Abstract
Objective
The Pediatric Bipolar Disorder (PBD) profile of the Child Behavior Checklist (CBCL), a parent completed measure that avoids clinician ideological bias, has proven useful in differentiating patients with Attention Deficit/Hyperactivity Disorder (ADHD). We used CBCL-PBD profiles to distinguish patterns of comorbidity and to search for quantitative trait loci (QTL) in a genome wide scan in a sample of multiple affected ADHD sibling pairs.
Method
540 ADHD subjects aged 5–18 years were assessed with the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-PL) and CBCL. Parents were assessed with the Schedule for Affected Disorders and Schizophrenia (SADS-LA) supplemented by the KSADS for disruptive behavioral disorders. Patterns of psychiatric comorbidity were contrasted based on the CBCL-PBD profile. A QTL variance component analysis was used to identify potential genomic regions that might harbor susceptibility genes for the CBCL-PBD quantitative phenotype.
Results
Bipolar spectrum disorders represented less than 2% of the overall sample. The CBCL-PBD classification was associated with increased generalized anxiety disorder (p=.001), oppositional defiant disorder (p=.008), conduct disorder (p=.003), and parental substance abuse (p=.005). A moderately significant linkage signal (multipoint maximum LOD score, MLS=2.5) was found on chromosome 2q.
Conclusions
The CBCL-PBD profile distinguishes a subset of ADHD patients with significant comorbidity. Linkage analysis of the CBCL-PBD phenotype suggests certain genomic regions that merit further investigation for genes predisposing to severe psychopathology.
Keywords: attention-deficit/hyperactivity disorder, bipolar disorder, child behavior checklist, quantitative trait loci, linkage analysis
Introduction
The Child Behavior Checklist (CBCL) has been proposed as a nonideological measure to characterize comorbidity in ADHD subjects.1,2 The CBCL is an empirically derived, highly valid and reliable, parent checklist of pediatric psychopathology that is comprised of several dimensions and scored according to established algorithms.3 As a parent completed measure, the CBCL provides a common metric across patients and studies that is free from clinician interpretation.4 Subsequent reports have proven the utility of this approach.5–9 Numerous studies have demonstrated that a CBCL Pediatric Bipolar Disorder (PBD) profile, defined by clinical elevations (T scores >70) on the CBCL Attention Problems (AP), Aggression (AGG), and Anxious/Depressed (A/D) subscales, may represent a susceptibility profile for PBD in children with ADHD. 4,5–7,9. The CBCL-PBD profile is highly heritable,5,10,11 and its genetic architecture has been established.10 It is clear from this previous work that the CBCL-PBD profile does not substitute for a categorical DSM IV diagnosis of bipolar disorder, but serves as a risk profile for severe psychiatric illness. 4,7,10
The sum of scales comprising the PBD profile also provides a putative quantitative trait suitable for genetic investigation. Unlike discrete traits, which are either present or absent in individuals, quantitative traits occur along continua within populations and provide greater measured variability among individuals which can improve power to detect underlying genes. The paucity of clear genetic findings in psychiatry, in spite of the well demonstrated heritability of many psychiatric disorders, has been attributed in part to their complex nature and the concomitant difficulty in measuring underlying liability with more quantitative approaches.12–14
We previously conducted genome wide linkage studies in our sample of multiple affected sibling pairs and identified several genetic regions associated with increased risk for ADHD, although quantitative trait locus (QTL) analysis of ADHD symptom scores yielded no significant linkages.15–18 In the current study, we used CBCL subscales to compare patterns of lifetime psychiatric comorbidity in the same sample of ADHD youth. Given that we knew rates of PBD in our sample were low, we assessed if subjects meeting CBCL-PBD criteria were characterized by other mood disorders or, alternatively, other patterns of comorbidity. Similarly, we assessed patterns of lifetime psychiatric diagnoses in parents based on their children’s CBCL-PBD status to determine if parents from CBCL-PBD families had increased rates of bipolar or other mood disorders, or alternatively, to assess other potential differences. Lastly, we used each ADHD proband’s quantitative CBCL-PBD phenotype to conduct a genome wide QTL analysis in search for genomic regions that might harbor risk genes influencing severe psychopathology in ADHD subjects.
Method
Subjects
Study subjects were 540 children and adolescents aged 5 – 18 years (mean=10.6, SD=3.2) and 519 of their parents ascertained from 270 families with ADHD affected sibling pairs. Families were included if at least one child met full DSM-IV ADHD criteria and a second child had a diagnosis of definite or probable ADHD, defined as being no more than one symptom short of full criteria, but with evidence of impairment in two settings. All families were English speaking. Mean socioeconomic status (SES) rank as defined by Hollingshead was 2.4 (SD=.9) (Hollingshead range=I–V).19
After receiving a full oral explanation of study requirements, subjects provided written informed consent/assent under procedures approved by the UCLA Institutional Review Board. Detailed descriptions of recruitment methods, screening, and subject assessment have been previously described.20,21 Briefly, lifetime psychiatric diagnoses were based on semi-structured diagnostic interviews conducted by clinical psychologists or highly trained interviewers with extensive experience and reliability training. Children and adolescents were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (KSADS).22 Adult parents were assessed using the Schedule for Affective Disorders and Schizophrenia – Lifetime Version (SADS-LA IV),23 supplemented with the KSADS Behavioral Disorders module for diagnosis of ADHD and disruptive behavior disorders.22 Direct interviews were supplemented with parent and teacher versions of the Swanson, Nolan, and Pelham, version IV (SNAP-IV) rating scale,24 as well as a parent completed CBCL and Teacher Report Form.3 Parents also completed current ratings of self and spouse behavior with the ADHD Rating Scale IV (ADHD-RS).25
Best estimate diagnoses were assigned using all available clinical information according to strict DSM-IV criteria,26 and reviewed by senior clinicians (JJM, JTM). The mean weighted κ for proband diagnoses was 0.84 (SD=.14), including values for ADHD (1.0), oppositional defiant disorder (.93), and conduct disorder (1.0). Mean weighted κ for parent diagnoses was .88 (SD=.10), including values for ADHD (.91), oppositional defiant disorder (.86), and conduct disorder (.83). The mean weighted κ comparing agreement between initial rater and best estimate diagnoses for 10 psychiatric conditions occurring in more than 5% of all subjects was .95 (SD=.03).
Procedures
Children and adolescents were assigned to categorical groups with or without the CBCL-PBD profile based on T scores using previously established thresholds (figure 1).4,10 The CBCL-PBD group (N=45) consisted of subjects with T score elevations > 70 on the Attention Problems, Aggressive Behavior, and Anxious/Depressed CBCL subscales. The CBCL-Attention Problems (AP) group (N=103) consisted of subjects with T score elevations > 70 on Attention Problems, but T score values ≤ 70 on Aggressive Behavior and Anxious/Depressed subscales. CBCL-comparison subjects (N=392) had values ≤ 70 on all three subscales. All probands had definite or probable ADHD. The total sample was 72% male and 77% Caucasian, with no differences between the three groups for age (F=.89, p=.41), sex ratio (χ2 = 4.20, p=.12), or race (χ2 = 1.55, p=.46). Additionally, families with at least one child meeting CBCL-PBD criteria were classified as CBCL-PBD families (N=42), while those with no child meeting CBCL-PBD criteria were classified as non-CBCL-PBD families (N=228). Finally, a quantitative CBCL-PBD phenotype was computed for all ADHD probands by summing raw scores on CBCL Attention Problems, Aggressive Behavior, and Anxious/Depressed subscales.
Figure 1.
Quantitative Trait Loci Analysis for CBCL Pediatric Bipolar Disorder Phenotype.
Genotype data for all ADHD probands and parents were obtained as previously described.15,17 Data were available for 423 highly polymorphic markers spanning all 22 autosomes. Denser sets of microsatellite and single-nucleotide polymorphism (SNP) markers (~ 2 or 3 cM density) were included for nine chromosomal regions chosen on the basis of increased linkage peaks in previous genome scan studies of ADHD.16
Data Analysis
Descriptive statistics were summarized and rates of DSM-IV disorders in ADHD probands and parents were computed. Sibling correlations on CBCL subscales were analyzed with Pearson’s r statistic. To minimize the number of comorbidity comparisons between groups, we limited consideration to those disorders occurring more than 5% in either the child or parent sample. We used t tests or chi-square analyses to assess demographic differences between CBCL defined categorical groups. Differences in rates of psychiatric comorbidities among CBCL-PBD, CBCL-AP, and CBCL-comparison groups, as well as between parents in CBCL-PBD and non-CBCL-PBD families, were assessed with chi-square analyses or Fisher’s exact tests. In consideration of multiple tests, significance was assessed at an alpha level < .01 for tests of comorbidity, with p values less than .05 and greater than .01 considered trends. All statistical tests were performed using SAS 9.1.27
Quantitative Trait Linkage Analysis
To asses whether any genomic regions harbored loci that influence CBCL-PBD risk in our ADHD sample, we used the maximum likelihood estimation of variance components (VCs) using the CBCL-PBD score as a quantitative trait. VC analyses were performed with Genehunter Version 2.1. A model of no dominance was assumed, since twin and family data support largely additive effects for both ADHD and the CBCL-PBD profile.10,28,29 VC analyses are known to be powerful tests of linkage due to consideration of almost all phenotypic variability within families. The approach is based on a decomposition of the CBCL-PBD phenotype as a linear function of the effects of the QTL, residual genetic, and random environmental effects.30 For all computations, we employed a 1 cM increment for IBD (inheritance-by-descent) scanning.
Results
Subjects’ mean (SD) age was 10.6 (3.2) years. Twenty seven percent of ADHD subjects had elevations (T > 70) on the AP scale, with 31% of these, or 8% of the entire sample, also showing elevations on AGG and AD scales. Sibling scores on the quantitative CBCL-PBD phenotype were highly correlated, with Pearson’s r equal to .44 (p<.0001). Of 270 families, 42 (16%) had at least one child meeting CBCL-PBD criteria. The occurrence of bipolar spectrum illness was low in the entire sample, with rates of bipolar I disorder of .9% and 1.9%; of bipolar II disorder of .7% and 2.2 %, and rates of cyclothymia of .2% and .4% in ADHD probands and parents, respectively. There were a total of 10 cases (1.9%) of bipolar spectrum disorders (bipolar I, bipolar II, and cyclothymia) among the ADHD probands – 5 (1.3%) in the CBCL-comparison group, 3 (6.6%) in the CBCL-PBD group and 2 (1.8%) in the CBCL-AP group. These samples were not sufficiently large for categorical comparisons.
Table 1 summarizes differences in the frequencies of psychiatric comorbidities occurring in more than 5% of the child or parent sample among CCL-PBD, CBCL-AP, and CBCL-comparison ADHD subjects, as well as between parents from CBCL-PBD versus non-CBCL-PBD families. The CBCL-PBD profile was associated with significantly higher rates of comorbid oppositional defiant disorder, conduct disorder, and generalized anxiety disorder. There were trends for increased obsessive compulsive disorder and Tourette’s disorder in the CBCL-PBD group, and increased rates, but not significant even at the trend level, of substance abuse disorders. There were no differences in rates of major depression or dysthymia. Parents in CBCL-PBD families had significantly higher rates of substance abuse, but showed no differences in mood disorders, anxiety disorders, or substance dependence.
Table 1.
Patterns of Psychopathology in ADHD Siblings and Their Parents.
| Lifetime Diagnosis* | CBCL Profiles | Pathology in One or Both Parents by Child CBCL-PBD Status** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n=392) |
↑PBD (n=45) |
↑AP (n=103) |
Total (n=540) |
Effect | No PBD (n=228) |
≥ 1 PBD (n=42) |
Total (n=270) |
Effect | |||
| n (%) | n (%) | n (%) | n (%) | Statistic | p value | n (%) | n (%) | n (%) | Statistic | p value | |
| ADHD | 138 (61) | 25 (60) | 163 (60) | Χ2=.02 | .9 | ||||||
| Conduct Disorder | 36 (9) | 11 (24) | 5 (5) | 52 (10) | G2=11.7 | .003 | 38 (17) | 7 (17) | 45 (17) | Χ2=0 | 1.00 |
| Dysthymia | 23 (6) | 4 (9) | 3 (3) | 30 (6) | G2=2.5 | .3 | 41 (18) | 12 (29) | 53 (20) | Χ2=2.5 | .1 |
| Generalized Anxiety | 57 (15) | 15 (33) | 9 (9) | 81 (15) | Χ2=15.1 | .001 | 34 (15) | 6 (14) | 40 (15) | Χ2=.01 | .9 |
| Major Depression | 62 (16) | 9 (20) | 15 (15) | 86 (16) | Χ2=.7 | .7 | 154 (68) | 29 (69) | 183 (68) | Χ2=.04 | .8 |
| OCD | 20 (5) | 7 (16) | 9 (9) | 36 (7) | G2=6.6 | .02 | 12 (5) | 5 (15) | 17 (6) | G2=3.9 | .05 |
| ODD | 183 (47) | 31 (69) | 43 (42) | 257 (48) | Χ2=9.7 | .008 | 28 (12) | 9 (21) | 37 (14) | Χ2=2.5 | .1 |
| PTSD | 13 (3) | 0 (0) | 1 (1) | 14 (3) | G2=4.5 | .1 | 36 (16) | 10 (24) | 46 (17) | Χ2=1.6 | .2 |
| Substance Abuse | 11 (3) | 1 (2) | 0 (0) | 12 (2) | G2=5.2 | .07 | 88 (39) | 26 (62) | 114 (42) | Χ2=7.9 | .005 |
| Substance Depend | 2 (.5) | 0 (0) | 0 (0) | 2 (.4) | G2=1.28 | .5 | 57 (25) | 15 (36) | 72 (27) | Χ2=2.1 | .1 |
| Tourette’s Disorder | 15 (4) | 5 (11) | 9 (9) | 29 (5) | G2=6.3 | .04 | 3 (1) | 1 (2) | 4 (2) | Χ2=2.1 | .1 |
CBCL - Child Behavior Checklist; PBD - CBCL Pediatric Bipolar Disorder Scale; AP - CBCL Attention Problem Scale; ADHD - Attention Deficit/Hyperactivity Disorder; OCD - Obsessive Compulsive Disorder; ODD - Oppositional Defiant Disorder; PTSD - Post Traumatic Stress Disorder
Includes all categories occurring in > 5% probands or parents.
Parent CBCL-PBD status based on having ≥ 1 child meeting CBCL-PBD criteria. G2 – Fisher’s Exact Test.
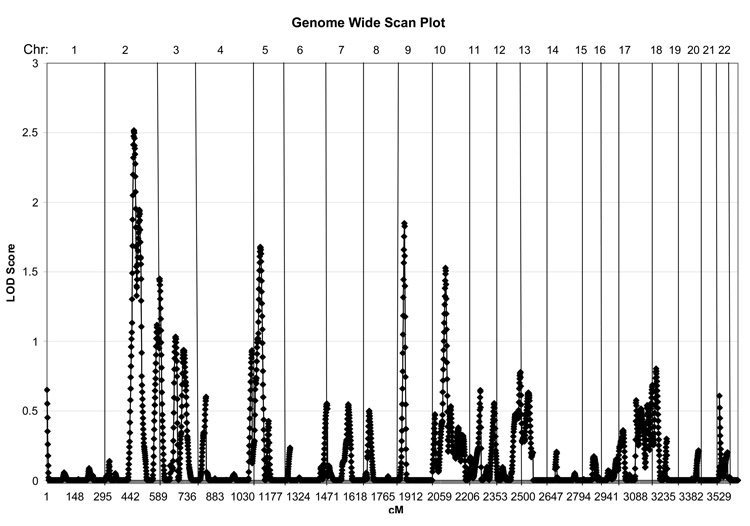
Multipoint linkage analyses of the PBD phenotype yielded suggestive linkage near marker D2S151 on chromosome 2q (figure 2). The multipoint maximum LOD score (MLS) of 2.5 exceeds the threshold (>2.2) for ‘suggestive’ linkage,31 with markers indicated in Table 2 that span the one-lod support interval. Modest linkage signals (MLS range 1.5–2) were found on chromosomes 5, 9, and 10, also suggesting regions of nominal linkage that may merit further study.
Table 2.
MLS Values (Single-point and Multipoint) for PBD Quantitative Trait Loci Analysis and Markers on Chromosome 2.
| Location | Single-Point | Multipoint | |||
|---|---|---|---|---|---|
| Marker | Cytogenetic | Marshfield (cM) | MLS | MLS | pa |
| D2S347 | 2q21 | 131.51 | 1.10 | 0.57 | 0.11 |
| D2S112 | 2q21 | 141.62 | 1.22 | 1.48 | 0.009 |
| D2S151 | 2q23 | 152.04 | 0.93 | 2.52 | 0.00007 |
| D2S142 | 2q24 | 161.26 | 1.02 | 1.68 | 0.005 |
| D2S2330 | 2q24 | 169.41 | 1.37 | 1.60 | 0.007 |
P values for multipoint MPS values are found by multiplying 2loge and determining significance from χ2 tables, taking into account the one-sided nature of the linkage test.38
Discussion
In a genetically enriched sample of ADHD affected sibling pairs, the CBCL-PBD profile identified a small (8%) but distinct subset of individuals with severe psychopathology. These results are consistent with an earlier report describing similarly increased rates of oppositional defiant, conduct, and anxiety disorders in clinical samples.2 Also in other reports, the CBCL-PBD profile identified increased ADHD, oppositional defiant disorder, conduct disorder, and suicidality in a population based twin sample,11 and increased delinquency and suicidality in an epidemiological sample.32 The increased rate of substance abuse in the CBCL-PBD sample, while not significant, is notable given that many subjects in our sample had not yet entered the age of risk for substance use initiation, but is also consistent with previous findings.33 Looking across our sample and others, it is clear that children and adolescents identified by the CBCL-PBD profile possess significant psychopathology. There remains an ongoing clinical imperative to recognize, characterize, and develop more specific treatment strategies for these severely ill youth.
Although the potential value of QTL analyses in ADHD genetics studies has been discussed,34 we are not aware of any prior studies that have successfully employed this approach using the quantitative CBCL-PBD phenotype. Our previous investigations of ADHD symptoms counts within ADHD affected sibling pairs revealed no significant evidence for linkage,15 in contrast to when the discrete ADHD phenotype was used.16 However, in the present study using the CBCL-PBD quantitative phenotype, we found suggestive evidence for linkage on chromosome 2 in a region that showed nominal linkage (MLS 1.0) in our first scan of ADHD on the discrete ADHD phenotype and later fell below that nominal level in an extended sample.15,16 Of particular related interest, is the recent finding of significant linkage on chromosome 2q22–q24, the same region underlying our peak linkage signal, with bipolar disorder in a study of 52 families of European descent.35 This region has not, to our knowledge, been previously examined in relationship to ADHD. These emerging results may support further study of this region and use of the CBCL-PBD measure as a susceptibility profile within ADHD to identify putative genes involved in that condition and its clinical heterogeneity.
Although the overall rates of bipolar spectrum illnesses in our sample were lower than in other reports, they still represent up to a two-fold elevation above adult rates usually described in community samples, but are not near the increased rates described with ADHD. 36 It is notable that the CBCL-PBD group did have rates of diagnosed bipolar spectrum illness 3–5 times greater than seen in the CBCL-comparison and CBCL-AP groups, although these differences were not testable given the low overall rates in the sample. To our surprise, the CBCL-PBD profile did not identify subjects or families characterized by increased rates of any DSM-IV mood disorder. This contrasts with numerous prior investigations 1,2,4,6–9 but is consistent with some reports.11,32 It is unclear why such large differences in ADHD comorbidity occur across extant studies. Our sample, which required the participation of both biological parents, might be biased towards healthier, better functioning families, although in previous reports we demonstrated expected rates of comorbid disorders and psychiatric impairment.20,21,37 There are subtle assessment differences between investigations. Some studies relied on the epidemiological version of the KSADS (KSADS-E) which contains fewer probes and allows less clinician discretion than the KSADS-PL used in our investigation.1,36 Other reports used the CBCL itself as the diagnostic phenotype,5,10,32 without consideration of DSM defined syndromes.
Another explanation for the relatively low overall rates of bipolar spectrum illness seen in our sample is the method employed at our site in interpreting the DSM. PBD has been defined by some investigators as phenomenologically dissimilar from the adult presentation, more typically characterized by chronic irritability and a relative lack of positive affective features and distinct episodes.33,38,39–41 Others have argued that this approach in community settings has led to inconsistent application of DSM criteria and a general tendency to assign a diagnosis of bipolar disorder to any child with impulse control deficits and affective instability. 42–44 One attempt to address the challenges of establishing valid symptomatic boundaries for PBD is a proposed differentiation of narrow versus broad bipolar phenotypes.44 The narrow phenotype, which informed our application of DSM criteria in this study, is defined by strict DSM criteria for mania or hypomania, including discrete episodes of elevated, expansive, or irritable mood. In contrast, the broad phenotype is defined by chronic, non-episodic illness that generally lacks the hallmark symptoms of grandiosity and hyperarousal. The predominance of anxiety, oppositional defiant disorder, and conduct disorder in our CBCL-PBD group is more suggestive of the chronic irritability described with the broad PBD phenotype. It is noteworthy that while the CBCL-PBD profile failed to differentiate ADHD probands or parents with higher rates of mood disorders, almost 70% of all families had evidence of lifetime parental depression. The overall high rates of depression in these parents have previously been described and might reflect the psychosocial stress of raising multiple siblings with ADHD, in addition to any underlying biological predisposition.20 In particular view of our linkage findings, the increased rates of psychoactive substance abuse in parents of CBCL-PBD families favors a biological etiology for these disorders that merits further investigation.
There should be no disagreement, however, over the existence of a distinct pediatric syndrome characterized by chronic irritability and affective instability. Our suggestive linkage findings provide supporting evidence of a biological basis for this subgroup of ADHD patients. As we develop future editions of the DSM, the nosological choice to be made is whether we should continue to describe these individuals as bipolar, while acknowledging developmental differences in younger patients, or instead operationalize a new diagnostic category that avoids confusion with the typical adult bipolar presentation. As previously proposed, the CBCL-PBD profile appears useful as a standardized measure in understanding subjects across studies and in potentially identifying biological susceptibility to severe comorbidity.
This study has several methodological limitations. First, in constructing our CBCL categories, we relied on previously described group thresholds. 4,10 Other groups have demonstrated that slightly lower cutoffs points or quantitative interpretations of the CBCL-PBD scale lead to increased sensitivity for detecting comorbid pathology.5,7 However, as our interest was in characterizing subjects who met the originally defined categorical CBCL-PBD cutoff, not in establishing the predictive power of the rating, we choose to use the more conservative threshold. This also reflected our wish to avoid repeated testing at various threshold levels in an attempt to force the data to fit some a priori expectation. Second, we relied on a model of additive effects in our QTL analysis, based on some prior studies.10,29 However, other work has suggested that ADHD might be inherited under a dominant model.45 While this remains a possibility, in QTL analysis the power to detect a dominant or recessive effect (if present) is generally greater than additive alone across a range of allele frequencies and residual polygenic effects.46 Thus, mis-specification of our inheritance model would, in fact, reduce the power available to detect genetic effects. Finally, the study data set has been used in previous genomewide scans for ADHD and reading disorder.15–18,47 Although linkage peaks identified in this study do not overlap with these previous scans, we have chosen not to emphasize nominal linkage peaks due to the increased probability of Type I error. Our findings need be considered with caution in light of the multiple phenotype testing in the sample. They are offered as preliminary data for comparison with other groups using this quantitative trait. Replication is needed before further investigation, i.e. fine mapping and SNP association studies, is warranted.
This study may be one of the first to identify a suggestive genetic association with such severe behavioral problems in children. It remains unclear if the CBCL-PBD profile serves to identify a more homogenous group of children with ADHD, or if it is also useful in indentifying severe psychopathology in individuals without ADHD. The question is relevant as one considers whether the identified individuals should be viewed as suffering from a severe ADHD subtype or a separate, but commonly comorbid, condition. Consensus on the diagnostic classification of these patients will enhance ongoing research and support directed treatment studies that will provide a basis for improved clinical management.
Acknowledgments
This work was supported by National Institute of Mental Health grants MH01969 (to JJM), HD40275 (to SKL), MH01805 (to JTM), MH071852 (to SFN), and MH58277 (to SLS).
The authors dedicate this article to the memory of Richard D. Todd, M.D., Ph.D. (1951-2008).
Footnotes
Disclosure: Dr. McGough has served as a consultant for Eli Lilly & Company, Janssen Pharmaceuticals and Shire Pharmaceuticals, and has received research support from Eli Lilly & Company, Janssen Pharmaceuticals, and Shire Pharmaceuticals. Dr. McCracken has served as a consultant for Abbott, Janssen, Pfizer Pharmaceuticals, Sanofi-Aventis, and Wyeth Pharmaceuticals. He has received research support from Aspect, Bristol Myers Squibb, Eli Lilly, and Janssen, and speaker honoraria from UCB. The other authors report no financial conflicts of interest.
References
- 1.Biederman J, Monuteaux MC, Kendrick E, Klein KL, Faraone SV. The CBCL as a screen for psychiatric comorbidity in paediatric patients with ADHD. Arch Dis Child. 2005;90:1010–1015. doi: 10.1136/adc.2004.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biederman J, Wozniak J, Kiely K, et al. CBCL clinical scales discriminate prepubertal children with structured interview-derived diagnosis of mania from those with ADHD. J Am Acad Child Adolesc Psychiatry. 1995;34:464–471. [PubMed] [Google Scholar]
- 3.Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington, Vermont: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 4.Mick E, Biederman J, Pandina G, Faraone SV. A preliminary meta-analysis of the child behavior checklist in pediatric bipolar disorder. Biol Psychiatry. 2003;53:1021–1027. doi: 10.1016/s0006-3223(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 5.Althoff RR, Rettew DC, Faraone SV, Boomsa DI, Hudziak JJ. Latent class analysis shows strong heritability of the child behavior checklist-juvenile bipolar disorder phenotype. Biol Psychiatry. 2006;60:903–911. doi: 10.1016/j.biopsych.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Dienes KA, Chang KD, Blasey CM, Adleman NE, Steiner H. Characterization of children of bipolar parents by parent report CBCL. J Psychiatric Res. 2002;36:337–345. doi: 10.1016/s0022-3956(02)00019-5. [DOI] [PubMed] [Google Scholar]
- 7.Faraone SV, Althoff RR, Hudziak JJ, Monuteaux M, Biederman J. The CBCL predicts DSM bipolar disorder in children: a receiver operating characteristic curve analysis. Bipolar Dis. 2005;7:518–524. doi: 10.1111/j.1399-5618.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 8.Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: Assessment and validity using the WASH-U-KSADS, CBCL, and TRF. J Affect Disord. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- 9.Hazell PL, Lewin TJ, Carr VJ. Confirmation that Child Behavior Checklist clinical scales discriminate juvenile mania from attention deficit/hyperactivity disorder. J Paidiatr Child Health. 1999;35:199–203. doi: 10.1046/j.1440-1754.1999.t01-1-00347.x. [DOI] [PubMed] [Google Scholar]
- 10.Hudziak JJ, Althoff RR, Derks EM, Faraone SV, Boomsma DI. Prevalence and genetic architecture of child-behavior checklist-juvenile bipolar disorder. Biol Psychiatry. 2005;58:562–568. doi: 10.1016/j.biopsych.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Volk H, Todd R. Does the child behavior checklist juvenile bipolar disorder phenotype identify bipolar disorder? Biol Psychiatry. 2007;62:115–120. doi: 10.1016/j.biopsych.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 12.Dean M. Approaches to identify genes for complex human diseases: lessons from Mendelian disorder. Hum Mutation. 2003;22:261–274. doi: 10.1002/humu.10259. [DOI] [PubMed] [Google Scholar]
- 13.Kendler KS. Psychiatric genetics; a methodologic critique. Am J Psychiatry. 2005;162:3–11. doi: 10.1176/appi.ajp.162.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Risch N. Searching for genetic determinants in the new millennium. Nature. 2000;405:847–856. doi: 10.1038/35015718. [DOI] [PubMed] [Google Scholar]
- 15.Fisher SE, Franks C, McCracken JT, et al. A genome-wide scan for loci involved in attention-deficit/hyperactivity disorder (ADHD) Am J Hum Genet. 2002;70:1183–1196. doi: 10.1086/340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogdie MN, Fisher SE, Yang M, et al. Attention deficit hyperactivity disorder: fine mapping support linkage to 5p13, 6q12, 16p13, and 17p11. Am J Hum Genet. 2004;75:661–668. doi: 10.1086/424387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogdie MN, Macphie IL, Minassian SL, et al. A genome-wide scan for attention-deficit/hyperactivity disorder in an extended sample: suggestive linkage on 17p11. Am J Hum Genet. 2003;72:1268–1279. doi: 10.1086/375139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smalley SL, Kustanovich V, Minassian SL, et al. Genetic linkage of attention-deficit hyperactivity disorder (ADHD) on chromosome 16p13 in a region implicated in autism. Am J Hum Genet. 2002;71:959–963. doi: 10.1086/342732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollingshead AB. Two-Factor Index of Social Position. New Haven: Yale University Press; 1957. [Google Scholar]
- 20.McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162:1621–1627. doi: 10.1176/appi.ajp.162.9.1621. [DOI] [PubMed] [Google Scholar]
- 21.Smalley S, McGough J, Del’Homme M, et al. Familial clustering of symptoms and disruptive behaviors in multiplex families with attention deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:1135–1143. doi: 10.1097/00004583-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (KSADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Fyer AJ, Mannuzza SM, Klein DF, Endicott J. Schedule for Affective Disorders and Schizophrenia – Lifetime Version Modified for the Study of Anxiety Disorders (SADS-LA), Updated for DSM IV (SADS-LA IV) New York: New York State Psychiatric Institute; 1995. [DOI] [PubMed] [Google Scholar]
- 24.Swanson JM. SNAP-IV Scale. Irvine, CA: University of California Irvine Child Development Center; 1995. [Google Scholar]
- 25.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale IV: Checklists, Norms, and Clinical Interpretation. New York: Guilford; 1998. [Google Scholar]
- 26.Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 27.SAS Version 9.1. Cary, NC: SAS Institute; 2006. [Google Scholar]
- 28.Smalley SL. Genetic influences in childhood-onset psychiatric disorders: autism and attention-deficit/hyperactivity disorder. Am J Hum Genet. 1997;60:1276–1282. doi: 10.1086/515485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derks EM, Dolan CV, Hudziak JJ, Neale MC, Boomsma DI. Assessment and etiology of attention deficit hyperactivity disorder and oppositional defiant disorder in boys and girls. Behav Genet. 2007;37:559–566. doi: 10.1007/s10519-007-9153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blangero J, Williams JT, Almasy L. Quantitative trail locus mapping using human pedigrees. Hum Biol. 2000;72:35–62. [PubMed] [Google Scholar]
- 31.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 32.Holtmann M, Bölte S, Goth K, et al. Prevalence of the Child Behavior Checklist-pediatric bipolar disorder phenotype in a German general population sample. Bipolar Disord. 2007;9:895–900. doi: 10.1111/j.1399-5618.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- 33.Wozniak J, Spencer T, Biederman J, Kwon A, Monuteaux M, Rettew J. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. J Affect Disord. 2004;82 suppl 1:S59–S69. doi: 10.1016/j.jad.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Todd RD. Genetics of attention deficit/hyperactivity disorder; Are we ready for molecular genetic studies? Am J Med Genet. 2000;96:241–243. doi: 10.1002/1096-8628(20000612)96:3<241::aid-ajmg1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 35.Abou Jamra R, Fuerst R, Kaneva R, et al. The first genomewide interaction and locus-heterogeneity linkage scan in bipolar affective disorder: strong evidence of epistatic effects between loci on chromosomes 2q and 6q. Am J Hum Genet. 2007;81:974–986. doi: 10.1086/521690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wozniak J, Biederman J, Kiely K, et al. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry. 1995;34:867–876. doi: 10.1097/00004583-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Smalley SL, McGough JJ, Moilanen IK, et al. Prevalence and psychiatric comorbidity of attention deficit hyperactivity disorder in an adolescent Finnish population. J Am Acad Child Adolesc Psychiatry. 2007;46:1575–1583. doi: 10.1097/chi.0b013e3181573137. [DOI] [PubMed] [Google Scholar]
- 38.Wozniak J, Biederman J, Kwon A, et al. How cardinal are cardinal symptoms in pediatric bipolar disorder? an examination of clinical correlates. Biol Psychiatry. 2005;58:583–588. doi: 10.1016/j.biopsych.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Carlson GA, Meyer SE. Phenomenology and diagnosis of bipolar disorder in children, adolescents, and adults: complexities and developmental issues. Dev Psychopathol. 2006;18:939–969. doi: 10.1017/S0954579406060470. [DOI] [PubMed] [Google Scholar]
- 40.Geller B, Luby J. Child and adolescent bipolar disorder: A review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36:1168–1176. doi: 10.1097/00004583-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Mick E, Spencer T, Wozniak J, Biederman J. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58:576–582. doi: 10.1016/j.biopsych.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 42.Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996–2004. Biol Psychiatry. 2007;62:107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pogge DL, Wayland-Smith D, Zaccario M, Borgaro S, Stokes J, Harvey PD. Diagnosis of manic episodes in adolescent inpatients: Structured diagnostic procedures compared to clinical chart diagnoses. Psychiatry Res. 2001;101:47–54. doi: 10.1016/s0165-1781(00)00248-1. [DOI] [PubMed] [Google Scholar]
- 44.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 45.Hudziak JJ, Derks EM, Althoff RR, Rettew DC, Boomsma DI. The genetic and environmental contributions to attention deficit hyperactivity disorder as measured by the Conners’ rating scales – revised. Am J Psychiatry. 2005;162:1614–1620. doi: 10.1176/appi.ajp.162.9.1614. [DOI] [PubMed] [Google Scholar]
- 46.Alcaïs A, Abel L. Linkage analysis of quantitative trait loci: sibpairs or sibships? Hum Hered. 2000;50:251–256. doi: 10.1159/000022925. [DOI] [PubMed] [Google Scholar]
- 47.Loo SK, Fisher SE, Francks C, et al. Genome-wide scan of reading ability in affected sibling pairs with attention-deficit/hyperactivity disorder: unique and shared genetic effects. Mol Psychiatry. 2004;9:485–493. doi: 10.1038/sj.mp.4001450. [DOI] [PubMed] [Google Scholar]