Abstract
Purpose
Microbial contamination of contact lenses is associated with corneal infection and inflammation. This study determined which microbiological, clinical and demographic factors are associated with bacterial contamination of a silicone hydrogel contact lens when worn for continuous wear.
Methods
Two hundred five normal subjects were enrolled in the Longitudinal Analysis of Silicone Hydrogel (LASH) Contact Lens Study and were fitted with lotrafilcon A lenses for monthly continuous wear and followed for 1 year. Lenses were aseptically removed after 1 week and 4 months of wear and cultured using an agar sandwich technique. Lids and conjunctiva were routinely cultured at baseline, and after 1 week and 4 months of continuous wear. Lenses and ocular sites were considered to have substantial microbial bioburden when they harbored pathogenic organisms or high levels of commensal organisms. Univariate and multivariate logistic regression analyses were conducted to examine whether substantial conjunctival or lid bioburden, subject demographics, lens wearing history, symptoms, and biomicroscopic signs were associated with lens bioburden.
Results
About one-third (32.4%) of subjects had substantial bacterial bioburden in either eye across multiple visits. Over half (53.2%) and about one-tenth (11.7%) of subjects had substantial lid and conjunctival bioburden, respectively, and 11.2% discontinued due to discomfort. The adjusted odds ratios (and 95% confidence intervals) for presence of substantial lens bioburden were 2.49 (1.17–5.30), 4.24 (1.45–12.40), and 4.11 (1.17–14.46) for substantial lid bioburden, substantial conjunctival bioburden, and lens discomfort, respectively.
Conclusions
Bacterial contamination of silicone hydrogel contact lenses is common during continuous wear. Substantial lens bioburden is associated with discomfort precluding successful continuous wear. The presence of substantial lid and conjunctival bioburden are associated with a 2.5 fold and over 4 fold greater risk of substantial lens bioburden and are likely the major routes of contamination.
Keywords: contact lenses, bacterial bioburden, continuous wear
Bacterial contamination of soft contact lenses is associated with microbial keratitis and corneal inflammatory events (CIEs).1–9 The association of significant contact lens bacterial bioburden with some CIEs has been reported in clinical studies 2–5, 8 and causation of CIEs by bacteria isolated from contact lenses worn during a CIE has been shown in animal models.10, 11
Many studies have attempted to define the sources of bacteria that are isolated from worn contact lenses during adverse events or asymptomatic wear. 12–18 To date, microorganisms that colonize lid margins13, 17, hands14, 16, lens cases12, 18, and the domestic water supply17 have been implicated as such sources. Furthermore, other studies have looked for associations between lens contamination and physical lens properties or patient factors.19–24 Only length of time since a lens was first used20, occasional wear23, avoidance of rinsing lenses prior to insertion23, and protein deposition19, 24 have been implicated, although in later studies no association was found between lens deposits and lens bacterial bioburden.20, 22
In the absence of contact lens wear, the cornea is considered to be sterile.25 However, the conjunctiva and lids harbor resident microorganisms that can populate the lens surfaces during uncomplicated wear. Normal ocular biota include coagulase negative staphylococci, Corynebacterium species, Micrococcus species, Bacillus species, and Propionibacterium species.17, 26–32 The frequency of isolation of conjunctival microorganisms during soft lens wear varies between 15–90% in the literature.28–30, 32, 33 However, most studies report the conjunctiva is sparsely populated with organisms at a frequency of about 30%.29, 32, 33 The lid margin is more often colonized and found to harbor organisms up to 70% of the time.32 Previous studies report conflicting results on the association between lens microbial contamination and conjunctival microbiota. Increased microbial loads on the conjunctiva have been reported during hydrogel lens wear,26, 30, 31 whereas others have found decreased29, 33, 34 or unchanged27, 28, 35 conjunctival microbiota with lens wear. Since conjunctival tissues and lid margins come into direct contact with soft lenses in vivo it is important to explore these sites as potential sources of lens contamination during asymptomatic or symptomatic wear. Indeed, Willcox17 and Hart13 have shown that during asymptomatic wear of low Dk soft lenses, the likely route for normal ocular microbiota to colonize lenses is via the lid margins. Similar studies have not been repeated in silicone hydrogel wearers.
The Longitudinal Analysis of Silicone Hydrogel (LASH) Contact Lens Study is a prospective cohort study of 205 lotrafilcon A extended contact lens wearers followed for one year for the development of a CIE. One of the key exploratory variables is bacterial contamination of study lenses. The aims of this report are to characterize the bacterial colonization of study lenses, lids, and conjunctiva; determine if associations exist between substantial lens and ocular surface bioburden; and to determine if other demographic and clinical factors are associated with substantial lens bioburden in the LASH Study. In short, the goal of this report is to characterize sources and risk factors for substantial lens bacterial bioburden during extended wear. The LASH Contact Lens Study affords a multitude of opportunities for examining the relationship between lens bioburden and other factors. It is the largest study of silicone hydrogel lens wearers with prospective assessment of substantial lens and ocular surface bioburden reported in the literature. Other studies have been able to assess associations between the colonization of lenses and eyelids, but the infrequency of conjunctival colonization has limited the ability to assess relationships between lens and conjunctival contamination. The large sample size of the LASH Contact Lens Study provides an opportunity to explore the associations between substantial lens microbial contamination and conjunctival bioburden, lid bioburden, demographic factors, and clinical covariates in a multivariate statistical model. Understanding these relationships is important for future analyses where these same covariates will be explored for their association with the development of a CIE. The information can also be used to postulate future mechanisms to prevent bacterial colonization of silicone hydrogel contact lenses.
METHODS
The LASH Study Cohort and Design
The LASH Contact Lens Study is a prospective cohort study of 205 subjects fit to the lotrafilcon A silicone hydrogel contact lens for up to 29 consecutive nights (30 days) of continuous wear (CW), with monthly disposal, and followed for 1 year at the Department of Ophthalmology & Visual Sciences at Case Western Reserve University in Cleveland, Ohio. Healthy myopic or hyperopic subjects with minimal or no astigmatism and no contraindications to continuous wear lens use were enrolled and monitored for the development of a CIE. Current lotrafilcon A extended lens wearers were excluded. Subject demographics are listed in Table 1.
Table 1.
Characteristics of 205 Subjects Enrolled in the LASH Contact Lens Study from October 2006 to February 2008.
| Parameter | Distribution Number (percent) |
|---|---|
| Age (mean 32.8, range 15–62 years) | |
| Under 21 | 26 (12.7%) |
| Between 21 and 50 | 163 (79.5%) |
| Over 50 | 16 (7.8%) |
| Previous Soft Lens Wear Experience | |
| Current or recent (within 12 months) users | 152 (74.1%) |
| Neophytes | |
| Never wore | 21 (10.3%) |
| Discontinued more than 12 months ago | 32 (15.6%) |
| Race | |
| Caucasian | 115 (56.1%) |
| African-American | 49 (23.9%) |
| Asian | 31 (15.1%) |
| Other | 10 (4.9%) |
| Education (Highest level achieved, of 203 reported) | |
| High School | 17 (8.4%) |
| Some college | 53 (26.1%) |
| 4 year college degree | 61 (30.1%) |
| Graduate work | 72 (35.5%) |
| Smoking Status (of 201 reported) | |
| Current | 21 (10.5%) |
| Never | 170 (84.6%) |
| Former | 10 (5.0%) |
| History of Previous Adverse Event (of 204 reporting) | |
| Yes | 90 (44.1%) |
| No | 93 (45.6%) |
| Not applicable | 21 (12.3%) |
| Discomfort | |
| Yes | 23 (11.2%) |
| No | 182 (88.8%) |
Enrolled neophyte subjects entered a 2 week daily wear phase. Thereafter, all daily wear adapted neophytes, and all other adapted subjects returned for visits after 1 week of EW, and then after 1, 4, 8 and 12 months of CW. One of the key exposure variables was substantial lens bioburden. In pursuit of this data, lenses and ocular surfaces were routinely cultured in the first four months of the study. Lids and conjunctival surfaces were cultured at baseline, and after 1 week and 4 months of wear. Lenses were cultured after 1 week and 4 months of wear. Lids, conjunctivae, and contact lenses were also cultured during any adverse event, or on the first visit following the development of a contact lens peripheral ulcer (CLPU) if diagnosed by an asymptomatic corneal scar. More frequent sampling was not performed since other groups have established no changes to resident lens and ocular surface biota over time during routine CW.36,37 In addition to demographic factors collected at baseline, biomicroscopic findings were collected at all visits. All subjects signed written informed consents prior to participation. This study was approved by the University Hospitals Case Medical Center Institutional Review Board and followed all the Tenets of the Declaration of Helsinki.
The data analyzed for this report include all visits up to and including the 4 month CW visit because all scheduled microbiology sampling occasions were completed by this time. Data collection was limited to the first four months of lens wear to allow accurate assessment of those subjects that discontinued lens wear within that time without creating sampling bias in the retained subjects. Data from unscheduled visits and/or adverse events in the first four months of lens wear are included since the goal of this report is to characterize multiple factors related to lens related bacterial bioburden in the first few months of extended lens wear as opposed to only assessing changes to normal ocular surface biota during asymptomatic wear.
Sampling of Contact Lens Microbiota
Methods for culturing contact lenses utilized an adaptation of an agar overlay technique which has been routinely used in extended wear clinical trials.3, 5, 6, 8, 17, 25. Lenses were aseptically removed from the subjects’ eyes using gloved hands and sent for microbiological analysis (within three hours of collection) in a vial containing 0.5 ml of unpreserved sterile saline. If the patient initiated lens removal because of a red or uncomfortable eye, they were instructed to bring the lenses in non-preserved Unisol saline (Alcon Laboratories, Fort Worth, TX) provided to them. Upon arrival at the microbiology laboratory the lens was aseptically removed and placed concave side down on a chocolate agar plate, and covered with 10 ml of molten agar. Plates were incubated in 5% CO2 for 48 hours at 35°C after which colony forming units (CFUs) were enumerated and colonies identified by use of Gram stain and standard biochemical methods. All plates were re-incubated for up to 5 days if no growth was seen by 48 hours. Coagulase-negative staphylococci were only identified further if they were associated with an adverse event.
The transport saline was not routinely sampled, as it was felt that loosely attached microbes that could “fall off” the lens during transportation to the microbiology laboratory were probably not contributory to the presence or absence of corneal inflammation which was the primary outcome in LASH Study. In fact, the gentle wash associated with the transport saline served to eliminate potential contaminants acquired during the sampling process.
Sampling of Ocular Site Microbiota
Lids and conjunctivae were sampled without anesthesia by use of calcium alginate swabs moistened with sterile non-preserved Unisol saline. After contact lenses were removed, samples were taken from the upper bulbar conjunctiva, avoiding contact with the lids, lashes, and tarsal conjunctiva. The swab was used to directly inoculate a chocolate agar plate. A second Unisol-moistened calcium alginate swab was passed along the lower lid margin, avoiding contact with the bulbar conjunctiva and lashes, and then immediately swabbed onto a chocolate agar plate. The choice of the sample sites were based on previous studies which performed similar analyses so that these results could be compared.17, 32 Plates were incubated and isolates identified as for contact lenses above.
Classification of Substantial Bioburden
Culture results were classified by the levels and potential pathogenicity of the organisms isolated, as follows: 0, no growth; 1, low numbers of commensal ocular biota or organisms of low pathogenicity; 2, high levels of commensal ocular biota or organisms of low pathogenicity; and 3, any level of pathogenic organisms. The term “substantial bioburden” used throughout this study refers to classification levels 2 or 3. The term “pathogenic species” refers to classification level 3. Determination of organisms defined as commensal or low virulence versus pathogenic species for the eye were based upon previous studies that utilized similar ordinal or binary classification schemes.32, 38, 39 In brief, substantial bioburden was present if a lens, lid, or conjunctiva harbored pathogenic organisms or high levels of commensal organisms. The presence of any Gram negative or selected Gram-positive bacteria (Staphylococcus aureus and streptococci), even in low numbers, qualified as substantial bioburden as these species are associated with ocular infection and inflammation, they are infrequently isolated in asymptomatic subjects, and are considered pathogenic.39 Additionally, the presence of commensal organisms qualifed as substantial bioburden if the number of CFU cultured from a particular site met or exceeded a predetermined magnitude. Determinations of CFU cut-points for classification of substantial bioburden secondary to commensal microbiota or low virulence organisms were adapted from the literature38 to our culturing technique. The last column in Tables 2–4 list the magnitude of the bacterial load required for classification of substantial bioburden, stratified by species.
Table 2.
Bacteria isolated from contact lenses and levels considered to be significant.
| Organism | Frequency of Isolation in right eye Number (%)n=341 lenses cultured | Frequency of Isolation in left eye Number (%)n=340 lenses cultured | Mean CFU per lens | Median CFU per lens (OD, OS) | Range CFU Per lens | Magnitude of CFU/lens Required For Definition of Substantial Bioburden (number of occasions that subjects met criteria in either eye) |
|---|---|---|---|---|---|---|
| CNS* | 107 (31.4%) | 111 (32.7%) | 14 | 3, 4 | 1–200 | >10 (45) |
| Staphylococcus aureus | 6 (1.8%) | 11 (3.2%) | 14 | 7.5, 3 | 1–50 | >0 (14) |
| Viridans group streptococcus | 4 (1.2%) | 6 (1.8%) | 28 | 6.5, 38.5 | 1–100 | >0 (1) |
| Corynebacterium* | 4 (1.2%) | 4 (1.2%) | 548 | 350, 750 | 85–1000 | >100 (4) |
| Serratia marcescens | 3 (0.9%) | 5 (1.5%) | 978 | 1000, 100 | 6–2000 | >0 (5) |
| Stenotrophomonas maltophilia | 1 (0.3%) | 0 | 500 | NA | NA | >0 (1) |
| Lactobacillus sp | 1 (0.3%) | 0 | 200 | NA | NA | >0 (1) |
| Haemophilus parainfluenzae | 1 (0.3%) | 0 | 1 | NA | NA | >0 (1) |
| Bacillus* | 1 (0.3%) | 2 (0.6%) | 75 | 100 | 2–100 | >10 (2) |
| Enterobacter asburiae | 1 (0.3%) | 1 (0.3%) | 100 | NA | 100 | >0 (1) |
| Enterobacter cloacae | 1 (0.3%) | 1 (0.29%) | 8 | NA | 3–12 | >0 (1) |
| Proteus mirabilis | 1 (0.3%) | 1 (0.3%) | 200 | NA | 200 | >0 (1) |
| Pseudomonas fluorescens | 0 | 1 (0.3%) | 33 | NA | NA | >0 (1) |
| Escherichia coli | 0 | 1 (0.3%) | 1000 | NA | NA | >0 (1) |
CNS=coagulase negative Staphylococcus species;
=normal microbiota or organism of low ocular pathogenicity
Table 4.
Bacteria isolated from conjunctiva and levels considered to be significant.
| Organism | Frequency of Isolation right eye Number (%)n=556 tissues cultured | Frequency of Isolation left eye Number (%)n=550 tissues cultured | Mean CFU per tissue | Median CFU per lid (OD, OS) | Range CFU per tissue | Magnitude of CFU/Conjunctival Swab Required For Definition of Substantial Bioburden (number of occasions that subjects met criteria in either eye) |
|---|---|---|---|---|---|---|
| CNS* | 93 (16.7%) | 93 (16.9%) | 7 | 2, 2 | 1–100 | >=20 (12) |
| Staphylococcus aureus | 9 (1.6%) | 7 (1.3%) | 5 | 2, 2 | 1–23 | >0 (13) |
| Viridans group streptococcus | 3 (0.5%) | 2 (0.4%) | 6 | 4.5, 7.5 | 3–13 | >0 (5) |
| Corynebacterium* | 1 (0.2%) | 0 | 2 | NA | na | >=20 (0) |
| Streptococcus agalactiae | 1 (0.2%) | 0 | 1 | NA | na | >0 (1) |
| Haemophilus parainfluenza | 1 (0.2%) | 0 | 1 | NA | na | >0 (1) |
| Bacillus* | 0 | 1 (0.2%) | 2 | NA | na | >=20 (0) |
CNS=coagulase negative Staphylococcus species;
normal microbiota or organism of low ocular pathogenicity
Classification of Other Covariates
Variables depicting subject demographics, symptoms, and biomicroscopic findings were treated as categorical variables. Demographic and symptomatic variables at baseline were categorized as follows: history of previous adverse events (yes/no or not applicable), previous lens wearing experience (never, current, or within the past 12 months), age (<21 or older), race (Caucasian, African-American, Asian, other), level of education (at least a 4 year college degree or not), and smoking status (never, ever, or current). Lens discomfort was defined as present if a subject complained of discomfort which precluded successful extended wear yet no fitting concerns were identified; otherwise lens discomfort was noted as absent. The following clinical and biomicroscopic variables were categorized as present or absent: dry eye (present if < 10 mm wet on Schirmer 1 test with anesthesia), blepharitis, meibomian gland dysfunction, and upper palpebral conjunctival lid roughness (all defined as present if ≥ Grade 1 on Efron or Cornea and Contact Lens Research Unit (CCLRU) Grading Scales). Lastly, lens deposits were classified as present if at least one mucoid or lipid deposit was identified on the front surface of lenses, and lens dryness was classified as present if multiple small (<0.1mm) discrete non-wetting areas or at least one area of non-wetting >0.1mm in size were noted during in vivo biomicroscopic evaluation.
Statistical Methods
Univariate analyses including chi-square tests, Fisher exact tests, and univariate logistic regression were used to examine initial associations between substantial lens bioburden and substantial lid bioburden, substantial conjunctival bioburden, subject demographics, symptoms, and biomicroscopic findings if noted on at least one visit including the baseline visit. Chi-square tests were performed to assess relationships between the time after eye opening and substantial lens and ocular surface bioburden and Spearman’s rank correlations were used to assess correlations between the number of hours subjects were awake with CFU counts of the most prevalent organisms. Factors that were significant at p<0.10 were considered for multivariate testing using logistic regression.
In view of conflicting results in the literature on the association between lens and conjunctival bioburden, this study approached model building for multivariable unconditional logistic regression with substantial lens bioburden as the outcome and substantial conjunctival bioburden as the main exposure variable. First, stratified analyses (chi-square) were used to detect confounding and effect modification of individual covariates on the relationship between substantial lens and conjunctival bioburden. Multivariable logistic regression was then used to control confounding variables. The analytic strategy for model building utilized hierarchical backward elimination. Likelihood Ratio Tests were used to compare nested models. Specifically, an initial assessment of interaction using the “chunk test”40 was implemented followed by stepwise removal of each variable and comparison of the resultant model to the gold standard model with all potential confounders present. All variables that were found to be significant upon univariate analyses as well as confounders and additional variables that improved the precision and validity of the overall model were retained in the final model. Significance was set at the 0.05 alpha level. All statistical analyses were performed using SAS (version 9.1.3). The strength of association for all factors in the final model was summarized using odds ratios and 95% confidence intervals. The goodness of fit of the final model was assessed using the Hosmer Lemeshow test.
Analyses were then repeated using stricter definitions for presence of exposure variables that were subjected to repeated assessments (lid and conjunctival bioburden). That is, a subject was required to have two positive responses (at least two occasions) of substantial lid or conjunctival bioburden to be classified as having a positive response for that variable.
RESULTS
Subjects
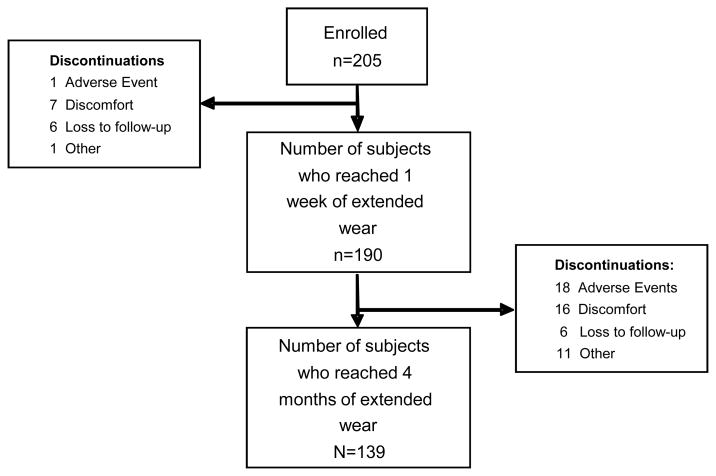
From October 2006 to February 2008, two hundred and five subjects were enrolled and contributed 793 person-visits of follow-up. Table 1 lists the subject demographics and the distribution of each stratified parameter. Sixty six subjects (32.2%) discontinued within the first four months of the study. Figure 1 tracks the reason for discontinuation between the baseline, 1 week, and 4 month visits. The majority of subjects (51/66, 77.3%) discontinued after the 1 week visit. Thus, lens cultures are not available for the 15 subjects that discontinued prior to the 1 week visit. These subjects were removed from the multivariate analysis. Twenty-one subjects (10.2%) experienced a CIE during the first four months of the study.
Figure 1.
Flow chart of subject retention and discontinuation in the first four months of The LASH Contact Lens Study.
Lens and Ocular Site Microbiology
Bacterial Analyses Across All Visits
Tables 2–4 list the organisms isolated from lenses, lid margins, and conjunctival tissues. These tables include the frequency of isolation of each species per site, the mean, median and range of CFUs found per species and site, the levels considered to be substantial in our analyses, and the frequency with which contamination by each species was considered substantial. Only bacteria were isolated (no fungi were isolated). Coagulase negative staphylococci are by far the most frequent organisms cultured from all sites which is not surprising as these are considered normal ocular microbiota.41 Although the incidence of lens contamination with Corynebacterium species and Serratia marcescens was low (approximately 1%), when these organisms were present they were found in very high numbers. Across all visits, if a site was culture positive, typically only one bacterial species was present. Specifically, the frequencies of lenses with single or mixed cultures were 35.0%, 2.0%, and 0.1% for one, two or three species, respectively; for lids 73.0%, 4.5%, and 0.3% for one, two or three species, respectively; and for conjunctivae 18.3%, 0.2%, and 0.1% for one, two or three species, respectively. Alternatively, across all visits, 62.3% of lenses were sterile and 22.5%, 8.9% and 6.3% of lenses harbored organisms classified as level 1 (low levels of normal microbiota or organisms of low virulence), 2 (high levels of normal microbiota or organisms of low virulence), or 3 (any level of pathogenic organisms), respectively; for lids 21.8% were sterile and 57.4%, 12.7%, and 8.0% harbored organisms classified as level 1, 2, or 3, respectively; and for conjunctivae 81.3% were sterile and 15.5%, 1.2% and 2.0% harbored organisms classified as type 1, 2, or 3, respectively.
To assess whether organisms cultured from lenses were the same species as those cultured from either lid or conjunctival swabs, only those visits in which lenses harbored organisms were assessed. On 257 occasions where lenses harbored organisms, 83.8% had the same species cultured from lids and lenses. Only 26.1% had the same species detected on lenses and conjunctival swabs.
The numbers of hours a subject was awake at the time of each study visit was categorized into intervals to assess whether early morning appointments had potentially higher microbial loads than mid-day or later appointments. The majority of visits (55.8%) occurred between 4 and 8 hours after the subject awoke, while 16.1% of visits occurred within 4 hours of waking, and 28.1% occurred after 8 hours of waking. Time awake was not associated with the degree of bacterial bioburden on lenses, lids or conjunctival swabs (p>0.05 on all comparisons). Furthermore, there were no correlations between the number of hours subjects were awake with CFU counts of the most prevalent organisms on lenses, lids, or conjunctival swabs (p>0.09 for all comparisons).
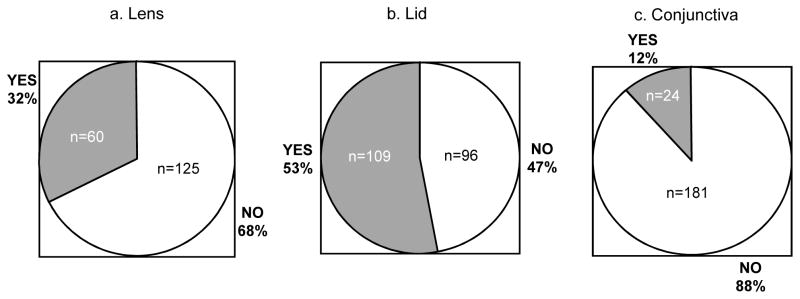
Figure 2 displays the percentage of subjects that harbored significant levels of bacterial bioburden (levels 2 or 3) at any visit in either eye in the first four months of lens wear on lenses, lids, or conjunctivae.
Figure 2.
Percentage of subjects with substantial bacterial bioburden in either eye at one or more visits.
Bacterial Analyses Stratified by Visit
Table 5 displays the percentage of subjects with culture positive sites in at least one eye, stratified by visit. Unscheduled visits occurred at any point in time during the study, usually initiated by subjects, and often concluded with an adverse event finding such as a CIE, contact lens papillary conjunctivitis, or other reasons for discomfort. The results are presented by various levels of bacterial presence and include the percentage of subjects with 1) any bacterial species present, 2) significant bacterial bioburden, and 3) any pathogenic bacterial species present. Excluding unscheduled visits, there is a trend toward diminished bioburden frequency at all sites with time, which was significant only for substantial conjunctival bioburden (p=0.0348). However, the frequency of lens bioburden significantly increases during unscheduled visits possibly driven by microbially related adverse events. This trend was not noticed for lid or conjunctival bioburden. The relationship between lens microbial contamination and adverse events (in particular CIEs) across the entire 12 month follow-up of this cohort will be explored in detail in future reports.
Table 5.
Number (percentage) of subjects with culture positive sites in at least one eye, stratified by visit.
| Baseline | 1 week EW | 4 month CW | p-value for trend | Unscheduled* | p-value for Comparison of Unscheduled vs 4 Month CW | |
|---|---|---|---|---|---|---|
| Lens | n=175 | n=135 | n=22 | |||
| Any bacterial species | NA | 88 (50.3%) | 58 (43.0%) | 0.2003 | 15 (68.2%) | 0.0279 |
| Significant bacterial bioburden | NA | 36 (20.6%) | 26 (19.26%) | 0.7746 | 10 (45.5%) | 0.0067 |
| Pathogenic species | NA | 15 (8.6%) | 10 (7.4%) | 0.7090 | 7 (31.8%) | 0.0006 |
| Lid | n=205 | n=182 | n=135 | n=24 | ||
| Any bacterial species | 187 (91.2%) | 157 (86.3%) | 120 (88.9%) | 0.3016 | 17 (70.8%) | 0.0182 |
| Significant bacterial bioburden | 67 (32.7%) | 58 (31.9%) | 33 (24.4%) | 0.2281 | 6 (25.0%) | 0.9535 |
| Pathogenic species | 29 (14.2%) | 25 (13.7%) | 10 (7.4%) | 0.1352 | 1 (4.2%) | 0.5643 |
| Conjunctiva | n=205 | n=182 | n=135 | n=24 | ||
| Any bacterial species | 61 (29.8%) | 61 (33.5%) | 32 (23.7%) | 0.3145 | 6 (25.0%) | 0.8909 |
| Significant bacterial bioburden | 18 (8.8%) | 6 (3.3%) | 5 (3.7%) | 0.0348 | 2 (8.3%) | 0.3083 |
| Pathogenic species | 10 (4.9%) | 5 (2.8%) | 3 (2.2%) | 0.3435 | 1 (4.2%) | 0.5751 |
49 unscheduled visits occurred, cultures were only taken during adverse events
Table 6 displays the assessment of repeated bioburden. That is, Table 6 lists the frequency with which a subject had the same or different level of bacterial bioburden at both the 1 week and 4 month CW visits. The majority of subjects were culture negative in both eyes at 1 week as well as at 4 months. However, the same is not true for culture positive results. In the 40 subjects that had substantial lens bioburden in at least one eye at one visit, only 27.5% (11/40) had substantial lens bioburden in at least one eye at the alternate visit; for lids, of 62 subjects with substantial bioburden at one visit, 27.5% (17/62) had substantial bioburden at the alternate visit; and for conjunctiva, of 8 subjects with substantial bioburden at one visit, 12.5% (1/8) had substantial bioburden at the alternate visit. Thus, significant microbiota appear to be transient as they do not routinely recur across multiple visits for a particular subject.
Table 6.
Frequency of similar bacterial bioburden at 1 week EW and 4 month CW.
| Number of subjects with culture negative results in both eyes at both visits (percent) | Number of subjects with culture positive results in at least one eye at both visits (percent) | Number of subjects with mixed results (percent) | |
|---|---|---|---|
| Lens n=129 | |||
| Significant bacterial bioburden | 89 (69.0%) | 11 (8.5%) | 29 (22.5%) |
| Pathogenic species | 110 (85.3%) | 1 (0.8%) | 18 (14.0%) |
| Lid n=133 | |||
| Significant bacterial bioburden | 71 (53.4%) | 17 (12.8%) | 45 (33.8%) |
| Pathogenic species | 106 (79.7%) | 4 (3.0%) | 23 (17.3%) |
| Conjunctiva n=133 | |||
| Significant bacterial bioburden | 125 (94.0%) | 1 (0.8%) | 7 (5.3%) |
| Pathogenic species | 128 (96.3%) | 1 (0.8%) | 4 (3.0%) |
Univariate Assessments of Lens Microbial Contamination
The following variables demonstrated preliminary associations with substantial lens bacterial bioburden: substantial lid bioburden, substantial conjunctival bioburden, Asian race (protective effect), and lens discomfort. As stated previously, 53.2% and 11.7% had substantial lid and conjunctival bioburden on at least one visit, respectively, and 15.1% of the subjects were Asian. Additionally, 11.2% of the subjects had discomfort which precluded successful CW. Table 7 displays the percentage of subjects with presence or absence of each of these variables stratified by presence or absence of substantial lens bioburden, the crude odds ratio, and the 95% confidence interval for each association. That is, each cell represents the column percentage of subjects with presence or absence of lens bioburden for the covariate listed along the top row of the table.
Table 7.
Column Percentages of Key Risk Factors and Univariate Association with Significant Lens Bioburden.
| Significant Lid Bioburden Number (percent) | Significant Conjunctival Bioburden Number (percent) | Asian Race Number (percent) | Discomfort Number (percent) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | ||
| Significant Lens Bioburden | No | 67 (80.7) | 58 (56.9) | 119 (73.0) | 6 (27.3) | 100 (64.5) | 25 (83.3) | 120 (69.8) | 5 (38.5) |
| Yes | 16 (19.3) | 44 (43.2) | 44 (27.0) | 16 (72.7) | 55 (35.5) | 5 (16.7) | 52 (30.2) | 8 (61.5) | |
| Crude Odds Ratio (95% CI) | 3.18 (1.62–6.22) | 7.21 (2.65–19.60) | 0.36 (0.13–1.00) | 3.69 (1.15–11.82) | |||||
Multivariate Analysis
Table 8 lists the final variables retained in the multivariate model and their adjusted odds ratios and 95% confidence intervals. No interactions were detected (Likelihood Ratio Test, p=0.1661). Similar to the results of the univariate analyses, the presence of substantial conjunctival and lid bioburden, and lens discomfort, were associated with an increased risk of substantial lens bioburden. Asian race was no longer significant in the multivariate model.
Table 8.
Multivariate Analysis for Significant Lens Bioburden
| Variable | P value | Odds Ratio | 95% confidence interval |
|---|---|---|---|
| Significant Lid Bioburden | |||
| No | Referent | ||
| Yes | 0.0179 | 2.49 | 1.17–5.30 |
| Significant Conjunctival Bioburden | |||
| No | Referent | ||
| Yes | 0.0022 | 4.24 | 1.45–12.40 |
| Race | |||
| Caucasian | Referent | ||
| Asian | 0.1123 | 0.40 | 0.13–1.24 |
| African American | 0.8324 | 0.91 | 0.40–2.10 |
| Other | 0.9875 | 1.01 | 0.22–4.70 |
| Discomfort | |||
| No | Referent | ||
| Yes | 0.0279 | 4.11 | 1.17–14.46 |
| Level of Education | |||
| High School or some college | Referent | ||
| College degree or beyond | 0.3786 | 0.71 | 0.33–1.53 |
Hosmer-Lemeshow Test for final model: p=0.3780
The repeated analysis where stricter definitions of bioburden were employed revealed similar trends. Specifically, substantial conjunctival bioburden (p=0.0194) and discomfort (p=0.0231) were associated with an increased risk of substantial lens bioburden. Asian race (p=0.0506) was associated with a decreased risk of substantial lens bioburden. Interestingly, repeated lid bioburden was not significant. Although similar trends were present, the odds ratios had wide confidence intervals and are not reported.
DISCUSSION
This study reports that within the first 4 months of CW with lotrafilcon A silicone hydrogel contact lenses about one-third of subjects harbor substantial bacterial bioburden on lenses worn in either eye. Alternatively, over half of subjects have lids with substantial bacterial bioburden, and slightly over one-tenth have substantial conjunctival bacterial bioburden. The presence of substantial and pathogenic bacterial bioburden in the absence of clinically apparent infection highlights the potency of the ocular defense mechanisms. The spectrum, frequency of isolation, and quantification of organisms reported herein is similar to that reported by others,5, 17, 20, 32, 33, 41 although this study shows a lower isolation frequency for Corynebacterium species on lids and conjunctiva than that reported by others.32, 33 The difference between Corynebacterium species isolation rates in this study (1% for conjunctiva, 2–5% for lids) compared to others (about 9% for conjunctiva and 11–17% for lids) is unknown but may be due to differences in culturing technique. Interestingly, this study found a greater diversity of bacteria on the silicone hydrogel study contact lenses (14 types) than either lids (11 types) or conjunctivae (7 types), a finding also reported by Willcox.17 In view of the fact that lens contamination is probably the greatest risk factor for corneal infection, the sources and factors leading to the contamination of microbes on lenses are of interest.
This study has identified strong associations between substantial bacterial bioburden found on lens surfaces and substantial bacterial bioburden found on lids and conjunctivae. The presence of substantial lid bioburden at some point in the first 4 months of lens wear is associated with a 2.5 fold higher risk of substantial lens contamination, compared to those subjects with no bioburden or consistently low levels of commensal lid organisms. The presence of substantial conjunctival bioburden at some point in the first 4 months of lens wear is associated with more than a 4 fold higher risk of substantial lens contamination, compared to those subjects with no bioburden or consistently low levels of commensal conjunctival organisms. We have also found that when bacteria are detected on the contact lens surface, the same species is usually (83.8% of visits) cultured from lids at the same visit, whereas the same species is less frequently (26.1% of visits) found on conjunctival swabs. The strong relationship between lens and lid cultures and lack of parallelism between lens and conjunctival cultures is likely a reflection of the swabbing technique. Although the entire contact lens was cultured, only about one fourth of the bulbar conjunctiva was cultured while the entire lower lid was cultured. Therefore, detection and enumeration of microbes found on the ocular surface at a single visit is at best semi-quantitative and repeated assessments of microbial load provide a better indication of bacterial bioburden and its association with lens bioburden or disease over time.
It is not surprising that lid and conjunctival bioburden are associated with contact lens bioburden; however, the directions of the associations are unclear. There are multiple possibilities that explain these relationships. First, the conjunctiva or lid may serve as the source of contamination to the lens as has already been hypothesized.13, 17 Alternatively, the reverse may be true as the lens may be the source of initial contamination (from the environment, air, or manipulation) which spills onto the ocular surface. Lastly, contact lens wear may disrupt the ecology of the ocular surface by altering the balance of the normal microbiota. For example, contact lens usage affects the balance between staphylococci and corynebacteria in conjunctival microbiota and causes the advance of staphylococci.42 The alteration of the normal microbiota by the contact lens may suppress or interfere with factors that inactivate, remove, or prevent foreign microbes from inoculating the eye. In fact, the effect of soft contact lens extended wear on the ocular microbiota is unclear. During asymptomatic soft contact lens wear, some authors have reported an increase in ocular microbiota with lens wear26, 30–32 while others have not.14, 27–29, 33–36 Specifically, an increase in numbers but not types of organisms were found on lid margins31, 32 or conjunctivae26, 32 with soft contact lens wear, and more potential pathogens have been recovered from the conjunctivae of extended wear users compared to daily wear users.32 In a study of lotrafilcon A lens wearers, the frequency of positive conjunctival cultures (CNS and Corynebacterium species) rose from 34% before lens wear to 90% after 30 day CW.30
The most likely route for contamination of contact lenses is from the skin to the lens. The evidence for this is that the majority of isolates found on contact lenses are coagulase negative staphylococci which are normal, ubiquitous skin microbiota. Leitch41 and Hart13 have reported significant decreases in the frequency of gram positive organisms isolated from the lower lid margin to lens to conjunctiva which confers the opinion that staphylococcal microbiota are likely dispersed from the facial skin onto the lens and then the ocular surface. Nonetheless, regardless of cause, it is important to ascertain the strength of the associations between lens and ocular surface bioburden. The associations reported herein have implications for future analyses (such as on the association between lens contamination and corneal adverse events) where ocular site contamination may confound the effect.
This study highlights a trend toward less bioburden on lids and conjunctivae with time. This is similar to results of McBride33 who found a sudden decrease in the percentage of positive cul-de-sac cultures with as little as 2 days of soft contact lens wear. They speculated that the lenses may have had an antibacterial effect or study subjects became more fastidious in ocular hygiene throughout her study. However, in the LASH Study, this trend is likely evidence of a survivor sampling bias as the more successful subjects (whom are hypothesized to have decreased bioburden) are retained in the study.
This study is the first to document a potential association between lens discomfort and microbial contamination of the contact lens. Subjects that reported discomfort which precluded successful CW were more than four times more likely to harbor substantial bacterial bioburden on their lenses compared to subjects who did not report discomfort. One potential explanation is that the discomfort prompted the subjects to remove, handle, and manipulate the lenses more frequently resulting in increased bacterial inoculation from their hands. Another possibility is that lenses with higher microbial loads caused a subtle inflammatory response which was perceived as discomfort. This latter hypothesis is supported by data of Fleiszig28 who found that the conjunctival microbiota of former lens wearers had a higher bioburden (mostly diptheroids) than current wearers or subjects who had never worn lenses. They hypothesized that former lens wearers were predisposed to alterations in the conjunctival microbiota which perhaps lead to their failure with contact lens wear.
In the univariate analyses and multivariate analysis where stricter definitions of ocular site bioburden were employed, Asians had a lower risk of lens bacterial bioburden compared to Caucasians as well as all other races combined. Only one other group has previously reported an association between race and bacterial bioburden.36 An Indian population was found to have a greater frequency of positive cultures and greater CFU counts on contact lenses compared with an Australian population; the authors postulated this reflected environmental differences of study sites.36 In this study, the association between Asian race and decreased bacterial bioburden may be positively confounded by hygiene practices or some unknown or unmeasured covariate because there is no other evidence that supports differing ocular biota in Asians outside of environmental differences. Indeed, the effect of race was not present in the final multivariate model. However, race should be controlled and explored further in future studies.
No association was found between time after awakening and bacterial counts. The hypothesis was that there may be a higher microbial load on lenses in the early morning hours as some bacteria may have proliferated overnight in a static (non-blinking) environment. Previous groups have attempted to determine correlations between time after lens insertion and number of bacterial CFU per lens and also found no correlation.22 Perhaps this is due to rapid clearing of organisms by the ocular surface as Ramachandran has shown that in the absence of contact lens wear, the increased bacterial load following eye closure dimishes within 3–5 hours after eye opening.39
This study was designed to primarily recover viable aerobic bacteria from lenses and ocular sites as anaerobes are rarely associated with contact lens related adverse events. However, the gram-positive anaerobic bacilli of the Propionbacterium genus are normal inhabitants of the eye,17, 32 usually nonpathogenic, and have been cultured off contact lenses worn during asymptomatic wear.17, 43 Therefore, one limitation of our study is that anaerobic cultures were not performed. However, the study was designed to maximize recovery of organisms most commonly found on lenses in vivo by assaying at 35 degrees C in 5% CO2, essentially the same conditions to which bacteria are exposed to during contact lens wear. Additionally, some potentially pathogenic strains, such as invasive or biofilm forming S. epidermidis strains44, 45 may have not have been distinguished from other avirulent commensal coagulase negative staphylococci because these were not subcategorized further in asymptomatic subjects. However, the potential for differential misclassification of bioburden is low since the inability to detect anaerobes or virulent S. epidermidis strains was consistent across all subjects. Lastly, aseptic removal techniques may not have been practiced if subjects initiated lens removal. However, this should not have impacted our findings related to discomfort, because only 4 of 13 subjects that had discomfort removed their lenses prior to culture, and only two of those had significant bioburden detected on the lens.
Only about one-fourth of subjects with significant lens or lid bioburden at one visit had persistent bioburden at a subsequent visit, and only one-eighth of subjects with substantial conjunctival bioburden at one visit had persistent bioburden at a subsequent visit. Thus, transient association of pathogenic levels bacteria on the lens and ocular surface is more common than persistent colonization. Others that have reported transient colonization of pathogens in lens wearers include Boost and Cho who noted that microbial contaminants on lenses, lens cases and suction holders of orthokeratology subjects were of a transient nature12 and Sweeney43 who noted that pathogenic colonization of bacteria on extended wear contact lenses is sporadic rather than a gradual accumulation over time. This study documents that substantial bacterial bioburden of the ocular surface is also transient and significantly increases the risk of (transient) lens bacterial bioburden.
In summary, substantial lens bioburden is associated with substantial conjunctival and lid bioburden and discomfort precluding successful continuous wear. These findings are important to the contact lens industry as new techniques are developed to limit bacterial presence on lens surfaces.46–48 Additionally, these associations are important to ascertain for future analyses on the effect of lens bacterial bioburden on CIEs where these variables may confound the effect.
Table 3.
Bacteria isolated from lid margins and levels considered to be significant.
| Organism | Frequency of Isolation in right eye Number (%)n=556 lids cultured | Frequency of Isolation in left eye Number (%)n=552 lids cultured | Mean CFU per lid | Median CFU per lid (OD, OS) | Range CFU per lid | Magnitude of CFU/lid Required For Definition of Substantial Bioburden (number of occasions that subjects met criteria in either eye) |
|---|---|---|---|---|---|---|
| CNS* | 419 (75.4%) | 399 (72.3%) | 35 | 20, 14 | 1–200 | >=100 (106) |
| Staphylococcus aureus | 35 (6.3%) | 35 (6.3%) | 24 | 12, 8 | 1–100 | >0 (51) |
| Viridans group streptococcus | 9 (1.6%) | 9 (1.6%) | 30 | 10, 5 | 1–100 | >0 (13) |
| Corynebacterium* | 2 (0.4%) | 5 (0.9%) | 16 | 17, 12 | 10–30 | >=100 (0) |
| Serratia marcescens | 0 | 1 (0.18%) | 3 | NA | NA | >0 (1) |
| Achromobacter xylosoxidans* | 1 (0.2%) | 1 (0.2%) | 13 | NA | 9–17 | >=20 (0) |
| Haemophilus parainfluenza | 1 (0.2%) | 0 | 2 | NA | NA | >0 (1) |
| Bacillus* | 0 | 1 (0.2%) | 17 | NA | NA | >=20 (0) |
| Enterobacter cloacae | 0 | 1 (0.2%) | 1 | NA | NA | >0 (1) |
| Chryseobacterium meningosepticum | 0 | 1 (0.2%) | 8 | NA | NA | >0 (1) |
| Moraxella catarrhalis | 0 | 1 (0.2%) | 23 | NA | NA | >0 (1) |
CNS=coagulase negative Staphylococcus species;
normal microbiota or organism of low ocular pathogenicity
Acknowledgments
This work was supported by the National Eye Institute (NIH Grant: NEI K23 EY015270-01), P30 EY 11373, CIBA Vision, Prevent Blindness Ohio, Research to Prevent Blindness, Ohio Lions Eye Research Foundation, and Alcon Laboratories. The work was performed while Dr. Szczotka-Flynn was a recipient of an American Optometric Foundation Ezell Fellowship. The clinicalTrials.gov identifier is NCT00727402.
Additional acknowledgements to Jonathan Lass MD, Sara Debanne PhD, Ajay Sethi PhD, and Desmond Fonn MOptom for their kind review of this manuscript.
References
- 1.Das S, Sheorey H, Taylor HR, Vajpayee RB. Association between cultures of contact lens and corneal scraping in contact lens related microbial keratitis. Arch Ophthalmol. 2007;125:1182–5. doi: 10.1001/archopht.125.9.1182. [DOI] [PubMed] [Google Scholar]
- 2.Holden BA, La Hood D, Grant T, Newton-Howes J, Baleriola-Lucas C, Willcox MD, Sweeney DF. Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. CLAO J. 1996;22:47–52. [PubMed] [Google Scholar]
- 3.Jalbert I, Willcox MD, Sweeney DF. Isolation of Staphylococcus aureus from a contact lens at the time of a contact lens-induced peripheral ulcer: case report. Cornea. 2000;19:116–20. doi: 10.1097/00003226-200001000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Keay L, Harmis N, Corrigan K, Sweeney D, Willcox M. Infiltrative keratitis associated with extended wear of hydrogel lenses and Abiotrophia defectiva. Cornea. 2000;19:864–9. doi: 10.1097/00003226-200011000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Sankaridurg PR, Sharma S, Willcox M, Naduvilath TJ, Sweeney DF, Holden BA, Rao GN. Bacterial colonization of disposable soft contact lenses is greater during corneal infiltrative events than during asymptomatic extended lens wear. J Clin Microbiol. 2000;38:4420–4. doi: 10.1128/jcm.38.12.4420-4424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sankaridurg PR, Sharma S, Willcox M, Sweeney DF, Naduvilath TJ, Holden BA, Rao GN. Colonization of hydrogel lenses with Streptococcus pneumoniae: risk of development of corneal infiltrates. Cornea. 1999;18:289–95. doi: 10.1097/00003226-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Sankaridurg PR, Vuppala N, Sreedharan A, Vadlamudi J, Rao GN. Gram negative bacteria and contact lens induced acute red eye. Indian J Ophthalmol. 1996;44:29–32. [PubMed] [Google Scholar]
- 8.Sankaridurg PR, Willcox MD, Sharma S, Gopinathan U, Janakiraman D, Hickson S, Vuppala N, Sweeney DF, Rao GN, Holden BA. Haemophilus influenzae adherent to contact lenses associated with production of acute ocular inflammation. J Clin Microbiol. 1996;34:2426–31. doi: 10.1128/jcm.34.10.2426-2431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stapleton F, Keay LJ, Sanfilippo PG, Katiyar S, Edwards KP, Naduvilath T. Relationship between climate, disease severity, and causative organism for contact lens-associated microbial keratitis in Australia. Am J Ophthalmol. 2007;144:690–8. doi: 10.1016/j.ajo.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Willcox MD, Hume EB. Differences in the pathogenesis of bacteria isolated from contact-lens-induced infiltrative conditions. Aust N Z J Ophthalmol. 1999;27:231–3. doi: 10.1046/j.1440-1606.1999.00189.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu PZ, Thakur A, Stapleton F, Willcox MD. Staphylococcus aureus causes acute inflammatory episodes in the cornea during contact lens wear. Clin Experiment Ophthalmol. 2000;28:194–6. doi: 10.1046/j.1442-9071.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 12.Boost MV, Cho P. Microbial flora of tears of orthokeratology patients, and microbial contamination of contact lenses and contact lens accessories. Optom Vis Sci. 2005;82:451–8. doi: 10.1097/01.opx.0000168587.72893.ec. [DOI] [PubMed] [Google Scholar]
- 13.Hart DE, Hosmer M, Georgescu M, Farris RL. Bacterial assay of contact lens wearers. Optom Vis Sci. 1996;73:204–7. doi: 10.1097/00006324-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Hart DE, Shih KL. Surface interactions on hydrogel extended wear contact lenses: microflora and microfauna. Am J Optom Physiol Opt. 1987;64:739–48. doi: 10.1097/00006324-198710000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Lipener C, Nagoya FR, Zamboni FJ, Lewinski R, Kwitko S, Uras R. Bacterial contamination in soft contact lens wearers. CLAO J. 1995;21:122–4. [PubMed] [Google Scholar]
- 16.Mowrey-McKee MF, Sampson HJ, Proskin HM. Microbial contamination of hydrophilic contact lenses. Part II: Quantitation of microbes after patient handling and after aseptic removal from the eye. CLAO J. 1992;18:240–4. [PubMed] [Google Scholar]
- 17.Willcox MD, Power KN, Stapleton F, Leitch C, Harmis N, Sweeney DF. Potential sources of bacteria that are isolated from contact lenses during wear. Optom Vis Sci. 1997;74:1030–8. doi: 10.1097/00006324-199712000-00025. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin-Borlace L, Stapleton F, Matheson M, Dart JK. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol. 1998;84:827–38. doi: 10.1046/j.1365-2672.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 19.Fowler SA, Greiner JV, Allansmith MR. Attachment of bacteria to soft contact lenses. Arch Ophthalmol. 1979;97:659–60. doi: 10.1001/archopht.1979.01020010315005. [DOI] [PubMed] [Google Scholar]
- 20.Hart DE, Reindel W, Proskin HM, Mowrey-McKee MF. Microbial contamination of hydrophilic contact lenses: quantitation and identification of microorganisms associated with contact lenses while on the eye. Optom Vis Sci. 1993;70:185–91. doi: 10.1097/00006324-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Iskeleli G, Bahar H, Unal M, Artunay O, Akova N, Torun MM. Microbiologic evaluation of frequent-replacement soft contact lenses. CLAO J. 2002;28:192–5. doi: 10.1097/01.ICL.0000024118.45191.9B. [DOI] [PubMed] [Google Scholar]
- 22.Mowrey-McKee MF, Monnat K, Sampson HJ, Smith CM, Davies GA, Mandt L, Proskin HM. Microbial contamination of hydrophilic contact lenses. Part I: Quantitation of microbes on patient worn-and-handled lenses. CLAO J. 1992;18:87–91. [PubMed] [Google Scholar]
- 23.Yung MS, Boost M, Cho P, Yap M. Microbial contamination of contact lenses and lens care accessories of soft contact lens wearers (university students) in Hong Kong. Ophthalmic Physiol Opt. 2007;27:11–21. doi: 10.1111/j.1475-1313.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 24.Barr JT, Lapple WJ, Snyder AC, Hsu JC, Tuovinen OH. Evaluation of contact lenses by microbial enumeration and protein determination. Am J Optom Physiol Opt. 1988;65:476–80. doi: 10.1097/00006324-198806000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Willcox MD, Harmis NY, Holden BA. Bacterial populations on high-Dk silicone hydrogel contact lenses: effect of length of wear in asymptomatic patients. Clin Exp Optom. 2002;85:172–5. doi: 10.1111/j.1444-0938.2002.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 26.Callender MG, Tse LS, Charles AM, Lutzi D. Bacterial flora of the eye and contact lens. Cases during hydrogel lens wear. Am J Optom Physiol Opt. 1986;63:177–80. doi: 10.1097/00006324-198603000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Elander TR, Goldberg MA, Salinger CL, Tan JR, Levy B, Abbott RL. Microbial changes in the ocular environment with contact lens wear. CLAO J. 1992;18:53–5. [PubMed] [Google Scholar]
- 28.Fleiszig SM, Efron N. Microbial flora in eyes of current and former contact lens wearers. J Clin Microbiol. 1992;30:1156–61. doi: 10.1128/jcm.30.5.1156-1161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hovding G. The conjunctival and contact lens bacterial flora during lens wear. Acta Ophthalmol (Copenh) 1981;59:387–401. doi: 10.1111/j.1755-3768.1981.tb03004.x. [DOI] [PubMed] [Google Scholar]
- 30.Iskeleli G, Bahar H, Eroglu E, Torun MM, Ozkan S. Microbial changes in conjunctival flora with 30-day continuous-wear silicone hydrogel contact lenses. Eye Contact Lens. 2005;31:124–6. doi: 10.1097/01.icl.0000141923.63458.df. [DOI] [PubMed] [Google Scholar]
- 31.Larkin DF, Leeming JP. Quantitative alterations of the commensal eye bacteria in contact lens wear. Eye. 1991;5:70–4. doi: 10.1038/eye.1991.14. [DOI] [PubMed] [Google Scholar]
- 32.Stapleton F, Willcox MD, Fleming CM, Hickson S, Sweeney DF, Holden BA. Changes to the ocular biota with time in extended- and daily-wear disposable contact lens use. Infect Immun. 1995;63:4501–5. doi: 10.1128/iai.63.11.4501-4505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBride ME. Evaluation of microbial flora of the eye during wear of soft contact lenses. Appl Environ Microbiol. 1979;37:233–6. doi: 10.1128/aem.37.2.233-236.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smolin G, Okumoto M, Nozik RA. The microbial flora in extended-wear soft contact-lens wearers. Am J Ophthalmol. 1979;88:543–7. doi: 10.1016/0002-9394(79)90512-9. [DOI] [PubMed] [Google Scholar]
- 35.Hovding G. Conjunctival and contact lens bacterial flora during continuous 'bandage' lens wear. Acta Ophthalmol (Copenh) 1982;60:439–48. doi: 10.1111/j.1755-3768.1982.tb03036.x. [DOI] [PubMed] [Google Scholar]
- 36.Gopinathan U, Stapleton F, Sharma S, Willcox MD, Sweeney DF, Rao GN, Holden BA. Microbial contamination of hydrogel contact lenses. J Appl Microbiol. 1997;82:653–8. doi: 10.1111/j.1365-2672.1997.tb03598.x. [DOI] [PubMed] [Google Scholar]
- 37.Keay L, Willcox MD, Sweeney DF, Morris CA, Harmis N, Corrigan K, Holden BA. Bacterial populations on 30-night extended wear silicone hydrogel lenses. CLAO J. 2001;27:30–4. [PubMed] [Google Scholar]
- 38.Baleriola-Lucas C, Fukuda M, Willcox MD, Sweeney DF, Holden BA. Fibronectin concentration in tears of contact lens wearers. Exp Eye Res. 1997;64:37–43. doi: 10.1006/exer.1996.0182. [DOI] [PubMed] [Google Scholar]
- 39.Ramachandran L, Sharma S, Sankaridurg PR, Vajdic CM, Chuck JA, Holden BA, Sweeney DF, Rao GN. Examination of the conjunctival microbiota after 8 hours of eye closure. CLAO J. 1995;21:195–9. [PubMed] [Google Scholar]
- 40.Kleinbaum DG, Klein M. Logistic Regression A Self-Learning Text. 2. New York: Springer-Verlag; 2002. [Google Scholar]
- 41.Leitch EC, Harmis NY, Corrigan KM, Willcox MD. Identification and enumeration of staphylococci from the eye during soft contact lens wear. Optom Vis Sci. 1998;75:258–65. doi: 10.1097/00006324-199804000-00022. [DOI] [PubMed] [Google Scholar]
- 42.Kozer-Bilgin L, Demir N, Altan-Yaycioglu R. Microbiological evaluation of contact lenses and contact lens disinfection solutions in an asymptomatic population and in medical personnel. Clao J. 1999;25:228–32. [PubMed] [Google Scholar]
- 43.Sweeney DF, Stapleton F, Leitch C, Taylor J, Holden BA, Willcox MD. Microbial colonization of soft contact lenses over time. Optom Vis Sci. 2001;78:100–5. doi: 10.1097/00006324-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Xu L, Wang J, Wen Y, Vuong C, Otto M, Gao Q. Conversion of Staphylococcus epidermidis strains from commensal to invasive by expression of the ica locus encoding production of biofilm exopolysaccharide. Infect Immun. 2005;73:3188–91. doi: 10.1128/IAI.73.5.3188-3191.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallas V, Stapleton F, Willcox MD. Bacterial invasion of corneal epithelial cells. Aust N Z J Ophthalmol. 1999;27:228–30. doi: 10.1046/j.1440-1606.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- 46.Mathews SM, Spallholz JE, Grimson MJ, Dubielzig RR, Gray T, Reid TW. Prevention of bacterial colonization of contact lenses with covalently attached selenium and effects on the rabbit cornea. Cornea. 2006;25:806–14. doi: 10.1097/01.ico.0000224636.57062.90. [DOI] [PubMed] [Google Scholar]
- 47.Weisbarth RE, Gabriel MM, George M, Rappon J, Miller M, Chalmers R, Winterton L. Creating antimicrobial surfaces and materials for contact lenses and lens cases. Eye Contact Lens. 2007;33:426–9. doi: 10.1097/ICL.0b013e318157f488. [DOI] [PubMed] [Google Scholar]
- 48.Zhu H, Kumar A, Ozkan J, Bandara R, Ding A, Perera I, Steinberg P, Kumar N, Lao W, Griesser SS, Britcher L, Griesser HJ, Willcox MD. Fimbrolide-coated antimicrobial lenses: their in vitro and in vivo effects. Optom Vis Sci. 2008;85:292–300. doi: 10.1097/OPX.0b013e31816bea0f. [DOI] [PubMed] [Google Scholar]