Abstract
The formation of stable adhesive contacts between pre- and post-synaptic neurons represents the initial step in synapse assembly. The cell adhesion molecule N-cadherin, the receptor tyrosine phosphatase DLAR, and the scaffolding molecule Liprin-α play critical, evolutionarily conserved roles in this process. However, how these proteins signal to the growth cone, and are themselves regulated, remains poorly understood. Using Drosophila photoreceptors (R cells) as a model, we evaluate genetic and physical interactions among these three proteins. We demonstrate that DLAR function in this context is independent of phosphatase activity, but requires interactions mediated by its intracellular domain. Genetic studies reveal both positive and, surprisingly, inhibitory interactions amongst all three genes. These observations are corroborated by biochemical studies demonstrating that DLAR physically associates via its phosphatase domain with N-cadherin in Drosophila embryos. Together, these data demonstrate that N-cadherin, DLAR, and Liprin-α function in a complex to regulate adhesive interactions between pre- and post-synaptic cells, and provide a novel mechanism for controlling the activity of liprin-α in the developing growth cone.
Keywords: Drosophila, neurodevelopment, photoreceptor, axon, target selection, cell adhesion, synapse
Introduction
During development stable, precise connections form between pre- and post-synaptic cells. The establishment of these contacts is dependent upon both instructive cues that determine which neurons connect, as well as permissive interactions that are necessary for the subsequent assembly of synapses. The molecular mechanisms that lead to the formation of stable contacts between neurons, and that catalyze synapse formation after an axon reaches its target, are only incompletely understood (reviewed in Huber et al., 2003; Shen, 2004). Indeed, it is unclear how a developing neuron transitions from a mode of axon extension toward a prospective target to the formation of a stable adhesive contact, to the assembly of a synapse. Here we examine genetic and physical interactions amongst a group of proteins that appear to play an evolutionarily conserved role in stabilizing the interactions between pre- and post-synaptic cells.
Classical cadherins, receptor tyrosine phosphatases and the synaptic scaffolding molecule liprin-α play critical roles in axon targeting and synapse formation in many systems (reviewed in Suzuki and Takeichi, 2008; Stryker and Johnson, 2007; Lilien and Balsamo, 2005). The scaffolding protein Liprin-α and the receptor tyrosine phosphatase LAR regulate synapse morphogenesis, and genetic interactions between these genes suggest that LAR recruits Liprin-α to synapses in both worms and flies (Zhen and Jin 1999; Kaufmann et al. 2002; Ackley et al. 2005). These proteins also mediate recruitment of AMPA receptors and cadherin-catenin complexes to synapses in cultured rat neurons (Wyszynski et al. 2002; Dunah et al. 2005). Genetic epistasis experiments demonstrate that DLAR requires Liprin-α for its function at the Drosophila neuromuscular junction and that both Liprin-α and LAR depend on each other for localization (Kauffman et al., 2002; Ackley et al. 2005). Indeed, LAR can bind Liprin-α via interactions between its D2 phosphatase domain and C-terminal sterile alpha motifs (SAM) on Liprin-α (Serra-Pages et al. 1995; Serra-Pages, et al. 1998). Additionally, Liprin-α interacts with the kinesin KIF1A and is required for anterograde transport and accumulation of synaptic vesicles pre-synaptically (Shin et al. 2003; Miller et al. 2005; Patel et al. 2006). Interestingly, LAR also has axon guidance functions in Drosophila and vertebrate motor neurons (Krueger et al. 1996; Sun et al. 2000; Uetani et al., 2006), but such a role has not been identified for Liprin-α. Together, these studies suggest that LAR may stabilize nascent synapses by recruiting Liprin-α associated synaptic vesicles.
Classical cadherins also regulate synaptic morphogenesis. In hippocampal neurons the cadherin associated protein β-catenin is required pre-synaptically for synaptic vesicle localization, a process regulated by BDNF through effects on catenin phosphorylation and cadherin association (Bamji et al. 2003). Cadherins also control dendritic spine morphogenesis and activity dependent remodeling (Togashi et al. 2002; Okamura et al. 2004). Indeed, dynamic regulation of vertebrate N-cadherin levels post-synaptically plays a key role in coupling synapse plasticity to morphological changes in dendritic spines (Tai et al., 2007). Finally, biochemical studies at both mature synapses, and in developing axons, have demonstrated direct and indirect associations between vertebrate N-cadherin, LAR, liprin-α, and β-catenin (Kypta et al. 1996; Brady-Kalnay et al. 1998; Dunah et al. 2005). While these studies raise the possibility that N-cadherin cooperates with LAR and Liprin-α during axon target selection and synapse formation, no in vivo functional interactions between N-cadherin and DLAR or liprin-a have been described. Here we examine genetic and physical interactions amongst these three proteins in an experimental context in which all three play critical roles.
The formation of precise connections between pre- and post-synaptic cells has been studied intensively in the Drosophila visual system, where individual photoreceptor axons are genetically programmed to make stereotyped projections to specific targets (reviewed in Clandinin and Zipursky, 2002). Photoreceptors (R cells) are organized into unit eyes called ommatidia. Each ommatidium contains eight R cells that enter the optic lobe in a common fascicle until they reach the brain, before making specific stereotyped connections with their neuronal targets. These axons choose their synaptic partners through two steps, the first of which requires ganglion specific targeting whereby R1–R6 axons terminate in the first optic ganglion, the lamina, while R7 and R8 axons terminate in a deeper ganglion, the medulla. In the second stage of target selection, within the lamina, R1–R6 axons make precise lateral connections innervating specific target neurons arranged in a regular columnar pattern. In the medulla, in contrast, R7 and R8 axons innervate distinct layers within the neuropil (Meinertzhagen and Hanson 1993). All of these targeting decisions are independent of visual input and neuronal activity, and are thus genetically programmed (Clandinin and Zipursky, 2000; Hiesinger et al., 2006). Finally, a variety of genetic screens have identified many loci that play important roles in different aspects of visual system development (reviewed in Mast et al., 2006).
The classical cadherin N-cadherin, the receptor tyrosine phosphatase DLAR and the scaffolding molecule liprin-α play important roles in late stages of target selection in both the lamina and the medulla (Lee et al., 2001; Clandinin et al., 2001; Maurel-Zaffran et al., 2001; Ting et al., 2005, Prakash et al., 2005; Choe et al., 2006; Hofmeyer et al., 2006). In particular, individual R1-6 axons mutant for N-cadherin, DLAR, or Liprin-α reach the lamina neuropil but fail to make short extensions to adjacent columns of target neurons. In the medulla, R7 axons mutant for these genes terminate in an inappropriate layer. While R1–R6 axons extend to their targets during a single developmental period during mid-pupal development (Meinertzhagen and Hanson, 1993; Clandinin and Zipursky, 2000), layer-specific targeting by R7 axons occurs in two temporally distinct steps (Ting et al., 2005). In particular, R7 axons initially project to the R7-temporary layer during early pupal development, before making a final extension to a deeper layer (Ting et al. 2005). Intriguingly while R7 axons need N-cadherin both to reach the R7-temporary layer, and to extend to their final layer, the initial extension to the R7 temporary layer is independent of liprin-α, and DLAR (which are only required for the final extension to the appropriate layer). These N-cadherin mediated interactions are thought to be purely adhesive in nature (Yonekura et al., 2007). Thus, while photoreceptor targeting phenotypes seen in adult N-cadherin, DLAR and liprin-a mutants are broadly similar, in both the lamina and the medulla, detailed phenotypic studies argue that targeting in the lamina and the medulla use developmentally distinct mechanisms.
Here we examine the functions of these three loci in detail, focusing on their roles in R1–R6 axon targeting in the lamina. In this context, DLAR function is independent of its catalytic activity, suggesting that it acts as a molecular scaffold. Moreover, while all three genes display similar loss-of-function phenotypes, genetic studies reveal that LAR and N-cadherin can also inhibit liprin-α activity. Single, double and triple mutant analyses reveal that these three genes have at least partially independent functions, while biochemical studies demonstrate that these proteins are physically associated. Thus, these studies describe a new model for these proteins’ functions at a specific identified synapse.
RESULTS
DLAR is cell-autonomously required in all R1–R6 cells for normal targeting
To make individual photoreceptors homozygous for a DLAR mutant chromosome in an otherwise wild-type background we used the MARCM method to generate somatic mosaics (Fig. 1; Lee and Luo, 1999; Prakash et al., 2005). This method allowed us to examine cell-autonomous defects in target selection but also provided a mechanism to express DLAR transgenes specifically in DLAR mutant cells. We scored extension of R1-6 axons in the lamina at 40% pupal development and categorized axon targeting as normal, aberrant (meaning that the axon either extended to multiple targets or to the wrong target, or was morphologically atypical), or failing to extend (meaning that the axon did not extend away from the ommatidial axon fascicle). Using this method, individual R1-6 cells homozygous for a control chromosome projected to appropriate targets 100% of the time (Figs. 1A, G; n=98). Among R1-6 axons mutant for the null allele DLAR2127 43% extended normally, 28% extended aberrantly, and 29% did not extend (Figs 1B, C, G; n=163). As these defects were not significantly differently across R cell subtypes, data from all R cells were pooled (Fig. 1G). These phenotypes are qualitatively identical to defects previously described using this method to examine N-cadherin and liprin-α mutant R1–R6 cells (Prakash et al., 2005; Choe et al., 2006). Thus DLAR is cell-autonomously required in all R1–R6 cells for normal target selection within the lamina.
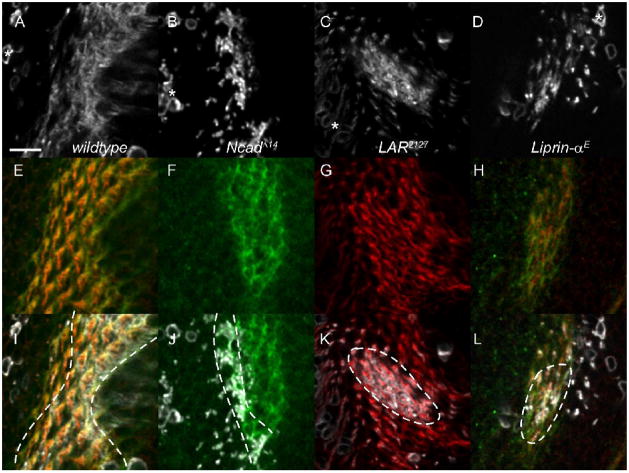
Figure 1. LAR is required cell autonomously in all R cells for target selection, and its function does not require phosphatase activity.
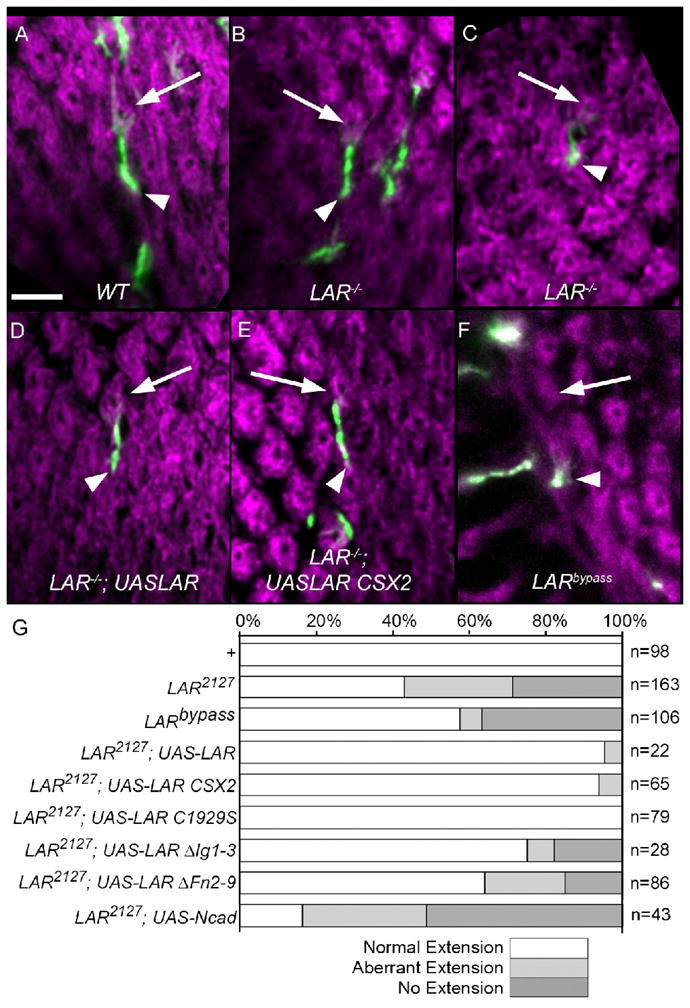
R4 cells homozygous for a wild-type chromosome (A), or for LAR2127 (B–E), or for the LARbypass allele (F). In (D), the homozygous cell expresses a wild-type LAR transgene under UAS control. In (E), the homozygous cell expresses a catalytically inactive LAR transgene, UAS-LAR CSX2. Marked clones expressed GFP and their projections were imaged in the lamina (green) at 42% pupal development. Photoreceptor axons form donut shaped cartridges (magenta). Each panel depicts an R4 axon, and the photoreceptor bundle it originates from (arrowhead) and its intended cartridge (arrow). Depending on genotype of the clone, the R4 axons extends normally (A, B, D, E), extends aberrantly (C), or does not extend at all (F). Scale 5 μm. (G) Quantification of targeting phenotypes for LAR mutant R cell clones, pooled across all R cell subtypes. R1-6 extensions in the lamina were categorized as normal (white bars), aberrant (light grey bars), or not extending (dark grey bars). For each genotype, n denotes the number of R cell axons scored. UAS-LAR CSX2 encodes a LAR transgene bearing inactivating C-S mutations in both phosphatase domains; UAS-LAR C1929S encodes a transgene bearing inactivating C-S mutation in the C terminal phosphatase domain; UAS-LAR ΔIg1-3 deletes the first 3 Ig domains; UAS-LAR ΔFN2-9 deletes 8 of the 9 Fibronectin Type III domains.
The DLAR extracellular domain is required for DLAR function in R1–R6 axons
Drosophila DLAR comprises an extracellular domain that contains three immunoglobulin-like and nine fibronectin type-III repeats, as well as an intracellular domain that consists of two phosphatase domains, D1 and D2 (Streuli et al. 1989). To identify which domains and functions of DLAR are essential in photoreceptor target selection, we used domain specific DLAR mutations and modified DLAR transgenes to perturb DLAR function (Krueger et al., 2003). As a prelude to structure function studies of DLAR, we first demonstrated that expressing a full length DLAR transgene in mutant R cells rescued the DLAR null phenotype nearly completely, with 95% of rescued mutant axons extending normally and 5% aberrantly (Figs. 1D, G; n=22); none of these axons failed to extend. However, only partial rescue was observed when photoreceptors expressed DLAR transgenes deleting either the immunoglobulin-like (ΔIg1-3) or eight of the nine fibronectin type-III repeats (ΔFn2-9) (Fig. 1G). Normal extension occurred in 75% (n=28) and 64% (n=86) of cases deleting the immunoglobulin and fibronectin domains of DLAR, respectively. This rescue by ΔIg1-3 or ΔFn2-9 was statistically significant (χ2, p<0.01) but intermediate relative to that obtained with the full length DLAR transgene. These data argue that both of these extracellular regions are required for target selection.
The DLAR D2 domain, but not phosphatase activity, is required for DLAR function
The DLAR D1 and D2 phosphatase domains contain a conserved cysteine essential for enzymatic activity, and are both catalytically active. The construct DLAR-CSX2 contains cysteine to serine mutations in both phosphatase domains that abrogate phosphatase activity (Krueger et al. 2003). Surprisingly, expressing this construct in photoreceptors homozygous mutant for DLAR2127 resulted in rescue of the mutant phenotype that was quantitatively indistinguishable with that seen using a wild-type transgene, with 94% of axons extending normally (Figs. 1E, G; n=65). Thus DLAR phosphatase activity is not essential for DLAR function in R1-6 cell axons. To examine this issue further, we next tested whether the cytoplasmic domain of DLAR was required for its function. To do this, we took advantage of the hypomorphic chromosomal allele DLARbypass, which is truncated after the D1 domain, and lacks the D2 domain (Krueger et al. 2003). Embryos homozygous for this allele retain normal expression of DLAR protein (Krueger et al., 2003). In MARCM clones, 37% of R cells failed to extend, 6% extended aberrantly, and 58% of homozygous R cells (Fig. 1G, n=106) extended correctly, displaying a strong phenotype that was nonetheless somewhat weaker than that associated with a null allele of DLAR, DLAR2127 (χ2, p<0.001). Thus, the intracellular domain of DLAR is required for DLAR function in R cell axons. Together, this data suggests that DLAR function in this context is independent of phosphatase activity but raises the possibility that DLAR may associate with another protein(s) (such as liprin-α) via its D2 domain, and that this association is critical to DLAR function in this context.
Liprin-α, LAR and N-cadherin display both positive and negative genetic interactions
If N-cadherin, DLAR, and Liprin-α have similar functions in R1–R6 target selection, these genes may also interact in other tissues and earlier developmental processes that contribute to the adult lethality of null mutations in these genes. We therefore took advantage of hypomorphic mutations in these genes, assembling a series of genetic backgrounds in which we tested pairwise genetic interactions among N-cadherin, DLAR, and Liprin-α for their effect on adult viability. Flies bearing heteroallelic combinations of null and hypomorphic alleles of DLAR or Liprin-α can survive to adulthood at low frequency and provide a sensitized background to test the effects of modifiers (Table 1). In crosses between flies carrying the hypomorphic allele DLAR451 and the loss of function allele DLAR2127, 8.9% of adult progeny are of the heteroallelic genotype DLAR451/DLAR2127. This heteroallelic class of progeny should form one-third of survivors if there is no lethal interaction between alleles. To test for genetic interactions with DLAR, we measured survival of the DLAR451/DLAR2127 progeny in genetic backgrounds in which the activities of either N-cadherin or Liprin-α had been reduced by 50% using null alleles of each. Here the percentage of DLAR451/DLAR451 flies dropped approximately tenfold, to 0.9% (χ2, p<0.0001) in genetic backgrounds in which a single copy of Liprin-α or N-caherin had been removed. This result suggests that both Liprin-α and N-cad enhance lethality associated with reduced DLAR activity.
Table 1. Genetic interactions between N-cadherin, LAR and Liprin-α in adult lethality.
Flies heterozygous for a null allele of LAR or Liprin-α were crossed to flies heterozygous for hypomorphic alleles of the same gene. In the F1 generation the percentage of flies heteroallelic for null and hypomorphic alleles of LAR or Liprin-α were scored. Heteroallelic progeny were of the genotypes LAR451/LAR2127, Liprin-αF/Liprin-αE, and Liprin-α1/Liprin-αE. To test for genetic interactions, these crosses were repeated with one parent also heterozygous for null alleles of NcadΔ14, LAR2127, or Liprin-αE.
| Heteroallelic genotype in F1 | % of F1 progeny with genotype | Number of F1 progeny scored |
|---|---|---|
| LAR451/LAR2127 | 8.9% | 1023 |
| LAR451/LAR2127, Liprin-αE/+ | 0.9% | 1657 |
| LAR451/LAR2127, NcadΔ14/+ | 0.9% | 1424 |
| Liprin-αF/Liprin-αE | 6.7% | 670 |
| Liprin-αF/Liprin-αE, LAR2127/+ | 22.8% | 1555 |
| Liprin-αF/Liprin-αE, NcadΔ14/+ | 25.2% | 1255 |
| Liprin-α1/Liprin-αE | 3.3% | 520 |
| Liprin-α1/Liprin-αE, LAR2127/+ | 13.9% | 1286 |
| Liprin-α1/Liprin-αE, NcadΔ14/+ | 17.4% | 772 |
| Liprin-αF/NcadΔ14, LAR2127/+ | 35.8% | 839 |
Crosses between flies bearing the hypomorphic allele Liprin-αF and the null allele Liprin-αE produce viable heteroallelic progeny of the genotype Liprin-αF/Liprin-αE 6.7% of the time (Table 1). Unexpectedly, when the activity of either N-cadherin or DLAR was reduced by removal of a single copy of either gene, the viability of this genetic background increased. In particular, Liprin-αF/Liprin-αE flies bearing a single wild-type copy of DLAR represented 22.8% of progeny (χ2, p<0.0001), while those flies bearing a single wild-type copy of N-cadherin represented 25.2% of progeny (χ2, p<0.0001). These data demonstrate that the loss of function alleles DLAR2127 and N-cadΔ14 suppress the adult lethality of Liprin-α. To test whether this effect reflected an allele-specific interaction with liprinF, we repeated the same experiment using a second hypomorphic allele, Liprin-α1. In particular, heteroallelic progeny of the genotype Liprin-α1/Liprin-αE represent 3.3% of progeny. Liprin-α1/Liprin-αE flies also carrying DLAR2127 represented 13.9% of progeny (χ2, p<0.0001), and those flies also carrying N-cadΔ14 represented 17.4% of progeny (χ2, p<0.0001). These results demonstrate that in genetic backgrounds in which Liprin-α activity is reduced, null alleles of N-cadherin and DLAR act as dominant suppressors, increasing viability of this genotype by at least 3-fold. These interactions are not allele specific since they occur with two distinct Liprin-α hypomorphs. Thus, they could reflect negative regulatory interactions that N-cadherin and DLAR exert when Liprin-α activity is reduced. Alternatively, these interactions could reflect a negative regulatory interaction that is revealed when only a fragment of the Liprin-α protein retained by both alleles is expressed (see Discussion).
DLAR, Liprin-α, and N-cadherin do not act in a linear genetic pathway
We next sought to evaluate genetic interactions between DLAR, Liprin-α, and N-cad during photoreceptor target selection, under conditions in which the activity of each protein is eliminated. Since null mutations in these genes are lethal, we bypassed the early requirements for these genes by creating somatic mosaics using the FLP/FRT system, expressing the FLP recombinase under the control of the eyeless promoter (eyFLP; (Newsome et al. 2000). We then combined this FLP source with the MARCM method (Lee and Luo, 1999), in which cells homozygous for control or mutant chromosomes do not express the Gal4 repressor Gal80. Finally, to visualize target selection by individual R cells we specifically labeled R4 axons using the construct E(spl)mδ-lacZ (Cooper and Bray 1999). In this genetic background, R cell clones are typically large, comprising at least 50–100 cells, having undergone considerable expansion prior to differentiation. Thus, these clones minimize the potential effects of perduring mRNA and protein on the phenotypes observed. Within this large group of mutant cells, only the R4 axons are then labeled; typical clones contained between 5–20 labeled R4 axons. In the lamina, R4 axons make stereotyped extensions, which could be visualized at 40% pupal development, immediately after the stage at which they reach their neuronal targets (Meinertzhagen and Hanson 1993). This approach, then, enabled us to examine the targeting behavior of hundreds of identical R cell axons, allowing us to quantitatively compare the behavior of single, double and triply mutant R4 cells.
This work, combined with previous studies examining N-cadherin and liprin-a demonstrated that R cell axons lacking any of these three genes frequently innervate inappropriate targets in the lamina and medulla, and that subtypes of R1-6 axons depend equivalently on each gene (Prakash et al. 2005; Ting et al. 2005; Choe et al. 2006). When the eye was made homozygous for a control chromosome, 100% of R4 axons targeted normally (Figs. 2A, I; n=113). As expected, when large R cell clones were made homozygous for null alleles of DLAR, Liprin-α, or N-cadherin R4 axons frequently failed to extend, or extended aberrantly (Figs. 2B–D, I). In particular, in clones homozygous for a molecular null allele of N-cadherin, only 10% of R4 axons extended normally, while 70% extended aberrantly, and 20% did not extend (Fig. 2I; n=104). In clones homozygous for a null allele of DLAR, 23% extended normally, 61% extended aberrantly, and 16% did not extend (Fig. 2I; n=172). In clones homozygous for a null allele of Liprin-α 21% extended normally, 49% extended aberrantly, and 30% did not extend (Fig. 2I; n=166). Thus, R4 targeting defects are quantitatively indistinguishable when photoreceptors were homozygous mutant for null alleles in DLAR or Liprin-α, but were somewhat more severely affected when homozygous for a null allele of N-cadherin (χ2, p<0.05). Moreover, consistent with the notion that perdurance effects play only a minor role in influencing phenotypic severity in these clonal studies, the results are quantitatively similar to those seen in single cell clones, having no effect on N-cadherin and liprin-α and affecting the expressivity of DLAR mutants only slightly (Prakash et al., 2005; Choe et al., 2006; this work). In photoreceptors homozygous for double mutant combinations of null alleles in DLAR, Liprin-α, and N-cadherin, targeting errors were more frequent than in single mutants. In N-cadherin DLAR double mutants (Figs. 2E, I, n=136), and N-cadherin Liprin-α double mutants (Figs. 2F, I, n=64), no R4 axons extended normally, while approximately equal numbers of axons extended abnormally and failed to extend. In Liprin-αE DLAR2127 5% of axons extended normally (Figs. 2G, I, n=140), a phenotype significantly worse than that seen in DLAR2127 and Liprin-α E single mutants (χ2, p<0.05). Finally, in triple mutant clones, 2 % of axons extended normally, 24% extended aberrantly, and 64% did not extend, but this distribution is not significantly different from any of the double mutant combinations (Figs. 2H, I, n=136). Together these data demonstrate that mutations in DLAR, Liprin-α, and N-cadherin act only additively, enhancing defects in R4 target selection. As all of these mutations are molecular null alleles, and the clones we generated are unlikely to display significant perdurance, these observations are inconsistent with N-cadherin, DLAR and liprin-α acting in a simple, linear genetic pathway. That is, such a linear pathway would predict that double and triple mutant combinations would be no more severe that single mutant backgrounds.
Figure 2. N-cadherin, LAR, and Liprin-α display additive genetic interactions in R4 axons.
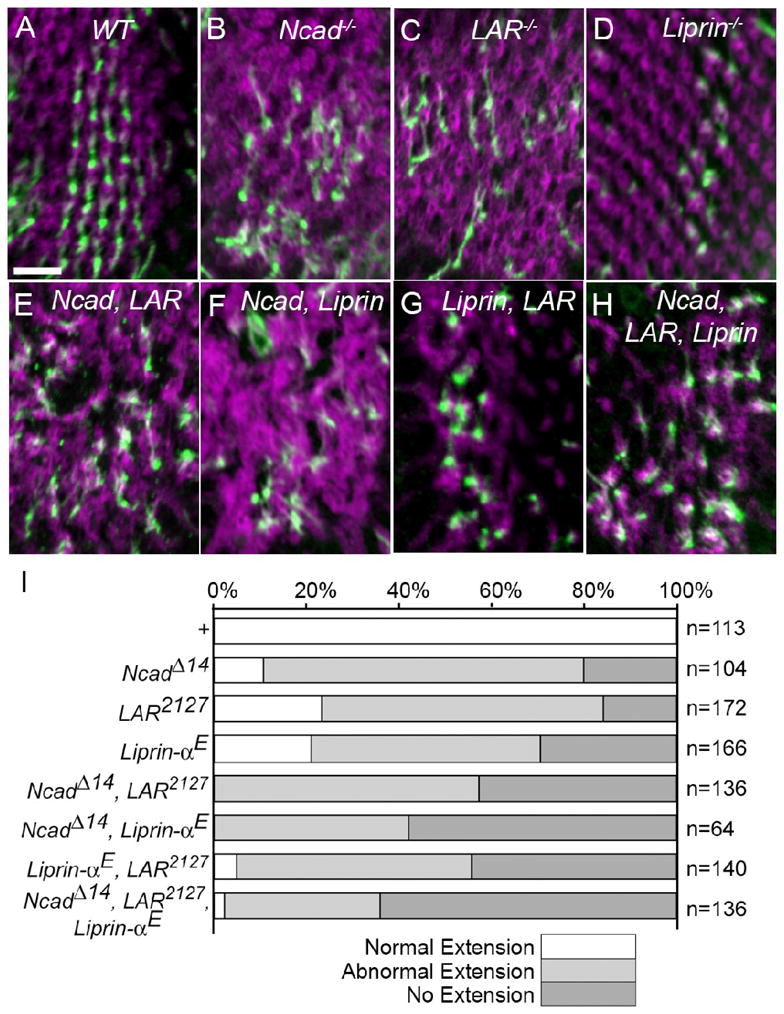
Somatic mosaics of the eye were generated using the eyFLP method. Patches of R cells were made homozygous for a control chromosome or one carrying the loss of function mutations N-cadherinΔ14, LAR2127, or Liprin-α E, singly or in combination, in an otherwise heterozygous (phenotypically wild-type) animal. At 42% pupal development wild-type photoreceptors (magenta) form regularly arranged donut shaped structures called cartridges (A), but these structures are disrupted in mutant patches (B–H). Individual R4 axons (green) from wild-type ommatidia make stereotyped unidirectional projections (A), but in mutant patches axons often fail to extend or project to the wrong target (B–H). Scale 10 μm. (I) Quantification of R4 axon targeting in N-cadherinΔ14, LAR2127, or Liprin-α single, double, or triple mutant somatic mosaic clones. R4 projections were scored as normal (white), abnormal (gray), or failing to extend (black). n denotes the number of R4 axons scored.
To further explore the genetic relationship between N-cadherin, DLAR and liprin-α we asked whether these proteins could substitute for each other’s function in R1-6 target selection. Using the MARCM method of generating somatic mosaics (Lee and Luo, 1999), we made individual photoreceptors homozygous for a null mutation in one of these genes, and expressed a transgene encoding a wild-type copy of a different gene. Expression of the DLAR transgene in N-cadherinΔ14 mutant photoreceptors did not rescue the mutant phenotype (data not shown). Meanwhile expression of a N-cadherin transgene in DLAR2127 or liprin-α1 mutant photoreceptors worsened the mutant phenotype, suggesting that N-cadherin levels are important for targeting and consistent with experiments demonstrating that expression of N-cadherin in a wild-type photoreceptor can cause targeting errors (Figure 1G and data not shown; Prakash et al, 2005). The inability of these proteins to substitute for each others’ function in R1-6 target selection argues against a simple linear genetic relationship between these genes, and demonstrates that N-cadherin, liprin-α and DLAR have both overlapping and distinct functions in the developing growth cone (see Discussion).
N-cadherin and DLAR localization in R1-6 growth cones does not change in N-cadherin, DLAR, or Liprin-α mutants
One possible function for N-cadherin, DLAR, or Liprin-α may be to transport or localize other proteins essential for target selection at the growth cone. To investigate this possibility we examined the localization of N-cadherin and DLAR in R1-6 growth cones at the developmental time point at which they are extending towards their targets in the lamina. Using the MARCM method for generating somatic mosaics (Lee and Luo, 1999), large patches of the eye weremade homozygous for wild-type (Fig. 3A, E, I), N-cadherinΔ14(Fig 3B, F, J), DLAR2127 (Fig 3C, G, K), or Liprin-α E (Fig 3D, H, L) and compared the localization of N-cadherin and DLAR with surrounding wild-type tissue. When photoreceptors were mutant for N- cadherin - or liprin-α, expression of DLAR was unchanged, while in photoreceptors mutant for DLAR or liprin-a, expression of N-cadherin was unchanged. Together, these data argue that N-cadherin and DLAR localization in the lamina plexus are independent of N-cadherin, DLAR, and Liprin-α activity.
Figure 3. Localization of N-cadherin or LAR in photoreceptor growth cones does not depend on other proteins involved in this step of targeting.
Somatic mosaic patches of photoreceptors in the eye were generated using the MARCM method. These mutant photoreceptors express GFP (white) and their processes can be visualized in the lamina. Approximate clone boundaries are marked by hashed lines in I-L. Occasionally, lamina unipolar neurons are also mosaic (*). At 28% pupal development R1-6 axons are beginning to extend towards their targets in the lamina and express N-cadherin (red) and LAR (green). Cells are made homozygous for wild-type (A, E, I), N-cadherinΔ14 (B, F, J), LAR2127 (C, G, K), or Liprin-αE (D, H, L).
N-cadherin and DLAR form a physical association
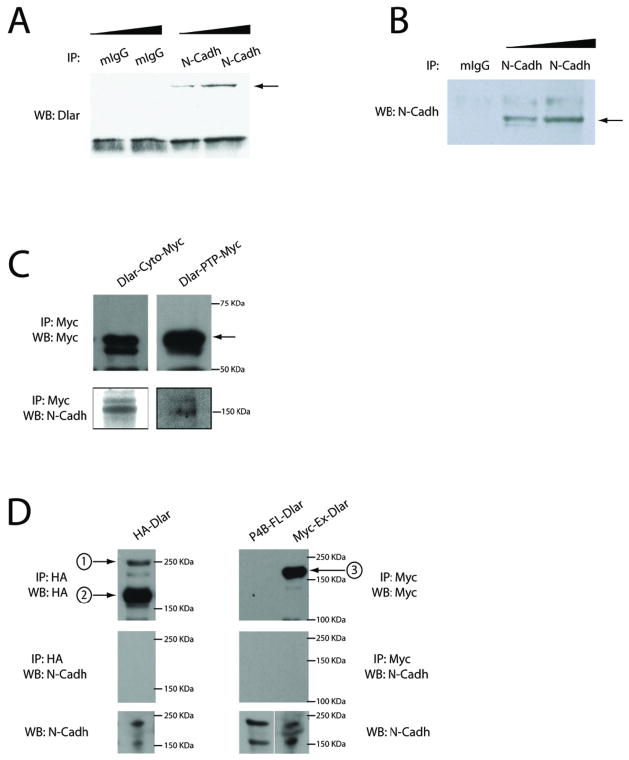
Previous studies had demonstrated that liprin-α is physically associated with LAR in a variety of cellular contexts, in both flies and mammals (Serra-Pages et al., 1995; Kaufmann et al., 2002; Dunah et al., 2005). The complex genetic interactions we observed raised the possibility that DLAR may also associate with N-cadherin. As part of a DLAR affinity purification and mass spectrometry screen in cell culture, we identified N-cadherin as a candidate DLAR-associated protein (A.G. and D.V.V., unpublished observations), consistent with previous observations in vertebrate cells (Kypta et al., 1996). As a first approach to confirming this interaction in vivo, we used antibodies directed against N-cadherin and DLAR to conduct co-immunoprecipitation experiments using wild-type embryo extracts. We were able to pull-down N-cadherin from these lysates and demonstrate that DLAR was quantitatively associated with it, suggesting these two proteins are associated in vivo (Figs. 4A, B). As DLAR undergoes post-translational cleavage, our antibodies directed against the extracellular portion of DLAR were unable to pull-down N-cadherin (data not shown). To examine the N-cadherin-DLAR interaction more closely, we used one untagged DLAR transgene (P4B), one HA-tagged full length DLAR transgene (Maurel-Zaffran et al., 2001), as well as 3 Myc-tagged DLAR transgenes (Krueger et al., 2003): one expressing just the extracellular domain, one expressing the entire cytoplasmic domain and one expressing just the phosphatase domains. We expressed these constructs in embryos using the pan-neuronal Elav promoter, then immuno-precipitated the corresponding fragments of DLAR using antibodies directed against HA or Myc tags, and detected co-immunoprecipitation of N-cadherin with the cytoplasmic domain of DLAR, and the phosphatase domain construct, but not with either the full-length transgene or the extracellular domain (Figs 4C, D). Thus, DLAR is physically associated with N-cadherin via its intracellular domain in vivo.
Figure 4. N-cadherin and DLAR are physically associated in vivo.
(A) Immunoprecipitation of DLAR with N-Cadherin. (A) N-Cadherin was immunoprecipitated from late stage embryonic extracts (Ncad), and the precipitate probed with antisera raised against DLAR or (B) N-Cadherin. (C) Embryonic extracts expressing either the entire cytoplasmic region (left panel) or the PTP domains of the Dlar receptor (right panel) associate with N-Cadherin. Myc was immunoprecipitated from lysates and probed for either Myc to identify Dlar (top panels) or for N-Cadherin (bottom panels). (D) The extracellular domain of Dlar is not sufficient to pull down N-Cadherin. Embryonic extracts expressing either full length HA tagged Dlar (left panel), an untagged full length Dlar (P4B) or a Myc tagged extracellular domain of the receptor were examined (right panel). The HA tagged full length receptor undergoes cleavage and after immuno-precipitating the receptor with HA antibody, two major bands are present, the first (1), being full length Dlar and the second (2) major band appearing to be the extracellular portion of Dlar, as identified by comparison to Myc tagged extracellular Dlar alone (3) (right panel). None of the three Dlar constructs tested were able to pull down N-Cadherin (middle panels). Bottom panels show that embryos expressing full length or extracellular Dlar express endogenous N-Cadherin.
DISCUSSION
Summary
These studies support three central conclusions. First, our data demonstrate that DLAR has functions independent of its catalytic activity to allow formation of stable contacts between R1–R6 axons and their targets. Second, our genetic and physical interaction studies demonstrate that all three proteins form a complex but do not act in a simple linear genetic pathway. Finally, our genetic data demonstrate that while N-cadherin, DLAR and liprin-α all have similar loss-of-function phenotypes, N-cadherin and DLAR also act as negative regulators of liprin-α. These genetic and physical interactions suggest a mechanism by which liprin-α activity can be precisely controlled.
Dissecting DLAR function in R1–R6 axons
Previous studies have demonstrated that DLAR controls target selection by R1-6 photoreceptor axons in the lamina and R7 axons in the medulla in somatic mosaic flies in which the entire eye is mutant for DLAR (Clandinin et al. 2001; Maurel-Zaffran et al. 2001) These studies demonstrated that DLAR is required cell-autonomously in R1 and R6 cells by examining targeting of single mutant axons in adult animals, but left open the possibility that targeting is initially normal in DLAR mutants. Indeed, such a late function has been described for DLAR in the targeting of R7 axons (Ting et al., 2005). To address this issue directly, we examined photoreceptor targeting immediately after R1-6 axons make their initial extension to their targets, in somatic mosaics in which individual photoreceptors were mutant. Such DLAR mutant photoreceptors fail to extend to their targets in 29% of all cases, while a further 28% extend aberrantly. These results are quantitatively comparable to previous studies of single adult R1 and R6 cells homozygous for DLAR, in which 48% of R cell axons failed to extend (Clandinin et al. 2001). As R1–R6 cell axons extend to their targets in the 10 hours preceding the time point we examined in this study (Clandinin and Zipursky, 2000), and reach their targets only 2 hours before we assess targeting phenotypes, these data argue that DLAR is required for the initial formation of stable contacts between R1–R6 axons and their targets. This contrasts with the function of DLAR in R7 axons, where the initial targeting of these axons to a distinct layer is independent of DLAR function, and the targeting defect in DLAR emerges during a second step in the targeting process (Ting et al., 2005). Of course, we cannot exclude the possibility that there are two molecularly distinct processes taking place during R1–R6 target selection in the lamina, but that they occur contemporaneously.
Our studies also shed light on the structural domain requirements of this DLAR function. Two extracellular matrix proteins, the laminin-nidogen complex and heparan sulfate proteoglycans (HSPGs) bind DLAR via its fibronectin type-III repeats (FNIII) and immunoglobulin-like domains (Ig), respectively (O’Grady et al. 1998; Aricescu et al. 2002; Johnson et al., 2006). We demonstrate that DLAR constructs missing either the FNIII or Ig domains partially rescue the DLAR mutant phenotype compared with full length DLAR. These data suggest that laminins and HSPGs function as partially redundant ligands for DLAR during R1-6 target selection. Consistent with this possibility, the HSPGs Syndecan and Dallylike genetically interact with DLAR and have complex guidance phenotypes in photoreceptors (Fox and Zinn 2005; Rawson et al. 2005; Johnson et al. 2006). The intracellular domain of DLAR contains two phosphatase domains, but only the membrane proximal D1 domain is catalytically active (Pot et al. 1991; Krueger et al. 2003). The D2 domain can bind to Liprin-α and the cytoskeletal regulators ena and Abl (Serra-Pages et al. 1995; Debant et al. 1996; Wills et al. 1999). Our data demonstrate that DLAR phosphatase activity is not required for normal target selection, but that the D2 domain has a critical function in R1–R6 axons. A similar requirement for the D2 domain but not phosphatase activity was also described for DLAR function in viability, though the cellular focus of DLAR activity in this context is unknown (Krueger et al. 2003). Our data argue that DLAR function in R cells is likely to be as a scaffold, linking its extracellular ligands to the recruitment of proteins like Liprin-α to the membrane. One possibility, then, is that this association between DLAR and liprin-α might then serve to recruit pre-synaptic components that stabilize nascent synaptic contacts, as has previously been suggested for DLAR-liprin-α interactions at the neuromuscular junction (Kauffmann et al., 2002). While DLAR may influence the localization of N-cadherin within the growth cone, we did not observe any gross changes in N-cadherin localization in DLAR mutant photoreceptors.
N-cadherin, DLAR and Liprin-α work together in a complex genetic pathway
Our analysis of double and triple mutant photoreceptors homozygous for molecular null alleles in each of these genes, combined with our biochemical studies, shed additional light on how these proteins promote downstream events in the growth cone. In particular, our results argue strongly against the possibility that these proteins act in a simple, linear pathway with a single output (Fig. 5). That is, the strong prediction from such a model would be that single, double and triple mutant combinations for null alleles would all be quantitatively equivalent, since eliminating any one component would completely block the pathway. This is not what we observe when we examine R cell targeting phenotypes in large clones: all double and triple mutant combinations are more severe that any of the single mutant clones. Furthermore, if these proteins acted in a linear pathway, we would predict that over-expression of one of these proteins could functionally substitute for absence of one of the others. However, we did not find any evidence that DLAR or N-cadherin could fulfill such a role. These results argue that while these proteins are physically associated in a complex, each gene has effects on axon targeting that are at least partially independent of one another. As N-cadherin is required both pre- and post-synaptically to mediate adhesive contacts between R cell axons and their targets (Prakash et al., 2005), while DLAR and liprin-α are required strictly pre-synaptically (Choe et al., 2006), one aspect of DLAR and liprin-α function could be to stabilize these initial adhesive interactions either directly or indirectly. Moreover, even when all three proteins are eliminated, R cell axon extension is not completely blocked, arguing that there is at least one additional pathway that stabilizes pre- and post-synaptic contacts.
Figure 5. Schematic illustration of the genetic and physical interactions between N-cadherin, DLAR and liprin-α.
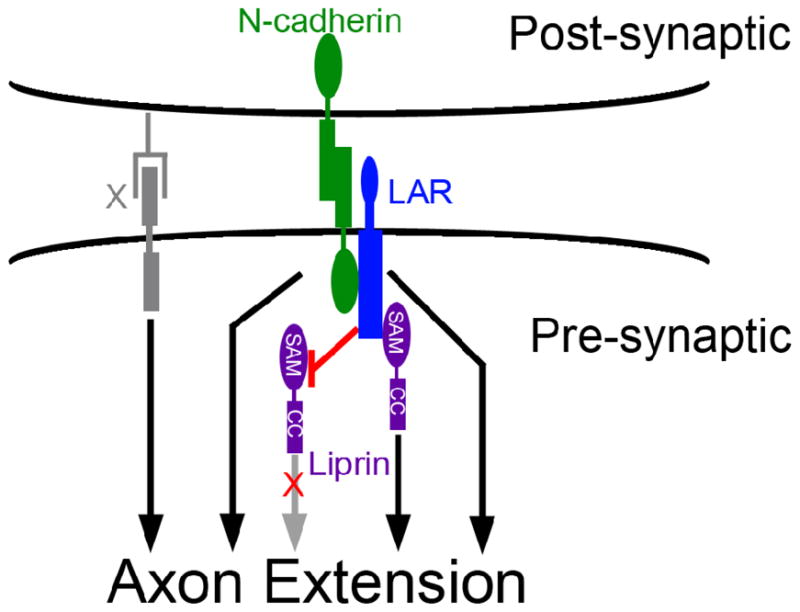
N-cadherin (green) is required both pre- and post-synaptically for R cell axons to make appropriate connections (Prakash et al., 2005), while DLAR and liprin-α are required strictly pre-synaptically (Choe et al., 2006). As N-cadherin, liprin-α and DLAR display additive interactions in double and triple mutant combinations, all three genes must have independent, positive effects on contact stabilization (black arrows). However, since reducing N-cadherin and DLAR activity can suppress the lethality associated with liprin-α hypomorphs, these two genes must also inhibit liprin-α (red arrow). We hypothesize that this inhibition is relieved by binding of the liprin-α SAM domain to DLAR. Finally, since even R cell axons homozygous for null alleles of all three genes still occasionally form contact with their targets, we infer a fourth adhesive factor, X (gray) that can act in parallel to stabilize R cell-target interactions.
N-cadherin and DLAR negatively regulate liprin-α
Loss of function mutations in N-cadherin, DLAR, and liprin-α all display similar targeting phenotypes in R cell axons, and double and triple mutant combinations display stronger phenotypes than single mutants. Thus, in a formal genetic sense, all three loci act as positive regulators of axon extension. However, our genetic interaction studies using heteroallelic combinations that reduce DLAR and liprin-α activity have uncovered unexpected complexity in the regulatory relationships between these proteins. In particular, while reducing liprin-α or N-cadherin activity in a hypomorphic DLAR background causes the expected phenotypic enhancement, reducing N-cadherin or DLAR activity in hypomorphic liprin-α backgrounds causes phenotypic suppression. Thus, depending precisely on how the interaction experiment is conducted, the net genetic interaction between N-cadherin, DLAR and liprin-α can either be positive, or negative. We propose two alternative models to reconcile these results. One possibility is that quantitative differences in liprin-a activity are critical to determining the sign of the interaction. In this view, when liprin-a activity is reduced, N-cadherin and DLAR act as negative regulators of liprin-a, but when liprin-a activity is either normal, or completely eliminated, N-cadherin and DLAR act as positive regulators. Inconsistent with this view, removing one copy of liprin-a, thereby reducing liprin-a activity by half, enhances the lethality associated with reduced DLAR function, arguing that at least under these conditions of reduced liprin-α activity, liprin-a and DLAR both act as positive regulators of viability. We therefore favor an alternate view in which qualitative differences in liprin-a activity cause the complex genetic interactions we see. In particular, the negative regulatory interactions between these three genes are only detected when liprin-a hypomorphic mutations are used. These hypomorphs result from premature nonsense mutations that truncate the Liprin-α protein either midway through the protein (at amino acid 590 in the case of liprin-α1) or immediately before the C-terminus, at amino acid 1165 (in the case of liprin-α F) (Choe et al., 2006). As neither of these alleles are phenotypically as severe as the null allele, the remaining protein fragments encoded by these alleles must be expressed in active forms. We therefore propose that the presence of the N-terminal fragment of liprin-a that is common to both hypomorphic alleles, in the absence of the C-terminus, unmasks a novel negative regulatory interaction between N-cadherin, DLAR and liprin-a.
This proposed function of a liprin-a fragment corresponds to an interesting division in the protein’s structure. Liprin-α consists of two broad domains, an N-terminal coiled-coiled domain and three C-terminal sterile-alpha motifs (SAM) that mediate unique interactions with other molecules (Serra-Pages et al. 1995; Serra-Pages et al. 1998). The coiled-coil domain mediates homodimerization and binds to the presynaptic active zone components RIM, ELKS and GIT1 (Schoch et al. 2002; Ko et al. 2003; Ko et al. 2003) and the SAM domain interacts with LAR (Serra-Pages et al. 1995). Both Liprin-α hypomorphs delete all or part of the SAM domain, preserving the coiled-coil domain: Liprin-αF is a nonsense mutation that removes the final SAM motif and Liprin-α1 is a nonsense mutation that preserves almost all of the coiled-coil domain but deletes all three SAM motifs (Choe et al. 2006). These data suggest that the ability of N-cadherin and DLAR to inhibit Liprin-α function reflects a direct or indirect interaction with the coiled-coil domain that can only be seen in the absence of the SAM domains. Since the SAM domains are required for the association of Liprin-α and DLAR, we infer that this inhibition normally occurs only when Liprin-α is not associated with DLAR, and that the binding of Liprin-α to DLAR likely serves to relieve this inhibition (Fig. 5). Such a mechanism would provide a means of regulating liprin-α function, allowing it to be active only when it associates with DLAR and N-cadherin. Intriguingly, recent work on the worm homolog of liprin-α has also revealed a prominent role for negative regulatory interactions (Patel and Shen, 2009). Regardless of the precise model that underlies the genetic interactions amongst these three loci, these studies highlight the complexity of the underlying molecular mechanisms by which the formation of stable contacts between pre- and post-synaptic cells is controlled.
MATERIALS AND METHODS
Genetics
Single cell MARCM studies on R1–R6 target selection were performed as described using a heat-shock inducible FLP recombinase, heat shocking animals for 30 minutes at 37°C during the third larval stage, labeling R cell axons using the pan-neural promoter elav-Gal4 driving expression of mCD8GFP (Prakash et al., 2005). In DLAR rescue experiments, this Gal4 element also drove expression of the corresponding DLAR transgene. In studies of N-cadherin and DLAR expression in the lamina, large contiguous patches of mosaic cells (10–20 ommatidia) were generated by heat shocking earlier during development, during the second larval stage, for 1 hour at 37°C. Large R cell clones studied using the MARCM method were generated by replacing the heat shock inducible FLP with one under the control of the eyeless promoter (eyFLP; Newsome et al., 2000), and by replacing elav-Gal4 with mδ-Gal4, which drives expression strongly in R4, and weakly in R3 and R7 (derived from Cooper and Bray, 1999; a gift from Paul Garrity; this work). The following alleles were used: N-cadherin Δ14 (Prakash et al., 2005), DLAR bypass (Krueger et al., 2003), DLARomb451 (Clandinin et al., 2001), DLAR 2127 (Maurel-Zaffran et al., 2001), Liprin-a1, Liprin-aE, Liprin-aF (all from Choe et al., 2006). All DLAR transgenes were from Krueger et al., 2003.
Histology and Imaging
Fly brains were dissected and stained as described (Clandinin et al., 2001). The following primary antibodies were used: mAB24B10 (anti-chaoptin, 1:50, Developmental Studies Hybridoma Bank), a Rat monoclonal raised against N-cadherin exon 8 (used at 1:20; Iwai et al, 1997), and the mouse anti-DLAR antibody 9D8 (1:10, a gift of Kai Zinn). The following secondary antibodies were also used: anti-rabbit IgG Alexa 488, anti-rat IgG Alexa 543, anti-mouse IgG Alexa 594, anti-mouse IgG Alexa (all from Invitrogen, and used at 1:200). Images were taken on a Leica TCS SP2 AOBS confocal microscope, using a 40 × 1.25NA oil lens, deconvolved using Huygens Professional (SVI), and rendered using Imaris (Bitplane). Figures were assembled in Photoshop (Adobe).
Biochemistry
0–19 h-old dechorionated embryos were used to immunoprecipitate N-cadherin following a previously described protocol (Oda et al., 1993). N-cadherin was immunoprecipitated on GammaBind Sepharose beads (GE Lifesciences) with N-Cadherin hybridoma culture supernatant (DN-In; gift from T. Uemura) used at a ratio of 1:10 or 1:5. Anti-DLAR hybridoma culture supernatant (108.3C; gift from K. Zinn) was used for immunoblotting. 1ug or 2ug of purified mouse IgG antibodies (Jackson Immunochemicals) were used for control immunoprecipitations. An aliquot of the N-Cadherin immunoprecipitate was immunoblotted separately with N-Cadherin hybridoma supernatant to ascertain the efficiency of the immunoprecipitation protocol.
All UAS-DLAR Drosophila strains used were crossed to Elav-Gal4 virgin females, embryos were collected and lysed in buffer (50mM Tris pH 7.5, 5% glycerol, 0.2 to 1% NP-40, 1.5mM MgCl2, 125mM NaCl, 25mM NaF, 1mM Na3VO2) supplemented with protease inhibitors (complete mini tablets, Roche). Embryos were lysed only before use and samples were pre-cleared with 30μl of Protein G before antibody incubations. Protein G beads were pre-bound to HA antibody (Covance) over-night at 4°C. Bound beads were then incubated with embryonic lysate for 2 hours at 4°C. For Myc IP: Myc antibody (Millipore) was used 1:500 to and incubated overnight at 4°C.. Next, 40μl or protein G (GE lifesciences) was added and samples were incubated for 2–3 hrs at 4°C. All samples (for HA and Myc IP) were washed 3 times in lysis buffer containing protease inhibitors (complete mini tablets, Roche) and boiled for 10 minutes in 2.5 × sample buffer containing 50mM DTT and beta-mercapto ethanol. Samples were then loaded onto either 6% polyacrylamide SDS gels (for identification of full length or extracellular Dlar) or onto a 7.5% gel (for identification of the cyroplasmic Dlar). Gels were then transferred onto nitrocellulose, blocked for 1hour in 5% milk in TBS-T and probed overnight with antibodies directed against Myc (1:1000, Millipore), HA (1:2500, Covance) or N-Cadherin (1:5 dilution of supernatant, DSHB). The membranes were washed 3× in TBS-T and incubated with 1:10,000 dilution of goat anti-mouse HRP secondary antibodies (Jackson labs) in 5% milk in TBS-T (for HA and Myc) or with 1:10,000 dilution of goat anti-rat HRP (Millipore; for N-Cadherin) blots for 1 hour at RT. Blots were visualized using ECL reagent (Pierce).
Acknowledgments
We thank Paul Garrity for giving us mδ-Gal4, and members of the T.R.C. laboratory for helpful discussions on this work. This work was supported in part by the National Institutes of Health Grant R01 EY015231 (to T.R.C.). S.P. was supported in part by the Stanford Medical Scientist Training Program. DVV, CID, AG, and KAD were funded by grants from the NINDS. T.R.C. is a Sloan Fellow, a Searle Scholar, and a recipient of a Burroughs-Wellcome Career Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackley BD, Harrington RJ, Hudson ML, Williams L, Kenyon CJ, Chisholm AD, Jin Y. The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J Neurosci. 2005;25:7517–28. doi: 10.1523/JNEUROSCI.2010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol Cell Biol. 2002;22:1881–1892. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–31. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Mourton T, Nixon JP, Pietz GE, Kinch M, Chen H, Brackenbury R, Rimm DL, Del Vecchio RL, Tonks NK. Dynamic interaction of PTPmu with multiple cadherins in vivo. J Cell Biol. 1998;141:287–96. doi: 10.1083/jcb.141.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KM, Prakash S, Bright A, Clandinin TR. Liprin-alpha is required for photoreceptor target selection in Drosophila. Proc Natl Acad Sci USA. 2006;103:11601–11606. doi: 10.1073/pnas.0601185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, Lee CH, Herman T, Lee RC, Yang AY, Ovasapyan S, Zipursky SL. Drosophila LAR regulates R1–R6 and R7 target specificity in the visual system. Neuron. 2001;32:237–248. doi: 10.1016/s0896-6273(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Afferent growth cone interactions control synaptic specificity in the Drosophila visual system. Neuron. 2000;28:427–436. doi: 10.1016/s0896-6273(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Making connections in the fly visual system. Neuron. 2002;35:827–41. doi: 10.1016/s0896-6273(02)00876-0. [DOI] [PubMed] [Google Scholar]
- Cooper MT, Bray SJ. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]
- Debant A, Serra-Pagès C, Seipel K, O’Brien S, Tang M, Park SH, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93:5466–5471. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Hueske E, Wyszynski M, Hoogenraad CC, Jaworski J, Pak DT, Simonetta A, Liu G, Sheng M. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat Neurosci. 2005;8:458–467. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- Fox AN, Zinn K. The heparan sulfate proteoglycan syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr Biol. 2005;15:1701–1711. doi: 10.1016/j.cub.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Hiesinger PR, Zhai RG, Zhou Y, Koh TW, Mehta SQ, Schulze KL, Cao Y, Verstreken P, Clandinin TR, Fischbach KF, Meinertzhagen IA, Bellen HJ. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol. 2006;16:1835–1843. doi: 10.1016/j.cub.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeyer K, Maurel-Zaffran C, Sink H, Treisman JE. Liprin-alpha has LAR-independent functions in R7 photoreceptor axon targeting. Proc Natl Acad Sci USA. 2006;103:11595–11600. doi: 10.1073/pnas.0604766103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Ann Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, Flanagan JG, Van Vactor D. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- Ko J, Kim S, Valtschanoff JG, Shin H, Lee JR, Sheng M, Premont RT, Weinberg RJ, Kim E. Interaction between liprin-alpha and GIT1 is required for AMPA receptor targeting. J Neurosci. 2003;23:1667–1677. doi: 10.1523/JNEUROSCI.23-05-01667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Na M, Kim S, Lee JR, Kim E. Interaction of the ERC family of RIM-binding proteins with the liprin-alpha family of multidomain proteins. J Biol Chem. 2003;278:42377–42385. doi: 10.1074/jbc.M307561200. [DOI] [PubMed] [Google Scholar]
- Krueger NX, Van Vactor D, Wan HI, Gelbart WM, Goodman CS, Saito H. The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila. Cell. 1996;84:611–22. doi: 10.1016/s0092-8674(00)81036-3. [DOI] [PubMed] [Google Scholar]
- Krueger NX, Reddy RS, Johnson K, Bateman J, Kaufmann N, Scalice D, Van Vactor D, Saito H. Functions of the ectodomain and cytoplasmic tyrosine phosphatase domains of receptor protein tyrosine phosphatase Dlar in vivo. Mol Cell Biol. 2003;23:6909–6921. doi: 10.1128/MCB.23.19.6909-6921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J Cell Biol. 1996;134:1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mast JD, Prakash S, Chen PL, Clandinin TR. The mechanisms and molecules that connect photoreceptor axons to their targets in Drosophila. Semin Cell Dev Biol. 2006;17:42–9. doi: 10.1016/j.semcdb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Maurel-Zaffran C, Suzuki T, Gahmon G, Treisman JE, Dickson BJ. Cell-autonomous and -nonautonomous functions of LAR in R7 photoreceptor axon targeting. Neuron. 2001;32:225–235. doi: 10.1016/s0896-6273(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Hanson TE. The development of the optic lobe. In: Martinez-Arias MBaA., editor. The Development of Drosophila Melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1993. pp. 1363–1491. [Google Scholar]
- Miller KE, DeProto J, Kaufmann N, Patel BN, Duckworth A, Van Vactor D. Direct observation demonstrates that Liprin-alpha is required for trafficking of synaptic vesicles. Curr Biol. 2005;15:684–9. doi: 10.1016/j.cub.2005.02.061. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–60. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Oda H, Uemura T, Shiomi K, Nagafuchi A, Tsukita S, Takeichi M. Identification of a Drosophila homologue of alpha-catenin and its association with the armadillo protein. J Cell Biol. 1993;121:1133–1140. doi: 10.1083/jcb.121.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady P, Thai TC, Saito H. The laminin-nidogen complex is a ligand for a specific splice isoform of the transmembrane protein tyrosine phosphatase LAR. J Cell Biol. 1998;141:1675–1684. doi: 10.1083/jcb.141.7.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Tanaka H, Yagita Y, Saeki Y, Taguchi A, Hiraoka Y, Zeng LH, Colman DR, Miki N. Cadherin activity is required for activity-induced spine remodeling. J Cell Biol. 2004;167:961–72. doi: 10.1083/jcb.200406030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Lehrman EK, Poon VY, Crump JG, Zhen M, Bargmann CI, Shen K. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat Neurosci. 2006;9:1488–98. doi: 10.1038/nn1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Shen K. RSY-1 is a local inhibitor of presynaptic assembly in C. elegans. Science. 2009;323:1500–1503. doi: 10.1126/science.1169025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot DA, Woodford TA, Remboutsika E, Haun RS, Dixon JE. Cloning, bacterial expression, purification, and characterization of the cytoplasmic domain of rat LAR, a receptor-like protein tyrosine phosphatase. J Biol Chem. 1991;266:19688–19696. [PubMed] [Google Scholar]
- Prakash S, Caldwell JC, Eberl DF, Clandinin TR. Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat Neurosci. 2005;8:443–450. doi: 10.1038/nn1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson JM, Dimitroff B, Johnson KG, Rawson JM, Ge X, Van Vactor D, Selleck SB. The heparan sulfate proteoglycans Dally-like and Syndecan have distinct functions in axon guidance and visual-system assembly in Drosophila. Curr Biol. 2005;15:833–838. doi: 10.1016/j.cub.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Serra-Pagès C, Kedersha NL, Fazikas L, Medley Q, Debant A, Streuli M. The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein co-localize at focal adhesions. EMBO J. 1995;14:2827–2838. doi: 10.1002/j.1460-2075.1995.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Pagès C, Medley QG, Tang M, Hart A, Streuli M. Liprins, a family of LAR transmembrane protein-tyrosine phosphatase-interacting proteins. J Biol Chem. 1998;273:15611–15620. doi: 10.1074/jbc.273.25.15611. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Südhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Shen K. Molecular mechanisms of target specificity during synapse formation. Curr Opin Neurobiol. 2004;14:83–88. doi: 10.1016/j.conb.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Shin H, Wyszynski M, Huh KH, Valtschanoff JG, Lee JR, Ko J, Streuli M, Weinberg RJ, Sheng M, Kim E. Association of the kinesin motor KIF1A with the multimodular protein liprin-alpha. J Biol Chem. 2003;278:11393–401. doi: 10.1074/jbc.M211874200. [DOI] [PubMed] [Google Scholar]
- Streuli M, Krueger NX, Tsai AY, Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci USA. 1989;86:8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker E, Johnson KG. LAR, liprin alpha and the regulation of active zone morphogenesis. J Cell Sci. 2007;120:3723–3728. doi: 10.1242/jcs.03491. [DOI] [PubMed] [Google Scholar]
- Sun Q, Bahri S, Schmid A, Chia W, Zinn K. Receptor tyrosine phosphatases regulate axon guidance across the midline of the Drosophila embryo. Development. 2000;127:801–12. doi: 10.1242/dev.127.4.801. [DOI] [PubMed] [Google Scholar]
- Suzuki SC, Takeichi M. Cadherins in neuronal morphogenesis and function. Dev Growth Differ. 2008;50(Suppl 1):S119–30. doi: 10.1111/j.1440-169X.2008.01002.x. [DOI] [PubMed] [Google Scholar]
- Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54:771–85. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Ting CY, Yonekura S, Chung P, Hsu SN, Robertson HM, Chiba A, Lee CH. Drosophila N-cadherin functions in the first stage of the two-stage layer-selection process of R7 photoreceptor afferents. Development. 2005;132:953–963. doi: 10.1242/dev.01661. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J Neurosci. 2006;26:5872–80. doi: 10.1523/JNEUROSCI.0386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills Z, Bateman J, Korey CA, Comer A, Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999;22:301–312. doi: 10.1016/s0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pagès C, Streuli M, Weinberg RJ, Sheng M. Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- Yonekura S, Xu L, Ting CY, Lee CH. Adhesive but not signaling activity of Drosophila N-cadherin is essential for target selection of photoreceptor afferents. Dev Biol. 2007;304:759–70. doi: 10.1016/j.ydbio.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M, Jin Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 1999;401:371–5. doi: 10.1038/43886. [DOI] [PubMed] [Google Scholar]