Abstract
Recent data indicate that cortical dopamine denervation results in dystrophic changes in the dendrites of pyramidal cells, including decreases in dendritic spine density and length. However, it is not known if the loss of signaling through specific dopamine receptors subserves these dendritic changes. We examined the dendritic structure of layer V pyramidal cells in the prefrontal cortex of D1, D2, and D4 dopamine receptor null mutant mice and their wild-type littermates. Decreased basal dendritic length and spine density were observed in the D1 knockout mice. Similarly, a decrease in basal dendritic spine density was uncovered in the D2 knockout mice relative to wild-type littermates. No changes in any dendritic parameter were observed in the D4 knockout mice. These observations suggest that the dystrophic changes observed in prefrontal cortical pyramidal cell dendrites are due to loss of signaling through D1 and possibly D2 receptors. The current data also suggest that caution should be exercised in the interpretation of behavioral, physiological and biochemical studies of the PFC in dopamine receptor knockout mice.
Keywords: dendritic spine, dopamine receptor, Golgi impregnation, Parkinson’s Disease, pyramidal cell, schizophrenia
Over the past 20 years a large number of studies have uncovered structural changes in the brains of schizophrenic persons. In particular, there is a decrease in cortical thickness and volume in the prefrontal cortex (PFC), but without an overall change in the numbers of neurons (Selemon and Goldman-Rakic, 1999). Because neuronal number is unchanged, elements in the neuropil, including dendrites, may be lost. Among the most replicated of postmortem findings is the decrease in the density of dendritic spines on pyramidal cell (PCs) in the PFC (Black et al., 2004;Broadbelt et al., 2002;Garey et al., 1998;Glantz and Lewis, 2000;Kolluri et al., 2005). Also reported is a decrease in the dopamine innervation of the PFC in schizophrenia that does not appear to be attributable to antipsychotic drug treatment (Akil et al., 1999), raising the possibility that the dystrophic changes in PC dendrites in the PFC may be related to a decrease in dopaminergic tone. We recently found that dopamine denervation of the rat PFC results in a decrease in both basal and apical dendritic spine density, basal dendritic length, and dendritic arborization in layer V PFC PCs in the rat (Wang and Deutch, 2008). This observation is consistent with the hypothesis that a decrease in dopamine signaling in the PFC results in dendritic remodeling of cortical neurons in schizophrenia. However, the receptors through which dopamine modulates dendritic morphology in the PFC are not known.
Dopamine has previously been shown to modulate dendritic structure in the striatum, where loss of signaling through the D2 receptor appears to be critical (Cepeda et al., 2001;Day et al., 2006;Rodriguez and Pickel, 1999). Three of the five dopamine receptor transcripts are expressed in the PFC of the rodent in low-to-moderate abundance (Bergson et al., 1995;Meador-Woodruff et al., 1991;Wang and Pickel, 2002). D1 mRNA-expressing PCs are found in layers V and VI, with a second band of D1 -expressing cells in layer II (Gaspar et al., 1995;Vincent et al., 1993). The distribution of D2 receptor-expressing PCs in the PFC is more restricted, being found mainly in layer V (Gaspar et al., 1995;Vincent et al., 1993). Finally, PCs in the PFC that express the D4 transcript are found in the deep layers (V and VI) (Noain et al., 2006). In situ hybridization histochemistry studies have not revealed any significant numbers of dopamine D3 or D5 receptor-expressing cells in the adult rat PFC, although RT-PCR studies have reported on the presence of these two dopamine receptors during embryonic and early postnatal development (Araki et al., 2007).
A recent study in the D1 null mutant (−/−) mouse noted dendritic abnormalities in the PFC, including an altered apical dendritic trajectory toward the pial surface (Stanwood et al., 2005); dendritic spine density was not assessed in this study. Animals treated chronically with the dopamine receptor antagonist haloperidol, which in vivo targets D2-like receptors almost exclusively, do not show any change in the length or spine density of basal dendrites of layer V PCs in the PFC (Wang and Deutch, 2008), suggesting that a loss of signaling through the D2 receptor does not elicit dendritic spine changes. We are not aware of any studies that have examined dendritic structure of PCs in animals treated with D1 or D4 receptor antagonists or in any dopamine receptor null mutant mice. To assess the role of DA receptors in denervation-induced dendritic spine remodeling in the PFC, we utilized mice with genetic deletion of D1, D2 or D4 DA receptors and their wild-type (wt) littermates, determining if the constitutive absence of these dopamine receptors results in morphological changes in layer V pyramidal neurons in the PFC.
Results
Total basal dendritic length
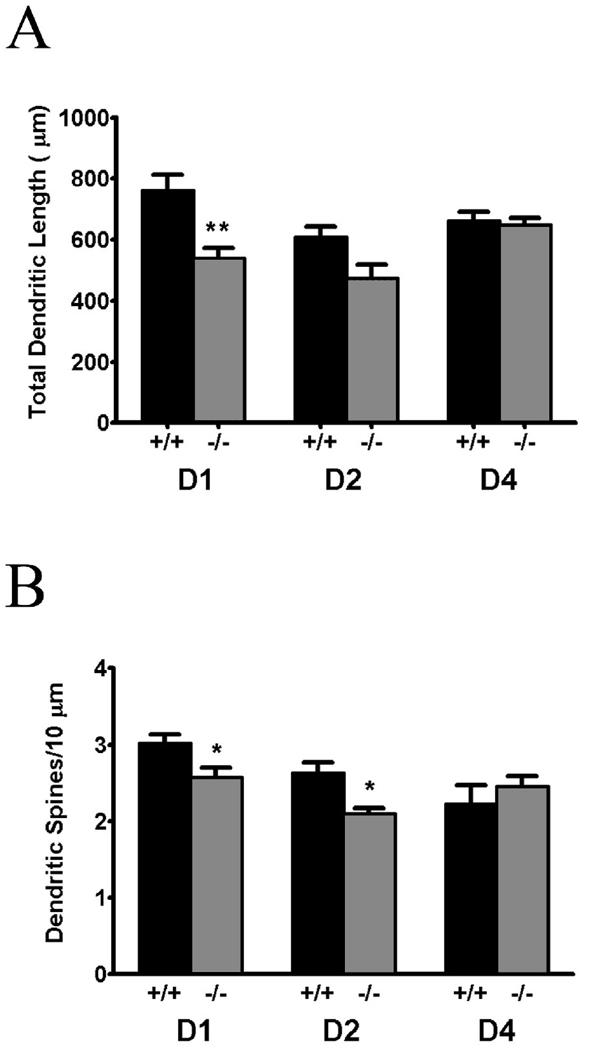
Dendritic length was significantly decreased in D1 knockout relative to wildtype littermates (t9=3.72, p=0.005; see Figure 1). In contrast, there was not a significant difference in total basal dendritic length in D2 knockout mice relative to wildtype controls, although a non-significant trend toward a decrease (p=.063) was noted (see Figure 1). No difference in total basal dendritic length was seen in D4 null mutants relative to controls. We did not determine total apical dendritic length because the apical dendrite of layer V pyramidal cells was often transected as it coursed toward the pial surface of the PFC.
Figure 1.
Total basal dendritic spine length (panel A) and basal dendritic density (panel B) are decreased in layer V pyramidal cells of the PFC in D1 knockout mice. There is also a significant decrease in total basal dendritic length in the D1 mutant, but not in the D2 knockout mouse, although a trend toward a decrease was noted. No change in the spine density of dendritic length was found in the D4 knockout. Data are expressed as mean ± SEM μm dendrite length (panel A) and number of spines/10 µm dendritic density (panel B). *, p < 0.05, ** p < 0.01 relative to wild-type controls
Basal dendritic spine density
ANOVA revealed that spine density on the basal dendrites of layer V pyramidal cells was significantly decreased in D1 −/− relative to D1 +/+ mice (F1,54 = 6.17, p=.016; see Figure 1 and Figure 2). A significant difference in spine density as a function of distance from the soma of the dendrite was also uncovered (F5,54 = 58.31, p<.001; Fig. 2), but no significant distance × genotype interaction was found. We also determined total number of spines per basilar dendritic tree. The total number of basal dendritic spines in the D1 knockout mouse was decreased by 42% (t9 = 4.32, p = .0019), and by 34% in the D2 null mutant (t6 = 3.34, p = .0157).
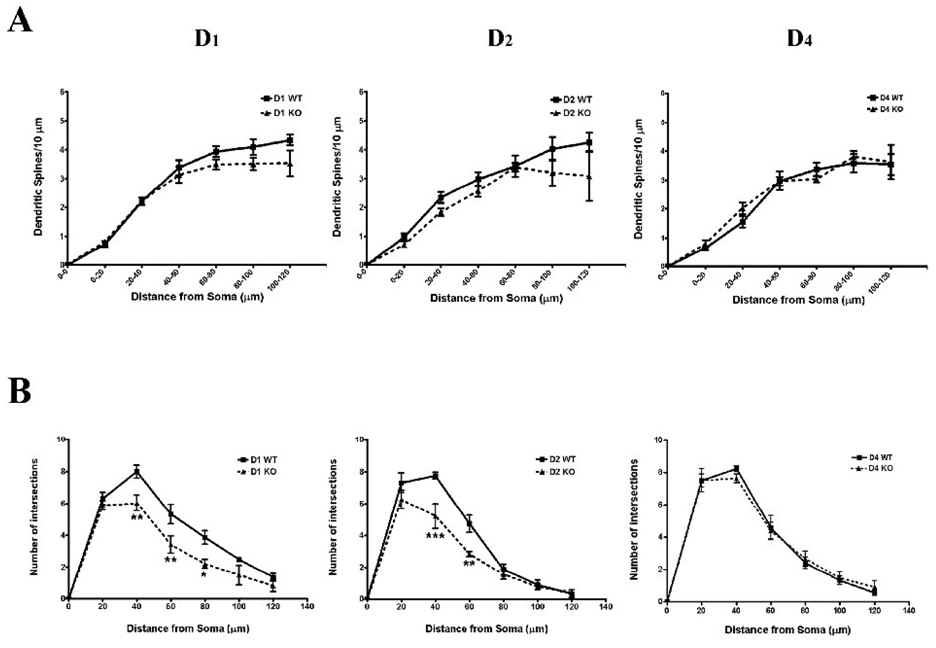
Figure 2.
Panel A shows changes in dendritic spine density as a function of distance along the basal dendrite. Despite the significant main effect of genotype in the D1 and D2 knockout mice, there was no genotype × distance interaction. In panel B the results of Sholl analyses revealed a decrease in basal dendritic complexity in the D1 and D2 but not D4 knockout mice. *, p < 0.05, ** p < 0.01, *** p < 0.001 relative to wild-type controls
Although we did not observe a significant decrease in total dendritic length in D2 knockout mice, a significant decrease in basal dendritic spine density was observed in these mice (F1,36 = 6.60, p=.015l; Figure 1 and 2), as was a decrease in spine density as a function of distance from the soma (F5,36 = 18.77, p<.001). Again, no significant genotype by distance interaction was uncovered.
There was no difference between D4 knockouts and wt controls in basal dendritic spine density (see Figure 1 and Figure 2).
Apical dendritic spine density
Because the apical dendrites of layer V PCs were usually transected as they coursed to the superficial layers, we were unable to measure dendritic spines on the entire extent of the apical dendrite. However, we could reliably reconstruct the first 100 µm of the apical dendrite, and therefore measured spine density on the apical dendrite at distances between 80–100 µm distal to the soma. We did not observe any differences across genotypes for the D1, D2, or D4 receptors. Apical dendritic spine density (mean ± SEM number of spines/10 µm dendrite) measurements were as follows. D1 receptor: 3.38 ± 0.33 (wildtype) vs 3.34 ± 0.32 (knockout) (t9=0.09, p=0.933); D2 receptor: 3.10 ± 0.45 vs 2.33 ± 0.62 (t6=1.01, p=0.35); and D4 receptor: 3.22 ± 0.41 vs 3.74 ± 0.92 (t8=0.52, p=0.618).
Dendritic branching
Sholl analyses to determine basal dendritic complexity at 20 µm intervals distal to the soma were conducted (see Figure 2 and Figure 3). In D1 −/− mice both significant main effects of genotype (F1,54 = 24.96, p<.001) and distance from the soma (F5,54 = 57.67, p<.001) were observed, but no significant interaction. Bonferroni t-tests revealed significantly less dendritic branching at distances between 40–80 µm distal to the soma. In D2 knockout mice a similar decrease in basal dendritic complexity was attributable to genotype (F1,36 = 16.02, p < .001; Fig. 2), as was a change in branching as a function of distance from the soma (F5,36 = 91.33, p < .001). A significant genotype × distance interaction was also observed in the D2 knockout mice (F5,36 = 3.22, p=.017), due to specific decreases in branching of relatively proximal dendrites (40 and 60 µm distal to the soma). Finally, the D4−/− did not differ significantly from wildtype controls in basal dendritic complexity, nor was there a significant genotype × distance interaction.
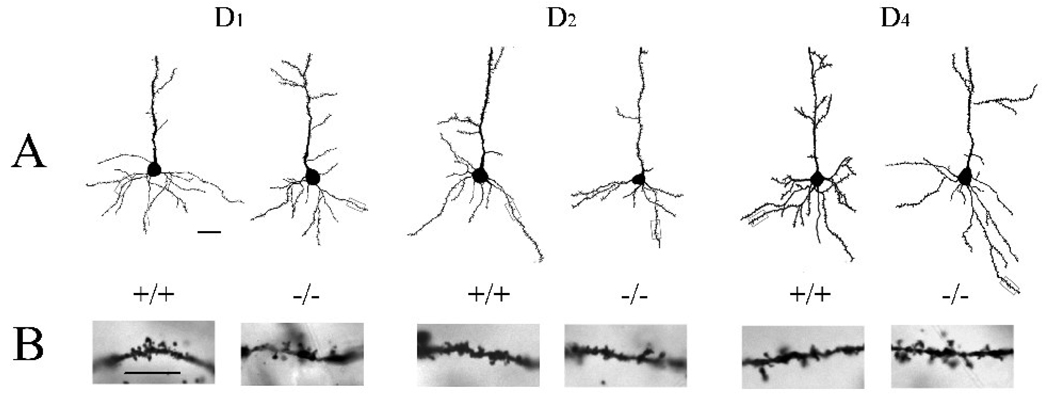
Figure 3.
Reconstructions of representative Layer V pyramidal neurons in the PFC of D1 (left two neurons), D2 (center two neurons), and D4 (right two neurons) wildtype (shown on the left of each pair of neurons) and null mutants (on the right of each pair of cells). The insets below each reconstructed neuron are photomicrographs of segments of the basal dendrites from that neurons. Scale bar = 30 µm in (A) and 10 µm in (B).
Discussion
D1 receptor knockout mice exhibited dystrophic changes in layer V pyramidal cells in the medial PFC, including a decrease in dendritic length and dendritic spine density. The PFC pyramidal cells of dopamine D2 null mutants also had fewer dendritic spines than wildtype mice, although there was not a significant decrease in the length of the basal dendritic tree. These data suggest that disruption of dopamine signaling through either the D1 or D2 receptor may account for the dystrophic changes in pyramidal cells that are seen secondary to dopamine denervation of the PFC. Genetic deletion of the third dopamine receptor that is expressed by layer V pyramidal cells in the PFC, the D4 receptor, did not result in any structural change to the dendritic tree of layer V PCs, indicating that the effects observed in the D1 and D2 knockout mice are not non-specific.
There is a paucity of data on structural changes in PFC neurons in dopamine receptor null mutant mice, although these animals have been used extensively in studies of cortical and corticostriatal function (Beaulieu et al., 2007;Glickstein et al., 2002; Glickstein and Schmauss, 2004;Huang et al., 2004;Trantham-Davidson et al., 2008;Waddington et al., 2005). We recently reported that the apical dendrites of D1 knockout mice fail to take a normal direct trajectory toward the pial surface, instead ascending through the cortical mantle in a tortuous path (Stanwood et al., 2005), but did not systematically examine dendritic spines or the organization of the basal dendritic tree. We are not aware of any other studies that have examined the dendritic structure of PFC pyramidal cells in D2 or D4 receptor knockout mice.
Although cells expressing both D1 and D2 mRNAs are most abundant in deep layers of the rodent PFC, cells expressing these two receptors appear to be mainly segregated (Gaspar et al., 1995), consistent with sub-laminar distributions of D1 and D2 mRNA-expressing PCs. This originally suggested to us that differences in pyramidal cell dendrites might be observed in the D1 and D2 knockouts, but probably not in both. However, we observed dystrophic changes in both D1 and D2 knockout mice.
The dystrophic changes in the dendrites of layer V PFC neurons in the D2 knockout mice were also unexpected because we had previously found that treatment of rats for three weeks with haloperidol, which in vivo is an selective D2 receptor antagonist (Zhang and Bymaster, 1999), does not change dendritic structure of PFC pyramidal cells (Wang and Deutch, 2008). It is unclear why we observed a decrease in spine density in the D2 knockout but failed to find any effect of pharmacological blockade of the D2 receptor. It is unlikely that the dose of haloperidol used our original study was too low, because the same dose of haloperidol gave in the drinking water resulted in an average of ~85% D2 receptor occupancy (Perez-Costas et al., 2008). It is also unlikely that the duration of the drug treatment was not sufficient because we previously found that disruption of the dopamine innervation of the PFC causes spine changes within three weeks.
Recent data suggest one other mechanism that might account for the dystrophic dendrites in the PFC of D2−/− mice but not mice treated with the D2 antagonist haloperidol. It is possible that release of dopamine is altered in the PFC of the D2 mutant mice, such that decreased signaling through the D1 receptor occurs and leads to changes in pyramidal cell morphology. Thus, Schmauss and colleagues demonstrated a blunted prefrontal cortical c-fos response to dopamine D1 agonist stimulation in mice lacking D2 or D3 receptors (Glickstein and Schmauss, 2004;Schmauss et al., 2002). Moreover, an elegant study by Kellendonk and colleagues (2006) revealed that transient D2 receptor overexpression in the striatum leads to activation of D1 receptors in the PFC, and suggested that the altered cortical D1 receptor response seen in the constitutive D2 receptor knockout is developmentally-mediated. The generation of conditional D2 receptor knockout mice will be required to test this hypothesis.
Our data point to the critical role of dopamine in determining dendritic morphology in cortical pyramidal cells. The dystrophic changes in the dendrites of PFC PCs of D1 and D2 receptor knockout mice are consistent with the hypothesis that the loss of dendritic spines seen in the PFC in schizophrenia may be linked to a decrease in cortical dopaminergic tone. However, our data do not exclude the possibility that the loss of dendrites on layer V PCs determines in part the decrease in dopamine tone in the PFC: layer V PCs project to and synapse with dopamine neurons in the ventral mesencephalon that in turn innervate the PFC (Carr and Sesack, 2000).
Dendritic spines, on which dopamine receptors are expressed, are the anatomical conduit by which most excitatory and some inhibitory inputs gain access to the pyramidal cell. As such, the loss of dendritic spines on prefrontal cortical PCs in the D1 and D2 knockout mice has broad implications for the use of these animals in studies of PFC function, including electrophysiological, biochemical, and behavioral studies. For example, a relatively large body of animal and human data has argued for the involvement of specific dopamine receptors in various cognitive functions. D1 dopamine receptors in the PFC are critically involved in working memory (Goldman-Rakic et al., 2004; McNab et al., 2000; Takahashi et al., 2008) and polymorphisms in the D1 dopamine receptor are associated with performance in a working memory task (Lane et al., 2008). In contrast, there are somewhat contrasting data on the involvement of the D2 dopamine receptor in working memory (Wang et al., 2004; Takahashi et al., 2008) ), but D2 receptors in the frontal cortex are necessary for executive function, including conditional associative memory (Bach et al., 2007) and tasks of cognitive flexibility (De Steno and Schmauss, 2009). Because dendritic spines are the major locus of expression of dopamine receptors, (Sesack et al., 2003; Negyessy et al., 2005; Bordelon-Glauser et al., 2008), changes in spine number would be predicted to impact cognition.
Interpretations of behavioral and functional studies of the PFC in various genetically manipulated mice, including dopamine receptor null mutants, usually fail to consider potential structural changes in pyramidal cells. It is likely that the observed changes in PC dendritic structure arise because of a lack of specific dopamine receptors during development. Because conditional dopamine receptor knockouts are not available, it is critical that caution be exercised in interpreting behavioral and physiological studies of neuronal plasticity in D1 and D2 knockout mice.
Experimental Procedure
Adult male D1 knockout (n=5) and wildtype (n=6) mice were originally obtained from Jackson Laboratories (Bar Harbor, ME), and then subsequently bred, maintained, and housed at Vanderbilt University. D2 and D4 knockout mice were generated as previously described (Kelly et al., 1998;Kruzich et al., 2004; Rubinstein et al., 1997). All mice were backcrossed at least 10 generations to a C57Bl/6J background. Four male D2 knockout and four male littermate wildtpe mice were used, with six D4−/− and +/+ mice used. Animals were raised under group housing conditions on a 12-hours light/dark circle and with unrestricted access to food and water. These studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals, with the approval and under the oversight of the Vanderbilt University Animal Care and Use Committee.
The mice were deeply anesthetized with isoflurane and sacrificed by decapitation. The brains were removed from the cranial vault. The sections were subjected to Golgi impregnation using the FD Rapid GolgiStain kit (FD NeuroTechnologies, MD), with empirically-determined modifications in the duration of incubation during the development step. Coronal 150 µm thick coronal sections cut on a freezing microtome and then mounted on gelatin subbed slides. All samples for a given receptor (e.g., D1 wildtype and D1 knockouts) were processed at the same time, in the same solutions, and incubated or developed in various solutions for the same durations. Sections were then washed, dried, and coverslipped in DPX.
Golgi-impregnated neurons in layer V of the prelimbic (area 32) area of the PFC were randomly selected and reconstructed using a computer-based neuron tracing system (Neurolucida, MicroBrightField, Inc., VT, USA); reconstructions were done by a person blind to the genotype of the animals. At least five randomly-selected pyramidal neurons were reconstructed from each animal provided they met the following criteria: 1) cells were located in layer V of the prelimbic cortex; 2) neurons were pyramidal in shape and appeared evenly impregnated under a 10x objective; 3) at least three basilar dendritic shafts were present. 4) soma and branches were not obscured by the processes of adjacent neurons. All neurons were reconstructed by the same investigator using a 63x oil immersion lens with digital doubling of objective magnification. After neurons were reconstructed and the average values for dendritic parameters (i.e., total basal dendritic length, total dendritic spine density and sholl analysis of basal dendritic complexity) were calculated by each individual animal, group differences in total basal dendritic length, total basal dendritic spine density and spine density as a function of distance (20 µm) from the soma were determined. The data were analyzed by means of two-way ANOVAs with Bonferroni t-tests when indicated by a significant interaction or main effect, or by Student’s t-tests.
Acknowledgements
This work was supported by the National Institutes of Health (RO1 MH077298 (AYD) and MH67497 (DKG)), the Vanderbilt Kennedy Center for Research on Human Development, and the National Parkinson Foundation Center of Excellence at Vanderbilt University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Illness of the National Institutes of Health, or the National Parkinson Foundation. The authors gratefully acknowledge the outstanding technical support of Katherine L. Suchand.
Abbreviations
- PCs
pyramidal cell
- PFC
prefrontal cortex
- wt
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Araki KY, Sims JR, Bhide PG. Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain Res. 2007;1156:31–45. doi: 10.1016/j.brainres.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc Natl Acad Sci USA. 2008;105:16027–16032. doi: 10.1073/pnas.0807746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Bordelon-Glausier JR, Khan ZU, Muly EC. Quantification of D1 and D5 dopamine receptor localization in layers I, III, and V of Macaca mulatta prefrontal cortical area 9: coexpression in dendritic spines and axon terminals. J Comp Neurol. 2008;508:893–905. doi: 10.1002/cne.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, Levine MS. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol. 2001;85:659–670. doi: 10.1152/jn.2001.85.2.659. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- De Steno DA, Schmauss C. A role for dopamine D2 receptors in reversal learning. Neuroscience. 2009;162:118–127. doi: 10.1016/j.neuroscience.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors have spatial working memory deficits. J Neurosci. 2002;22:5619–5629. doi: 10.1523/JNEUROSCI.22-13-05619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein SB, Schmauss C. Effect of methamphetamine on cognition and repetitive motor behavior of mice deficient for dopamine D2 and D3 receptors. Ann N Y Acad Sci. 2004;1025:110–118. doi: 10.1196/annals.1316.014. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Huang YY, Simpson E, Kellendonk C, Kandel ER. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci U S A. 2004;101:3236–3241. doi: 10.1073/pnas.0308280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Suchland KL, Grandy DK. Dopamine D4 receptor-deficient mice, congenic on the C57BL/6J background, are hypersensitive to amphetamine. Synapse. 2004;53:131–139. doi: 10.1002/syn.20043. [DOI] [PubMed] [Google Scholar]
- Lane HY, Liu YC, Huang CL, Hsieh CL, Chang YL, Chang L, Chang YC, Chang WH. Prefrontal executive function and D1, D3, 5-HT2A and 5-HT6 receptor gene variations in healthy adults. J Psychiatry Neurosci. 2008;33:47–53. [PMC free article] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, Bunzow JR, Akil H, Civelli O, Watson SJ., Jr Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5:231–242. [PubMed] [Google Scholar]
- Negyessy L, Goldman-Rakic PS. Subcellular localization of the dopamine D2 receptor and coexistence with the calcium-binding protein neuronal calcium sensor-1 in the primate prefrontal cortex. J Comp Neurol. 2005;488:464–475. doi: 10.1002/cne.20601. [DOI] [PubMed] [Google Scholar]
- Noain D, Avale ME, Wedemeyer C, Calvo D, Peper M, Rubinstein M. Identification of brain neurons expressing the dopamine D4 receptor gene using BAC transgenic mice. Eur J Neurosci. 2006;24:2429–2438. doi: 10.1111/j.1460-9568.2006.05148.x. [DOI] [PubMed] [Google Scholar]
- Perez-Costas E, Guidetti P, Melendez-Ferro M, Kelley JJ, Roberts RC. Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J Neural Transm. 2008;115:745–753. doi: 10.1007/s00702-007-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Pickel VM. Enhancement of N-methyl-D-aspartate (NMDA) immunoreactivity in residual dendritic spines in the caudate-putamen nucleus after chronic haloperidol administration. Synapse. 1999;33:289–303. doi: 10.1002/(SICI)1098-2396(19990915)33:4<289::AID-SYN6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- Schmauss C, Glickstein SB, Adlersberg M, Hsiung SC, Tamir H. A single dose of methamphetamine rescues the blunted dopamine D(1)-receptor activity in the neocortex of D(2)- and D(3)-receptor knockout mice. Ann N Y Acad Sci. 2002;965:21–27. doi: 10.1111/j.1749-6632.2002.tb04148.x. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Parlaman JP, Levitt P. Anatomical abnormalities in dopaminoceptive regions of the cerebral cortex of dopamine D1 receptor mutant mice. J Comp Neurol. 2005;487:270–282. doi: 10.1002/cne.20548. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Takano H, Arakawa R, Okumura M, Otsuka T, Kodaka F, Hayashi M, Okubo Y, Ito H, Suhara T. Differential contributions of prefrontal and hippocampal dopamine D(1) and D(2) receptors in human cognitive functions. J Neurosci. 2008;28:12032–12038. doi: 10.1523/JNEUROSCI.3446-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Kroner S, Seamans JK. Dopamine modulation of prefrontal cortex interneurons occurs independently of DARPP-32. Cereb Cortex. 2008;18:951–958. doi: 10.1093/cercor/bhm133. [DOI] [PubMed] [Google Scholar]
- Vincent SL, Khan Y, Benes FM. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 1993;13:2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington JL, O'Tuathaigh C, O'Sullivan G, Tomiyama K, Koshikawa N, Croke DT. Phenotypic studies on dopamine receptor subtype and associated signal transduction mutants: insights and challenges from 10 years at the psychopharmacology-molecular biology interface. Psychopharmacology (Berl) 2005;181:611–638. doi: 10.1007/s00213-005-0058-8. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- Wang HD, Deutch AY. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33:1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Zhang W, Bymaster FP. The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology (Berl) 1999;141:267–278. doi: 10.1007/s002130050834. [DOI] [PubMed] [Google Scholar]