Abstract
To define the factors that modulate regulatory T (Treg) cells in the tumor setting, we co-cultured various tumor cells with either purified Treg cells, or with unfractionated splenocytes. We found that Treg expansion occurred only with unfractionated splenocytes, suggesting that accessory cells and/or factors produced by them play an essential role in tumor-induced Treg expansion. We performed gene expression profiling on tumor-associated Treg cells to identify candidate signaling molecules and studied their effects on tumor-induced Treg expansion. We inadvertently discovered that IL-12 treatment blocked Treg expansion in an IL-12 receptor-dependent fashion. Additional studies showed that IL-12 acts by stimulating Interferon-gamma mediated inhibition of Treg cell proliferation, which may partially account for the anti-tumor effects of IL-12. Furthermore, IL-12 treatment was found to decrease IL-2 production, which may lead to interferon-gamma independent inhibition of Treg cells, as IL-2 is required for their survival and expansion. Mechanistic studies revealed that Interferon-gamma signaling directly causes cell cycle arrest in Treg cells. This study demonstrates that an IL-12-Interferon-gamma axis can suppress tumor-induced Treg proliferation. This mechanism may counteract the ability of Treg cells to promote tumor growth in vivo.
Keywords: Regulatory T cells, Cytokine, Interleukin 12, Interferon-gamma, Tumor clearance
Introduction
Clinical observations and animal models have established that the immune system responds to tumors, even though they are derived from the host. Despite the observations that activated, tumor-specific immune cells exist in cancer patients and animal models, the immune system often fails to prevent tumor formation and metastasis (1). Thus, established tumors must have developed strategies to inhibit or evade the immune system.
Sakaguchi and colleagues first established the essential role of CD4+CD25+ regulatory T (Treg) cells in the maintenance of peripheral tolerance to self-antigens (2, 3). More recent studies have implicated Treg cells in inducing tolerance to tumors (4, 5). Mounting evidence indicates that tumors can promote the expansion, recruitment, and activation of Treg cells (6). Increased numbers of CD4+CD25+Foxp3+ Treg cells have been found either in the circulation or in the tumors of patients with various cancers (lung, breast, colorectal, esophageal, gastric, ovarian, pancreatic, melanoma, hepatocellular, leukemia, and lymphoma) (7). In addition, large numbers of Treg cells in tumors and malignant ascites were shown to be correlated with worse clinical outcomes in several types of cancer (8). Likewise, studies using in vivo mouse models have shown that Treg cell proliferation/activation induced by tumors or self-antigens can help tumors to escape immunosurveillance (9, 10). Furthermore, antigen-based tumor vaccines have also been found to activate Treg cells, which can considerably dampen antitumor immune responses (11).
Extensive investigations have improved our understanding of the molecular and cellular basis for the development, activation, and function of Treg cells. Antigen-TCR signals are not only determinants for thymic selection and differentiation of Treg cells (12, 13), but they also control their peripheral activation and suppressive efficacy (14, 15). Although Treg cells predominantly recognize self-antigens and suppress self-reactive T cells (12, 13, 16), it has also been shown that they can respond to non-self antigens, such as viral antigens, allo-antigens, and foreign antigens in transgenic mouse models (17-19). Cytokine signals also regulate Treg cell development, function, and homeostasis. IL-2 has been shown to be indispensable for Treg expansion, activation, and maintenance (20, 21). Other gamma-chain cytokines (IL-4, IL-7, and IL-15) can also support Treg cell development and function (22, 23). IL-10 has been reported to be a soluble mediator of Treg suppressive function (5, 7). TGF-β has been shown to promote the proliferation of natural Treg cells, and also the “conversion” of non-regulatory T cells into induced Treg cells (5, 7).
We recently demonstrated that Treg cells in the tumor environment (but not Treg cells in the peripheral lymphoid tissues) utilize granzyme B to suppress anti-tumor immunity (24), which suggests that Treg cells are subject to regulation by local factors during anti-tumor immune responses. To identify such factors, we utilized the Foxp3-GFP reporter mice in this study, and developed an in vitro co-culture system to study Treg cells in the tumor setting. We found that tumor cells indirectly drive Treg cell proliferation via accessory immune cells. We also discovered, inadvertently, that IL-12 profoundly inhibits tumor-induced Treg cell proliferation; this inhibition is partially dependent on Interferon-gamma. This study provides definitive evidence that an IL-12-Interferon-gamma axis can inhibit Treg cell proliferation. These findings demonstrate that Treg cells are subject to cytokine-mediated inhibition, and support a notion that cytokines with such a property may have the potential to serve as an adjuvant for tumor vaccine-based immunotherapy.
Materials and Methods
Animals and tumor cells
Wild type (WT) 129/SvJ, and WT and IFNγR1-/- mice in the C57Bl/6J strain were obtained from the Jackson Laboratory. WT 129/SvPas mice were obtained from the Charles River Laboratory. The IL12Rβ2-/- mice in the C57Bl/6J strain were maintained as described (25). The IFNγR1-/- mice in the 129/SvPas mice were obtained from Dr. Herbert Virgin. The RMAS lymphoma cells, originally derived from C57BL/6 mice (H-2b, I-Ab), were obtained from Dr. Wayne Yokoyama and maintained in complete K10 medium (24). The primary APL samples are cryopreserved cells harvested from the spleens of 3 independent leukemic mCG-PML-RARα transgenic mice in pure C57BL/6 strain (26, 27). All mice were bred and maintained in SPF housing, and all experiments were conducted in accordance with Roswell Park Cancer Institute and Washington University School of Medicine animal care guidelines, using protocols approved by the animal studies committee.
Reagents and antibodies
The purified CD25 antibody (PC61) and rat IgG1 isotype control (HRPN) were purchased from Bio Express. The CD4+CD25+ Treg isolation and Pan T cell isolation kits, and T cell depletion (CD90) microbeads were purchased from Miltenyi Biotec, and T cell isolation or depletion was performed by following the manufacturer’s instruction manuals. Antibodies used include anti-mouse CD3 (145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD25 (7D4, PC61), CD16/32 (2.4G2) (BD Biosciences), Foxp3 (eBioscience, FJK-16s).
In vitro tumor-splenocytes co-cultures
4 × 106 unfractionated splenocytes were harvested from naïve mice and mixed with 1.2 × 105 RMAS tumor cells (3000RAD-irradiated) in 2 ml of K10 medium in tissue culture treated 12-well plates for 4-5 days. To study exogenous cytokine effect, various doses of cytokines were added in the beginning of the co-culture experiments. At the beginning and the end of co-cultures, absolute numbers of various T cell compartments were obtained by multiplying the percentages (determined by flow cytometry as described below, and represented in Figure S1) by the total numbers of live cells harvested from the cultures. T cell expansion is presented as fold change by the equation below:
Intracellular staining and flow cytometry
1×106 cells were washed and resuspended in staining buffer (PBS, 0.5% bovine serum albumin, 0.5 mM EDTA). Samples were labeled with primary-conjugated antibodies against cell surface markers (anti-mouse CD3, CD4, CD8, CD25) (BD Biosciences). Samples were fixed, permeabilized (Foxp3 staining kit, eBioscience), and stained with primary-conjugated anti-Foxp3 antibody (FJK-16s; eBioscience) diluted at 1:400 in staining buffer. Sample data were acquired on a Cytek-modified FACScan (Becton Dickinson) flow cytometer, and analyzed using FlowJo (TreeStar) software.
CFSE labeling of naive CD4+CD25+ Treg cells and mixing co-cultures
CD4+CD25+ Treg cells were isolated from the resting spleens of WT mice, IL12Rβ2-/- mice, and IFNγR1-/- mice using the CD4+CD25+ Treg isolation kit, according to the manufacturer’s instructions (Miltenyi Biotec). The purified Treg cells were labeled at 37°C in PBS with 150 nM of CFSE for 15 minutes and mixed into WT splenocytes, or IL12Rβ2-/- splenocytes, or IFNγR1-/- splenocytes cultured with irradiated RMAS cells for 4-5 days.
Bioluminescence imaging of in vivo tumor clearance
RMAS cells were transduced with a ΔU3 retroviral construct that drives the expression of a fusion cDNA encoding click beetle red luciferase (CBR) and green fluorescence protein (GFP) as previously described (24). CBR luciferase-expressing RMAS clones were injected IP into WT mice in 200 μl RPMI 1640 on day 0, and bioluminescence imaging was performed 2 hours later and variously through 10 after injection. For imaging, mice were injected IP with D-luciferin (150 μg/g body weight), anesthetized (2% isoflurane) and placed in an IVIS 50 imaging system (Caliper) (exposure time, 1-30 sec; binning, 2-8; no filter; f/stop, 1; FOV, 12 cm) (28). Regions of interest (ROI) were defined manually over the whole body using Living-Image software (Igor Wavemetrics) for determining tumor burden signal intensities. Data were expressed as photon flux (photons/sec) across all mice receiving the same treatment.
Flow-based in vitro cell death assay
Naïve, unfractionated splenocytes from Foxp3-GFP knock-in mice were co-cultured with irradiated RMAS cells as described above for 4 days. Lymphocytes were harvested from the co-cultures and stained with primary-conjugated antibodies against cell surface markers (CD3, CD4, and CD8) and 7-AAD to assess cell death in Treg and other T cell compartments by flow cytometry.
Results
Tumor cells induce Treg cell expansion in the presence of accessory splenocytes
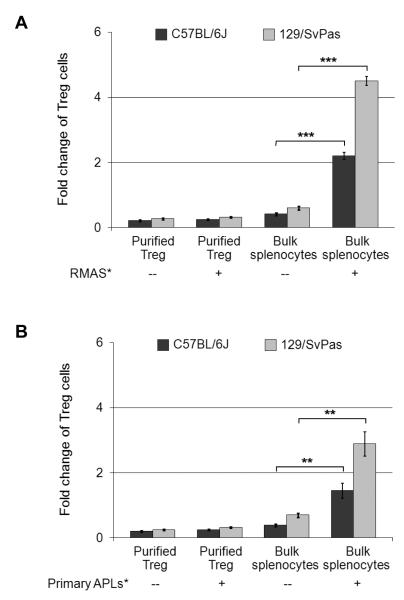
To identify factors that may modulate Treg cell function, we explored several approaches to study Treg cells in vitro in a tumor relevant setting. First, both purified Foxp3-GFP+ cells (from Foxp3-GFP reporter mice) and CD4+CD25+ cells failed to proliferate, and the majority of them died when co-cultured with RMAS lymphoma cells (live or lethally irradiated) for 4-5 days (Figure 1A). Secondly, when unfractionated splenocytes (which contain lymphocytes and accessory cells) were co-cultured with live tumor cells, the tumor cells outgrew the lymphocytes (data not shown). Finally, we found that co-culturing irradiated RMAS cells with unfractionated splenocytes induces significant expansion of CD4+Foxp3+ Treg cells, but not of CD4+Foxp3− or CD8+ T cells (Figures 1A and 2); this expansion occurs in K10 medium without any exogenous growth factors. To rule out an RMAS-specific effect, and to determine whether primary tumor cells can induce Treg expansion in the cultures, we used cryopreserved primary tumor cells derived from the splenic tumors of mice with acute promyelocytic leukemia (APL) (26, 27). Similar amounts of Treg expansion occurred with 3 independent primary APL tumor samples in the co-cultures (Figure 1B).
Figure 1. Irradiated RMAS and primary APL tumor cells induce Treg expansion when co-cultured with unfractionated splenocytes, but not purified Treg cells.
A. 1 × 106 CD4+CD25+ T cells were isolated from the resting spleens of WT 129/SvPas or C57BL/6J mice, and cultured in 1 ml regular K10 medium only or with 1 × 106 irradiated (3,000 Rads) RMAS tumor cells. In addition, 4 × 106 unfractionated splenocytes were cultured in 2 ml regular K10 medium only or with 1.2 × 105 irradiated RMAS tumor cells. B. Instead of RMAS cells, cryopreserved primary APL tumor cells harvested from the spleens of 3 independent leukemic mCG-PML-RARα transgenic mice were irradiated (3,000 Rads) and used at the same doses in the co-cultures. At the beginning and the end of co-cultures, absolute numbers of CD4+Foxp3+ Treg cells were obtained by multiplying the percentages (determined by flow cytometry as described in the Methods, and represented in Figure S1) by the total numbers of live cells harvested from the cultures. Treg expansion is presented as fold change by the equation: Fold change = (Absolute numbers of Treg cells at the end of co-culture) / (Absolute numbers of Treg cells at the beginning of co-cultures). Data points are shown as mean ± SD; Two-tailed t-tests were used to determine statistical significance (**P<0.01, ***P<0.001).
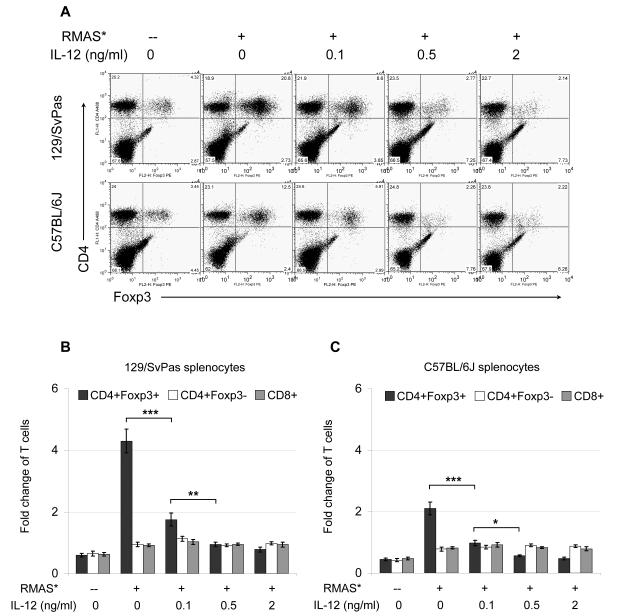
Figure 2. IL-12 treatment inhibits tumor-induced Treg expansion.
1.2 × 105 irradiated RMAS cells were co-cultured with 4 × 106 unfractionated splenocytes for 5 days in the presence of the indicated dose of recombinant murine IL-12. A. Representative flow cytometric plots show the percentages of CD4+Foxp3+ Treg cells and CD4+Foxp3− effector T cells in the end of the 5-day co-cultures. B. A summary of 3 independent co-culture experiments with 129/SvPas splenocytes is shown. C. A summary of 3 independent co-culture experiments with C57B/6J splenocytes is shown. Treg expansion is presented as fold-change of the final yield over initial input as described in Figure 1. Data points are shown as mean ± SD; Two-tailed t-tests were used to determine statistical significance (*P<0.05, **P<0.01, ***P<0.001).
Notably, RMAS cells and the primary APL cells used in this study (derived from B6 mice, and sharing MHC I and II with 129 mice, but expressing different minor histocompatibility antigens) induced greater Treg expansion when co-cultured with 129/Sv splenocytes (up to 4-fold expansion), compared with B6 splenocytes (~2 fold expansion, Figure 1A). Both 129/SvJ and 129/SvPas splenocytes supported similar amounts of Treg expansion.
Non-T accessory cells and IL-2 are required for tumor-induced Treg expansion
Because tumor-induced Treg expansion did not occur with purified Treg cells, we performed a series of experiments to define the cells and/or factors that promote tumor-induced Treg expansion. First, we used MACS separation techniques to fractionate total splenocytes into Pan T cells vs. T cell-depleted (TcD) accessory cells. Treg expansion occurred only when both non-T splenocytes and irradiated tumor cells were added to the cultures (Figures S2A and S2B). Secondly, since IL-2 and TGF-β have been shown to be relevant for Treg expansion, we used monoclonal antibodies to neutralize IL-2 and TGF-β in the tumor-splenocyte co-cultures. IL-2 neutralization completely blocked Treg expansion (Figures S2C and S2D), whereas TGF-β neutralization had no effect. These results suggest that the accessory non-T splenocytes and IL-2 are both required for tumor-induced Treg expansion in vitro.
Profiling of Treg gene expression in vivo to identify candidate cytokines that may modulate Treg expansion
From the co-culture experiments, we hypothesized that tumor cells may indirectly drive the expansion of Treg cells via antigen presentation and cytokine production by other splenocytes. To identify factors that may modulate Treg cell function in tumor-bearing mice in vivo, we utilized an array-based approach. We performed gene expression profiling on Treg cells using Affymetrix MOE430v2 arrays. Naïve Treg cells were purified from the resting spleens of Foxp3-GFP reporter mice using high speed flow sorting on the GFP signal. Tumor-associated Treg cells were purified from the tumor ascites of Foxp3-GFP reporter mice injected 5 days earlier with RMAS cells, while SpleenT Treg cells were purified from the spleens of the same tumor-bearing mice. RMA normalization was performed for all microarray data. 2-group comparison (t-test) of Ascites vs. Naïve samples, with multiple test corrections (adjusted p-value / FDR (false discovery rate) = q-value) was performed. Using these approaches, we have identified 419 probesets with q<0.005 and linear fold change ≥ 2 (Ascites:Naïve) and 114 probesets with q<0.005 and linear fold change ≤ 0.5 (Ascites:Naïve) (Figure S8 and Supplemental Table). By comparing cytokine receptor expressions of tumor-associated Treg cells to that of naïve or SpleenT Treg cells, we identified several candidate cytokines, as shown in Figure S3. IL-2 has been shown to be essential for Treg homeostasis and function (15, 29, 30); as expected, IL-2 receptor α (CD25) is upregulated in tumor-associated Treg cells. IL-7 receptor is also up-regulated, while IL-10 receptor mRNA abundance in Treg cells is unchanged. Neuropilin-1, a known VEGF receptor that has been proposed as a Treg cell surface marker, is down-regulated in the tumor-associated Treg cells (31). In addition, other pathways involved in Treg function are also differentially regulated. For example, granzyme B is the third most up-regulated gene (39-fold), consistent with our previous report that tumor-associated Treg cells activate granzyme B to suppress anti-tumor immune response (24). Multiple cell division genes (Cdc6, Cdca5, Ccna2, Cdca8) are among the top 30 up-regulated genes (Supplemental Table), suggesting that tumor-associated Treg cells may undergo active proliferation. These profiles suggest a complex network in which multiple pathways may influence the expansion and/or function of tumor-associated Tregs cells.
IL-12 treatment blocks tumor-induced Treg cell expansion
We tested the candidate cytokines for their activity in the tumor-splenocyte co-culture system. IL-2 treatment, at doses between 0.1-20 ng/ml, mildly increases Treg expansion (data not shown). Though VEGF blockade was shown to reduce intratumoral Treg cells and enhance GM-CSF stimulated tumor rejection (32), VEGF treatment revealed no effect on Treg expansion at doses ranging from 1ng/ml to 1000 ng/ml. Likewise, most of the other candidates, such as IL-10 and TNFα, show no effect on Treg expansion, even though multiple concentrations (directed by their levels in the tumor ascites) were tested individually and in combination with 2 to 3 candidates (data not shown).
Remarkably, addition of IL-12 to the culture, at doses between 0.1-2 ng/ml, significantly inhibited the expansion of CD4+Foxp3+ Treg cells in a dose-dependent fashion (Figure 2A), but had no significant effect on the numbers of CD4+Foxp3− or CD8+ T cells (Figure 2). Similar IL-12 effects were observed when the primary APL tumor cells were used in the co-culture assay (data not shown), ruling out a tumor cell type-specific effect. Notably, this finding is consistent with a previous report that IL-12 treatment reduced CD4+CD25+ T cell infiltration in a lung tumor model (33).
IL-12 signaling inhibits Treg expansion indirectly
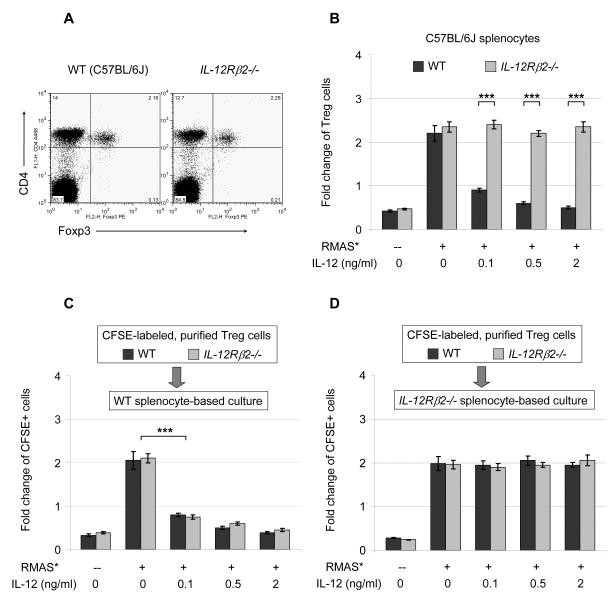
To further validate the IL-12 phenotype, we analyzed Treg expansion in IL-12 receptor β2-deficient (IL-12Rβ2-/-) mice. Treg cell numbers in the resting spleens of IL-12Rβ2-/- mice are comparable to that of strain-matched wild-type (WT) mice (Figure 3A). Using IL-12Rβ2-/- splenocytes, we found that these cells are completely resistant to IL-12 mediated suppression (Figure 3B). However, the lack of detectable IL-12 receptor mRNA in Treg cells suggested an indirect mechanism of IL-12 action (Figure S3). To test this hypothesis, we used CFSE to label CD4+CD25+ Treg cells purified from WT and IL-12Rβ2-/- mice, and measured the expansion of CFSE+ Treg cells mixed with WT splenocytes or IL-12Rβ2-/- splenocytes in co-culture assays. In WT co-cultures, WT Treg cells and IL-12Rβ2-/- Treg cells are equally susceptible to IL-12 mediated inhibition (Figure 3C); in IL-12Rβ2-/- co-cultures, both WT Treg cells and IL-12Rβ2-/- Treg cells are equally resistant to IL-12 mediated inhibition (Figure 3D). These data suggest an indirect mechanism, in which certain IL-12 responsive cell types and/or cytokines mediate the inhibitory effect on Treg cells.
Figure 3. IL-12 signaling blocks Treg expansion indirectly.
A. Representative flow cytometric plots show the percentages of CD4+Foxp3+ and CD4+Foxp3− T cells in the spleens of naïve WT C57BL/6J (B6) mice and strain-matched IL-12 receptor β2-deficient (IL12Rβ2-/-) mice. 10 mice in each genotype were examined in 3 independent experiments, with similar results. B. RMAS tumor cells were cultured with WT B6 splenocytes or IL12Rβ2-/- splenocytes for 5 days. Summary data from 2 independent experiments are shown. C and D. Purified WT and IL12Rβ2-/- Treg cells were labeled with CFSE and mixed into co-cultures of tumor cells with either WT splenocytes (C) or IL12Rβ2-/- splenocytes (D) for 5 days, and expansion of the CFSE-labeled Treg cells are summarized for 2 independent experiments. Treg expansion is presented as fold-change of the final yield over initial input as described in Figure 1. Data points are shown as mean ± SD; Two-tailed t-tests were used to determine statistical significance (*P<0.05, **P<0.01, ***P<0.001).
Interestingly, a subset of Treg cells were found to retain high levels of CFSE, while other subpopulations of Treg cells appeared to be more tumor-reactive and diluted CFSE signal to lower levels by proliferating more extensively (Figure S4).
Interferon-gamma signaling partially accounts for IL-12 mediated Treg inhibition
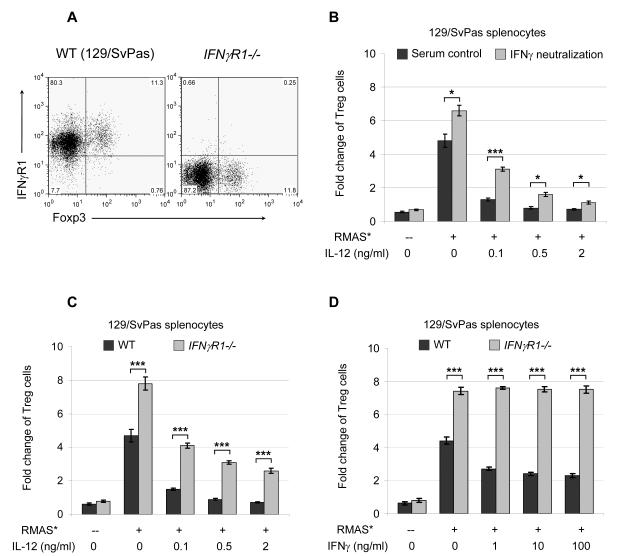
It has previously been shown that Interferon-gamma (IFNγ) produced by CD8+ T cells may regulate the generation/activation of CD4+CD25+ Treg cells in vivo (34). IL-12 is known to induce IFNγ production by other immune cells, including CD4+ and CD8+ T cells (35, 36). As expected, we found that Treg cells do express IFNγ receptor 1 mRNA and protein (Figures S3 and 4A). Therefore, we used an IFNγ neutralizing antibody (H22) to determine whether IFNγ is responsible for IL-12 mediated Treg inhibition. IFNγ neutralization increased Treg cell expansion by more than 30% in WT co-cultures in the absence of exogenous IL-12 (Figure 4B), suggesting that a basal level of IFNγ (produced by other immune cells in the cultures) inhibits Treg cell expansion. In addition, IFNγ neutralization partially abolished IL-12 mediated Treg inhibition. Similarly, in the absence of exogenous IL-12, Treg cells in IFNγ receptor 1-deficient (IFNγR1-/-) co-cultures proliferated >60% more efficiently than Treg cells in strain-matched WT co-cultures; Treg cells in the IFNγR1-/- co-cultures are more resistant to IL-12 mediated inhibition (Figure 4C). To determine the IFNγ levels that inhibit Treg expansion, we performed ELISA using cell-free media harvested from the cultures. Splenocytes in the culture secreted a basal level of IFNγ (0.2-0.9 ng/ml) in the absence or presence of tumor cells (Figure S5A). Exogenous IL-12 treatments enhanced IFNγ secretion to 5-17 ng/ml. Furthermore, adding 1-100 ng/ml exogenous IFNγ to WT co-cultures inhibits Treg expansion by 30-50% (Figure 4D). As expected, Treg cells in the IFNγR1-/- co-cultures are completely resistant to IFNγ mediated inhibition (Figure 4D), but partially resistant to IL-12 inhibition (Figure 4C). Together, these data show that IFNγ signaling partially accounts for IL-12 mediated inhibition of tumor-induced Treg expansion.
Figure 4. IFNγ signaling partially accounts for IL-12 mediated Treg inhibition.
A. Representative flow cytometric plots for IFNγ receptor 1 expression in naive WT 129/SvPas splenocytes and strain-matched IFNγ receptor 1-deficient (IFNγR1-/-) splenocytes. Shown are cells in the CD4+ gates. 6 mice in each genotype were examined in 2 independent experiments, with similar results. B. An IFNγ neutralizing antibody, H22, or its isotype control, PIP, was added to a final concentration of 50 μg/ml. A summary of 2 independent co-culture experiments is shown. C. RMAS tumor cells were cultured with WT 129/SvPas splenocytes or IFNγR1-/- splenocytes for 5 days. A summary of 3 independent experiments is shown. D. Indicated doses of IFNγ were used to treat the co-cultures. A summary of 2 independent experiments is shown. Treg expansion is presented as fold-change of the final yield over initial input (as described in the Methods and Figures 1 and S1). Data points are shown as mean ± SD; Two-tailed t-tests were used to determine statistical significance (*P<0.05, **P<0.01, ***P<0.001).
However, these data also suggest that there may be additional component(s) that mediate IL-12 inhibition of Treg expansion. Because neutralization of IL-2 completely blocks Treg expansion (Figure S2), we tested whether IL-12 treatment could influence IL-2 secretion. Interestingly, higher levels of IL-2 were detected in the splenocyte-only cultures (210-320 pg/ml) compared to the tumor-splenocyte co-cultures (30-70 pg/ml), where the higher levels of IL-12, in the absence of tumor cells, did not correlate with significant Treg expansion. Nevertheless, IL-12 treatment significantly reduced IL-2 secretion in the co-cultures (Figure S5B), which may account for the IFNγ-independent inhibition of Treg cells.
Interferon-gamma signaling inhibits Treg proliferation directly
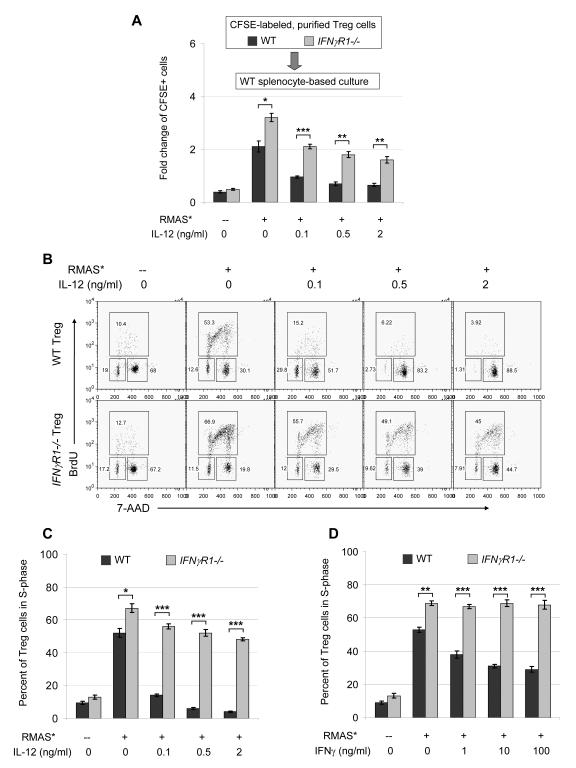
To determine whether IFNγ receptor-mediated signaling in Treg cells directly inhibits proliferation, we used CFSE to label CD4+CD25+ Treg cells purified from WT or IFNγR1-/- mice, and compared their proliferative activity in the WT splenocyte-based co-cultures. IFNγR1-/- Treg cells proliferate significantly faster than WT Treg cells in the absence of exogenous IL-12 (Figure 5A), and are significantly more resistant to IL-12 treatment. These data suggest that IFNγ receptor-dependent signaling in Treg cells directly inhibits proliferation, and is required for IL-12 mediated maximal inhibition of Treg cell expansion. In addition, cell cycle analyses revealed that IL-12 treatment significantly reduces proliferating Treg cells in the S-phase (Figures 5B and 5C). In contrast, IFNγR1-/- Treg cells display significantly more cells in S-phase in the absence of exogenous IL-12, and are more resistant to the cell cycle arrest caused by IL-12 treatment. Furthermore, IFNγ treatment directly reduced proliferating Treg cells in the S-phase (Figure 5D), though to a lesser extent compared to IL-12 treatment that induced more intensive inhibition due to an additional mechanism, which may be caused by reduced secretion of IL-2 (Figure S5B). Interestingly, these treatments appear to ‘trap’ more Treg cells in the G2/M phase (Figure 5B). It has been reported that disruption of T cell receptor (TCR) signaling (37), or IL-2 deprivation (38) can cause G2/M arrest in CD4+ T cells, and that IFNγ treatment can also induce G2/M arrest in human cell lines (39). Our data suggest that lack of stimulation may account for G2/M accumulation in the splenocyte only cultures, whereas IL-12 treatment apparently resulted in an elevation of IFNγ and a reduction of IL-2 (Figure S5) that may jointly lead to increased G2/M arrest.
Figure 5. IFNγ signaling directly inhibits Treg proliferation.
A. Purified WT and IFNγR1-/- Treg cells were labeled with CFSE and mixed with WT splenocytes and irradiated RMAS cells for 4 days, and expansion of the CFSE-labeled Treg cells are shown as summary data from 2 independent experiments. Treg expansion is presented as fold-change of the final yield over initial input (as described in the Methods and Figures 1 and S1). B. Representative cell cycle analysis of CD4+FoxP3+ Treg cells in cultures of irradiated RMAS cells and WT 129/SvPas splenocytes or IFNγR1-/- splenocytes. On day 5, BrdU was added to the cultures to a final concentration of 10 μM and incubated for 4 hours before cells were harvested for analysis. C. Summary data of 2 independent experiments for cell cycle analyses, showing the percentage of Treg cells in S-phase. D. Instead of IL-12, indicated doses of IFNγ were used to treat the co-cultures. Shown are summary data of 2 independent experiments for cell cycle analyses. Data points are shown as mean ± SD; Two-tailed t-tests were used to determine statistical significance (*P<0.05, **P<0.01, ***P<0.001).
IL-12 treatment inhibits Treg expansion and enhances tumor clearance in vivo in an IFNγ-dependent fashion
To study the impact of IL-12 on tumor growth in vivo, we first tested whether IL-12 could directly influence tumor cell proliferation or survival. We used various doses of IL-12 (0.1, 0.5, 1, 2, and 5 ng/ml) to treat RMAS cells for 2 weeks in culture. Fresh IL-12 was replenished at each passage of the culture. These treatments showed no effect on RMAS cell proliferation or survival, ruling out a direct effect of IL-12 on tumor growth.
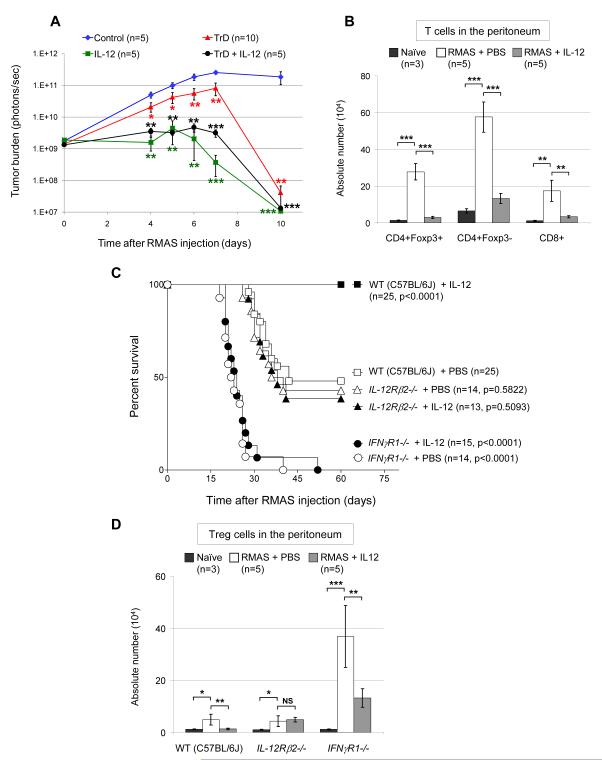
To test whether IL-12 mediated inhibition of Treg cell proliferation has an impact on tumor clearance in vivo, we compared the effect of IL-12 treatment with that of Treg cell depletion. We utilized luciferase-expressing RMAS cells and performed bioluminescence imaging to monitor mice injected intraperitoneally (IP) with these cells. Daily IL-12 treatment (100 ng per mouse) significantly enhances tumor clearance in (Figures S6 and 6A). As expected, Treg cell depletion also increases tumor clearance. Notably, IL-12 treatment enhances tumor clearance more efficiently than Treg cell depletion, perhaps because it also activates immune effector cells (33). Finally, the combination of Treg depletion with IL-12 treatment appears to have a similar effect as IL-12 treatment alone (Figure 6A), perhaps because IL-12 treatment alone has blocked Treg expansion in vivo. To test this hypothesis, we measured absolute T cell numbers in the peritoneum of naïve mice vs. mice IP-injected with RMAS cells. CD4+Foxp3+ Treg cells are increased about 20-fold in the tumor-bearing 129/SvJ mice, and IL-12 treatment almost completely blocks the increase (Figure 6B). Similar tumor-induced increases in the populations of CD4+Foxp3− and CD8+ effector T cells were also detected. IL-12 treatment also reduced the number of effector T cells, perhaps because it reduced tumor burden, which drove the increase of effector T cells in this model.
Figure 6. IL-12 treatment inhibits Treg expansion and enhances tumor clearance in vivo in an IFNγ-dependent fashion.
A. Four days prior to RMAS injection, 129/SvJ WT mice received a single IP injection of 400 μg PC61 CD25 antibody to deplete Treg cells (TrD), or were injected with an isotype control antibody. From day 0 to day 7 after IP injection of 2 × 106 luciferase-expressing RMAS cells, these mice were injected IP daily with equal volumes of PBS while a third set of mice received 100 ng of IL-12 IP daily. A fourth group of mice received both Treg depletion (TrD) and IL-12 treatment. Bioluminescence imaging was performed to monitor tumor burden. Shown are summary data of 2 independent experiments. B. Total cells were harvested by flushing the peritoneal cavity of naïve 129/SvJ WT mice or mice that were IP-injected with 1 × 106 RMAS cells 5 days earlier, and treated with 100 ng IL-12 or equal volume of PBS control daily for 5 days. Cells were stained with primary conjugated antibodies directed against CD4, CD8, or Foxp3, and analyzed by flow cytometry. Absolute numbers of T cells were obtained by multiplying the percentage of the indicated T cell compartments by the total cell numbers harvested from the peritoneum. C. Kaplan-Meier survival curves of C57BL/6J WT mice versus strain-matched IL12Rβ2-/- mice and IFNγR1-/- mice, after intravenous injection with 1 × 104 RMAS cells. From day 0 to day 40, these tumor-challenged mice received 50 ng of IL-12 IP or equal volume of PBS IP every other day. Shown are summary data of 3 independent experiments. D. C57BL/6J WT, strain-matched IL12Rβ2-/-, and IFNγR1-/- mice were IP-injected with 1 × 104 RMAS cells. From day 0 to day 12, these tumor-challenged mice received 50 ng of IL-12 IP or equal volume of PBS IP every other day. On day 14, total cells were harvested from the peritoneal cavity and analyzed by flow cytometry as described above in panel B to acquire the absolute numbers of CD4+Foxp3+ Treg cells. Shown are summary data of 2 independent experiments. Data points are shown as mean ± SD; Two-tailed t-tests were used to determine statistical significance (*P<0.05, **P<0.01, ***P<0.001).
To define the effect of IL-12 and IFNγ on tumor growth in vivo, we used RMAS cells to challenge IL-12Rβ2-/- and IFNγR1-/- mice that are in syngeneic C57BL/6J background. We injected mice with a tumor dose that caused about 50% death of WT mice. As shown in Figure 6C, all the WT mice treated by exogenous IL-12 were rescued from death caused by tumor growth; endogenous IL-12 was not sufficient to impact tumor growth since IL-12Rβ2-/- mice showed a survival rate similar to that of WT mice. However, all the IFNγR1-/- mice succumbed to tumor growth, indicating that endogenous IFNγ is required for tumor immunity in this system. Furthermore, IL-12 treatment did not improve the survival of the IFNγR1-/- mice, suggesting that IL-12′s anti-tumor effect is dependent on IFNγ signaling. We also examined the effect of IL-12 and IFNγ on Treg expansion in vivo. As shown in Figure 6D, Treg expansion was observed in WT mice after tumor challenge, which was completely blocked by exogenous IL-12 treatment. Treg cells in IL-12Rβ2-/- mice expanded to levels comparable to that in WT animals, suggesting that endogenous IL-12 was not sufficient to control Treg expansion. As expected, significantly higher levels of Treg expansion were observed in IFNγR1-/- mice, which was partially inhibited by IL-12 treatment. However, this partial inhibition of Treg expansion by exogenous IL-12 did not improve the survival of IFNγR1-/- mice, perhaps because IFNγR1 deficiency disabled effector lymphocytes, which require normal IFNγ function to clear the tumor cells (Figure 6C).
In summary, these in vivo observations now confirm the in vitro data, and strengthen the role of the IL-12-IFNγ axis in regulating Treg cells and tumor immunity.
Discussion
In this study, we developed an in vitro system to study factors that influence Treg cell expansion in the tumor setting. Irradiated tumor cells preferentially induce Treg cells to expand, with little effect on T effector cells. Although we do not know the specific antigens that cause Treg expansion, our data suggest that both accessory cells and IL-2 are required for Treg expansion. We also tested the effects of candidate cytokines on Treg cell expansion; most cytokines had no effect, but IL-12 inhibited tumor-induced Treg expansion in an Interferon-gamma dependent fashion. Mechanistic studies revealed that Interferon-gamma signaling directly inhibits Treg cell proliferation. Our findings support a model in which IL-12 stimulates immune effector cells to produce more IFNγ, which then leads to the growth arrest of Treg cells. The inhibition of Treg cells may be critical in the early phase of an adaptive immune response, when accessory cells start to present tumor antigens, and also produce cytokines like IL-12 and IFNγ. These cytokines may work in a positive feedback loop to ensure a potent adaptive immune response, which could end prematurely should Treg cells also become activated.
A previous report suggested that IL-12 treatment induced the apoptotic cell death of intra-tumoral CD4+CD25+ T cells in vivo (33). In that study, Annexin V was used to measure apoptosis; local IL-12 treatment increased the percentage of CD4+CD25+ T cells that stained positive for Annexin V. However, CD25 is probably not a reliable Treg marker for in vivo mechanistic studies (40), especially when IL-12 is used to stimulate effector T cells during an anti-tumor response. It is possible that the observed death in CD4+CD25+ T cells was in fact the activation-induced death of effector T cells, rather than Treg cells. To clarify this issue, we used splenocytes from Foxp3-GFP knock-in mice in co-culture assays, and used 7-AAD to measure cell death. No significant cell death was observed in the CD4+GFP+ Treg cell compartment (Figure S7); however, IL-12 treatment did increase cell death in the CD4+GFP− effector T cell compartment. Because cells can lose GFP when membrane integrity is lost, this data alone cannot exclude Treg cell death that occurs in this setting. However, our cell cycle analyses suggest that the anti-proliferative mechanism is important.
Our data show that IL-12 indirectly inhibits Treg cells, and that IFNγ signaling accounts for at least part of this phenotype. While an additional, probably IL-2 dependent mechanism may also account for part of IL-12 mediated inhibition of Treg proliferation, it is also possible that cellular components are involved. Purified Treg cells do not expand when co-cultured with tumor cells; Non-T accessory cells in the co-cultures are required to promote Treg expansion, presumably by presenting tumor antigens and/or by producing growth factors. Therefore, it is certainly possible that IL-12 can prevent Treg expansion by altering the function of an as yet unidentified population of accessory cells.
In summary, we show here that Treg inhibition by IL-12 may underlie some of its anti-tumor effects. Although the initial trials were suspended a decade ago because of dose- or regimen-related toxicities (41, 42), this cytokine has recently been revitalized for clinical studies in cancer patients (43-45). Since Treg cell proliferation (induced by tumors or even vaccines) can considerably dampen tumor immunity (9-11), this study provides mechanistic evidence to support the notion that IL-12, by inhibiting Treg cell proliferation, may serve as an adjuvant for tumor vaccine-based immunotherapy.
Supplementary Material
Acknowledgments
Financial support: This work was supported by N.I.H. grants DK49786 (T.J.L.), P50 CA94056 (D.P.W.) from the National Institutes of Health, and a basic research fellow scholar award (X.C.) from American Society of Hematology.
Footnotes
Conflict-of-interest disclosure: The authors have no special/competing interests to disclose.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16:115–23. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 5.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 6.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 8.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–74. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bui JD, Uppaluri R, Hsieh CS, Schreiber RD. Comparative analysis of regulatory and effector T cells in progressively growing versus rejecting tumors of similar origins. Cancer Res. 2006;66:7301–9. doi: 10.1158/0008-5472.CAN-06-0556. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa H, Kato T, Tawara I, et al. Accelerated chemically induced tumor development mediated by CD4+CD25+ regulatory T cells in wild-type hosts. Proc Natl Acad Sci U S A. 2005;102:9253–7. doi: 10.1073/pnas.0503852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–36. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–10. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–77. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–10. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- 15.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa H, Kato T, Tawara I, et al. Definition of target antigens for naturally occurring CD4(+) CD25(+) regulatory T cells. J Exp Med. 2005;201:681–6. doi: 10.1084/jem.20041959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bushell A, Jones E, Gallimore A, Wood K. The generation of CD25+ CD4+ regulatory T cells that prevent allograft rejection does not compromise immunity to a viral pathogen. J Immunol. 2005;174:3290–7. doi: 10.4049/jimmunol.174.6.3290. [DOI] [PubMed] [Google Scholar]
- 18.Tanchot C, Vasseur F, Pontoux C, Garcia C, Sarukhan A. Immune regulation by self-reactive T cells is antigen specific. J Immunol. 2004;172:4285–91. doi: 10.4049/jimmunol.172.7.4285. [DOI] [PubMed] [Google Scholar]
- 19.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–9. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 21.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–76. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 22.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–11. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burchill MA, Yang J, Vang KB, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–21. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Russell TD, Yan Q, Fan G, et al. IL-12 p40 homodimer-dependent macrophage chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor beta 1. J Immunol. 2003;171:6866–74. doi: 10.4049/jimmunol.171.12.6866. [DOI] [PubMed] [Google Scholar]
- 26.Pollock JL, Lane AA, Schrimpf K, Ley TJ. Murine acute promyelocytic leukemia cells can be recognized and cleared in vivo by adaptive immune mechanisms. Haematologica. 2005;90:1042–9. [PubMed] [Google Scholar]
- 27.Pollock JL, Westervelt P, Walter MJ, Lane AA, Ley TJ. Mouse models of acute promyelocytic leukemia. Curr Opin Hematol. 2001;8:206–11. doi: 10.1097/00062752-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Gross S, Piwnica-Worms D. Real-time imaging of ligand-induced IKK activation in intact cells and in living mice. Nat Methods. 2005;2:607–14. doi: 10.1038/nmeth779. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 30.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–6. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 31.Bruder D, Probst-Kepper M, Westendorf AM, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–30. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Lalani AS, Harding TC, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. 2006;12:6808–16. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 33.Kilinc MO, Aulakh KS, Nair RE, et al. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol. 2006;177:6962–73. doi: 10.4049/jimmunol.177.10.6962. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa H, Kato T, Tawara I, et al. IFN-gamma controls the generation/activation of CD4+ CD25+ regulatory T cells in antitumor immune response. J Immunol. 2005;175:4433–40. doi: 10.4049/jimmunol.175.7.4433. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 36.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–8. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 37.Yu XZ, Zhu L, Davis JE, Tso JY, Hansen JA, Anasetti C. Induction of apoptosis by anti-CD3 epsilon F(ab’)2 in antigen receptor transgenic murine T cells activated by specific peptide. J Immunol. 1996;157:3420–9. [PubMed] [Google Scholar]
- 38.Deng G, Podack ER. Suppression of apoptosis in a cytotoxic T-cell line by interleukin 2-mediated gene transcription and deregulated expression of the protooncogene bcl-2. Proc Natl Acad Sci U S A. 1993;90:2189–93. doi: 10.1073/pnas.90.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivo C, Levy F, Pilatte Y, et al. Control of cell cycle progression in human mesothelioma cells treated with gamma interferon. Oncogene. 2001;20:1085–93. doi: 10.1038/sj.onc.1204199. [DOI] [PubMed] [Google Scholar]
- 40.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 41.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 42.Orange JS, Salazar-Mather TP, Opal SM, et al. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med. 1995;181:901–14. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Younes A, Pro B, Robertson MJ, et al. Phase II clinical trial of interleukin-12 in patients with relapsed and refractory non-Hodgkin’s lymphoma and Hodgkin’s disease. Clin Cancer Res. 2004;10:5432–8. doi: 10.1158/1078-0432.CCR-04-0540. [DOI] [PubMed] [Google Scholar]
- 44.Ansell SM, Geyer SM, Maurer MJ, et al. Randomized phase II study of interleukin-12 in combination with rituximab in previously treated non-Hodgkin’s lymphoma patients. Clin Cancer Res. 2006;12:6056–63. doi: 10.1158/1078-0432.CCR-06-1245. [DOI] [PubMed] [Google Scholar]
- 45.Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–85. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.