Abstract
Background
Cardiovascular risk factors are associated with a higher risk of developing dementia. Studies in older populations, however, have often failed to show this relationship. We assessed the association between cardiovascular risk factors measured in midlife and risk of being hospitalized with dementia and determined whether this association was modified by age and ethnicity.
Methods
We studied 11,151 participants in the population-based Atherosclerosis Risk in Communities cohort, aged 46-70 (23% African Americans) in 1990-1992, when participants underwent a physical exam and cognitive testing. Hospitalizations with dementia were ascertained through December 2004.
Results
During follow-up, 203 cases of hospitalization with dementia were identified. Smoking (hazard ratio (HR), 95% confidence interval (CI): 1.7, 1.2-2.5), hypertension (HR, 95% CI: 1.6, 1.2-2.2) and diabetes (HR, 95% CI: 2.2, 1.6-3.0) were strongly associated with dementia, both in whites and African-Americans. These associations were stronger when risk factors were measured at younger age than at older age. In analyses including updated information on risk factors during follow-up, the HR of dementia in hypertensive versus non-hypertensive participants was 1.8 at age <55 years compared to 1.0 at age 70+ years. Parallel results were observed for diabetes (HR 3.4 in <55, 2.0 in ≥70), smoking (4.8 in <55, 0.5 in ≥70), and hypercholesterolemia (HR 1.7 in <55, 0.9 in ≥70)
Conclusion
In this prospective study, smoking, hypertension, and diabetes were strongly associated with subsequent risk of hospitalization with dementia, particularly in middle aged individuals. Our results emphasize the importance of early lifestyle modification and risk factor treatment to prevent dementia.
Keywords: dementia, cardiovascular risk factors, incidence
Introduction
Dementia is one of the major public health problems affecting the elderly in developed countries. Estimates in the United States (US) show that 1 in 6 individuals older than 70 have dementia, with prevalence increasing exponentially with advanced age.[1, 2] As the elderly population grows, the number of affected individuals, as well as the costs and social burden associated with dementia, will soar. United States population projections suggest that the number of dementia cases in 2050 will increase 3-fold compared to the year 2000.[3]
The most common forms of dementia in the general population are Alzheimer's disease and vascular dementia.[4] Alzheimer's disease is the diagnosis given in approximately 70% of total dementia cases, while 20% are labeled as vascular dementia, the rest being ascribed to miscellaneous etiologies.[1] Nonetheless, pathological evidence has shown considerable overlap between Alzheimer's disease and vascular dementia,[5] suggesting that Alzheimer's disease could be considered a vascular disease or has a vascular component.[6] Some degree of vascular involvement, particularly microvascular disease, is generally present in Alzheimer's disease cases, though to what degree alterations in the microvasculature are caused by or predispose to Alzheimer's disease remains unresolved.[7]
Different epidemiologic studies have found that the presence of cardiovascular risk factors, including hypertension, diabetes, obesity or smoking, increases the risk of developing subsequent dementia, including Alzheimer's disease.[8-12] These observations further advance the case for a vascular involvement in the pathogenesis of dementia in general, and Alzheimer's disease in particular. However, randomized trials of anti-hypertensive medications for the prevention of dementia in older individuals have been disappointing, showing no protective effect.[13] This apparent contradiction could be attributed to a significant predictive and etiologic role of cardiovascular risk factors in midlife but not in older ages. A recent review of prospective studies assessing the association of smoking and dementia showed that smoking predicted the risk of dementia only if measured in midlife.[14] The same phenomenon has been described for the link between hypertension and dementia.[15] The studies included in these two reviews, however, compared results from different populations and did not address directly whether age modified the effect of cardiovascular risk factors on dementia. Further evidence derived from prospective studies in which vascular risk factors and cognition were measured both in middle and older age is required.
Until now, studies on cardiovascular risk factors and dementia have examined mostly white populations, with the exception of the Honolulu Asia Aging Study, which studied Japanese-Americans residing in Hawaii.[16] The prevalence of both dementia and some cardiovascular risk factors is higher among African-Americans,[2, 17, 18] but the role of vascular risk factors in dementia incidence in African-Americans is largely unstudied.
Our objective was to evaluate the association of cardiovascular risk factors with the incidence of dementia defined through hospital discharge codes in whites and African-Americans enrolled in the Atherosclerosis Risk in Communities (ARIC) study, a population-based prospective study in the US, and to determine whether the age at which the risk factors were measured modified this association.
Methods
Study population
The ARIC study is a population-based cohort including 15,792 men and women, aged 45-64, recruited during 1987-1989 from four communities in the US (Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; suburbs of Minneapolis, Minnesota).[19] Its main objective was to investigate the major determinants of atherosclerosis in the general population. ARIC participants were examined at baseline and three more times approximately every three years (last exam 1996-1998). Response rates among survivors for the successive examinations were 93%, 86% and 80%. Additionally, annual phone follow-up calls are made to all surviving cohort members (93% response rate on average).
The second ARIC examination, conducted in 1990-1992, included a cognitive assessment. For the purpose of this analysis, we restricted our sample to ARIC participants who attended the second examination and were white from Minnesota, Washington County, and Forsyth County communities, or African-American from Jackson and Forsyth County (n=14,257). Ninety-one participants were excluded for failing to meet racial/ethnic criteria. Individuals meeting any of the following criteria at the second examination were excluded: history of coronary heart disease, stroke, or transient ischemic attack (n=1,537), missing information on any of the study covariates (n=488), and scoring below sex- and race-specific 5th percentile in any of the cognitive tests to exclude potential prevalent cases of dementia (n=1,492) (see below).
The study was approved by the Institutional Review Boards at each participating center. ARIC participants provided written informed consent.
Exposures measurement
In each study exam, ARIC participants gave detailed information on lifestyles, use of medication and history of previous disease. They underwent a physical examination, including blood pressure, weight, and height measurements. Blood pressure was taken with a random-zero sphygmomanometer; the mean of the last 2 of 3 measurements was used for analysis. Hypertension was defined as having a systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or having used antihypertensive medication in the two weeks before the examination. Height and weight were measured with the participants wearing a scrub suit and no shoes. Body mass index was calculated as weight in kilograms divided by height in meters squared.
Educational level and occupation were determined in the first ARIC examination. Education was categorized in six levels: grade school or less, high school but no degree, high school graduate, vocational school, college, and graduate or professional school. Occupation was classified in 8 broad categories: managerial and professional specialty; technical, sales, and administrative support; service; farming, forestry, and fishing; precision production, craft, and repair; operator, fabricators, and laborers; homemakers; and retired. In exams 2 and 4, ARIC participants underwent a cognitive function assessment. Three neuropsychological tests, Delayed Word Recall Test,[20] Digit Symbol Substitution Test,[21] and Word Fluency Test,[22] were administered by trained interviewers. The Delayed Word Recall Test is a memory test that specifically evaluates verbal learning and recent memory, the Digit Symbol Substitution Test assess sustained attention and psychomotor speed, and the World Fluency Test measures the flexibility of verbal thought processes.
Fasting blood samples were obtained in each examination, and plasma, serum, and DNA isolated. Hypercholesterolemia was defined by total blood cholesterol level ≥240 mg/dL or the participant taking cholesterol-lowering drugs in the 2 weeks prior to the examination. Diabetes was defined as fasting blood glucose ≥126 mg/dL, or non-fasting blood glucose ≥200 mg/dL, or a self-reported history of or treatment for diabetes. Genotyping of the APOE polymorphisms was performed using the TaqMan assay (Applied Biosystems, Foster City, CA) with variants at codons 130 and 176 (formerly 112 and 158) assayed separately. The data from these two codons were combined to generate the six APOE genotypes. The ABI 7900 and Sequence Detection System software (Applied Biosystems) was used for allele detection and genotype calling.[23] Genotypes were classified as: ε2/ε2+ε2/ε3, ε3/ε3 (reference), ε2/ε4+ε3/ε4, ε4/ε4.
Outcome ascertainment
The main outcome of interest for this analysis was incident dementia identified through ARIC follow-up for all hospitalizations by December 31, 2004 and chart abstraction of all hospital discharge codes. Hospitalizations in ARIC are identified by participant or proxy report in the annual follow-up and by surveillance of local hospital discharge lists. The ICD-9 codes used to define dementia referred to Alzheimer's disease (331.0), vascular dementia (290.4) or any other code that could have been used for dementia of other etiology (290.0, 290.1, 290.2, 290.3, 290.9, 294.1, 294.2, 294.8, 294.9, 331.1, 331.2, 331.8, 331.9). First occurrence of any of these codes was considered the event time. A rough assessment of the validity of this outcome was performed, first, computing age, sex and race-specific incidence rates of a first hospitalization with dementia and comparing them to previous studies, and, second, estimating the association between APOE genotype, a well-established risk factor for dementia, and the outcome. Because we excluded ARIC participants with low scores in any of the cognitive tests at exam 2, we assumed cases to be new (incident) events.
Statistical analysis
Specific incidence rates of dementia were estimated by dividing the number of new cases of dementia hospitalization in each age- (in 5-year periods), sex-, and race-specific group by the number of corresponding person-years. Follow-up for each study participant was defined as the time between exam 2 and occurrence of hospitalization with dementia, death, loss to follow-up, or December 31, 2004, whichever occurred earlier. We estimated hazard ratios (HR) of dementia and their 95% confidence intervals (CI) by presence of cardiovascular risk factors at baseline using Cox proportional hazard models, with time from exam 2 to dementia hospitalization as the dependent variable. Initial models adjusted for age, sex, race, educational level, occupation, study center, and scores in the cognitive assessment. A second model adjusted additionally for the presence of other cardiovascular factors. Analyses were repeated not including baseline cognitive scores as covariates. Finally, APOE genotype, considered as a potential confounder for the association between cardiovascular disease and risk of dementia, was included in the model. We ran stratified analyses by race, sex, age (<60, ≥60 at baseline) and APOE genotype (ε4 allele present or not), and estimated potential interactions with these variables using the likelihood ratio test.
To estimate the difference in associations by age at baseline and to take advantage of the repeated measures in the follow-up, we created two additional cohorts using exams 3 and 4 as baseline, excluding individuals who had a dementia hospitalization before the exam, additionally applying the same exclusion criteria as for the main analysis, and using information on risk factors measured at those examinations. Subsequently, we pooled together the three cohorts in one Cox model, including the same potential confounders as in the main analysis. Because each study participant could be included more than once, we used a robust variance estimator to take into account within-individual correlations.[24] We stratified the analysis by age in the following categories: <55, 55-59, 60-64, 65-69, and ≥70. We additionally tested whether, in non-demented survivors at exam 4, cardiovascular risk factors measured in exam 2 were better predictors of dementia hospitalization than measurements in exam 4.
Results
From 14,348 participants attending the second examination, 11,151 met inclusion criteria. Figure 1 shows each of the reasons for exclusion. Selected characteristics of the included study participants are presented in table 1. As reported previously, the prevalence of hypertension, obesity and diabetes was higher in African-Americans compared to whites.
Figure 1.
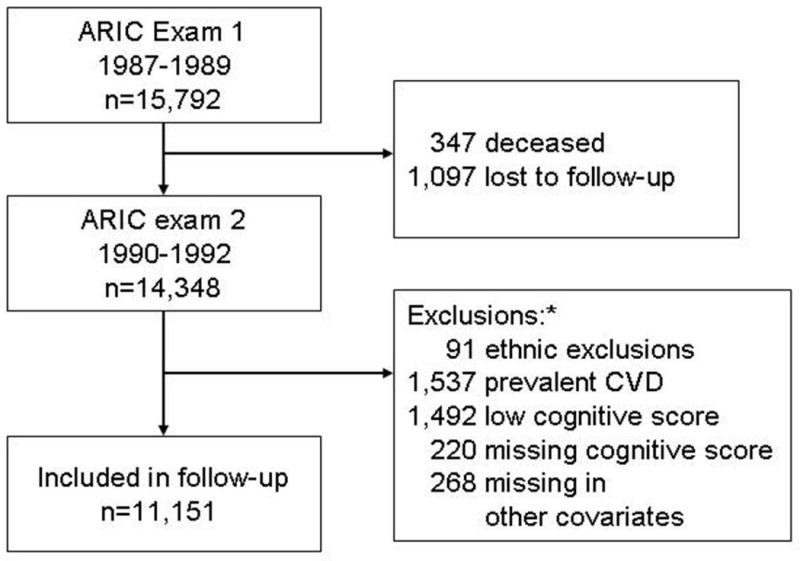
Study participants flow diagram.
ARIC: Atherosclerosis Risk in Communities study. CVD: cardiovascular disease
* Study participants could meet more than one exclusion criteria
Table 1.
Selected characteristics of individuals included in the follow-up at exam 2. ARIC study, 1990-1992
| Whites | African-Americans | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| N | 4713 | 3819 | 1696 | 923 |
| Age (years) | 56.6 (5.6) | 57.1 (5.6) | 55.6 (5.6) | 55.7 (5.6) |
| [range] | 47 – 70 | 47 – 68 | 46 – 70 | 47 – 70 |
| Education (%): | ||||
| Less than high school | 11.5 | 11.5 | 31.7 | 33.6 |
| Completed high school | 52.0 | 39.2 | 31.7 | 27.9 |
| College education | 36.5 | 49.2 | 36.6 | 38.6 |
| Current smoker (%) | 20.5 | 20.4 | 20.2 | 33.5 |
| BMI (kg/m2) | 26.8 (5.5) | 27.6 (4.0) | 31.1 (6.5) | 28.1 (5.0) |
| Hypertension (%) | 25.5 | 29.0 | 53.1 | 48.9 |
| Diabetes (%) | 8.7 | 11.9 | 22.9 | 20.8 |
| Hypercholesterolemia (%) | 26.9 | 18.9 | 25.1 | 20.3 |
| Results of cognitive tests: | ||||
| Delayed word recall | 7.2 (1.2) | 6.6 (1.3) | 6.6 (1.4) | 6.1 (1.4) |
| Digit symbol substitution | 53.4 (10.0) | 47.9 (9.9) | 35.2 (12.5) | 30.5 (11.7) |
| Word fluency | 37.4 (10.7) | 35.8 (11.1) | 30.4 (11.8) | 29.6 (13.2) |
Values refer to means (standard deviation) unless otherwise stated
We identified 203 cases of hospitalization with dementia during 142,625 person-years of follow-up (average follow-up: 12.8 years). Mean age at first dementia hospitalization was 71.7 (standard deviation: 5.9). Figure 2 depicts the age, sex, and race-specific incidence rates of hospitalization with dementia. Rates increased exponentially with age in men and women, and in African-Americans and whites. Overall, African-Americans had higher rates than whites. Scores in the Delayed Word Recall and Digit Symbol Substitution tests (p-value<0.0001 for both tests), but not in the Word Fluency Test (p-value=0.74), were predictors of the risk of dementia (figure 3). The proportion of individuals with cardiovascular disease as a primary diagnosis in hospitalizations with dementia (19%) was lower than that observed in the entire ARIC sample (29%).
Figure 2.
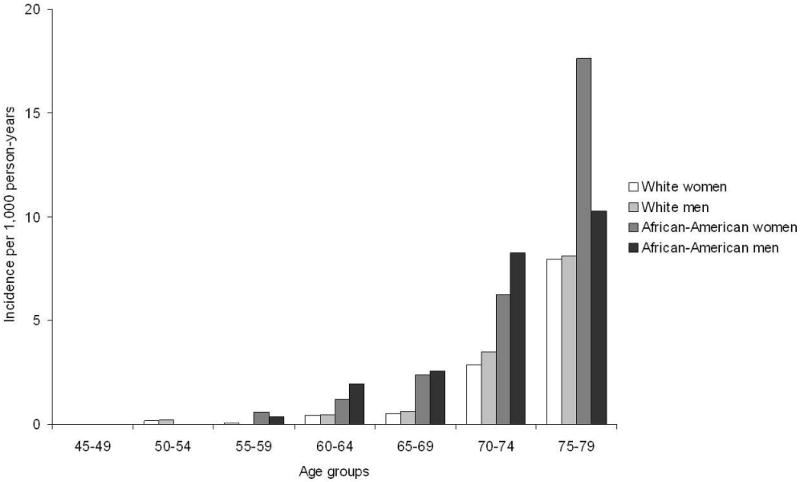
Incidence of hospitalization with dementia by age, sex and race. ARIC study, 1990-2004.
Age-adjusted hazard ratio (HR) (95% confidence interval) is 2.5 (1.9, 3.3) for African-Americans vs. whites and 1.0 (0.7, 1.3) for men vs. women.
Figure 3.
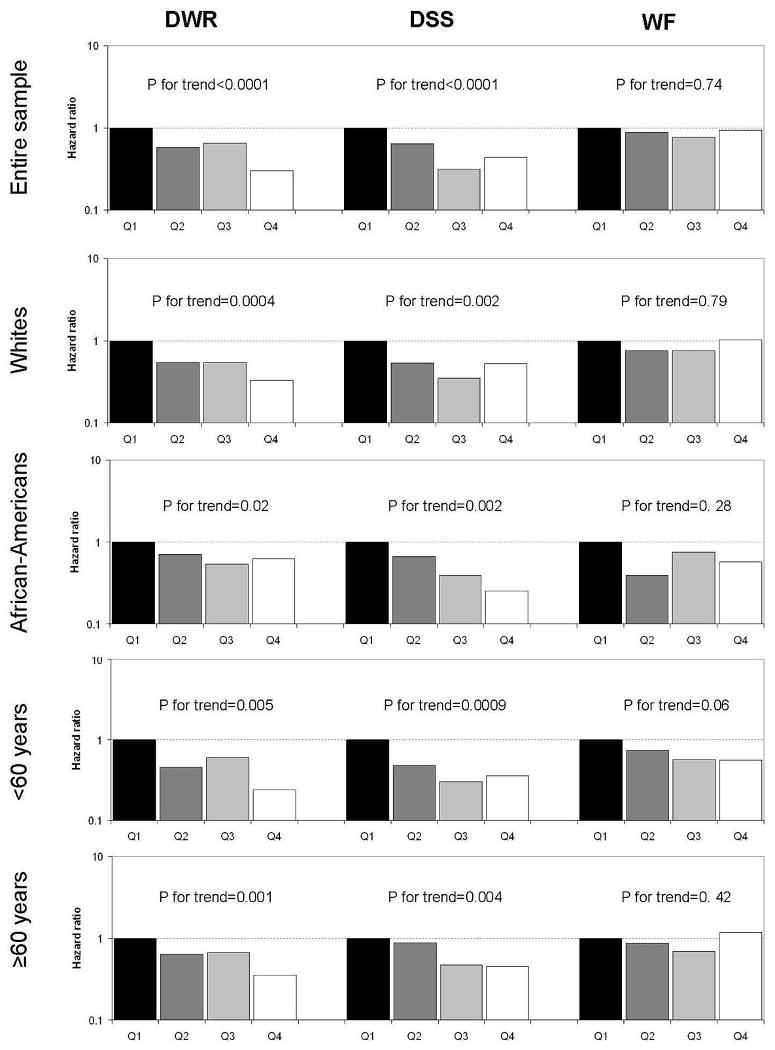
Hazard ratios of dementia hospitalization by quartiles of cognitive score at exam 2 for the entire sample, and for race and age groups separately. Analyses adjusted for age, sex, race and study center. DWR: Delayed Word Recall Test; DSS: Digit Symbol Substitution Test; WF: Word Fluency Test. P-value for trend estimated for cognitive score as a continuous variable.
Presence of the APOE ε4 allele was associated with an increased risk of dementia. Individuals with at least one ε4 allele had an 80% increase in the incidence of dementia compared to those without ε4 allele (HR 1.8, 95% CI 1.4, 2.4). This association followed a dose-response model. Compared to individuals with ε3/ε3 genotype, the HR (95% CI) of dementia hospitalization for ε3/ε4 and ε4/ε4 genotypes were 1.4 (0.9-2.1) and 4.6 (2.5-8.7) in whites and 1.8 (1.1-3.0) and 2.9 (1.2-6.9) in African-Americans (p-trend<0.001 and p-interaction=0.32).
Associations between the main cardiovascular risk factors and dementia hospitalization are shown in table 2. Smoking, hypertension, and diabetes were associated with a higher risk of the outcome, while there was no association between obesity/overweight or hypercholesterolemia, and dementia. Results were similar across APOE genotype categories, when baseline cognitive scores were not included in the analysis as covariates or when participants with cardiovascular disease at baseline were included (not shown).
Table 2.
Hazard ratios (HR) and 95% confidence intervals (CI) of hospitalization with dementia according to presence of selected cardiovascular risk factors at exam 2. ARIC study, 1990-2004.
| Cases | Person-years | HR (95% CI)1 | |||
|---|---|---|---|---|---|
| Entire sample | Whites | African-Americans | |||
| Hypercholesterolemia | |||||
| No | 147 | 109,385 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 56 | 33,240 | 1.0 (0.7, 1.4) | 0.9 (0.6, 1.3) | 1.2 (0.7, 2.0) |
| BMI | |||||
| <20 | 5 | 3,808 | 0.9 (0.4, 2.3) | 0.9 (0.3, 2.6) | 0.5 (0.1, 4.3) |
| 20-<25 | 52 | 41,955 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 25-<30 | 80 | 56,910 | 1.0 (0.7, 1.4) | 1.2 (0.8, 1.9) | 0.6 (0.3, 1.1) |
| 30+ | 66 | 39,952 | 1.0 (0.6, 1.4) | 1.0 (0.6, 1.8) | 0.7 (0.3, 1.1) |
| Cigarette smoking | |||||
| Never | 82 | 59,593 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Past | 66 | 53,626 | 1.0 (0.7, 1.3) | 0.9 (0.6, 1.4) | 0.9 (0.5, 1.6) |
| Current | 55 | 29,406 | 1.7 (1.2, 2.5) | 1.6 (1.0, 2.6) | 1.8 (1.0, 3.2) |
| Hypertension | |||||
| No | 96 | 96,892 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 107 | 45,732 | 1.6 (1.2, 2.2) | 1.6 (1.1, 2.3) | 1.7 (1.0, 2.8) |
| Diabetes | |||||
| No | 143 | 125,111 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 60 | 17,514 | 2.2 (1.6, 3.0) | 1.9 (1.2, 3.1) | 2.8 (1.7, 4.5) |
Cox proportional hazards model adjusted for age, gender, race, study center, educational level, occupation, cognitive test scores at exam 2, other variables in the table and APOE genotype
Associations between cardiovascular risk factors and dementia were similar in whites and African-Americans (table 2), and men and women (data not shown). Age, however, interacted significantly with the presence of cardiovascular risk factors: the associations were stronger among individuals younger than 60 at baseline (table 3). Figure 4 presents results from an analysis that combines data using exams 2, 3 and 4 as separate baseline measures to classify individuals in the appropriate age group at each visit and update their risk factor information. The relative hazard of dementia associated to the different cardiovascular risk factors, with the exception of obesity, decreased with increasing age. Finally, in an analysis restricted to non-demented survivors in exam 4, exam 2 measurements were slightly better predictors of dementia hospitalization than exam 4 measurements: the HR (95% CI) of dementia for exam 2 vs. exam 4 measurements were 1.7 (1.0-2.8) vs. 1.5 (0.9-2.6) (smoking), 1.5 (1.0-2.2) vs. 1.4 (0.9-2.1) (hypertension), and 2.3 (1.5-3.6) vs. 2.0 (1.3-3.1) (diabetes). Average age at visit 2 and visit 4 for these ARIC participants was 56.4 and 62.5 respectively.
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) of hospitalization with dementia according to presence of selected cardiovascular risk factors at exam 2, stratified by age at that examination. ARIC study, 1990-2004.
| Age <60 years | Age ≥60 years | ||||||
|---|---|---|---|---|---|---|---|
| Cases | Person-years | HR (95% CI)1 | Cases | Person-Years | HR (95% CI)1 | P for interaction2 | |
| Hypercholesterolemia | |||||||
| No | 43 | 76,569 | 1 (ref.) | 104 | 32,816 | 1 (ref.) | |
| Yes | 20 | 20,551 | 1.4 (0.8, 2.4) | 36 | 12,689 | 0.9 (0.6, 1.3) | 0.03 |
| BMI | |||||||
| <20 | 1 | 2,534 | 1.1 (0.1, 8.7) | 4 | 1,274 | 0.9 (0.3, 2.4) | |
| 20-<25 | 12 | 28,642 | 1 (ref.) | 40 | 13,313 | 1 (ref.) | |
| 25-<30 | 17 | 37,772 | 0.7 (0.3, 1.5) | 63 | 19,139 | 1.1 (0.7, 1.6) | |
| 30+ | 33 | 28,172 | 1.3 (0.6, 2.7) | 33 | 11,780 | 0.7 (0.4, 1.2) | 0.04 |
| Cigarette smoking | |||||||
| Never | 22 | 40,422 | 1 (ref.) | 60 | 19,171 | 1 (ref.) | |
| Past | 19 | 34,869 | 1.1 (0.6, 2.1) | 47 | 18,757 | 0.9 (0.6, 1.3) | |
| Current | 22 | 21,829 | 2.2 (1.2, 4.1) | 33 | 7,577 | 1.6 (1.0, 2.5) | 0.44 |
| Hypertension | |||||||
| No | 25 | 69,413 | 1 (ref.) | 71 | 27,479 | 1 (ref.) | |
| Yes | 38 | 27,706 | 2.0 (1.2, 3.5) | 69 | 18,026 | 1.4 (1.0, 2.0) | 0.002 |
| Diabetes | |||||||
| No | 39 | 86,282 | 1 (ref.) | 104 | 38,829 | 1 (ref.) | |
| Yes | 24 | 10,838 | 2.7 (1.5, 4.7) | 36 | 6,676 | 1.9 (1.3, 2.8) | 0.05 |
Cox proportional hazards model adjusted for age, gender, study center, educational level, occupation, cognitive test and the other variables in the table
Likelihood ratio test comparing models with and without an interaction term between age (continuous variable) and the corresponding risk factor
Figure 4.
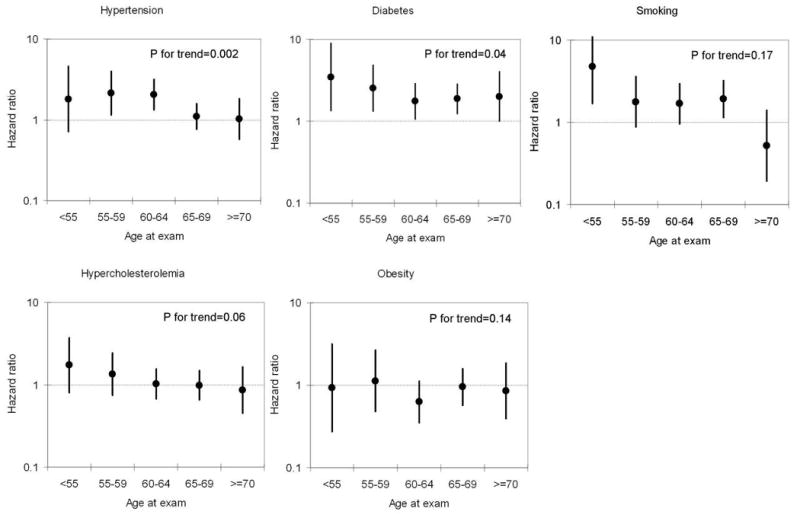
Hazard ratios and 95% confidence intervals (CI) of dementia associated with the presence of each cardiovascular risk factor, by age at study exam. Cox proportional hazards model adjusted for age, gender, race, study center, educational level, occupation, cognitive test scores at exam 2 and cardiovascular risk factors. P for trend corresponds to an interaction term between age as a continuous variable and the corresponding risk factor. Smoking: current vs. never smokers. Obesity: >=30 kg/m2 vs. 20-25 kg/m2
Discussion
In this population-based prospective cohort study, we found that hypertension, diabetes, and smoking measured in midlife were strongly associated with an increased risk of being hospitalized with dementia later in life. The associations were similar in whites and African-Americans, and persisted after adjustment for dementia risk factors including APOE genotype and cognitive function at baseline. Associations were stronger when risk factors were measured at a younger age. Results from previous observational studies conducted in Caucasian and Asian populations have shown that cardiovascular risk factors, including hypertension, diabetes, obesity/overweight and smoking, are associated with a higher risk of developing dementia.[8-12, 25-28] The present study extends the results to African-Americans.
Cardiovascular risk factors can lead to vascular disease in the brain by causing multi-infarcts, manifested as classic vascular dementia, as well as small vessel disease (lacunes and smaller infarcts, white matter lesions, and arteriolar changes).[29, 30] The burden of cerebral small vessel disease, in turn, is a major contributor to cognitive decline and dementia.[31, 32] Diabetes, hypertension, and smoking were the factors more strongly associated with the risk of dementia hospitalization in this sample, probably as a consequence of their potential role on microvascular disease and occurrence of white matter lesions.[30, 33]
In the present study, cardiovascular risk factors measured in midlife were stronger predictors of dementia hospitalization than factors measured in older age. This observation, which confirms our a priori hypothesis, has several possible explanations. First, cardiovascular risk factors could have a cumulative effect, with increased risk of dementia if an individual has suffered hypertension or diabetes, or has smoked for a longer period of time. Second, midlife measures could better predict future risk of dementia if individuals susceptible to develop dementia caused by vascular risk factors did so before reaching older age. As a result, older samples would be somewhat depleted of individuals vulnerable to cardiovascular risk factors.[14] Third, a diagnosis of dementia could be more easily overlooked in older individuals, with a greater number of co-morbidities, than in younger individuals. Finally, cardiovascular risk factors may lead to secondary changes including illness (e.g. heart failure, weight loss) and behavioral modification (e.g. smoking cessation before or after the onset of a vascular event). Such changes and better control of risk factors in older than younger individuals would diminish associations of cardiovascular risk factors measured at older age with subsequent risk. Although control rates of diabetes are higher among individuals older than 60 in the US,[34] the opposite pattern has been observed for hypertension.[35] Additionally, our knowledge of the effect of medications for hypertension and diabetes in older individuals is sparse. Independently of the underlying mechanism, our results suggest that, for prevention of dementia, control of cardiovascular risk factors starting in midlife is likely to be more important in the prevention of dementia than control starting later on. Our results may also help explain the lack of cognitive benefit found in most [36-39] but not all [40] antihypertensive clinical trials whose follow-up was only 3-5 years.
The study has a number of limitations including the endpoint ascertainment. Cases were individuals with dementia who were hospitalized and dementia was included among their discharge diagnoses. Dementia hospitalizations likely underestimate disease. In fact, the observed age-specific rates are lower than those reported in the Cardiovascular Health Study or the Northern Manhattan study.[2, 41] This could be problematic if the probability of hospitalization with dementia depended on the presence of cardiovascular risk factors. Smokers, hypertensives or diabetics have a higher risk of hospitalization due to cardiovascular, renal or lung diseases, creating a positive association between these risk factors and dementia hospitalization. Conversely, the presence of multiple diagnoses in smokers, hypertensives or diabetics may decrease the probability of the dementia diagnosis being recorded among the discharge codes and, thereby, creating a downward bias in the association. Several reasons, however, support the validity of dementia hospitalization as an adequate proxy for dementia incidence. First, different studies have found a high positive predictive value for dementia ICD discharge code.[42, 43] Second, the strong association of poor cognitive scores with dementia hospitalization, a factor unlikely to lead by itself to hospitalization, suggests that our study is identifying true cases of dementia. Finally, an overall higher risk of hospitalization in individuals with cardiovascular risk factors could not easily explain the interaction between these factors and age.
Our study has several strengths. Cardiovascular risk factors were assessed before the age of higher risk of dementia, reducing the risk of reverse causation. Also, measurements of cognitive function allowed the exclusion at baseline of individuals with possible early dementia. Other covariates that could act as confounders were determined in the different study visits, including sociodemographic variables, lifestyles and APOE genotype. Finally, the ARIC cohort comprises a large, representative, and racially diverse sample, facilitating the generalizability of the results.
In conclusion, we have observed that hypertension, diabetes and smoking in midlife are associated with a higher risk of dementia hospitalization. We present one of the only studies demonstrating this association in African-Americans, showing that they have a higher risk of dementia hospitalization as well as higher level of cardiovascular risk factors. Our results directly demonstrate that cardiovascular risk factors in midlife play an important role in the development of dementia at older age despite a weaker association when the risk factors are measured at older age. These results suggest that smoking cessation and prevention or control of hypertension and diabetes starting in midlife may have the added benefit of decreasing dementia hospitalization risk. Physiologic pathways and intermediate cerebral changes detectable by non-invasive imaging involved in the vascular pathophysiology of dementia should be studied, since clinical trials might require a decade or more of treatment to fully realize a benefit on dementia prevention.
Acknowledgments
The ARIC study is carried out as a collaborative study supported by the National Heart, Lung and Blood Institute [contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022]. The funding institution had no input into the data collection, conduct, or management of the study. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
None of the authors have any potential conflict of interest. Alvaro Alonso had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its licencees, to permit this article (if accepted) to be published in JNNP and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence (http://jnnp.bmjjournals.com//ifora/licence.pdf).
References
- 1.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29:125–32. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 3.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 4.Borenstein Graves A. Alzheimer's disease and vascular dementia. In: Nelson LM, Tanner CM, Van Den Eeden SK, McGuire V, editors. Neuroepidemiology: from principles to practice. New York: Oxford University Press; 2004. pp. 102–30. [Google Scholar]
- 5.Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 6.Launer LJ. Demonstrating the case that Alzheimer's disease is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 7.Bailey TL, Rivara CB, Rocher AB, et al. The nature and effects of cortical microvascular pathology in aging and Alzheimer's disease. Neurol Res. 2004;26:573–8. doi: 10.1179/016164104225016272. [DOI] [PubMed] [Google Scholar]
- 8.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 9.Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County Study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 10.Luchsinger JA, Tang MX, Stern Y, et al. Diabetes mellitus and Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–41. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 11.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 12.Anstey KJ, von Sanden C, Salim A, et al. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–78. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 13.McGuinness B, Todd S, Passmore P, et al. The effects of blood pressure lowering on development of cognitive impairment and dementia in patients without apparent prior cerebrovascular disease. Cochrane Database Syst Rev. 2006;19:CD004034. doi: 10.1002/14651858.CD004034.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Hernán MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19:448–50. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- 15.Staessen JA, Richart T, Birkenhager WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49:389–400. doi: 10.1161/01.HYP.0000258151.00728.d8. [DOI] [PubMed] [Google Scholar]
- 16.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men : the Honolulu-Asia Aging Study. Arterioscler Thromb Vasc Biol. 2000;20:2255–60. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 17.Demirovic J, Prineas R, Loewenstein D, et al. Prevalence of dementia in three ethnic groups: the South Florida Program on Aging and Health. Ann Epidemiol. 2003;13:472–8. doi: 10.1016/s1047-2797(02)00437-4. [DOI] [PubMed] [Google Scholar]
- 18.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 19.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 20.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–5. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. The Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corp; 1981. [Google Scholar]
- 22.Lezak MD. Neuropsychological assessment. 3rd. New York: Oxford University Press; 1995. [Google Scholar]
- 23.Volcik KA, Barkley RA, Hutchinson RG, et al. Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC Study participants. Am J Epidemiol. 2006;164:342–8. doi: 10.1093/aje/kwj202. [DOI] [PubMed] [Google Scholar]
- 24.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. Journal of the American Statistical Association. 1989;84:1074–8. [Google Scholar]
- 25.Rosengren A, Skoog I, Gustafson D, et al. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–6. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 26.Akomolafe A, Beiser A, Meigs JB, et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol. 2006;63:1551–5. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- 27.Whitmer RA, Sidney S, Selby J, et al. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 28.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes - systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–9. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 29.Longstreth WT, Jr, Bernick C, Manolio TA, et al. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–25. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 30.Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC study. Neuroepidemiology. 1997;16:149–62. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 31.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 32.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–4. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 33.Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315–22. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ong KL, Cheung BMY, Wong LYF, et al. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999-2004. Ann Epidemiol. 2008;18:222–9. doi: 10.1016/j.annepidem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Ong KL, Cheung BMY, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 36.SHEP Cooperative Research Group. Prevention of stroke by hypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 37.Prince MJ, Bird AS, Blizard RA, et al. Is the cognitive function of older patients affected by antihypertensive treatment? Results from 54 months of the Medical Research Council's treatment trial of hypertension in older adults. BMJ. 1996;312:801–5. doi: 10.1136/bmj.312.7034.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–75. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 39.Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–86. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment New evidence from the Systolic Hypertension in Europe (Syst-Eur) Study. Arch Intern Med. 2002;162:2046–52. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- 41.Tang MX, Cross P, Andrews H, et al. Incidence of Alzheimer's disease in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 42.Jin YP, Gatz M, Johansson B, et al. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology. 2004;63:739–41. doi: 10.1212/01.wnl.0000134604.48018.97. [DOI] [PubMed] [Google Scholar]
- 43.Phung TKT, Andersen BB, Hogh P, et al. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2007;24:220–8. doi: 10.1159/000107084. [DOI] [PubMed] [Google Scholar]


