Abstract
Two-dimensional electron spin-echo envelope modulation (HYSCORE) analysis of the uniformly 15N-labeled archaeal Rieske-type [2Fe-2S] ferredoxin (ARF) from Sulfolobus solfataricus P1 has been conducted in comparison with the previously characterized high-potential protein homologs. Major differences among these proteins were found in the HYSCORE lineshapes and intensities of the signals in the (++) quadrant, which are contributed from weakly coupled (non-coordinated) peptide nitrogens near the reduced clusters. They are less pronounced in the HYSCORE spectra of ARF than those of the high-potential protein homologs, and may account for the tuning of Rieske-type clusters in various redox systems.
Keywords: EPR, ESEEM, HYSCORE, Rieske, ferredoxin, [2Fe-2S] cluster, archaea
1. Introduction
Proteins containing Rieske-type [2Fe-2S](His)2(Cys)2 clusters are involved in a wide range of biological electron transfer reactions such as aerobic respiration, photosynthesis, and biodegradation of various alkene and aromatic compounds [1–6]. Rieske proteins from quinol-oxidizing cytochrome bc1/b6f complexes contain a high-potential [2Fe-2S] cluster (with midpoint redox potential (Em) of ~+150 to +490 mV), whereas the archaeal and bacterial Rieske-type ferredoxins have a relatively low-potential cluster (~−150 to −50 mV). The available crystallographic structures indicate that these proteins are structurally related and that a lower potential cluster tends to have less extensive hydrogen bonding network around the cluster [7–9]. The combined density functional theory/continuum electrostatics analysis further suggests a contribution of negatively charged residues in the low-potential homolog [10]. Thus, versatility of the cluster Em’s might have been achieved in the modular evolution of the cluster binding domain by accumulative natural mutations of the local non-coordinated residues around the tuneable cluster.
Pulsed electron paramagnetic resonance (EPR) techniques such as electron spin-echo envelope modulation (ESEEM) and electron-nuclear double resonance (ENDOR) probe coupling between electron and nuclear spins, and have become popular tools in the detailed analyses of various proteins with paramagnetic centers, often aided by isotopic labeling and other physicochemical methods [11–14]. The tuneable [2Fe-2S] cluster in Rieske-type proteins is hydrogen bonded with multiple backbone peptide nitrogens (Np’s) [7–9], some of which can be potentially resolved and quantitatively analyzed by the ESEEM measurement of the hyperfine (HF) frequencies of nuclei (such as 1H, 2H, 14N, and 15N) that interact with the effective S=1/2 electron spin of the reduced cluster.
In our previous study, we have established the heterologous overexpression system in Escherichia coli for two hyperthermophile Rieske-type protein homologs with the specific aim of exploring the differences in their cluster environments: (i) an archaeal low-potential Rieske-type ferredoxin (ARF) from Sulfolobus solfataricus strain P1 (Em,7 ~−60 mV) with homology to oxygenase-associated Rieske-type ferredoxins (DDBJ-EMBL-GenBank code, AB047031) and (ii) an archaeal high-potential Rieske protein called sulredoxin (SDX) from Sulfolobus tokodaii strain 7 (Em,acid pH ~+190 mV) with weak homology to cytochrome bc-associated Rieske proteins (DDBJ-EMBL-GenBank code, AB023295) [15–18]. Particular effort was devoted to analyzing the comparative, two-dimensional four-pulse ESEEM (also called hyperfine sublevel correlation, HYSCORE) spectra of 14N(natural abundance, N/A)-ARF and SDX, which have shown two major factors affecting the spectral differences from the “strongly coupled (coordinated)” 14Nδ of histidine ligands [17]: (i) the variation of the Nδ quadrupole couplings that are influenced by the changes in coordination geometry of histidine imidazole ligands to the reduced cluster, and (ii) the variation of the Nδ HF couplings that are affected to a lesser degree by the changes of the ligand geometry and the differences in the polypeptide environment. Additionally, we suggested a possible different interaction of the reduced cluster with certain backbone Np’s in these proteins [17]. However, the powder-type 14N HYSCORE spectra provided limited information about the “weakly coupled (non-coordinated)” remote Nε (of the histidine ligands) and Np’s, due to the influence of nuclear quadrupole interaction requiring special relations between the nuclear Zeeman frequency and HF coupling [19]. These weakly coupled nitrogens can be better resolved by the orientation-selected HYSCORE analysis of 15N-labeled proteins, because 15N does not contain the quadrupole moment. Currently, 15N HYSCORE characterization is available only for the high-potential Rieske proteins [18,20,21], although several 14N studies have been reported for the low-potential homologs with emphasis on the strong couplings from histidine Nδ ligands [17,22–24].
Here we report the 15N HYSCORE investigation of a low-potential Rieske-type ferredoxin for the first time, characterizing the coordinated and non-coordinated nitrogen nuclei around the reduced [2Fe-2S] cluster in the uniformly 15N-labeled ARF (15N-ARF). We discuss the similarities and variations of 15N HYSCORE features among different types of the Rieske protein family.
2. Experimental procedures
2.1. Materials and sample preparation
Escherichia coli strain JM109 (TaKaRa, Japan) used for cloning was grown in Lauria-Bertani (LB) medium, with 50 μg/ml kanamycin when required. Water was purified by a Millipore Milli-Q purification system. Other chemicals mentioned in this study were of analytical grade.
The uniformly 15N-labeled, recombinant ARF (DDBJ-EMBL-GenBank code, AB047031) from the hyperthermoacidophilic archaeon Sulfolobus solfataricus P1 was prepared as reported previously, using the combinations of the pTrc99A vector (Amersham Biosciences)/E. coli CodonPlus(DE3)-RIL host strain (Stratagene)/M9 salt-based synthetic medium system [15].
2.2. ESEEM and HYSCORE analyses
X-band pulsed EPR measurements were carried out by using an X-band Bruker ELEXSYS E580 spectrometer with an Oxford CF 935 cryostat at 10–11 K. ESEEM experiments with two-pulse and two-dimensional four-pulse sequences were employed, with appropriate phase cycling schemes to eliminate unwanted features from experimental echo envelopes, as previously described in detail [17]. Spectral processing of ESEEM patterns, including subtraction of relaxation decay (fitting by polynoms of 3–6 deg), apodization (Hamming window), zero filling, and fast Fourier transformation (FT), was performed using Bruker WIN-EPR software.
3. Results and discussion
3.1. Strongly coupled (coordinated) 15Nδ1,2 in the (+−) quadrant of the HYSCORE spectra
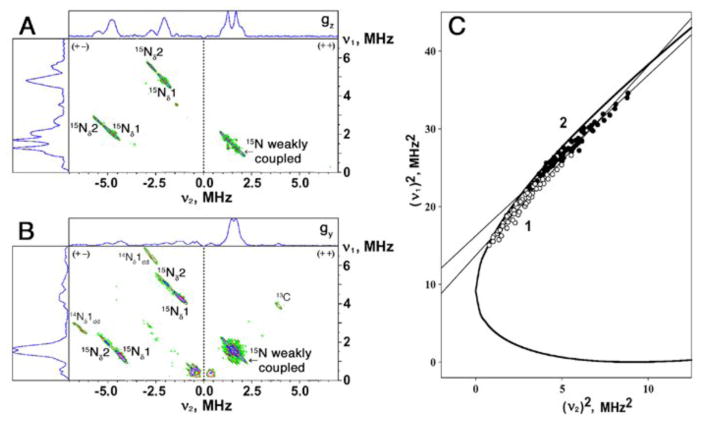
Strong antiferromagnetic coupling between the electron spins of two irons of the biological [2Fe-2S] cluster produces an EPR-silent (S=0) ground state in the oxidized Fe3+-Fe3+ form and a paramagnetic S=1/2 ground state in the reduced Fe3+-Fe2+ form. Dithionite-reduced Rieske-type [2Fe-2S] cluster in ARF is characterized by the anisotropic EPR spectrum, as a result of a rhombic g-tensor (gz,y,x=2.02, 1.90, 1.81) [15]. The two-dimensional HYSCORE spectrum consists of non-diagonal cross-peaks, whose coordinates are nuclear frequencies from electron spin ms=+1/2 and −1/2 manifolds belonging to the same nucleus [11]. Because 15N with nuclear spin I=1/2 has only two nuclear frequencies, each 15N may produce only a single pair of the cross-features which are located symmetrically relative to the diagonal line in the (+−) or (++) quadrant of the HYSCORE spectrum (depending on the 15N HF coupling strength). The cross-features produced by different types of 15N could be successfully resolved in the orientation-selected HYSCORE spectra of 15N-ARF measured at different points of the EPR line (Fig. 1A,B). In the (+−) quadrant, two pairs of cross-peaks with a contour parallel to the diagonal line are detected, which are attributed to the two coordinated histidine 15Nδ1,2 to the reduced cluster with the HF couplings of the order 6 and 8 MHz. As shown in Fig. 1C, the frequency coordinates of the data points from the cross-peaks in the (+−) quadrant were measured across the entire EPR line at different external magnetic field positions, and then plotted in the coordinates (ν1)2-versus-(ν2)2 after recalculating their frequencies for a common νI=1.511 MHz [25,26]. In this representation, all data points fell along two straight lines, described by the following equation:
where and .
Fig. 1.
HYSCORE spectra in contour presentation of the reduced Rieske-type [2Fe-2S] cluster in the uniformly 15N-labeled ARF, recorded at the gz (A) and gy (B) areas of the EPR line. The (ν1)2-versus-(ν2)2 plot for recalculated frequencies at a common νI=1.511 MHz (C) [25], where all data points for the cross-peaks correlating 15Nδ1 (open circle) and 15Nδ2 (filled circle), respectively, of 15N-ARF fell along straight line with slope and intercept: Q1=2.44 (S.E. 0.07), G1=13.7 (S.E. 0.2) MHz2 (for 15Nδ1) and Q2=2.07 (S.E. 0.04), G2=16.2 (S.E. 0.2) MHz2 (for 15Nδ2). These parameters gave the anisotropic HF tensor 15a=6.5 MHz, 15T=1.5 MHz for 15Nδ1, and 15a=7.9 MHz, 15T=1.6 MHz for 15Nδ2 (see Table 1). The heavy curve (C) is defined by |ν1+ν2|=2νI. Magnetic field, time τ, and microwave frequency, respectively: 342.5 mT (near gz), 136 ns, 9.695 GHz (A); 363.1 mT (near gy), 136 ns, 9.695 GHz (B).
The slope and intercept of each line from the linear regression fit determine the isotropic and anisotropic parts of the HF tensors (in the axial approximation) for each strongly coupled (coordinated) 15Nδ (15Nδ1, open circle; 15Nδ2, filled circle) of 15N-ARF (Fig. 1C and Table 1). These values are very similar to those reported for other Rieske-type proteins by the orientation-selected 15N HYSCORE [18,20,21] (Table 1) and 15N Q-band ENDOR [27]. On the basis of the previous 14N HYSCORE analyses of Rhodobacter sphaeroides cytochrome bc1 complex [28] and 14N(N/A)-ARF and SDX [17], two strong couplings 15Nδ1 and 15Nδ2 were tentatively assigned as the His44Nδ and His64Nδ ligands, respectively, of ARF (Table 1). One of these ligand residues, His64, can be substituted by cysteine to accommodate a fairly stable, oxidized [2Fe-2S](Cys)3(His)1 cluster in the ARF scaffold [15].
Table 1.
Isotropic and anisotropic parts of HF tensors for strongly coupled histidine 15Nδ ligands detected in the (+−) quadrant, and HF couplings of weakly coupled 15N nuclei currently resolved in the (++) quadrant of 15N HYSCORE spectra of the selected Rieske-type proteins
| Parameters | ARF | SDX | Rhodobacter sphaeroides Rieske protein fragment | |||
|---|---|---|---|---|---|---|
| (+−) quadrant | Nδ1a (His44)b | Nδ2a (His64)b | Nδ1a (His44)b | Nδ2a (His64)b | Nδ1a (His131)b | Nδ2a (His152)b |
| Q | 2.44 | 2.07 | 2.64 | 2.11 | 2.40 | 2.13 |
| G, MHz2 | 13.7 | 16.2 | 13.3 | 16.3 | 13.8 | 15.8 |
| a, MHz | 6.5 | 7.9 | 6.0 | 7.8 | 6.6 | 7.6 |
| T, MHz | 1.5 | 1.6 | 1.2 | 1.3 | 1.6 | 1.5 |
| (++) quadrantc,d | ||||||
| gz, MHz | 0.43; 1.03e | 0.3; 1.03e | 0.36; 1.13e | |||
| gx, MHz | 0.49; 1.1 | 0.42; 1.04 | 0.43; 1.22 | |||
| gy, MHzd | 0.25; 1.22 | 0.31; n.r.f | n.r.f; 1.01 | |||
| references | this work | [18] | [21] | |||
The terminology for 15Nδ1,2 is based on Ref. [18].
In R. sphaeroides cytochrome bc1 complex, the isotropic HF constant of one of two histidine 14Nδ ligands (14aiso ~5MHz; equivalent to 15Nδ2 in Table 1) in the presence of the Qo-site occupant, stigmatellin, is different from the configurations in the presence of myxothiazol, suggesting that the Nδ2, at which the changes identified occur, likely belongs to His152 involved in the interaction with the Qo-site occupants [28]. Tentative assignments of Nδ1,2 in Table 1 are made based on this previous observation in conjunction with the amino acid sequence homology, and should not be taken as definitive.
The positions of the peak maxima in this quadrant were determined with the accuracy ~0.03 MHz.
HYSCORE spectra recorded at the low- and high-field edges near the maximal and minimal g values give “single-crystal-like” patterns from the reduced cluster, whose gz and gx axes are directed along the external magnetic field. In contrast, the resonance condition at the intermediate gy value is fulfilled by many different, yet well-defined orientations.
The relative ESEEM intensity of the largest coupling ~1.03 MHz in 15N-ARF is only ~70% of that of the equivalent couplings in the high-potential protein homologs including SDX (see Fig. 2B).
Not resolved.
3.2. Weakly coupled 15Nε in the (++) quadrant of the HYSCORE spectra
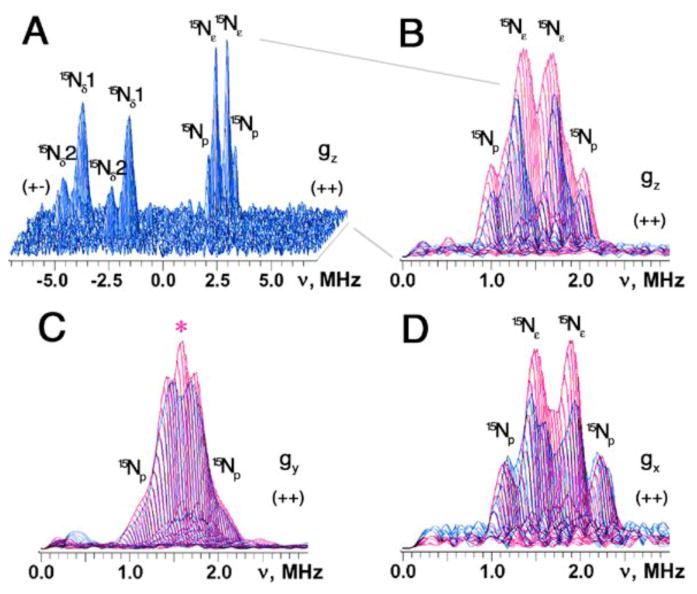
The (++) quadrant of the 15N-ARF spectra contains features centered symmetrically around the diagonal point with 15N Zeeman frequency and attributed to weakly coupled (non-coordinating) 15N nuclei near the reduced cluster (Fig. 1A,B). These features were best resolved in the “single-crystal-like” HYSCORE spectra recorded at the low- and high-field edges near the maximal and minimal g values (Fig. 2A,B,D). Near the gz area (low-field edge), two superimposed but relatively well-resolved pairs of the cross-features are detected at [2.01; 0.98] MHz (15Np) and [1.71; 1.28] MHz (15Nε), with the splittings of 1.03 and 0.43 MHz, respectively (Figs. 1A, 2A). Near the gx area (high-field edge), they are at [2.23; 1.13] MHz (15Np) and [1.92; 1.43] MHz (15Nε), with the splittings of 1.1 and 0.49 MHz, respectively (Fig. 2D). The similar splittings were also observed at some intermediate positions between the low- and high-field edges (e.g., see Figs. 1B, 2C), indicating their predominantly isotropic characters. This HYSCORE spectral pattern (but with small variations in their values) is reminiscent of those reported for the high-potential Rieske protein homologs [18,20,21] (Table 1).
Fig. 2.
HYSCORE spectra in 3D presentation of the uniformly 15N-labeled ARF recorded near the gz area (A) and superimposed stacked HYSCORE spectra in the (++) quadrant of 15N-ARF (blue) and 15N-SDX (red), recorded near the gz (B), gy (C), and gx (D) areas. At least two superimposed but well-resolved pairs of the cross-peaks are clearly detected at [2.0; 0.92] MHz (15Np) and [1.7; 1.2] MHz (15Nε) with the splittings of 1.1 and 0.5 MHz, respectively, near gz (A). Additional contribution to the 15N ESEEM amplitude in the (++) quadrant of the spectra (e.g., marked with red asterisk in panel C) is evident for 15N-SDX (red) [and other high-potential Rieske proteins; not shown] [18,20,21], when the stacked spectra (with zero projection angles) were re-scaled and superimposed after normalizing the relative scales of the cross-peak intensities from two Nδ ligands in the (+−) quadrant (B–D). The same small τ-value (τ=136 ns; slightly exceeding the dead time of the instrument) was chosen for the measurement of these 15N HYSCORE spectra, which allows the preferable observation of the undistorted lineshape of the cross-peaks as well as the minimization of the suppression effect on the ESEEM amplitudes [34]. Magnetic field, and microwave frequency, respectively: 342.5 mT (15N-ARF) and 344.3 mT (15N-SDX) (near gz), 9.695 GHz (A,B); 363.1 mT (15N-ARF) and 361.6 mT (15N-SDX) (near gy), 9.695 GHz (C); 387.0 mT (15N-ARF) and 386.0 mT (15N-SDX) (near gx), 9.695 GHz (D).
The isotropic HF coupling of the directly coordinated Nδ of the imidazole ring to a paramagnetic metal center is about 20 times larger than that of the (non-coordinated) remote Nε in various model complexes and metalloproteins [14,29]. This property is probably owing to the analogous spin density transfer phenomenon from the metal ion over the imidazole ring to the remote Nε, which is also sensitive to the protonation state of the Nε [14,29]. Thus, the intense pair of the cross-features with the smaller splitting of ~0.3–0.5 MHz in the HYSCORE spectra of these proteins (e.g., Nε in Fig. 2A) are consistent with those from the protonated form of the remote Nε’s of two histidine ligands to the reduced cluster (Table 1). The nuclear magnetic resonance (NMR) assignments of the HF-shifted resonances for His47Nε and His64Nε of a closely related Rieske-type ferredoxin component (T4moC) of the Pseudomonas mendocina toluene 4-monooxygenase complex [30,31] suggest that the two 15Nε nuclei of ARF are expected to have very similar HF couplings. They probably remain unresolved in the 15N X-band HYSCORE spectra where the estimated difference is comparable with the individual spectral line-widths.
3.3. Variations of other weakly coupled nitrogens among Rieske-type proteins
The (N/O)-H···S hydrogen bond network around the biological iron-sulfur clusters is one of the most important themes in modulating their redox properties. In the case with the N-H···S hydrogen bonds with the bridging and terminal sulfur atoms of the reduced iron-sulfur cluster system, the s- and p-orbitals of the nitrogens carry unpaired spin density transferred from the reduced cluster through chemical bonds (including hydrogen bonds). These spin densities can be observable as HF couplings in the ESEEM spectra [18–21,32].
In the (++) quadrant of the 15N-ARF spectra, the largest HF coupling of ~1.1 MHz is clearly resolved (Fig. 2), which is comparable to those previously detected in the ESEEM spectra of the plant and vertebrate [2Fe-2S](Cys)4 ferredoxins (~0.7 and ~1.1 MHz for 14Np, or ~1 and ~1.5 MHz for 15Np, respectively) [32] and the high-potential Rieske protein homologs (~1.1 MHz for 15Np) [18,20,21] (Table 1). The equivalent HF coupling ~1.1 MHz in the 15N HYSCORE spectra of the R. sphaeroides high-potential Rieske protein has been determined to come from Leu132Nα [21]. Notably, the NMR analysis of T4moC, which is closely related to ARF, showed the maximal chemical shift of ~426 ppm for the HF-shifted Gln4815Nα (equivalent to Lys45Nα in ARF [DDBJ-EMBL-GenBank code, AB047031] and Leu132Nα in the R. sphaeroides Rieske protein [21]) in the reduced protein (with the largest change of chemical shift by ~300 ppm upon reduction of the cluster) [30,31]. In the 1.48-Å structure of the Cys84Ala/Cys85Ala double mutant of T4moC (1vm9.pdb), this Np is hydrogen-bonded with the bridging sulfide S1 of the [2Fe-2S] cluster (Gln48Nα-S1 distance, 3.4 Å) [33]. Based on these considerations, we tentatively assigned the largest HF coupling of ~1.1 MHz in the (++) quadrant of the HYSCORE spectra to the 15Np nucleus (presumably Lys45Nα) of ARF (Fig. 2), which holds some unpaired spin density transferred from the reduced Rieske-type cluster via the N-H···S hydrogen bonding. In retrospect, the previously observed cross-features P1 and P2 in the 14N(N/A)-ARF spectra [17] can be re-assigned as the double quantum-double quantum (dq-dq) and double quantum-single quantum (dq-sq) features, respectively, of the same Np nucleus with the coupling ~1.1 MHz (for 15N).
The NMR analysis of T4moC also showed the presence of other HF-shifted Np’s, such as Ala66Nα (equivalent to Leu63Nα in ARF [DDBJ-EMBL-GenBank code, AB047031]) which is hydrogen bonded with the terminal Cys64Sγ ligand [30,31]. In principle, the 15N HYSCORE spectra can potentially provide information about all nitrogens involved in the measurable magnetic interactions with the unpaired electron spin of the reduced cluster, contrary to the 14N HYSCORE spectra. The 15Nδ1,2 couplings giving cross-peaks in the (+−) quadrant of the spectra (Fig. 2A) vary only slightly among different Rieske-type proteins (Table 1), suggesting that they should produce small changes in the corresponding relative intensities under the same experimental settings. The relative cross-peak intensities contributed from two 15Nδ ligands in the (+−) quadrant were therefore normalized after re-scaling and used as the internal references for the comparison of the spectral intensities of the aggregate 15Nε/15Np peaks in the (++) quadrant. Close inspection of the resulting 15N HYSCORE spectra of different Rieske-type proteins indicates the substantial variations in the lineshapes and relative intensities of their doublet components and the area around the diagonal point, in the (++) quadrant (Fig. 2B–D). Thus, although the present 15N-ARF spectra have apparently resolved the remote 15Nε and the largest 15Np couplings with the splittings 0.3–0.5 and ~1.1 MHz, respectively, like those reported for the high-potential protein homologs [18,20,21] (Table 1), these variations clearly indicate additional contributions of non-equivalent weak HF couplings from other 15N nuclei to the ESEEM amplitude in this particular region. Their possible candidates may be 15Np(s) of other non-coordinating residues around the reduced cluster and the terminal cyteine ligands, most of which should not give resolved cross-peaks in the corresponding 14N HYSCORE spectra [17,22–24]. This is important, because the overlap of these additional signals (weak couplings with narrow lineshapes), especially in the cases of the high-potential protein homologs, would interfere with the 15Nε splitting that is currently measured directly from the cross-peak positions (Fig. 2B–D). Because the ESEEM amplitudes are complicated functions of spin Hamiltonian operator parameters and experimental settings [11,12], deconvoluting each of these signals is practically difficult. Their further resolution and assignments would therefore require extensive site-specific isotope labeling of the residues near the cluster.
4. Concluding remarks
The HF couplings of the remote Nε of the histidine ligands to the reduced Rieske-type [2Fe-2S] cluster give only weak peaks that are masked by those from weakly coupled Np’s in ESEEM spectra. The best way to detect these nuclei with the X-band experiments for the future functional study is through substitution of 14N by 15N. The 15N HYSCORE characterization of dithionite-reduced 15N-ARF provides the first resolution of 15Nε and one of 15Np nuclei in a low-potential Rieske-type ferredoxin, which gave very similar HF couplings as those reported for the high-potential protein homologs [18,20,21] (Table 1). These features probably reflect the common structural framework and physical nature of the biological iron-sulfur clusters of this functionally versatile class, regardless of the cluster Em’s.
Significant variations were found among different Rieske-type proteins in the (++) quadrant of the corresponding, orientation-selected 15N HYSCORE spectra, where the weak HF couplings with narrow lineshapes from other 15Np’s appear to overlap with the 15Nε splitting and may interfere with its cross-peak positions. These weak couplings are less pronounced (but also present) in the 15N-ARF spectrum, indicating less contribution from these extra non-coordinated (probably peptide) nitrogens in the reduced ARF cluster system.
Acknowledgments
This work was supported in part by JSPS Grants-in-aid 15770088, 18608004 and 21659111 (T.I.), by JSPS Grant BSAR-507 (T.I.), by NSF Grant 9910113 (S.A.D.), and by NIH Grant GM62954 (S.A.D.).
Abbreviations
- ARF
archaeal Rieske-type ferredoxin from Sulfolobus solfataricus
- ENDOR
electron nuclear double resonance
- EPR
electron paramagnetic resonance
- ESEEM
electron spin-echo envelope modulation
- FT
Fourier transform
- Em
redox potential
- HF
hyperfine
- HYSCORE
hyperfine sublevel correlation
- NMR
nuclear magnetic resonance
- Np
peptide backbone nitrogen
- SDX
sulredoxin (a high-potential Rieske protein from Sulfolobus tokodaii)
- T4moC
Rieske-type ferredoxin component of toluene 4-monooxygenase complex
References
- 1.Mason JR, Cammack R. The electron-transport proteins of hydroxylating bacterial dioxygenases. Annu Rev Microbiol. 1992;46:277–305. doi: 10.1146/annurev.mi.46.100192.001425. [DOI] [PubMed] [Google Scholar]
- 2.Trumpower BL, Gennis RB. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu Rev Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- 3.Link TA. The structures of Rieske and Rieske-type proteins. Adv Inorg Chem. 1999;47:83–157. [Google Scholar]
- 4.Berry EA, Guergova-Kuras M, Huang LS, Crofts AR. Structure and function of cytochrome bc complexes. Annu Rev Biochem. 2000;69:1005–1075. doi: 10.1146/annurev.biochem.69.1.1005. [DOI] [PubMed] [Google Scholar]
- 5.Crofts AR. The cytochrome bc1 complex: function in the context of structure. Annu Rev Physiol. 2004;66:689–733. doi: 10.1146/annurev.physiol.66.032102.150251. [DOI] [PubMed] [Google Scholar]
- 6.Cramer WA, Zhang H, Yan J, Kurisu G, Smith JL. Transmembrane traffic in the cytochrome b6f complex. Annu Rev Biochem. 2006;75:769–790. doi: 10.1146/annurev.biochem.75.103004.142756. [DOI] [PubMed] [Google Scholar]
- 7.Iwata S, Saynovits M, Link TA, Michel H. Structure of a water soluble fragment of the ‘Rieske’ iron-sulfur protein of the bovine heart mitochondrial cytochrome bc1 complex determined by MAD phasing at 1.5 Å resolution. Structure. 1996;4:567–579. doi: 10.1016/s0969-2126(96)00062-7. [DOI] [PubMed] [Google Scholar]
- 8.Colbert CL, Couture MMJ, Eltis LD, Bolin J. A cluster exposed: structure of the Rieske ferredoxin from biphenyl dioxygenase and redox properties of Rieske Fe-S proteins. Structure. 2000;8:1267–1278. doi: 10.1016/s0969-2126(00)00536-0. [DOI] [PubMed] [Google Scholar]
- 9.Hunsicker-Wang LM, Heine A, Chen Y, Luna EP, Todaro T, Zhang YM, Williams PA, McRee DE, Hirst J, Stout CD, Fee JA. High-resolution structure of the soluble, respiratory-type Rieske protein from Thermus thermophilus: analysis and comparison. Biochemistry. 2003;42:7303–7317. doi: 10.1021/bi0342719. [DOI] [PubMed] [Google Scholar]
- 10.Klingen AR, Ullmann GM. Negatively charged residues and hydrogen bonds tune the ligand histidine pKa values of Rieske iron-sulfur proteins. Biochemistry. 2004;43:12383–12389. doi: 10.1021/bi0488606. [DOI] [PubMed] [Google Scholar]
- 11.Dikanov SA. Two-dimensional ESEEM spectroscopy. In: Atta-ur-Rahman, editor. New Advances in Analytical Chemistry. Gordon and Breach; Amsterdam: 2000. pp. 523–568. [Google Scholar]
- 12.Prisner T, Rohrer M, MacMillan F. Pulsed EPR spectroscopy: biological applications. Annu Rev Phys Chem. 2001;52:279–313. doi: 10.1146/annurev.physchem.52.1.279. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman BM. Electron-nuclear double resonance spectroscopy (and electron spin-echo envelope modulation spectroscopy) in bioinorganic chemistry. Proc Natl Acad Sci USA. 2003;100:3575–35778. doi: 10.1073/pnas.0636464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mims WB, Peisach J. ESEEM and LEFE of metalloproteins and model compounds. In: Hoff AJ, editor. Advanced EPR: Applications in Biology and Biochemistry. Elsevier; Amsterdam: 1989. pp. 1–57. [Google Scholar]
- 15.Kounosu A, Li Z, Cosper NJ, Shokes JE, Scott RA, Imai T, Urushiyama A, Iwasaki T. Engineering a three-cysteine, one-histidine ligand environment into a new hyperthermophilic archaeal Rieske-type [2Fe-2S] ferredoxin from Sulfolobus solfataricus. J Biol Chem. 2004;279:12519–12528. doi: 10.1074/jbc.M305923200. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki T, Kounosu A, Kolling DRJ, Crofts AR, Dikanov SA, Jin A, Imai T, Urushiyama A. Characterization of the pH-dependent resonance Raman transitions of archaeal and bacterial Rieske [2Fe-2S] proteins. J Am Chem Soc. 2004;126:4788–4789. doi: 10.1021/ja031976p. [DOI] [PubMed] [Google Scholar]
- 17.Dikanov SA, Shubin AA, Kounosu A, Iwasaki T, Samoilova RI. A comparative, two-dimensional 14N ESEEM characterization of reduced [2Fe-2S] clusters in hyperthermophilic archaeal high- and low-potential Rieske-type proteins. J Biol Inorg Chem. 2004;9:753–767. doi: 10.1007/s00775-004-0571-y. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki T, Kounosu A, Uzawa T, Samoilova RI, Dikanov SA. Orientation-selected 15N-HYSCORE detection of weakly coupled nitrogens around the archaeal Rieske [2Fe-2S] center. J Am Chem Soc. 2004;126:13902–13903. doi: 10.1021/ja045898x. [DOI] [PubMed] [Google Scholar]
- 19.Dikanov SA, Tyryshkin AM, Felli I, Reijerse EJ, Hüttermann J. C-band ESEEM of strongly coupled peptide nitrogens in reduced two-iron ferredoxin. J Magn Reson, Ser B. 1995;108:99–102. doi: 10.1006/jmrb.1995.1110. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki T, Kounosu A, Samoilova RI, Dikanov SA. 15N HYSCORE characterization of the fully deprotonated, reduced form of the archaeal Rieske [2Fe-2S] center. J Am Chem Soc. 2006;128:2170–2171. doi: 10.1021/ja0562393. [DOI] [PubMed] [Google Scholar]
- 21.Dikanov SA, Kolling DRJ, Endeward B, Samoilova RI, Prisner TF, Nair SK, Crofts AR. Identification of hydrogen bonds to the Rieske cluster through the weakly coupled nitrogens detected by electron spin echo envelope modulation spectroscopy. J Biol Chem. 2006;281:27416–27425. doi: 10.1074/jbc.M604103200. [DOI] [PubMed] [Google Scholar]
- 22.Shergill JK, Joannou CL, Mason JR, Cammack R. Coordination of the Rieske-type [2Fe-2S] cluster of the terminal iron-sulfur protein of Pseudomonas putida benzene 1,2-dioxygenase, studied by one- and two-dimensional electron spin-echo envelope modulation spectroscopy. Biochemistry. 1995;34:16533–16542. doi: 10.1021/bi00051a001. [DOI] [PubMed] [Google Scholar]
- 23.Dikanov SA, Xun L, Karpiel AB, Tyryshkin AM, Bowman MK. Orientationally-selected two-dimensional ESEEM spectroscopy of the Rieske-type iron-sulfur cluster in 2,4,5-trichlorophenoxyacetate monooxygenase from Burkholderia cepacia AC1100. J Am Chem Soc. 1996;118:8408–8416. [Google Scholar]
- 24.Dikanov SA, Davydov RM, Xun L, Bowman MK. CW and pulsed EPR characterization of the reduction of the Rieske-type iron sulfur cluster in 2,4,5-trichlorophenoxyacetate monooxygenase from Burkholderia cepacia AC1100. J Magn Reson Ser B. 1996;112:289–294. doi: 10.1006/jmrb.1996.0144. [DOI] [PubMed] [Google Scholar]
- 25.Dikanov SA, Bowman MK. Determination of ligand conformation in reduced [2Fe-2S] ferredoxin from cysteine β-proton hyperfine couplings. J Biol Inorg Chem. 1998;3:18–29. [Google Scholar]
- 26.Dikanov SA, Davydov RM, Gräslund A, Bowman MK. Two-dimensional ESEEM spectroscopy of nitrogen hyperfine couplings in methemerythrin and azidomethemerythrin. J Am Chem Soc. 1998;120:6797–6805. [Google Scholar]
- 27.Gurbiel RJ, Doan PE, Gassner GT, Macke TJ, Case DA, Ohnishi T, Fee JA, Ballou DP, Hoffman BM. Active site structure of Rieske-type proteins: electron nuclear double resonance studies of isotopically labeled phthalate dioxygenase from Pseudomonas cepacia and Rieske protein from Rhodobacter capsulatus and molecular modeling studies of a Rieske center. Biochemistry. 1996;35:7834–7845. doi: 10.1021/bi960380u. [DOI] [PubMed] [Google Scholar]
- 28.Samoilova RI, Kolling D, Uzawa T, Iwasaki T, Crofts AR, Dikanov SA. The interaction of the Rieske iron-sulfur protein with occupants of the Qo-site of the bc1 complex, probed by electron spin echo envelope modulation. J Biol Chem. 2002;277:4605–4608. doi: 10.1074/jbc.C100664200. [DOI] [PubMed] [Google Scholar]
- 29.Dikanov SA, Samoilova RI, Smieja JA, Bowman MK. Two-dimensional ESEEM study of VO2+ complexes with imidazole and histidine: histidine is a polydentante ligand. J Am Chem Soc. 1995;117:10579–10580. [Google Scholar]
- 30.Xia B, Pikus JD, Xia W, McClay K, Steffan RJ, Chae YK, Westler WM, Markley JL, Fox BG. Detection and classification of hyperfine-shifted 1H, 2H, and 15N resonances of the Rieske ferredoxin component of toluene 4-monooxygenase. Biochemistry. 1999;38:727–739. doi: 10.1021/bi981851a. [DOI] [PubMed] [Google Scholar]
- 31.Skjeldal L, Peterson FC, Doreleijers JF, Moe LA, Pikus JD, Westler WM, Markley JL, Volkman BF, Fox BG. Solution structure of T4moC, the Rieske ferredoxin component of the toluene 4-monooxygenase complex. J Biol Inorg Chem. 2004;9:945–953. doi: 10.1007/s00775-004-0594-4. [DOI] [PubMed] [Google Scholar]
- 32.Dikanov SA, Samoilova RI, Kappl R, Crofts AR, Hüttermann J. The reduced [2Fe-2S] clusters in adrenodoxin and Arthrospira platensis ferredoxin share spin density with protein nitrogens, probed using 2D ESEEM. Phys Chem Chem Phys. 2009;11:6807–6819. doi: 10.1039/b904597j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moe LA, Bingman CA, Wesenberg GE, Phillips GNJ, Fox BG. Structure of T4moC, the Rieske-type ferredoxin component of toluene 4-monooxygenase. Acta Cryst Sect D. 2006;62:476–482. doi: 10.1107/S0907444906006056. [DOI] [PubMed] [Google Scholar]
- 34.Dikanov SA, Tyryshkin AM, Bowman MK. Intensity of cross-peaks in HYSCORE spectra of S = 1/2, I = 1/2 spin systems. J Magn Reson. 2000;144:228–242. doi: 10.1006/jmre.2000.2055. [DOI] [PubMed] [Google Scholar]