Abstract
Gonadal hormones modulate fear acquisition, but less is known about the influence of gonadal hormones on fear extinction. We assessed sex differences and the influence of gonadal hormone fluctuations and exogenous manipulations of estrogen and progesterone on acquisition, extinction learning and extinction recall in a 3-day auditory fear conditioning and extinction protocol. Experiments were conducted on males and naturally cycling female rats. Regarding female rats, significant differences in fear extinction were observed between subgroups of females, depending on their phase of the estrous cycle. Extinction that took place during the proestrus (high estrogen/progesterone) phase was more fully consolidated, as evidenced by low freezing during a recall test. This suggests that estrogen and/or progesterone facilitate extinction. In support of this, injection of both estrogen and progesterone prior to extinction learning in female rats during the metestrus phase of the cycle (low estrogen/progesterone) facilitated extinction consolidation, and blockade of estrogen and progesterone receptors during the proestrus phase impaired extinction consolidation. When comparing male to female rats without consideration of the estrous cycle phase, no significant sex differences were observed. When accounting for cycle phase in females, sex differences were observed only during extinction recall. Female rats that underwent extinction during the metestrus phase showed significantly higher freezing during the recall test relative to males. Collectively, these data suggest that gonadal hormones influence extinction behavior possibly by influencing the function of brain regions involved in the consolidation of fear extinction. Moreover, the elevated fear observed in female relative to male rats during extinction recall suggests that gonadal hormones may in part play a role in the higher prevalence of anxiety disorders in women.
Keywords: estrous cycle, conditioned fear, anxiety, estrogen, progesterone
Introduction
Fear extinction is the gradual decrease of conditioned fear responses, such as freezing, to a conditioned stimulus that was previously paired with an aversive unconditioned stimulus such as a footshock. Evidence now points to the involvement of the amygdala, ventromedial prefrontal cortex (vmPFC), and hippocampus in the learning and consolidation of conditioned fear extinction in both rodents and humans (for reviews, see Quirk and Mueller 2008; Milad et al. 2006b; Myers and Davis 2007; Bouton 2004). Most studies that examine fear extinction have predominantly utilized male rodents and the human studies have averaged data from both women and men. Sex differences in learning and memory have been reported in a variety of behavioral paradigms including conditioned fear acquisition. Data from these studies indicate that gonadal hormones influence the acquisition of fear conditioning (Maren et al. 1994; Gupta et al. 2001; Wiltgen et al. 2001; Aguilar et al. 2003; Jasnow et al. 2006). Fewer studies have focused on sex differences and the influence of gonadal hormones on fear extinction. Some reports have shown that gonadal hormones facilitate the extinction of passive avoidance (Rivas-Arancibia and Vazquez-Pereyra 1994), conditioned taste aversion (Yuan and Chambers 1999), and extinction of contextual fear (Chang et al. 2009). Thus, there is evidence to suggest that gonadal hormones may influence or contribute to sex differences in conditioned fear extinction and its recall.
It has been shown that the amygdala, vmPFC, and hippocampus are sexually dimorphic in humans (Goldstein et al. 2001) and contain high levels of estrogen and progesterone receptors in rodents (Pilgrim and Hutchison 1994; Ostlund et al. 2003). It has also been reported that the function of these brain regions exhibits a different pattern of activation in response to emotional stimuli in men and women, and that they are influenced by the phase of the menstrual cycle in healthy women (Andreano and Cahill 2009; Cahill et al. 2004; Goldstein et al. 2005a; Protopopescu et al. 2005). Moreover, the function of these brain regions appears to be deficient in several anxiety disorders such as PTSD, and fear extinction is shown to be impaired in PTSD (Rauch et al. 2006; Milad and Rauch 2007; Liberzon et al. 2003; Pitman et al. 2001; Milad et al. 2008; Orr et al. 2000). Several epidemiological studies indicate that the prevalence of anxiety disorders such as post-traumatic stress disorder (PTSD) is higher in women (Pigott 2003; Breslau et al. 1998; Kinrys and Wygant 2005). Collectively, these data raise an important question that this study was designed to address: do gonadal hormones and fluctuations thereof influence fear extinction?
Using a three-day auditory fear conditioning and extinction paradigm, we assessed extinction learning and recall at different phases of the estrous cycle in naturally cycling female rats. We focused on two specific phases of the estrous cycle: the proestrus and metestrus phases. The former is characterized by elevated estrogen/progesterone levels while the latter is characterized by low levels of these hormones. We then examined the influence of exogenous administration of estrogen and/or progesterone in naturally cycling female rats during the metestrus phase. Finally, we administered pharmacological agents to block estrogen or progesterone receptors prior to extinction learning while rats were in the proestrus phase. Given that it has recently been shown that estrogen facilitates extinction of context conditioning (Chang et al. 2009) and that progesterone and its metabolites appear to have anxiolytic effects (Akwa et al. 1999), we predicted that elevated levels of gonadal hormones would facilitate extinction learning and recall.
Experimental Procedures
Subjects
A total of 22 male and 150 female Sprague Dawley rats (approximately 300 grams and 8 weeks of age) were housed individually at the Massachusetts General Hospital Center for Comparative Medicine and handled for 5 minutes/day for 2 days following their arrival. Rats were maintained on a 12h light/dark cycle and restricted to ~15g/day of laboratory rat chow, with free access to water. They were transported to a holding room in our laboratory in the morning and returned to the facility at the end of each day. The procedures were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital, in compliance with National Institutes of Health guidelines.
Behavioral Apparatus
All experimental procedures were performed in Plexiglas chambers (Coulbourn Instruments, Whitehall, Pennsylvania) of 25 × 29 × 29 cm inside sound-attenuated boxes (Med Associates, Burlington, Vermont). Each contained a single overhead house light, video camera, and a speaker mounted on the wall through which tone presentations were delivered. Each chamber held a metal bar apparatus that, when pressed, would deliver food pellets (Bioserve, Frenchtown, New Jersey) at a rate predetermined by a computer program (GraphicState 3.03, Coulbourn Instruments, Whitehall, Pennsylvania), and floors consisting of stainless-steel bars capable of delivering a mild electric shock. The conditioned stimulus (CS) was a 4kHz tone with an intensity of 80 dB and a duration of 30s, during which an LED indicator light would turn on (The LED light was only visible to the video camera recording the behavior of the rats). The unconditioned stimulus (US) was a 0.6mA, 0.5s footshock that co-terminated with the tone presentation during conditioning.
Behavioral Procedure
For approximately 10 days prior to the experiment, rats were trained to press a bar for food in order to establish a consistent level of activity against which to measure freezing (Quirk 2002). Rats that failed to reach predetermined learning criteria at a variable interval averaging 60s were not included and did not undergo any phase of the experiment. For all female rats included in the experiment, vaginal smears were taken daily to confirm that their estrous cycles were proceeding normally, as previously described (Becker et al. 2005). Vaginal epithelial cells were examined via microscope daily for at least 10 consecutive days; slides were dyed using DipQuick counter stain kit (Jorgensen Laboratories, Loveland, Colorado). Various randomly-selected samples were blindly re-examined by collaborators to ensure proper and consistent readings. Swabs were performed between 8:00 and 10:00 A.M. to maintain consistency, and rats were returned to their home cages for 60 minutes prior to the start of the experimental procedure.
Experiment 1: influence of estrous cycle phase on conditioning, extinction learning, and extinction recall
It is documented that the estrous cycle in female rats is a four-day cycle consisting of four discernable phases: estrus, metestrus, diestrus, and proestrus (Becker et al. 2005). To assess the influence of the estrous cycle on the different phases of the experiment, we focused on two specific phases of the cycle: proestrus (Pro) and metestrus (Met). The former is characterized by elevated estrogen and progesterone levels, whereas the latter is characterized by low estrogen and progesterone levels (Becker et al. 2005). On Day 1, 56 female rats were placed in the conditioning chambers, where they received 5 non-reinforced presentations of the tone alone (Habituation) followed by 7 tone-footshock pairings (Conditioning) at an average inter-trial interval of 3 minutes. Rats were immediately returned to their home cages following the conditioning phase. On Day 2, rats were returned to the chambers and presented with 20 non-reinforced tone trials (Extinction Learning), and on Day 3, they received 15 presentations of the tone alone (Extinction Recall). All rats underwent all experimental phases in the same chamber in which they had been trained to press the bar, and sessions were run 24 hours apart.
Given that female rats normally fall in a different phase of the estrous cycle on a daily basis (Becker et al. 2005), all female rats underwent the experimental procedures as described above regardless of the phase of the estrous cycle. Upon completion of the experiments, post-hoc separation of all female rats was conducted (determined by vaginal swabs) as follows: rats that underwent conditioning while either in the proestrus (n= 8) or metestrus phases (15), rats that underwent extinction learning while in proestrus (10) or metestrus (11), and rats that underwent extinction recall while in proestrus (7) or metestrus (11). Six rats did not fully progress to a new phase of the cycle during the 24 hours between tests (but were otherwise cycling normally); these rats were ultimately included in two of the aforementioned groups. All rats continued to receive vaginal swabs for at least 3 days following the experiment to confirm normal cycling.
Experiment 2: influence of exogenous administration of hormones on extinction recall
For this experiment, the cycling patterns of a new group of 58 female rats were monitored and the experiment was scheduled such that all rats underwent extinction learning during the metestrus phase. To further investigate which natural hormone variations may modulate the differences in fear behavior observed in experiment 2, 4 different groups of rats were treated as follows: a group was administered estradiol (n=13, 15 μg/kg, Sigma-Aldrich, St. Louis, MO, dissolved in saline), another progesterone (n=14, 4 mg/kg, Sigma-Aldrich, St. Louis, MO, dissolved in saline), the third received a combination of estradiol and progesterone using the same doses above (n=13), and the last group received vehicle (n= 18, saline). The volume of injection was the same for all groups (0.1 ml); all administered subcutaneously (s.c.) 30 minutes prior to extinction learning. On day 3, all groups underwent the extinction recall test.
Experiment 3: influence of hormone antagonists on extinction learning and recall
This experiment was implemented identically to experiment 2, but these 36 female rats underwent extinction learning while in the proestrus phase. Thirty minutes prior to extinction learning, rats received s.c. injections of either the progesterone antagonist Mifepristone (n=13, 30 mg/kg in propylene glycol, Sigma-Aldrich, St. Louis, MO), the nonselective estrogen antagonist Fulvestrant (n=12, 30mg/kg in sesame oil, Sigma-Aldrich, St. Louis, MO) or vehicle (n=11). All injection volumes were 0.1mL. On day 3, all groups underwent the extinction recall test. It has been reported that the half-life for Mifepristone in rats is approximately 1 hour (Heikinheimo et al. 1994) whereas the half-life for Fulvestrant is approximately 4 hours (Berry et al. 1990). Thus, all tests conducted on day 3 where done in a drug-free state.
Experiment 4: male vs. female
This experiment was conducted to examine the potential differences between male and female rats during all phases of the experiment. Twenty-two male rats underwent the same conditioning and extinction protocol described above. A comparison across each phase of the experiment was conducted between male rats vs. all naturally cycling female rats without any pharmacological manipulations regardless of the cycle phase (rats from experiment 1). A post-hoc comparison was then conducted between male rats vs. female rats that underwent conditioning and extinction training at the metestrus and proestrus phases of the experiment to assess whether estrous cycle phase may contribute to sex differences.
Behavioral Analysis
Freezing behavior – the absence of all movement apart from respiration – is a known fear response in rats and was used as the measure of conditioned fear. Freezing during the tone presentation was evaluated from digital videos using motion-sensing computer software (FreezeScan, Clever Systems, Reston, Virginia), and time spent freezing was recorded as a percentage of the total 30s duration of the tone. Freezing data were analyzed with repeated-measures analysis of variance (ANOVA) or student t-test when appropriate using statistical analysis software (SPSS Inc., Chicago, Illinois.). Data are shown as means for all 5 trials of habituation, the last 5 trials of conditioning (3-7), averaged in blocks of 5 trials during the entire extinction learning phase, and the average of the first 5 trials during the extinction recall test. Thus, all data shown represent blocks of 5 trials. Error bars represent standard error of the mean (S.E.).
Results
Experiment 1: influence of estrous cycle phase on conditioning, extinction learning, and extinction recall
Pro/Met during fear conditioning
Rats that underwent fear conditioning during the proestrus phase (Pro_Cond group) were compared to those that underwent conditioning during the metestrus phase (Met_Cond group). Student’s t-test conducted on the acquisition phase and extinction recall phase revealed no significant difference in either phase (t(22) = 1.20, p = 0.24, t(22) = 0.95, p = 0.35, for conditioning and extinction recall, respectively). Repeated measures ANOVA conducted on the extinction learning phase revealed a significant main effect of trial (F(3,63) = 20.32, p < 0.001), indicating intact extinction learning for both groups, but no significant main effect of group (F(1,21) = 0.99, p = 0.33), and no significant interaction (F(3,63) = 0.64, p = 0.59) was observed. These data are shown in figure 1A.
Figure 1. Extinction recall is significantly facilitated when female rats undergo extinction learning during the proestrus phase of the cycle (high estrogen and progesterone).
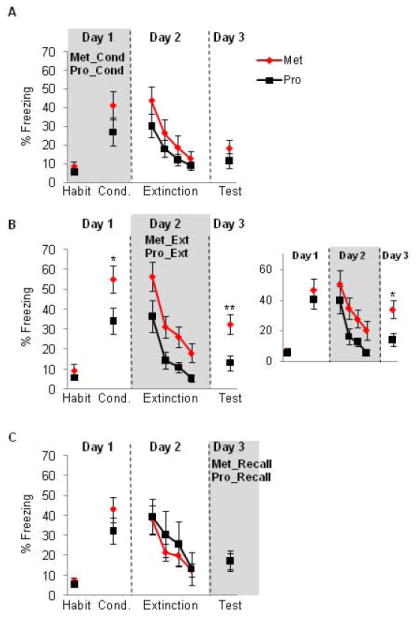
(A) Freezing for female rats that underwent conditioning training during either the metestrus (Met) or proestrus (Pro) phases of the estrous cycle. (B) Freezing for female rats that underwent extinction training during either the metestrus or proestrus phases of the estrous cycle. Note the significant facilitation of extinction recall (day 3) in the proestrus group. B-Inset: freezing for a sub-group of female rats after matching for acquisition levels. Significant difference in extinction recall remained in these sub-groups. (C) Freezing for female rats that underwent extinction recall during either the metestrus or proestrus phases of the estrous cycle. Habit = habituation trials; Cond. = conditioning trials. Trials 3-7 of the conditioning trials are shown for this figure and all remaining figures. Data represent means averaged over 5 trials ± S.E.M. *p < .05 compared to controls, **p < .01. Proestrus = black symbols; metestrus = red symbols.
Pro/Met during Extinction Learning
Rats that underwent extinction learning while in proestrus (Pro_Ext group) were compared with those that underwent extinction learning in metestrus (Met_Ext group). On Day 1, Pro_Ext group showed significantly lower freezing during the last 5 trials of the conditioning phase relative to Met_Ext group (t(20) = 2.16, p = 0.04, figure 2B). On Day 2, ANOVA with repeated measures revealed significant main effects of trial (F(3,57) = 23.12, p < 0.001), significant main effect of group (F(1,19) = 9.67, p = 0.006) but no significant interaction (F(3,57) = 0.23, p = 0.87), showing that Pro_Ext group had significantly lower freezing levels relative to Met_Ext group (figure 1B). The lack of significant interaction between groups indicates that the rate of extinction learning is equivalent in both groups. On day 3, the Pro_Ext group showed significantly lower freezing levels relative to the Met_Ext group during the first 5 trials (t(20) = 3.08, p = 0.005, see figure 1B).
Figure 2. Administration of estrogen and progesterone facilitated extinction recall whereas blockade of estrogen or progesterone receptors impaired extinction recall.
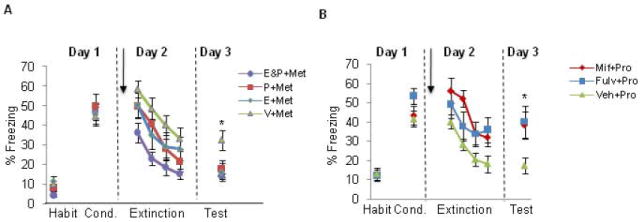
(A) Freezing levels for female rats receiving either: estradiol and progesterone (E&P+Met), estradiol (E+Met), progesterone (P+Met), or vehicle (V+Met) groups. Females underwent the extinction learning phase while in the metestrus (Met) phase. (B) Freezing levels shown for female rats receiving Fulvestrant (Fulv+Pro), Mifepristone (Mif+Pro), or vehicle (Veh+Pro). Females underwent the extinction learning phase while in the proestrus (Pro) phase. Note the impaired extinction recall in both treated groups relative to vehicle injected females. Arrows indicate s.c. injections, which occurred 30 minutes prior to extinction learning. Habit = habituation trials; Cond. = conditioning trials. Data represent means averaged over 5 trials ± S.E.M. *p < .05 compared to controls.
To test if the difference between the Pro_Ext and Met_Ext groups during the extinction recall was due to the between-group difference during fear acquisition, we used three different analytical approaches. First, we measured percent fear recovery, which is calculated based on the first 2 trials during extinction recall divided by the average of the first 2 trials during extinction learning multiplied by 100. This measure has been frequently used to assess between group differences during extinction recall test (for example, see Quirk et al. 2000; Lebron et al. 2004; Milad and Quirk 2002), especially when there is a between-group difference during fear conditioning. This measure revealed that Pro_Ext exhibited significantly lower % fear recovery relative to Met_Ext group (41.8% and 75.4% for Pro_Ext and Met_Ext, respectively, p = 0.0034). Second, we artificially matched for fear acquisition levels between groups by excluding those with especially high acquisition from the Met_Ext group (remaining n=8) and those with especially low acquisition from the Pro_Ext group (remaining n=8). The difference in freezing during extinction recall between these two sub-groups remained significant (t(15) = 2.60, p=0.019, see inset in figure 1B). Third, we repeated the statistical analysis (for the entire sample, i.e. no exclusions) co-varying for the acquisition levels in both groups. After co-varying for the acquisition levels, the differences in recall remained statistically significant (p < 0.05). It is, therefore, unlikely that the significant between-group differences we observed during extinction recall are due to pre-existing differences during fear acquisition.
Pro/Met during Extinction Recall
Finally, rats that underwent extinction recall test in the proestrus phase (Pro_Recall group) were compared with those that underwent extinction recall test in the metestrus phase (Met_Recall group). Student’s t-test conducted on the acquisition phase and extinction recall phase revealed no significant differences between groups in these phases (t(17) = 1.24, p = 0.23, t(17) = 0.82, p = 0.98, for fear acquisition and extinction recall, respectively). Repeated measures ANOVA conducted on the extinction learning phase revealed significant main effect of trial (F(3, 51) = 11.55, p < 0.001) but no significant main effect of group (F(1, 17) = 0.25, p = 0.61) or interaction (F(3, 51) = 0.39, p = 0.75) (data are shown in figure 1C).
Experiment 2: influence of exogenous administration of hormones on extinction recall
The following experiments were conducted to assess the effects of exogenous manipulations of gonadal hormones on fear extinction. As described in the methods section, all female rats underwent extinction learning during the metestrus phase of the cycle and received either estradiol (E+Met group), progesterone (P+Met group), estradiol+progesterone (E&P+Met group), or vehicle (Veh+Met group) 30 minutes prior to extinction learning. One-way ANOVA conducted on the acquisition phase revealed no significant differences between groups during fear conditioning (F(3,57) = 0.19, p = 0.90). Repeated-measures ANOVA conducted on the extinction learning phase revealed a significant main effect of trial (F(3,162) = 39.81, p < 0.001), and a significant main effect of group (F(3,54) =3.61, p = 0.019), but no significant interaction (F(9,162) = 0.54, p = 0.84). Post-hoc comparisons revealed that the E&P+Met group exhibited significantly lower freezing behavior during this phase relative to the Veh+Met group (p = 0.002), whereas freezing levels in the E+Met and P+Met groups did not statistically differ from the Veh+Met group (p = 0.16, and p = 0.13, for E+Met and P+Met, respectively). These results suggest that while the groups did not differ in the rate at which they extinguished, rats receiving both estrogen and progesterone, which would simulate a hormonal state of the proestrus phase, show significantly less freezing during extinction learning. These results suggest a potential anxiolytic effect when both estrogen and progesterone are used in combination. On Day 3, one-way ANOVA revealed significant main effect of group (F(3,57) = 4.19, p = 0.010). Post-hoc analysis of the extinction recall test revealed that Veh+Met group exhibited significantly higher freezing levels during this test compared to all remaining groups (p = 0.009, p = 0.013, and p = 0.004, for E+Met, P+Met, and E&P+Met groups, respectively). These data are shown in figure 2B.
Experiment 3: influence of hormone antagonists on extinction learning and recall
To corroborate the findings from experiments 1-2, we explored whether blocking estrogen or progesterone receptors would produce the opposite effect of exogenous administration of estrogen and progesterone. As described in the methods section, all female rats underwent extinction learning phase during the proestrus phase of the cycle. Rats received either the non-selective estrogen receptor antagonist fulvestrant (Fulv+Pro group), progesterone antagonist mifepristone (Mif+Pro group), or vehicle (Veh+Pro group) 30 minutes prior to extinction learning. One-way ANOVA conducted on the acquisition phase revealed no significant differences between groups during fear conditioning (F(2,35) =2.01, p = 0.15). Repeated-measures ANOVA conducted on the extinction learning phase revealed a significant main effect of trial (F(6,99) = 17.38, p < 0.001), and a significant main effect of group (F(2,33) =3.75, p = 0.034), but no significant interaction (F(6,99) = 1.18, p = 0.32). Post-hoc comparisons revealed that the Mif+Pro group showed significantly higher freezing levels relative to the Veh+Pro group (p = 0.014). Freezing levels in the Fulv+Pro group were not statistically significant relative to the Veh+Pro (p = 0.055). These results suggest that while the groups did not differ in the rate at which they extinguished, rats receiving the progesterone antagonist show significantly more freezing during extinction learning compared to those receiving vehicle; blockade of estrogen receptors at this phase showed a marginal effect. On Day 3, one-way ANOVA revealed significant main effect of group (F(2,33) = 3.36, p = 0.047) and post-hoc analysis indicated that both Fulv+Pro and Mif+Pro groups showed higher levels of freezing compared to Veh+Pro group (p = 0.029, and p = 0.033, for Fulv+Pro and Mif+Pro groups, respectively). These data are shown in figure 2B.
Experiment 4: male vs. female
Female rats that underwent experiment 1 were grouped regardless of cycle phase and compared to male rats that underwent the same experimental procedures. The male group exhibited higher freezing levels relative to the female group but this difference was not statistically significant (t(76) = 1.67, p = 0.095) (see figure 3a). On Day 2, Repeated measures ANOVA revealed a significant main effect of trial (F(3,228) = 44.9, p < 0.0001), indicating significant extinction learning within session for both groups. However, no significant main effect of group (F(1,76) = 0.76, p= 0.38) and no interaction (F(3,228) =1.43, p= 0.24) were observed, indicating that male and female groups did not significantly differ during the extinction learning phase. On Day 3, there was no difference between male and female groups in extinction recall (t(76) = 0.82, p = 0.59). Thus, these data indicate a marginal increase in fear acquisition in the male rats and lack of any significant differences between males and female groups during extinction learning and recall. These data are shown in figure 3a.
Figure 3. Comparison between males and females at various stages of the experiment.
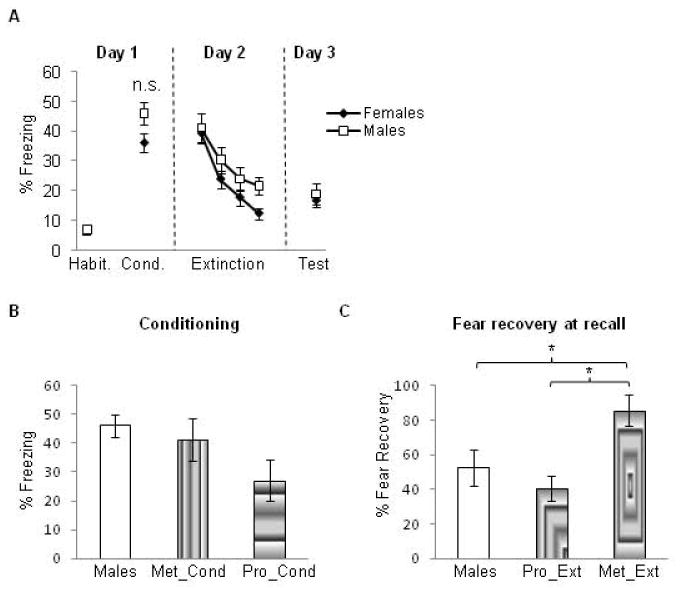
(A) No sex differences were observed between male and female rats at any phase of the experiment. Habit = habituation trials; Cond. = conditioning trials. Data represent means averaged over 5 trials ± S.E.M. Males = white symbols; females = black symbols. (B) Freezing values in the male rats during fear conditioning versus female rats that underwent conditioning during the proestrus (Pro_cond) and metestrus (Met_cond) phases. No significant differences were observed among all three groups in this test. (C) Percent recovery exhibited by male rats during extinction recall versus female rats that underwent extinction learning either during the proestrus (Pro_Ext) or metestrus (Met_Ext) phases. Male rats show low fear recovery that was comparable to that shown by the Pro_Ext group, which was significantly lower than that exhibited by the Met_Ext group. *p < .05
To examine potential sex differences that may be due to cycle phase, we conducted post-hoc analyses comparing freezing values in the male group (described above) during fear conditioning to female rats that underwent conditioning during the proestrus (Pro_Cond group from experiment 1) and to female rats that underwent fear conditioning during the metestrus phase (Met_Cond group from experiment 1). These analyses revealed that the male group and Met_Cond group showed similar levels of freezing, both of which were higher than those exhibited by the Pro_Cond group. This difference, however, was not statistically significant (one-way ANOVA, F(2,42) = 1.98, p = 0.15, see figure 3b).
To assess potential sex differences during extinction recall that might be due to cycle phase, we conducted additional post-hoc analyses. In these analyses, we used the percent recovery measure because this measure normalizes any differences in fear acquisition among groups. Given that the most robust difference between females was observed when they were separated by cycle phase during extinction learning (Pro_Ext and Met_Ext groups, figure 1b), percent recovery from these groups was compared to that obtained from the male group. To obtain a sample size that was comparable to the male group, we included all vehicle-injected females that underwent extinction learning during either proestrus or metestrus (experiments 2 and 3) in these post-hoc analyses (their behavior did not differ from their counterparts in the Pro_Ext and Met_Ext groups). One-way ANOVA of the percent fear recovery revealed a significant main effect of group (F(2,67) = 6.96, p = 0.002). Post-hoc analysis showed that the male group exhibited low fear recovery that was comparable to that exhibited by the Pro_Ext group (p = 0.362); the percent recovery for both of these groups was significantly lower than that exhibited by the metestrus group (85.5% + 8.9) (p = 0.01 for males vs. Met_Ext, and p = 0.001 for Pro_Ext vs. Met_Ext, see figure 3c).
Discussion
The data gathered in the present study show that naturally cycling gonadal hormones influence fear extinction in that elevated gonadal hormone levels during the proestrus phase appear to facilitate extinction recall. This was further supported by 1) the fact that administration of exogenous estrogen and progesterone prior to extinction learning on Day 2 facilitated extinction recall tested on Day 3, and 2) the fact that blockade of estrogen and progesterone receptors prior to extinction learning impaired extinction recall. Furthermore, no differences were observed between male and female rats when not taking the cycle phase into consideration. However, after dividing the female rats based on the cycle phase, we observed marginal yet not significant sex differences during fear acquisition. Interestingly, during extinction recall, the fear recovery in female rats that underwent extinction learning during the metestrus phase was significantly higher when compared to fear recovery in males. Fear recovery in male rats and in females that underwent extinction learning during the proestrus phases were comparable. To our knowledge, this is the first report to indicate that in naturally cycling female rats, gonadal hormone variance during the estrous cycle modulates auditory fear extinction recall.
The objective of administering estrogen and progesterone was to determine which of these two hormones would modulate extinction learning and recall. Our data indicate that both estrogen and progesterone appear to enhance extinction recall. Notably, the combined estrogen and progesterone injections in the same animals seemed to have an additive effect, specifically during extinction learning. The decreased fear expression during extinction learning when elevated gonadal hormones are present is consistent with the reported anxiolytic effects of these hormones (Lund et al. 2005; Hiroi and Neumaier 2006; Walf and Frye 2005). More importantly, the facilitated extinction consolidation induced by gonadal hormones is consistent with recent reports showing that estrogen administration facilitates extinction of contextually conditioned fear (Chang et al. 2009) and memory formation in other tasks (Leuner et al. 2004). We speculate that the facilitative effects of estrogen on fear extinction are mediated by the estrogen receptor beta (ER-beta), as several studies have shown that the anxiolytic effects of estrogen are mediated by the ER-beta (Toufexis et al. 2007; Lund et al. 2005; Tomihara et al. 2009). This view is supported by a recent study showing that extinction of context conditioning was facilitated through the activation of ER-beta in the hippocampus (Chang et al. 2009).
As for progesterone, its administration to ovarectomized rats facilitated contextual and cued fear conditioning, and enhanced cognitive performance in a variety of other behavioral tasks in mice (Frye and Walf 2008). Moreover, progesterone is metabolized into allopregnanolone (Engin and Treit 2007) which appears to have anxiolytic effects that are mediated via the amygdala, vmPFC, and hippocampus (Akwa et al. 1999; Engin and Treit 2007). In other tasks, progesterone facilitates extinction of cocaine self administration (Jackson et al. 2006). We note that the progesterone receptor antagonist used in the present study, mifepristone, also blocks glucocorticoid receptors. Given that several studies have shown that glucocorticoids may be involved in fear extinction (Yang et al. 2006; Gourley et al. 2009), it is possible that the impaired extinction recall observed after mifepristone injections in the present study may be due to the blockade of the glucocorticoid receptors. Nonetheless, our other experiments involving natural hormone variations and exogenous progesterone administration show that progesterone is indeed playing a role in facilitating extinction recall.
The mechanisms by which estrogen and progesterone modulate associative learning are not entirely clear. High levels of gonadal hormone receptors are expressed in the vmPFC, hippocampus and amygdala (Pilgrim and Hutchison 1994; Ostlund et al. 2003); all of these brain regions have been implicated in fear extinction in both rodents and humans (Quirk and Mueller 2008; Milad et al. 2006a). These receptors are expressed on inhibitory interneurons in these brain regions, suggesting that activation of estrogen receptors may have a modulatory role on the output of the vmPFC, amygdala, and hippocampus (Murphy et al. 1998; Blurton-Jones and Tuszynski 2002). The interplay between gonadal hormones and neural activity has been most extensively studied in the hippocampus (Shors et al. 2001). In this brain region, estrogen enhances synaptogenesis and long-term potentiation (LTP) (McEwen 2002), increases the formation of dendritic spines (Murphy et al. 1998; Good et al. 1999), increases cell proliferation (Tanapat et al. 2005), and increases neural excitability (Foy et al. 1999; Terasawa and Timiras 1968). GABA receptor agonists block the effects of estrogen on dendritic spines (Segal and Murphy 2001). Thus, estrogen appears to reduce the inhibitory tone of GABAergic neurons. On the other hand, progesterone increases GABAergic inhibition and reduces dendritic spines in the hippocampus (Segal and Murphy 2001; Woolley and McEwen 1993). It remains unclear as to whether or not similar mechanisms of action of these hormones are also found in the amygdala and vmPFC, structures critical for fear acquisition and extinction.
Sex differences in the acquisition of cued and contextual fear conditioning have been demonstrated. The findings of these studies, however, are not entirely consistent, most likely due to differences in experimental protocol, dose, and duration of hormonal manipulations. Some studies have shown increased fear acquisition in male relative to female rats in a contextual fear conditioning paradigm (Gupta et al. 2001; Wiltgen et al. 2001). Others, however, reported no sex differences in contextual fear conditioning and reported enhanced fear acquisition to cued conditioning in female relative to male rats (Wiltgen et al. 2005). Maren et al.,(1994) observed sex differences during fear acquisition only during contextual fear and not during cued fear conditioning. Thus, our results are consistent with reports indicating lack of significant differences between male and female rats during cued fear conditioning. The lack of any sex differences remained even when considering the estrous cycle phase in females. Interestingly, sex differences emerged during extinction recall when taking the cycle phase into account. Male rats exhibited intact extinction recall that was comparable to female rats that underwent extinction learning while estrogen and progesterone levels were elevated. On the other hand, female rats that underwent extinction learning while estrogen and progestereon levels were low exhibited high freezing levels during this test. This is somewhat surprising as male rats might be expected to be at similar hormonal levels to metestrus rats.
The significantly elevated fear recovery in the Met_Ext group relative to male rats during extinction recall test may have clinical implications. Epidemiological data suggest that the prevalence of anxiety disorders is higher in women relative to men (Pigott 2003; Breslau et al. 1998; Kinrys and Wygant 2005). Thus, the differences in prevalence and symptoms between women and men across the majority of anxiety disorders, point to brain-based differences in processing emotional stimuli in men and women (Cahill 2003; Goldstein et al. 2005b). The data gathered in the present study support this view and indicate that there may be an interaction between natural variance, as well as artificial manipulation of gonadal hormones, and the brain circuitry involved in fear inhibition (Protopopescu et al. 2005). Such interaction should be further investigated, which could lead to hypotheses regarding how sex differences in anxiety disorders might be influenced by gonadal hormones. The influence of gonadal hormones on fear extinction in healthy humans has been shown in a preliminary study (Milad et al. 2006a). Perhaps by concentrating exposure therapy at a particular phase of the cycle and/or administration of estrogen/progesterone in conjunction with exposure therapy, this line of research could help improve current treatments for anxiety disorders. It must be noted, however, that the experimental evidence presented herein is based on rodent’s data with an intact hormonal system. Speculations and translation of these findings into humans should be considered with caution. This is especially important given that it remains unclear how contraceptives (containing estrogen and progesterone) may influence the processes of fear learning and extinction in women.
Acknowledgments
The work was supported by a grant from the National Institute of Mental Health (K01 MH080346) to M.R.M. The authors would like to thank Dr. David Rubinow and Dr. Gregory J. Quirk for helpful comments on the manuscript.
List of abbreviations used in main text
- ANOVA
Analysis of Variance
- Cond
Conditioning
- E
Estradiol
- ER
Estrogen Receptor
- Ext
Extinction
- Fulv
Fulvestrant
- GABA
Gamma-Aminobutyric Acid
- LED
Light-emitting Diode
- LTP
Long-Term Potentiation
- Met
Metestrus
- Mif
Mifepristone
- PTSD
Post-Traumatic Stress Disorder
- Pro
Proestrus
- P
Progesterone
- S.E.
Standard Error of the Mean
- S.C.
Subcutaneous
- Veh
Vehicle
- vmPFC
Ventromedial Prefrontal Cortex
Footnotes
Disclosure\Conflict of interest None of the authors have any conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Aguilar R, Gil L, Gray JA, Driscoll P, Flint J, Dawson GR, et al. Fearfulness and sex in F2 Roman rats: males display more fear though both sexes share the same fearfulness traits. Physiol Behav. 2003;78:723–732. doi: 10.1016/s0031-9384(03)00043-x. [DOI] [PubMed] [Google Scholar]
- 2.Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 3.Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- 4.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 5.Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blurton-Jones M, Tuszynski MH. Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J Comp Neurol. 2002;452:276–287. doi: 10.1002/cne.10393. [DOI] [PubMed] [Google Scholar]
- 7.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 8.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 9.Cahill L. Sex- and hemisphere-related influences on the neurobiology of emotionally influenced memory. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1235–1241. doi: 10.1016/j.pnpbp.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learn Mem. 2004;11:261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus. 2009 doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- 12.Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol. 2007;18:461–470. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- 13.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 14.Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008;90:171–177. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005a;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein JM, Jerram M, Poldrack R, Anagnoson R, Breiter HC, Makris N, et al. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology. 2005b;19:509–519. doi: 10.1037/0894-4105.19.4.509. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 18.Good M, Day M, Muir JL. Cyclical changes in endogenous levels of oestrogen modulate the induction of LTD and LTP in the hippocampal CA1 region. Eur J Neurosci. 1999;11:4476–4480. doi: 10.1046/j.1460-9568.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- 19.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- 21.Heikinheimo O, Pesonen U, Huupponen R, Koulu M, Lahteenmaki P. Hepatic metabolism and distribution of mifepristone and its metabolites in rats. Hum Reprod. 1994;9(Suppl 1):40–46. doi: 10.1093/humrep/9.suppl_1.40. [DOI] [PubMed] [Google Scholar]
- 22.Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behav Brain Res. 2006;166:93–100. doi: 10.1016/j.bbr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 24.Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Kinrys G, Wygant LE. Anxiety disorders in women: does gender matter to treatment? Rev Bras Psiquiatr. 2005;27(Suppl 2):S43–S50. doi: 10.1590/s1516-44462005000600003. [DOI] [PubMed] [Google Scholar]
- 26.Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- 27.Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberzon I, Britton JC, Phan KL. Neural correlates of traumatic recall in posttraumatic stress disorder. Stress. 2003;6:151–156. doi: 10.1080/1025389031000136242. [DOI] [PubMed] [Google Scholar]
- 29.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 30.Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- 31.McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 32.Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, et al. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav Neurosci. 2006a;120:1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- 33.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 35.Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- 36.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol Psychol. 2006b doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 39.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 40.Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann N Y Acad Sci. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- 41.Pigott TA. Anxiety disorders in women. Psychiatr Clin North Am. 2003;26:621–vii. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 42.Pilgrim C, Hutchison JB. Developmental regulation of sex differences in the brain: can the role of gonadal steroids be redefined? Neuroscience. 1994;60:843–855. doi: 10.1016/0306-4522(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 43.Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry. 2001;62(Suppl 17):47–54. [PubMed] [Google Scholar]
- 44.Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci U S A. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Rivas-Arancibia S, Vazquez-Pereyra F. Hormonal modulation of extinction responses induced by sexual steroid hormones in rats. Life Sci. 1994;54:L363–L367. doi: 10.1016/0024-3205(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 50.Segal M, Murphy D. Estradiol induces formation of dendritic spines in hippocampal neurons: functional correlates. Horm Behav. 2001;40:156–159. doi: 10.1006/hbeh.2001.1688. [DOI] [PubMed] [Google Scholar]
- 51.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- 53.Terasawa E, Timiras PS. Electrical activity during the estrous cycle of the rat: cyclic changes in limbic structures. Endocrinology. 1968;83:207–216. doi: 10.1210/endo-83-2-207. [DOI] [PubMed] [Google Scholar]
- 54.Tomihara K, Soga T, Nomura M, Korach KS, Gustafsson JA, Pfaff DW, et al. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2009;96:300–306. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toufexis DJ, Myers KM, Bowser ME, Davis M. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. J Neurosci. 2007;27:9729–9735. doi: 10.1523/JNEUROSCI.2529-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 57.Wiltgen BJ, Sanders MJ, Behne NS, Fanselow MS. Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behav Neurosci. 2001;115:26–32. doi: 10.1037/0735-7044.115.1.26. [DOI] [PubMed] [Google Scholar]
- 58.Wiltgen BJ, Sanders MJ, Ferguson C, Homanics GE, Fanselow MS. Trace fear conditioning is enhanced in mice lacking the delta subunit of the GABAA receptor. Learn Mem. 2005;12:327–333. doi: 10.1101/lm.89705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 60.Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- 61.Yuan DL, Chambers KC. Estradiol accelerates extinction of a conditioned taste aversion in female and male rats. Horm Behav. 1999;36:1–16. doi: 10.1006/hbeh.1999.1520. [DOI] [PubMed] [Google Scholar]


