Ksp-Cre(+)/Ptenflox/flox mouse; 2) laser-captured urothelial carcinoma tissue from the same mouse; 3) tail DNA from the same mouse, and 4) tail DNA from a mouse homozygous for the wild-type Pten allele.
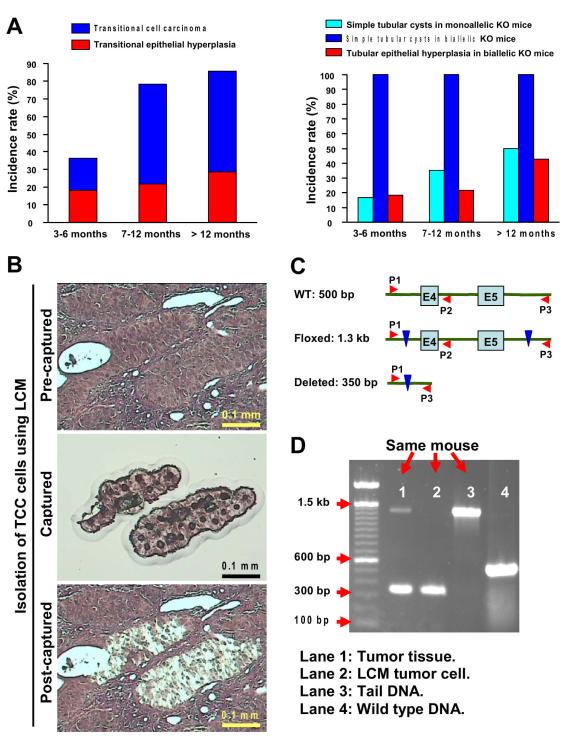
Figure 5. Pathological progression in the transitional epithelia and tubular epithelia of Pten deletion mice.
A, pathological alterations of the transitional epithelia were observed only in Ksp-Cre/Ptenflox/flox mice (left panel; the animal numbers for 3-6 months old, 7-12 months old, and >12 months old were 11, 23, and 7, respectively). Simple tubular cysts (Figure 4D, left panel) were observed in both Ksp-Cre/Ptenflox/+ and Ksp-Cre/Ptenflox/flox mice, at different incidence rates (right panel). Hyperplasia of tubular epithelia (Figure 4D, right panel) occurred only in Ksp-Cre/Ptenflox/flox mice (right panel). The mice with Pten heterozygous deletion did not develop tubular epithelial hyperplasia. B, urothelial carcinoma cells from a Ksp-Cre/Ptenflox/flox mouse were isolated using LCM. C, schematic diagram of the PCR strategy used to verify loss of Pten in the murine tumor. Three primers (P1, P2, and P3) were used for the PCR amplification of exons 4 and 5 of the Pten gene in the urothelial carcinoma cells isolated as indicated in B. D, PCR amplification confirmed the deletion of exons 4 and 5 of the Pten gene in the urothelial carcinoma of the involved mouse kidney. Four DNA samples were analyzed. Lane 1: total urothelial carcinoma tissue from a Ksp-Cre(+)/Ptenflox/flox mouse; Lane 2: laser-captured urothelial carcinoma tissue from the same mouse; Lane 3: tail DNA from the same mouse, and Lane 4: tail DNA from a mouse homozygous for the wild-type Pten allele.
Statistical analyses
Genes differentially expressed between urothelial carcinoma and the other tumor types, were identified using a moderated t-statistic as implemented in the limma BioConductor/R package (36). Significance values were adjusted using the false discovery rate (FDR) method to compensate for multiple testing. A two-sided Student’s t-test was used to determine if the expression of the genes associated with pathway activation or repression were deregulated in each individual tumor sample when compared to the median expression of the same genes in the non-diseased samples. A chi-squared test was used to compare the incidence rates of predicted PI3K/AKT activation from gene expression data. A Fisher’s exact test was used for comparison of the somatic activating mutation rates of PIK3CA between TCC and other types of kidney tumors.
RESULTS
Unique gene expression profile was revealed in human urothelial carcinoma of the renal pelvis
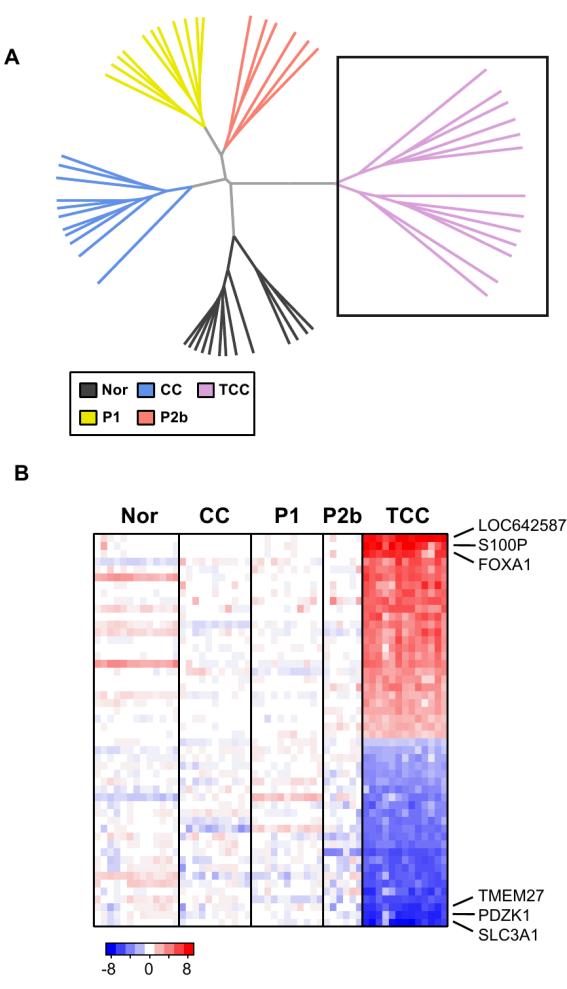
Gene expression profiling was performed on a set of renal urothelial carcinomas to gain insight into the molecular genetic defects associated with these tumors. Genes that are overexpressed in urothelial carcinoma relative to normal kidney cortex and other kidney tumors were identified. The expression levels of several genes, e.g., S100P, Rab25, various keratins, and forkhead transcription factors, were consistent with previous gene expression profiling studies of urothelial carcinoma (37, 38). In agreement with its unique pathological appearance, the gene expression profiling of urothelial carcinoma suggests that this carcinoma is a distinct subtype of kidney tumor (Fig. 1).
Figure 1. Gene expression differences between urothelial carcinoma and other kidney tumors.
Gene expression profiling data was obtained from non-diseased kidney cortex (Nor, n=13), clear cell (CC, n=11), papillary type 1 (P1, n=11), papillary type 2b (P2b, n=6), and urothelial carcinomas or transitional cell carcinoma (TCC, n=13) using the Affymetric HG-U133 Plus 2.0 chipset. A, The gene expression data was filtered using an interquartile range (IQR) filter to identify the most variable genes (n=7634), and the samples were organized using Euclidian distance and complete linkage hierarchical clustering. B, The 50 most significantly up and down-regulated genes in the urothelial carcinoma samples were identified and the expression values plotted as a heatmap (P < 1e-22). Red indicates the gene has increased expression in the urothelial carcinoma samples compared to the other samples. Blue indicates the gene has decreased expression.
AKT pathway was prominently activated in urothelial carcinoma of the renal pelvis
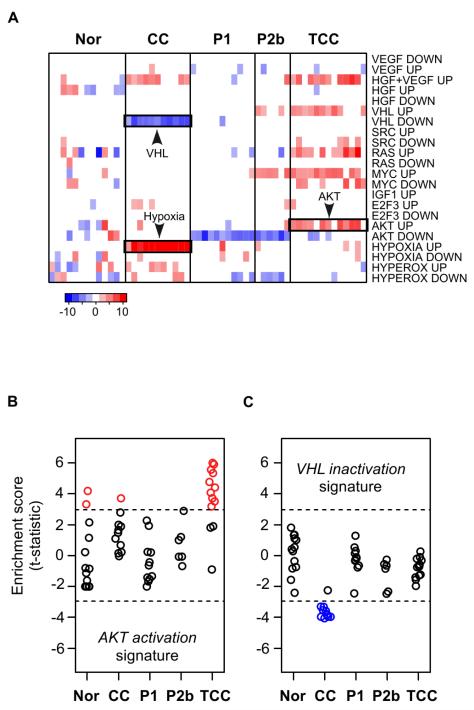
The gene expression data was also examined for evidence of signal transduction defects using gene set enrichment analysis (26, 27, 39, 40). Sets of genes that are regulated by known oncogenes and tumor suppressors were evaluated for deregulation in the urothelial carcinoma samples (Fig. 2A). This analysis revealed that a set of genes over-expressed following activation of PI3K/AKT in tissue culture cells was also significantly over-expressed in 10 of 13 urothelial carcinoma samples (76.9%) (Fig. 2B). Clear cell RCCs, which represent the majority of adult kidney tumors, are associated with biallelic inactivation of the VHL gene (41). Consistent with VHL inactivation, a set of VHL-regulated genes were significantly down-regulated in the clear cell RCC samples (Fig. 2C). The VHL-regulated genes were not significantly deregulated in the urothelial carcinoma samples, suggesting that defects in AKT signaling, but not VHL signaling, are associated with development of urothelial carcinoma of renal pelvis.
Figure 2. Prediction of AKT activation from gene expression data.
A, Gene expression profiling data were analyzed using a parametric gene set enrichment approach for evidence of deregulated signal transduction pathways. As described in the Methods, for each signaling pathway we generated lists of genes that exhibited altered expression in cells that were treated or mutated to affect that indicated pathway. For each pathway, the “up” gene signature is the list of genes that have increased expression in the treated/mutant cells relative to control cells (for example, MYC transfected versus mock transfected cells). Likewise, the “down” signature indicates the set of genes that show decreased expression relative to control cells. “GOF” and “LOF” indicate if the signature components are associated with gain of function or loss of function of each pathway, respectively. Plotted is the resulting summary statistic (t-statistic) for each gene list. Red indicates a significant number of genes in the list have increased expression in the tumor samples relative to the normal kidney while blue indicates a significant number of genes in each list have decreased expression. Only the most significant data are displayed (P < 0.005). B and C, predictions of signal transduction pathway abnormalities were generated using the P-GSEA approach. In this approach, each sample receives a t-statistic (t) and a significance value (P) that indicates that the gene signature is present in the tumor gene expression profile. Shown are t-statistics generated from genes signatures indicative of AKT pathway activation and VHL pathway inactivation in the different tumor subtypes. The dashed-line highlights the samples that have the most significant gene set enrichment (P < 0.005).
PIK3CA mutations were found only in urothelial carcinoma of the renal pelvis
Activating mutations in the catalytic subunit of PI3K (PIK3CA) are common occurrences in cancer. To determine if activating mutations in PIK3CA are associated with the predicted frequent activation of the PI3K/AKT pathway in renal pelvic urothelial carcinoma, sequence analysis of PIK3CA was performed on 22 human renal pelvic urothelial carcinomas and 87 cases of other types of renal tumors. Mutations of PIK3CA were found in 4 (18.2%) urothelial carcinoma cases (one tumor with K111E mutation in exon 1, two with E545K, and one with E542K in exon 9). Of these, the mutations of E545K and E542K in exon 9 occur in a hotspot of sequence mutation and are known activating mutations (13, 42-44). Therefore, at least a 13.6% (3/22) frequency of an activating PI3KCA mutation was found in the urothelial carcinoma samples (Supplemental Table 1). In contrast, no mutation was found in the 87 cases of other renal neoplasms; thus, the prevalence of activating mutations in PIK3CA is significantly higher in renal pelvic urothelial carcinoma (P = 0.01).
LOH at the PTEN gene locus in urothelial carcinoma of the renal pelvis
In addition to DNA sequence mutations, LOH of PTEN is a well-known event in many malignancies and serves to activate the PI3K/AKT pathway. To determine if LOH of PTEN occurs in renal pelvic urothelial carcinoma, we examined 8 pairs of matched normal/tumor tissues. LOH at the PTEN gene locus was found in 2 cases, a 25% frequency (Supplemental Table 1).
Loss of PTEN protein and elevation of phosphorylated mTOR in urothelial carcinoma of the renal pelvis
To determine the protein levels of two important components of the PI3K/AKT pathway, PTEN and mTOR, IHC staining of human urothelial carcinoma of renal pelvis was performed, followed by semi-quantitative scoring using a 0-12 scale system. Figure 3 shows an absence of PTEN protein in a renal pelvic urothelial carcinoma and elevated expression of phosphorylated AKT and phosphorylated mTOR. Of the 11 cases analyzed, 36.4% of the urothelial carcinoma s had loss of PTEN expression and 63.6% had elevation of phosphorylated mTOR (Supplemental Table 1).
Figure 3. Evidence for AKT pathway activation in human urothelial carcinomas at the protein level.
Human urothelial carcinoma tissue was sectioned and subjected to immunohistochemical staining for PTEN, phospho-AKT, and phospho-mTOR. The absence of PTEN in the tumor cells (T) was accompanied by elevated levels of phospho-AKT and phospho-mTOR.
The mice with homozygous Pten deletion harbored typical renal pelvic urothelial carcinomas
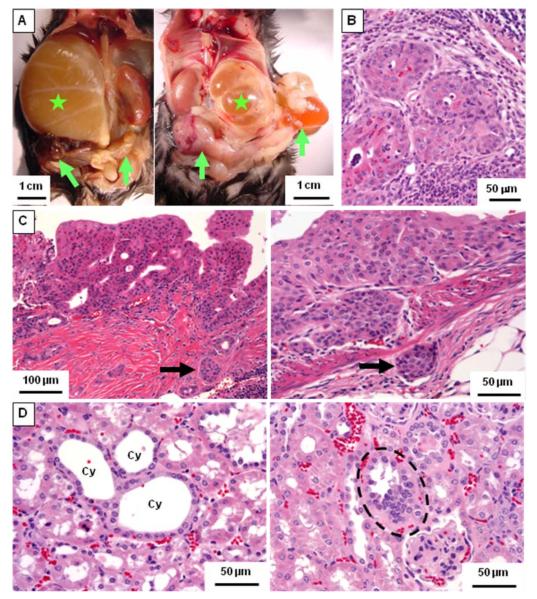
To verify the role of the PI3K/AKT pathway in the tumorigenesis of human renal pelvic urothelial carcinoma, mice were generated that carried a conditional deletion of the Pten gene specifically in the renal epithelium, using the Ksp-Cre/lox system. While neither transitional epithelial hyperplasia nor urothelial carcinoma was found in wild-type or monoallelic Pten knock-out (Ksp-Cre/Ptenflox/+) mice, typical urothelial carcinomas of renal pelvis were found in homozygous Pten deletion (Ksp-Cre/Ptenflox/flox) mice (Fig. 4). It was common in homozygous Pten deletion mice older than one year that urothelial carcinoma involving the uretero-pelvic junction obstructed urine outflow and caused hydronephrosis (Fig. 4A). Moreover, urothelial carcinoma in this animal model invaded through the muscular layer of the renal pelvis and into the surrounding fat tissue (Fig. 4C). Importantly, renal lymph node metastases were also found in 15.8% of the animals with urothelial carcinoma (Fig. 4B). The incidence rates of urothelial carcinoma of renal pelvis and precancerous transitional epithelial hyperplasia in homozygous Pten deletion mice increased along with age (Fig. 5A). The incidence of renal pelvic urothelial carcinoma was 18.2% in the mice younger than 6 months and increased to 57.1% in mice older than one year.
Figure 4. Animal models of homozygous Pten deletion in urogenital epithelia.
A, autopsy picture of Ksp-Cre/Ptenflox/flox mice at the age of 483 days (left panel, male) and 624 days (right panel, female). Stars denote the hydronephrotic kidneys caused by urothelial carcinoma. The left kidney of the male mouse in left panel is also abnormally enlarged. Arrows indicate adenocarcinomas in both seminal vesicles (left panel) and endometrial carcinoma (right panel). B-D, H&E-stained tissue sections from Ksp-Cre/Ptenflox/flox mice. B, metastatic cancer cells in the left renal lymph node consistent with the urothelial carcinoma in the kidney. C, a renal pelvic urothelial carcinoma with a papillary architecture invades into the muscularis (arrow in left panel). A renal pelvic urothelial carcinoma in another mouse invades through the muscular layer and into the surrounding fat tissue (arrow in right panel). D, tubular cystic abnormality (left panel; Cy, individual cysts) and hyperplasia of tubular epithelial cells (dashed circle in left panel) are noted.
To confirm the inactivation of Pten in the murine urothelial carcinomas, tissue was isolated using LCM (Fig. 5B) followed by PCR analysis to characterize the Pten locus in the tumors (Fig. 5C). This analysis confirmed the deletion of Pten exons 4 and 5 in the mouse urothelial carcinoma tissues (Fig. 5D).
Activation of the Akt pathway in mouse urothelial carcinomas of renal pelvis
IHC staining of PTEN, mTOR, and S6K (a downstream kinase regulated by mTOR), was performed on murine urothelial carcinoma tumor sections to determine the status of these proteins. Figures 6A clearly show that the absence of Pten was accompanied by elevated expression of phosphorylated mTOR and phosphorylated S6K.
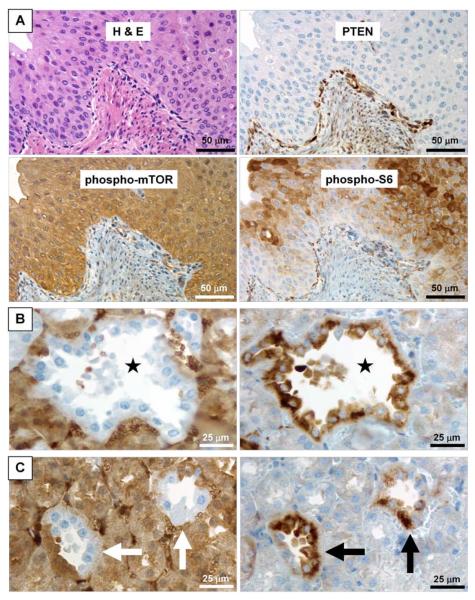
Figure 6. Activation of the Akt pathway in murine renal pelvic urothelial carcinoma and polycystic renal dysplasia following inactivation of Pten.
A, continuous sections of a murine renal pelvic urothelial carcinoma are routinely stained with H&E as well as immunohistochemically stained with anti-PTEN, anti-phospho-mTOR, and anti- phospho-S6K antibodies. Absence of PTEN in the tumor is accompanied by elevated expression of phospho-mTOR and accumulation of phospho-S6. B, continuous sections of a kidney from a homozygous Pten deletion were stained with anti-PTEN Ab (left panel) and anti-phospho-S6K Ab (right panel). Note that the absence of PTEN is consistent with the presence of phospho-S6K in the epithelium of a renal cyst (star), indicating the activation of Akt pathway in the involved epithelial cells. C, continuous sections of a kidney from a homozygous Pten deletion mouse were stained with anti-PTEN Ab (left panel) and anti-phospho-S6K Ab (right panel). Note that the mosaic absence of PTEN in two renal tubules (white arrows in left panel) is consistent with the mosaic presence of phospho-S6K in these tubules (black arrows in right panel).
Polycystic renal dysplasia and genital carcinomas following Akt pathway activation
Since the Ksp-cadherin promoter is expressed in the epithelial cells of the kidney as well as the developing genitourinary tract (33), inactivation of Pten in our animal model also resulted in some structural abnormalities in the renal parenchyma and the genital organs.
Polycystic tubular abnormalities, consisting chiefly of simple tubular cysts which were lined by a single layer of epithelial cells (Fig. 4D, left panel), occurred in all kidneys of homozygous Pten deletion mice in every age group (Fig. 5A, right panel). This abnormality was present in 50% or less of the kidneys of heterozygous Pten deletion mice in every age group and increased in frequency as the animals aged. The consistency of the absence of Pten protein and the presence of phosphorylated S6K protein (Fig. 6B and 6C) in these tubular epithelial cells confirmed the activation of Akt pathway in this alteration.
Hyperplasia of tubular epithelia in the renal parenchyma (Fig. 4D, right panel) only occurred in the homozygous Pten deletion mice, and the frequency also increased with age (Fig. 5A, right panel).
Systemic homozygous inactivation of Pten results in early embryonic death (45, 46). It has been reported that systemic heterozygous inactivation of Pten induces neoplasms in multiple organs including the endometrium (45, 47). Consistent with these findings, endometrial carcinomas and seminal vesicle carcinomas were found in our study in 43.5% of the homozygous Pten deletion mice (Fig. 4A) and were associated with elevated expression of phosphorylated mTOR in the tumor cells (Supplemental Fig. S1).
DISCUSSION
We conducted a comprehensive study to elucidate the role of the PI3K/AKT pathway activation in the development of renal pelvic urothelial carcinoma. We first identified differentially expressed genes consistent with the activation of PI3K/AKT pathway in human renal pelvic urothelial carcinoma using high-throughput gene expression profiling. Subsequently, we found 13.6% of these human tumors contained activating somatic PIK3CA mutations and 25% had LOH in and around the PTEN locus. In addition, 54.5% of these human urothelial carcinomas had significantly decreased or absent expression of PTEN protein, while 100% displayed increased phospho-mTOR expression. These data all support a key role for the PI3K/AKT pathway in human renal pelvic urothelial carcinoma. Finally, we were able to demonstrate induction of renal pelvic urothelial carcinoma highly similar to that of humans by means of a homozygous tissue-specific Pten deletion and activation of Akt and mTor signaling in a murine model.
Patients with upper-tract urothelial carcinoma are usually elderly. A study involving more than 5000 patients between 1985 and 1996 placed the mean age of urothelial carcinoma development as 70 years old (48). Consistent with this observation, our renal-specific Pten knock-out mice exhibit increasing prevalence of renal pelvic urothelial carcinoma with age, from 18.2% when younger than 6 months to 57.1% when older than 12 months (Fig. 5A, left panel). The late occurrence of renal pelvic urothelial carcinoma in both humans and animal models implies that genetic or environmental factors, in addition to PI3K/AKT pathway activation, may be involved in the initiation of renal urothelial carcinoma. Our mouse model may be a unique tool for addressing this issue.
The identification of AKT pathway activation in urothelial carcinoma suggests that targeting this kinase or its targets could provide therapeutic benefits for the majority of patients with this deadly disease. It has been reported that the members of HNPCC families have a 14-fold greater risk of developing urothelial carcinoma relative to the general population with the same ethnic background (8). HNPCC is caused by germline mutations in the mismatch repair genes. Mismatch repair deficiency in this setting results in the cellular phenotype known as microsatellite instability, which particularly affects mononucleotide repeat tracts. In subsets of HNPCC-related colorectal cancers and endometrial cancers, somatic mutations targeting the 6A tracts in exons 7 and 8 of PTEN have been found, resulting in upregulation of the AKT pathway (9). Therefore, based on these and our current report, we propose that intervention against AKT, or toward downstream targets such as mTOR, might also be an effective cancer prevention approach for individuals in HNPCC families. For example, rapamycin and its analogs are currently being tested in clinical trials in a variety of settings and could be rapidly integrated into the treatment of renal pelvic urothelial carcinoma.
To our knowledge, our animal model is the first that can generate spontaneous renal pelvic urothelial carcinoma. Interestingly, urothelial carcinoma of the renal pelvis accompanied by autosomal dominant polycystic kidney disease has been reported, but with an unclear molecular mechanism (49, 50). In our conditional Pten knock-out mice, renal pelvic urothelial carcinoma was commonly accompanied by polycystic renal dysplasia, mimicking the clinical manifestations of this human disease. Therefore, our model could be very useful in fully elucidating the related molecular mechanism(s) of urothelial carcinoma with polycystic kidney disease.
A pathological similarity has been observed between urothelial carcinoma of upper tract and bladder urothelial carcinoma, but the patterns of disease relapse are very different. Bladder urothelial carcinoma occurs in 15-50% of patients following urothelial carcinoma of upper tract, while the latter only occurs in 2-4% of bladder urothelial carcinoma patients with a longer relapse-free survival (3). In our study, no alteration of the transitional epithelium in the bladder was observed, although Cre recombinase has been reported to be expressed in the developing genitourinary tract of Ksp-cre transgenic mice (33). This implies that important differences in tumorigenic susceptibility exist in different parts of the transitional epithelia that line the urinary tract.
In summary, this study reports a comprehensive translational study involving a high through-put screening technology and the development of an animal model to systematically identify a uniquely activated molecular pathway in urothelial carcinoma of renal pelvis, and to further confirm the carcinogenic role of this pathway in the initiation of the malignancy. We found that the AKT pathway was activated in the majority of human renal pelvic urothelial carcinomas, which might be partly due to activating mutations of PIK3CA and the loss of PTEN. Conditional knock-out of the Pten gene results in renal pelvic urothelial carcinoma in mice, which confirms the etiological effect of AKT pathway activation in this malignancy. Importantly, our report implicating the up-regulation of AKT and downstream effectors such as mTOR in the development of urothelial carcinoma lends support to the testing of mTOR inhibitors in treating human renal pelvic urothelial carcinoma in various settings, both sporadic and heritable.
Supplementary Material
Acknowledgments
We thank Tak Mak (University of Toronto, ON, Canada) for providing Pten-flox mice; David Nadziejka and Vanessa Fogg for technical editing of the manuscript; and Bryn Eagleson, Sylvia Marinelli, and the VARI vivarium staff for expert animal husbandry. We thank the former and current staff members in the Laboratory of Cancer Genetics, VARI, who have helped in the establishment of the Gene Expression Omnibus. These colleagues include Min-Han Tan, John Ditlev, Mark Betten, Masayuki Takahashi, David Petillo, and Zhong-Fa Zhang. We thank Cassandra Zylstra, Daniel Robinson, and other members of the Williams laboratory for assistance in genotyping mice, and members of the VAI Germline Modification core of Pamela Swiatek for the preparation of DNA from mouse tail biopsies. We also thank Sabrina Noyes for manuscript preparation and submission.
This study was funded by The Gerber Foundation, the Hauenstein Foundation, the Michigan Economic Development Corporation, and the Michigan Technology Tri-Corridor (Michigan Animals Models Consortium grant 085P1000815). Charis Eng is a Doris Duke Distinguished Clinical Scientist and is partially funded by the National Cancer Institute (1P01CA124570-01A1).
REFERENCES
- 1.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 2.Kirkali Z, Tuzel E. Transitional cell carcinoma of the ureter and renal pelvis. Crit Rev Oncol Hematol. 2003;47:155–69. doi: 10.1016/s1040-8428(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 3.Raman JD, Scherr DS. Management of patients with upper urinary tract transitional cell carcinoma. Nat Clin Pract Urol. 2007;4:432–43. doi: 10.1038/ncpuro0875. [DOI] [PubMed] [Google Scholar]
- 4.Ataus S, Onal B, Tunc B, et al. Factors affecting the survival of patients treated by standard nephroureterectomy for transitional cell carcinoma of the upper urinary tract. Int Urol Nephrol. 2006;38:9–13. doi: 10.1007/s11255-005-3151-3. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi E, Horiguchi Y, Nakashima J, et al. Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J Urol. 2005;174:2120–3. doi: 10.1097/01.ju.0000181801.22474.8b. discussion 4. [DOI] [PubMed] [Google Scholar]
- 6.Wu CF, Pang ST, Chen CS, Chuang CK, Chen Y, Lin PY. The impact factors on prognosis of patients with pT3 upper urinary tract transitional cell carcinoma. J Urol. 2007;178:446–50. doi: 10.1016/j.juro.2007.03.115. dicussion 50. [DOI] [PubMed] [Google Scholar]
- 7.Mandal AK, Sindjic M, Sommers SC. Kidney pathology in endemic nephropathy. Clin Nephrol. 1987;27:304–8. [PubMed] [Google Scholar]
- 8.Sijmons RH, Kiemeney LA, Witjes JA, Vasen HF. Urinary tract cancer and hereditary nonpolyposis colorectal cancer: risks and screening options. J Urol. 1998;160:466–70. [PubMed] [Google Scholar]
- 9.Zhou XP, Kuismanen S, Nystrom-Lahti M, Peltomaki P, Eng C. Distinct PTEN mutational spectra in hereditary non-polyposis colon cancer syndrome-related endometrial carcinomas compared to sporadic microsatellite unstable tumors. Hum Mol Genet. 2002;11:445–50. doi: 10.1093/hmg/11.4.445. [DOI] [PubMed] [Google Scholar]
- 10.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95:1432–7. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–71. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 12.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 13.Ikenoue T, Kanai F, Hikiba Y, et al. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 2005;65:4562–7. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 14.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 15.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 16.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 18.Shao X, Tandon R, Samara G, et al. Mutational analysis of the PTEN gene in head and neck squamous cell carcinoma. Int J Cancer. 1998;77:684–8. doi: 10.1002/(sici)1097-0215(19980831)77:5<684::aid-ijc4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–62. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 20.Tokunaga E, Kimura Y, Mashino K, et al. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer. 2006;13:137–44. doi: 10.2325/jbcs.13.137. [DOI] [PubMed] [Google Scholar]
- 21.Chung JH, Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65:8096–100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 22.Shen WH, Balajee AS, Wang J, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–70. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi M, Yang XJ, Sugimura J, et al. Molecular subclassification of kidney tumors and the discovery of new diagnostic markers. Oncogene. 2003;22:6810–8. doi: 10.1038/sj.onc.1206869. [DOI] [PubMed] [Google Scholar]
- 24.Yang XJ, Tan MH, Kim HL, et al. A molecular classification of papillary renal cell carcinoma. Cancer Res. 2005;65:5628–37. doi: 10.1158/0008-5472.CAN-05-0533. [DOI] [PubMed] [Google Scholar]
- 25.Dai M, Wang P, Boyd AD, et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005;33:e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furge KA, Chen J, Koeman J, et al. Detection of DNA copy number changes and oncogenic signaling abnormalities from gene expression data reveals MYC activation in high-grade papillary renal cell carcinoma. Cancer Res. 2007;67:3171–6. doi: 10.1158/0008-5472.CAN-06-4571. [DOI] [PubMed] [Google Scholar]
- 27.Bild AH, Yao G, Chang JT, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2005;439:353–7. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 28.Chi JT, Wang Z, Nuyten DS, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerritsen ME, Tomlinson JE, Zlot C, Ziman M, Hwang S. Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br J Pharmacol. 2003;140:595–610. doi: 10.1038/sj.bjp.0705494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–11. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 31.Tiwari G, Sakaue H, Pollack JR, Roth RA. Gene expression profiling in prostate cancer cells with Akt activation reveals Fra-1 as an Akt-inducible gene. Mol Cancer Res. 2003;1:475–84. [PubMed] [Google Scholar]
- 32.Scharl A, Vierbuchen M, Conradt B, Moll W, Wurz H, Bolte A. Immunohistochemical detection of progesterone receptor in formalin-fixed and paraffin-embedded breast cancer tissue using a monoclonal antibody. Arch Gynecol Obstet. 1990;247:63–71. doi: 10.1007/BF02390663. [DOI] [PubMed] [Google Scholar]
- 33.Shao X, Johnson JE, Richardson JA, Hiesberger T, Igarashi P. A minimal Ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol. 2002;13:1824–36. doi: 10.1097/01.asn.0000016443.50138.cd. [DOI] [PubMed] [Google Scholar]
- 34.Qian CN, Knol J, Igarashi P, et al. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem. 2005;280:3938–45. doi: 10.1074/jbc.M410697200. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki A, Yamaguchi MT, Ohteki T, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–34. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 36.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Kaygusuz G, Wang L, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007;31:673–80. doi: 10.1097/01.pas.0000213438.01278.5f. [DOI] [PubMed] [Google Scholar]
- 38.Mor O, Nativ O, Stein A, et al. Molecular analysis of transitional cell carcinoma using cDNA microarray. Oncogene. 2003;22:7702–10. doi: 10.1038/sj.onc.1207039. [DOI] [PubMed] [Google Scholar]
- 39.Kim HS, Skurk C, Maatz H, et al. Akt/FOXO3a signaling modulates the endothelial stress response through regulation of heat shock protein 70 expression. Faseb J. 2005;19:1042–4. doi: 10.1096/fj.04-2841fje. [DOI] [PubMed] [Google Scholar]
- 40.Sweet-Cordero A, Mukherjee S, Subramanian A, et al. An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nat Genet. 2005;37:48–55. doi: 10.1038/ng1490. [DOI] [PubMed] [Google Scholar]
- 41.Kenck C, Wilhelm M, Bugert P, Staehler G, Kovacs G. Mutation of the VHL gene is associated exclusively with the development of non-papillary renal cell carcinomas. J Pathol. 1996;179:157–61. doi: 10.1002/(SICI)1096-9896(199606)179:2<157::AID-PATH557>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 42.Isakoff SJ, Engelman JA, Irie HY, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–1000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 43.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005;102:802–7. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuels Y, Diaz LA, Jr., Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–73. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki A, de la Pompa JL, Stambolic V, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–78. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 47.Podsypanina K, Ellenson LH, Nemes A, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci U S A. 1999;96:1563–8. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164:1523–5. [PubMed] [Google Scholar]
- 49.Grubb RL, 3rd, Collyer WC, Kibel AS. Transitional cell carcinoma of the renal pelvis associated with hypercalcemia in a patient with autosomal dominant polycystic kidney disease. Urology. 2004;63:778–80. doi: 10.1016/j.urology.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Miwa S, Fuse H, Hirano S, Masuda S. Transitional cell carcinoma of the renal pelvis in a long-term hemodialysis patient with autosomal dominant polycystic kidney. Int J Urol. 2001;8:572–4. doi: 10.1046/j.1442-2042.2001.00372.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.