Abstract
Converging evidence from basic and clinical studies suggests a role for proinflammatory cytokines in cancer-related fatigue, although the etiology of elevated inflammatory processes is unclear. We examined single nucleotide polymorphisms (SNPs) in the promoters of cytokine genes as genetic risk factors for cytokine-related fatigue in 33 fatigued and 14 non-fatigued breast cancer survivors, focusing on promoter sequence polymorphisms in IL1B and IL6 associated with differential expression of proinflammatory cytokines. Predictors of fatigue included presence of at least one cytosine at IL1B −511 (95%CI = 0.91–16.6, p = .007) and homozyosity for either variant of the IL6 −174 genotype (G/G or C/C; 95%CI = 1.12–17.9, p = .027). Associations between fatigue status and IL1B genotype remained significant after covariate adjustment for demographic, biobehavioral and treatment-related factors. These findings provide preliminary evidence that polymorphisms in IL1B may serve as a potential risk factor for persistent fatigue in the aftermath of cancer.
Keywords: Fatigue, Cancer, Proinflammatory cytokines, Cytokine gene polymorphisms
1. Introduction
Persistent, medically unexplained fatigue is one of the most common and disabling sequelae of breast cancer treatment (Bower et al., 2000, 2006). Experimental studies documenting effects of proinflammatory cytokines on central nervous system function and “sickness behavior” (Dantzer et al., 2008; Kent et al., 1992) have led our group and others to examine proinflammatory cytokine activity as a potential mechanism for cancer-related fatigue. We have shown elevations in plasma and cellular markers of inflammation in breast cancer survivors with persistent post-treatment fatigue, including elevations in circulating levels of IL-1 receptor antagonist (IL-1ra) and soluble IL-6 receptor (sIL-6r) as well as increases in ex vivo monocyte production of IL-6 following LPS stimulation (Bower et al., 2002, 2003, 2007; Collado-Hidalgo et al., 2006). However, the etiology of chronic inflammatory processes and associated symptoms of fatigue has not been determined.
The present study was designed to test the hypothesis that variations in cytokine-related fatigue are influenced by genetic polymorphisms in the regulatory regions (promoters) of genes that encode proinflammatory cytokines. In particular, we focus on genes related to the proinflammatory cytokines IL-1β and IL-6, given evidence of alterations in these systems among fatigued breast cancer survivors (Bower et al., 2002, 2003, 2007; Collado-Hidalgo et al., 2006) and other cancer populations with fatigue (Schubert et al., 2007). Single nucleotide polymorphisms (SNPs) that influence quantitative gene expression levels have been identified in the promoters of IL1B (−511 bases upstream of the transcription start site) (Giovine et al., 1992) and IL6 (−174) (Fishman et al., 1998; Olomolaiye et al., 1998). Recent evidence suggests a relationship between promoter polymorphisms in several proinflammatory cytokine genes and breast cancer susceptibility and prognosis (Hefler et al., 2005; Smith et al., 2004), as well as treatment-related changes in breast appearance (Andreassen et al., 2005). However, links with behavioral symptoms have not yet been examined. Based on the a priori hypothesis that polymorphisms affecting proinflammatory cytokine levels should influence the risk of fatigue, we tested promoter polymorphisms in IL1B and IL6 as candidate genes for fatigue in breast cancer survivors.
2. Methods
2.1. Participants
Breast cancer survivors were recruited from the Los Angeles area through tumor registry listings, newspaper advertisements, flyers, and other media coverage. Eligibility criteria included: (1) originally diagnosed with early-stage breast cancer (Stage 0, I, or II) between 1 and 5 years previously; (2) completed all cancer treatment with the exception of tamoxifen/aromatase inhibitors; (3) no evidence of cancer recurrence; and (4) no chronic medical conditions involving the immune system or regular use of immunosuppressive medications. From the 314 women screened for eligibility, we identified 33 survivors who experienced significant fatigue. Fatigue status was determined using the vitality subscale of the SF-36, where scores below 50 represent limitations or disability related to fatigue (Ware et al., 1992). Women were classified as fatigued if their vitality score was ≤55, although the majority (n = 31) scored at or below 50. We have previously shown that women scoring at or below 50 on the SF-36 vitality subscale show significant alterations in behavioral, immune, and neuroendocrine parameters, supporting the validity of this classification system (Bower et al., 2000, 2002, 2003, 2005a,b, 2007). A control group of 14 non-fatigued survivors was also identified who scored at or above 70 on the SF-36 vitality subscale.
A subset of study participants (n = 26 fatigued, 14 non-fatigued) was examined in a previous study that focused on circulating and cellular markers of proinflammatory cytokine activity (Collado-Hidalgo et al., 2006). In the previous study, a slightly more stringent criterion was used for determining fatigue status (i.e., score ≤50 on the SF-36 vitality scale over two to three assessments).
2.2. Procedure
Participants completed self-report questionnaires and provided blood samples for evaluation of genomic DNA. Height and weight were measured for determination of body mass index (BMI). The UCLA Institutional Review Board approved study procedures, and written consent was obtained from all participants.
2.3. Measures
Self-report questionnaires were used to assess demographic and medical characteristics, including age, ethnicity, menopausal status, and cancer treatments. Fatigue symptoms were assessed with the Multidimensional Fatigue Symptom Inventory (MFSI; Stein et al., 1998, 2004), a validated measure of cancer-related fatigue, and depressive symptoms were assessed with the Beck Depression Inventory II (BDI-II) (Beck et al., 1996).
Genomic DNA was extracted from peripheral blood leukocytes and amplified by PCR. PCRs were performed on a Bio-Rad iCycler using established primers for IL6 −174 (G/C) (DeMichele et al., 2003), and a newly developed primer pair designed using Primer 3 software for IL1B −511 (C/T) (see Table 1). PCR products spanning IL6 −174 (G/C) and IL1B −511 (C/T) were assayed by restriction fragment length polymorphism using the enzymes NlaIII or AvaI, respectively.
Table 1.
Primer sequences used for genotyping IL1B −511 (C/T) and IL6 −174 (G/C)
| Primer name | Sequence 5′–3′ |
|---|---|
| IL-1B-511 Forward | GTCTTGCAGGGTTGTGTGAG |
| IL-1B-511 Reverse | GCCAATAGCCCTCCCTGT |
| IL-6-174 Forward | ATGCCAAGTGCTGAGTCACTA |
| IL-6-174 Reverse | TCGAGGGCAGAATGAGCCTC |
2.4. Statistical analyses
Relationships between fatigue status (fatigued/non-fatigued) and genotypes were analyzed using SAS v9.1 (SAS Institute, Cary, NC). Simple associations were assessed by chi-square analysis (SAS PROC FREQ), and multivariate logistic regression analyses (SAS PROC LOGISTIC) analyzed relationships while controlling for demographic, medical, or biobehavioral confounds. Strength of relationships are expressed as odds ratios (OR) with 95% confidence intervals (95% CI).
3. Results
Fatigue status in this sample was confirmed with the Multidimensional Fatigue Symptom Inventory, with the fatigued group scoring significantly higher on the general fatigue scale (mean = 12.5, SD = 5.1) than the non-fatigued group (mean = 3.9, SD = 3.6; t(45) = −5.7, p < .0001). Demographic, medical, and treatment-related characteristics of fatigued and non-fatigued survivors are shown in Table 2. There were significant group differences in depressive symptoms (t(45) = −2.8, p = .007) and age (t(45) = 2.6, p = .01). None of the other group differences were significant (all ps > .10).
Table 2.
Demographic and treatment-related characteristics of study participants
| Characteristic | Fatigued (n = 33) | Non-fatigued (n = 14) |
|---|---|---|
| Age (mean ± SD)* | 54.1 ± 8.3 | 61.1 ± 8.5 |
| Ethnicity | ||
| White | 23 | 10 |
| Hispanic | 3 | 1 |
| African-American | 2 | 2 |
| Asian | 4 | 0 |
| Other | 1 | 1 |
| Body mass index (mean ± SD) | 28.2 ± 6.2 | 26.3 ± 6.4 |
| Treated with chemotherapy | 20 | 5 |
| Tamoxifen or aromatase inhibitor use | 19 | 9 |
| Years since diagnosis (mean ± SD) | 2.8 ± 1.2 | 2.2 ± .71 |
| BDI-II score (mean ± SD)* | 11 ± 7.6 | 4.9 ± 4.2 |
p < .05.
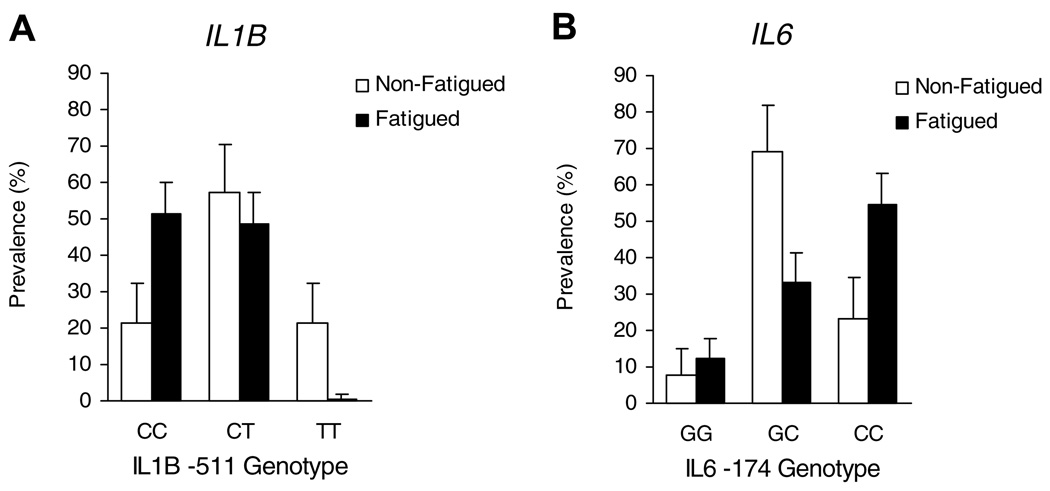
Analysis of the IL1B −511 (C/T) polymorphism revealed a substantial over-representation of CC alleles among fatigued survivors and a substantial under-representation of TT alleles (Fig. 1A). Increased prevalence of C vs. T nucleotides at IL1B −511 yielded a linear dose–response relationship (p = .008), and the prevalence of at least one cytosine at this position was substantially greater among fatigued participants (100%) than among non-fatigued (78.6%; 95% CI = 0.91–16.6, p = .007).
Fig. 1.
Prevalence of polymorphisms in the interleukin-1 beta gene (IL1B) and the interleukin-6 gene (IL6) in fatigued and non-fatigued breast cancer survivors. Significant predictors of fatigue status included presence of at least one cytosine at IL1B −511 (95%CI = 0.91–16.6, p = .007) and homozyosity for either variant of the IL6 −174 genotype (95%CI = 1.12–17.9, p = .027) by nonparametric chi-square analysis.
Fatigued breast cancer survivors showed an elevated occurrence of homozygosity for both the variant C allele and the wildtype G allele of the IL6 −174 (G/C) polymorphism, with approximately twofold greater representation of both GG and CC alleles in the fatigued group compared to non-fatigued controls (Fig. 1B). Only 33.3% of fatigued survivors were heterozygous for IL6 −174, compared to 69.2% of non-fatigued controls (95% CI = 1.12–17.9; p = .027).
To determine whether IL1β −511 and IL6 −174 rSNPs each constitute independent predictors for post-cancer fatigue, we carried out multivariate logistic regression analyses predicting fatigue status from both polymorphisms in parallel. Results showed that each polymorphism was independently associated with fatigue status, with fatigue continuing to show significant association with homozygosity at IL6 −174 (p = .024) and the prevalence of cytosine at IL1B −511 (p = .021). Consistent results were obtained in analyses restricting fatigue to individuals who scored at or below 50 on the SF-36 vitality scale.
To determine whether associations between genotype and fatigue status were independent of other demographic and clinical confounds (i.e., age, ethnicity, menopausal status, body mass index, depressive symptoms, and cancer treatments), we first identified confounds that were significantly or nearly significantly associated with one or more of the measured polymorphisms. Analyses controlling for these variables (i.e., age, depressive symptoms, mastectomy, breast reconstruction, and breast irradiation) continued to show significant relationships between fatigue and the IL1B −511 polymorphism, with the exception that controlling for depressive symptoms resulted in a near-significant association between fatigue and cytosine frequency at IL1B −511 (p = .052). However, relationships between IL6 −174 homozygosity and fatigue were rendered non-significant by control for age, and by control for several treatment-related characteristics (all p > .05). Analyses controlling for ethnicity (white vs. non-white) continued to show significant associations between each genotype and fatigue status.
As previously reported, fatigued women in the parent study from which these women were drawn had elevated plasma levels of IL-1ra and soluble IL-6R relative to non-fatigued survivors, as well as elevations in monocyte intracellular production of IL-6 following ex vivo LPS stimulation (Collado-Hidalgo et al., 2006). Consistent with results from the previous study, fatigued women in this sample showed significantly higher levels of sIL-6R (p = .028) and marginally significantly higher levels of IL-1ra (p = .074). There were no significant differences in plasma levels of IL-6, again consistent with results from the parent study.
Thus, exploratory analyses were conducted to evaluate whether IL1B −511 and IL6 −174 rSNPs were associated with alterations in these parameters using Spearman rank correlation coefficients. We found significant correlations between circulating concentrations of IL-1ra and the frequency of G alleles at IL6 −174 (r = .35, p = .019), and between circulating concentrations of sIL-6R and the frequency of C alleles at IL1B −511 (r = .33, p = .027). There was also a trend towards an association between frequency of G alleles at IL6 −174 and increased ex vivo monocyte production of IL-6 (r = .26, p = .078). However, we found no association between fatigue genotypes and circulating levels of IL-6.
4. Discussion
This study is the first to identify a relationship between cytokine gene regulatory polymorphisms and post-treatment fatigue in breast cancer survivors. In particular, we found that fatigued survivors showed an increased prevalence of cytosine at IL1B −511 controlling for demographic and clinical confounds; results for IL6 −174 were less reliable and appeared to be confounded with age and clinical variables in this sample. These results build on our previous findings of elevated inflammatory activity among fatigued breast cancer survivors, including increases in circulating inflammatory markers and functional alterations in proinflammatory cytokine response to lipopolysaccharide (Bower et al., 2002, 2007; Collado-Hidalgo et al., 2006). It can be hypothesized that presence of these genetic risk factors, particularly polymorphisms in IL1B, leads to enhanced production of proinflammatory cytokines triggered by the tumor and/or by cancer treatment, with subsequent effects on fatigue via cytokine regulation of central nervous system function (Dantzer et al., 2008).
Previous studies have shown that each of the genotypes assessed in this study is associated with increased expression of its respective cytokine (e.g., Brull et al., 2001; Burzotta et al., 2001; Giovine et al., 1992). In this cohort, we examined the association between these genotypes and circulating levels of IL-1ra and sIL-6R, given our previous findings of elevations in these markers among fatigued breast cancer survivors (Collado-Hidalgo et al., 2006). The IL1B −511 and IL6 −174 genotypes were positively correlated with circulating levels of these inflammatory markers, although not with circulating levels of IL-6. Of note, we also find no association between fatigue status and plasma IL-6 in this sample (Collado-Hidalgo et al., 2006). The IL6 −174 genotype was also associated with a marginally elevated cytokine production response to LPS. These findings require confirmation in a larger sample, but lend preliminary support for the hypothesis that SNPs in proinflammatory cytokine genes may influence cancer-related fatigue via activation of the proinflammatory cytokine network. Why this activation persists into the post-treatment period in these otherwise healthy survivors is unclear, but may be due to alterations in cellular components of the immune system (Bower et al., 2003) or in immune regulatory systems, such as the HPA axis (Bower et al., 2005a,b, 2007).
The primary limitation of this study was the small sample size, which renders the findings preliminary. In addition, because our study did not include a control group of women with no cancer history, we cannot determine whether the association between these cytokine polymorphisms and fatigue was specific to breast cancer diagnosis and treatment. Given that proinflammatory cytokines can trigger behavioral changes in healthy individuals (Reichenberg et al., 2001), it is possible that polymorphisms associated with increased cytokine expression may also confer risk for fatigue in non-cancer populations. Future research should evaluate the association between polymorphisms in cytokine-related genes and fatigue in women with and without a cancer history to determine whether this association is specific to cancer.
A growing number of studies have examined cytokine promoter SNPs in breast cancer susceptibility and prognosis, with mixed results (Balasubramanian et al., 2006; Hefler et al., 2005). The role of cytokine gene polymorphisms in cancer-related behavioral disturbances has received minimal attention, although there is preliminary evidence that promoter polymorphisms of IL-1β and IFN receptor may increase risk for depression in non-cancer populations (McCulley et al., 2004; Yoshida et al., 2005). Our results, though preliminary, suggest a novel role for cytokine SNPs in cancer-related fatigue and provide further support for an inflammatory etiology for persistent fatigue in breast cancer survivors. These findings may have clinical relevance by shedding light on risk factors for post-treatment fatigue, and by suggesting avenues for targeted treatment (e.g., anti-cytokine therapies).
Acknowledgments
This research was supported by the Cousins Center for Psycho-neuroimmunology in the UCLA Semel Institute for Neuroscience and Human Behavior, the Breast Cancer Research Foundation, and the Jonsson Comprehensive Cancer Center at UCLA. Dr. Collado-Hidalgo was supported by a Post-Graduate Training program in Psychoneuroimmunology Grant (T32-MH-19925) from the National Institute of Mental Health. Dr. Bower was supported in part by a career development award from the National Cancer Institute (K07 CA90407). Dr. Ganz was supported in part by an American Cancer Society Clinical Research Professorship.
References
- Andreassen CN, Alsner J, Overgaard J, Herskind C, Haviland J, Owen R, Homewood J, Bliss J, Yarnold J. TGFB1 polymorphisms are associated with risk of late normal tissue complications in the breast after radiotherapy for early breast cancer. Radiother. Oncol. 2005;75:18–21. doi: 10.1016/j.radonc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Balasubramanian SP, Azmy IA, Higham SE, Wilson AG, Cross SS, Cox A, Brown NJ, Reed MW. Interleukin gene polymorphisms and breast cancer: a case control study and systematic literature review. BMC Cancer. 2006;6:188. doi: 10.1186/1471-2407-6-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. BDI-II Manual. second ed. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom. Med. 2005a;67:277–280. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom. Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL, Cole SW. T-cell homeostasis in breast cancer survivors with persistent fatigue. J. Natl. Cancer Inst. 2003;95:1165–1168. doi: 10.1093/jnci/djg0019. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav. Immun. 2007;21:251–258. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J. Clin. Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005b;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Brull DJ, Montgomery HE, Sanders J, Dhamrait S, Luong L, Rumley A, Lowe GD, Humphries SE. Interleukin-6 gene −174g>c and −572g>c promoter polymorphisms are strong predictors of plasma interleukin-6 levels after coronary artery bypass surgery. Arterioscler. Thromb. Vasc. Biol. 2001;21:1458–1463. doi: 10.1161/hq0901.094280. [DOI] [PubMed] [Google Scholar]
- Burzotta F, Iacoviello L, Di Castelnuovo A, Glieca F, Luciani N, Zamparelli R, Schiavello R, Donati MB, Maseri A, Possati G, Andreotti F. Relation of the −174 G/C polymorphism of interleukin-6 to interleukin-6 plasma levels and to length of hospitalization after surgical coronary revascularization. Am. J. Cardiol. 2001;88:1125–1128. doi: 10.1016/s0002-9149(01)02046-x. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GC, Johnson RW, Kelley K. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMichele A, Martin AM, Mick R, Gor P, Wray L, Klein-Cabral M, Athanasiadis G, Colligan T, Stadtmauer E, Weber B. Interleukin-6 −174G->C polymorphism is associated with improved outcome in high-risk breast cancer. Cancer Res. 2003;63:8051–8056. [PubMed] [Google Scholar]
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J. Clin. Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovine FS, Takhsh E, Blakemore AIF, Duff GW. Single base polymorphism at −511 in the human interleukin-1{beta} gene (IL1{beta}) Hum. Mol. Genet. 1992;1:450. doi: 10.1093/hmg/1.6.450. [DOI] [PubMed] [Google Scholar]
- Hefler LA, Grimm C, Lantzsch T, Lampe D, Leodolter S, Koelbl H, Heinze G, Reinthaller A, Tong-Cacsire D, Tempfer C, Zeillinger R. Interleukin-1 and interleukin-6 gene polymorphisms and the risk of breast cancer in caucasian women. Clin. Cancer. Res. 2005;11:5718–5721. doi: 10.1158/1078-0432.CCR-05-0001. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol. Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- McCulley MC, Day IN, Holmes C. Association between interleukin 1-beta promoter (−511) polymorphism and depressive symptoms in Alzheimer’s disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;124:50–53. doi: 10.1002/ajmg.b.20086. [DOI] [PubMed] [Google Scholar]
- Olomolaiye O, Wood NA, Bidwell JL. A novel NlaIII polymorphism in the human IL-6 promoter. Eur. J. Immunogenet. 1998;25:267. [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav. Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Smith KC, Bateman AC, Fussell HM, Howell WM. Cytokine gene polymorphisms and breast cancer susceptibility and prognosis. Eur. J. Immunogenet. 2004;31:167–173. doi: 10.1111/j.1365-2370.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J. Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Yoshida K, Alagbe O, Wang X, Woolwine B, Thornbury M, Raison CL, Miller AH. Promoter polymorphisms of the interferon-alpha receptor gene and development of interferon-induced depressive symptoms in patients with chronic hepatitis C: preliminary findings. Neuropsychobiology. 2005;52:55–61. doi: 10.1159/000086605. [DOI] [PubMed] [Google Scholar]