Abstract
Background
The outcome literature of subthalamic nuclei (STN) deep brain stimulation (DBS) suggests that cognitive declines are commonly reported following surgery. We hypothesized that differences in electrode position and surgical trajectory may lead to a differential neuropsychological outcome.
Methods
We conducted a standardized evaluation of the location of the DBS electrode tip and the active electrodes, the surgical trajectory through which they were placed, and their relation to neuropsychological change scores (mental status, verbal memory, verbal fluency, and psychological measures) in 17 bilateral STN DBS patients using 6 month post-surgical magnetic resonance imaging data.
Results
Declines in mental status scores were related to electrodes that were more posterior-laterally placed within the frontal quadrant in either hemisphere or those located superiorally in the left hemisphere. Electrodes that were closer to the approximated STN and more superiorally located in the left hemisphere were associated with verbal learning declines at 6-months following surgery. In the right hemisphere, the electrodes that were located more in the lateral direction were related to verbal short-term memory declines; while for verbal long-term memory declines were found for electrodes located more posterior-laterally in the left hemisphere. Declines in verbal fluency scores were more variable with associations found between change scores and electrodes in the lateral and superior directions in the left hemisphere and those electrodes closer to the approximated STN and more superiorally and posteriorally located in the right hemisphere. In contrast, semantic fluency declines were only related to right hemisphere electrodes located more superiorally. Declines in mood were related to those electrodes located further away from the approximated STN, particularly those located more inferiorally and laterally in the left hemisphere. Anxiety change scores were not associated withy the location of the electrodes.
Conclusions
The results provide preliminary evidence that 6-months following bilateral STN DBS cognitive and emotional changes may be related to the surgical trajectory and electrode placement.
Keywords: Parkinson’s disease, Deep Brain Stimulation, Neuropsychology, Cognition, Neuroimaging, Magnetic Resonance Imaging
Advances in neurosurgical, radiological, and electrophysiological techniques have made it possible to produce marked improvements in the motor symptoms of advanced Parkinson’s disease (PD). (1–8) Deep brain stimulation (DBS) has been shown to be an effective and adaptable treatment for the control of PD-related motor symptoms.(4;5;9) The neuropsychological literature on the outcome of DBS suggests that the cognitive declines associated with the surgery are minimal compared to the robust motor improvements.(4;10;11) However, a large amount of variability exists in the literature regarding the cognitive sequelae following surgery. Further elucidation of the cognitive consequences could lead potentially to a clearer understanding of the surgical mechanisms of DBS.
Several studies reported that DBS patients failed to demonstrate cognitive changes on measures tapping memory, visuospatial, executive, and psychomotor functioning three months following implantation of subthalamic nuclei (STN) stimulators.(1;12;13) Significant improvements in delayed verbal recall, psychomotor speed, working memory, and anxiety symptoms following DBS, with only subtle deficits in semantic fluency (14); (15) Although these researchers found significant improvements following DBS, several other centers have reported cognitive declines, particularly when subgroups of patients, most notably older patients, are identified (16;17) Declines in numerous aspects of cognition following implantation of STN stimulators, including working memory, speed of mental processing, set switching, verbal and nonverbal memory, and verbal fluency, have recently been reported. Morrison et al.(18) reported that the surgery itself reduced attention, language, and verbal learning ability, with STN stimulation further reducing verbal recall. Our center reported declines in verbal recall 6-months following STN DBS surgery and information processing speed, naming, verbal fluency, and verbal memory declines 2 years following surgery as compared to a PD control group. (19) The cognitive findings following DBS suggest that verbal fluency and verbal memory abilities, which are believed to be dependent upon intact functioning of the frontostriatal neural system, may decline following implantation of STN stimulators. (19)
One hypothesis for the variability in the DBS cognitive outcome literature is the differences in surgical procedures across centers, including the precise placement of the electrodes within the STN and the surgical trajectory. The position of the DBS electrodes can vary from patient to patient based on several factors, including brain size, brain atrophy and planned surgical trajectory. The differences in electrode position may lead to a differential outcome both motorically and neuropsychologically. Tsai and colleagues(20) reported on the location of bilateral STN electrodes in 38 PD patients. They divided patients into a group with and a group without neuropsychological side effects based on the Mini-Mental Status Examination changes one year following DBS. These authors reported that that the neuropsychological effects of chronic STN-DBS were related to the more anteriorly located electrodes within the ventral STN. In addition to the electrode placement, the surgical trajectory may also be a source of cognitive variability. Neurosurgeons may vary the surgical trajectory of STN DBS based on the patient’s brain organization, including the size of the ventricles, degree of brain atrophy and surface vasculature. Although the microelectrodes and DBS electrodes follow the same pathway, it is not known if the surgical trajectory may lead to deficits.
We investigated the relationship between neuropsychological performance following bilateral STN DBS for the treatment of PD and DBS electrode position and surgical trajectory on post-operative magnetic resonance imaging (MRI). This study focused on mental status, verbal memory, verbal fluency, and semantic fluency declines following DBS surgery as these are the most robust cognitive changes reported in the literature following the surgery. We also investigated psychological variables including depression and anxiety changes and their relations with differences in the electrode position and surgical trajectory.
Research Design and Methods
Participants
Eighteen bilateral STN DBS patients were evaluated at baseline and 6 months following DBS placement on a comprehensive neuropsychological evaluation. A pre-operative CT scan with the stereotactic frame was performed on the day of surgery and a post-operative MRI was performed 6 months following implantation according to Medtronic, Inc. guidelines. The Baylor College of Medicine Institutional Review Board approved the study and informed consent was obtained from each patient.
Inclusion and Exclusion Criteria
PD patients are considered eligible for DBS if: (1) clinical findings are consistent with idiopathic PD; (2) they have had a history of response to levodopa therapy; and (3) they have had evidence of persistent advanced disease (i.e., disabling motor fluctuations, levodopa-induced dyskinesias, or freezing), and a Hoehn and Yahr (H&Y) Parkinson’s disease staging score of 3 or more during their “off” period. Patients are excluded from DBS if they: (1) have Mini-Mental State Examination (MMSE) scores less than 24; (2) have had psychiatric complications that might interfere with compliance (e.g., psychosis, severe major depression); (3) have an H&Y score of 5 during their “on” periods, (4) have a medical contraindication to surgery or stimulation (e.g., uncontrolled hypertension, advanced coronary artery disease), or (5) have an intracranial abnormality that would contraindicate surgery based on preoperative MRI (e.g., stroke, tumor). All patients included in this study were followed at the Baylor College of Medicine Parkinson’s Disease and Movement Disorders Clinic and were maintained on an optimal level of anti-Parkinson’s disease medication for at least one month prior to surgery.
Procedures
Neurosurgical procedures
DBS patients underwent simultaneous STN stimulation using deep brain stimulation leads and implantable pulse generators (Medtronic, Inc.) by one neurosurgeon (RS). DBS is divided into two stages: Stage I – Implantation of DBS leads and Stage II - Implantation of implantable pulse generators (IPG’s). In Stage I, stereotactic guidance, microelectrode recording and macroelectrode stimulation was used to determine the optimal site of placement for the quadripolar DBS lead. This lead was connected to a hand-held pulse generator. The STN target is generally located approximately 12 mm (10–14 mm) lateral from the midsagittal plane, 3 mm behind the midcommissural point, and 4 mm (0–6 mm) below the AC-PC line (coordinates: anteroposterior, 5.1± 0.72 1/12th of AC-PC length; extrema, 2.88–7.08. Vertical, −1.29±0.83 1/8th of HT; extrema, −3.29–0.19. Laterality, 12.1±2.05 from midline; extrema 9.00–15.2).(21) Stage II, the leads were tunneled subcutaneously to the infraclavicular region where an implantable pulse generator was placed in an infraclavicular pocket.
Neurological and Medication evaluations
All patients were evaluated in their “off” and “on” medication states using the Unified Parkinson’s Disease Rating Scale (UPDRS) and H&Y at baseline and 6 months post-DBS. All formal clinical neurological evaluations were performed by neurologists specializing in movement disorders. Medication and dosage changes were recorded across these time periods.
Radiological evaluation
Pre-operative CT images were acquired on a General Electric (Excite) scanner in an axial plane (40 slices), using a slice thickness of 1.25 mm and a field of view of 24 cm. The position of DBS electrodes was investigated 3 to 6 months postoperatively by MRI studies performed on a 1.5T General Electric (Excite) scanner, 12M5 software version. T1-weighted images were used to determine the end of the DBS lead and the surgical track through which the lead was placed. The stimulator was turned off with the amplitude parameter set to 0.0. The MRI protocol used was designed to provide the optimal images to visualize the STN, limit the electrode “shadow,” and reduce MRI-related distortions and deformations. This included T1-weighted images with acquired in a coronal plane (60–70 slices), with 3.0 mm slice thickness, and a 20 cm field of view. The electrode position was evaluated by one rater (EW) on the StealthStation™.
Neuropsychological Evaluation
The neuropsychological test battery for DBS patients was selected to assess the pattern of cognitive deficits previously shown to be impaired in advanced Parkinson’s disease.(22) The DBS patients were tested with their stimulators in the “on” position. The patients took their scheduled dosage of medications throughout testing. Standardized test administration procedures were used. We report on a subset of the neuropsychological measures focusing on general mental status, verbal memory, verbal fluency, and semantic fluency which are the most common cognitive declines reported following surgery. The clinical neuropsychological tests were administered in a standard, pre-determined order to mitigate the presentation of confounding material during memory delay periods. The Rey Auditory Verbal Learning Test (RAVLT) and verbal fluency “CFL” and “PRW” versions were used with alternate forms utilized to reduce the influence of practice effects. For the Mini-Mental Status Examination (MMSE), Dementia Rating Scale-II (DRS), verbal fluency (3 trials), and semantic fluency measures total scores were the metrics of analysis. For the RAVLT, the total learning score over 5 trials, short-term recall, and long-term recall scores were used as the metrics of analysis. For psychological evaluation, the Beck Depression Inventory (BDI) total score and the State-Trait Anxiety Inventory (State and Trait total scores; STAI) were administered and analyzed. Difference scores were calculated for each measure by subtracting the 6-month raw score minus the baseline raw score. Consequently, each difference score represents the change in performance from before to after surgery.
MRI Analysis
Each data set was imported onto a StealthStation™ and into the Framelink 5.1™ software, and proper orientation of the data set was confirmed. Next, the post-operative MRI was co-registered to the pre-operative CT using the automated StealthMerge Registration. Co-registration was visually confirmed on 8–10 slices to ensure accuracy (see Figure 1A). Next, the 9 rods of the stereotactic frame visible on CT were marked in order to orient the two imaging series to the stereotactic frame coordinates. These were marked at the level of the STN. In all cases, the mean rod marking error (comparison of the marked rods to the computer’s model of the stereotactic localizer) did not exceed 2 mm. The volumes were then realigned to the intercommissural fissure and two additional midline points in the coronal plane, and the anterior to posterior commissure (AC-PC) line to correct for head rotation, twist and tilt prior to generating triplanar data sets. Next, the AC-PC midpoint was established and marked; this was the coordinate that was equidistant from AC and PC in all three planes (see Figure 1B). Next, using all three planes in addition to a “probe’s eye view” as a reference, the tip of the DBS lead within each patient’s brain was determined for both the right and left hemispheres. Finally, the entry point on the surface of the patient’s brain through which the DBS lead was placed was determined for both right and left hemispheres. Starting at the most inferior slice where the lead tip was evident in the axial plane, the surgical pathway was traced superiorly, with continual reference to the pathway of the lead in all three planes until the point of entry at the brain’s surface. The surgical trajectory intersection with brain structures, location, surgical trajectory angle, and distance off or past the approximated STN was calculated for the electrode tip and the active electrode in each hemisphere.
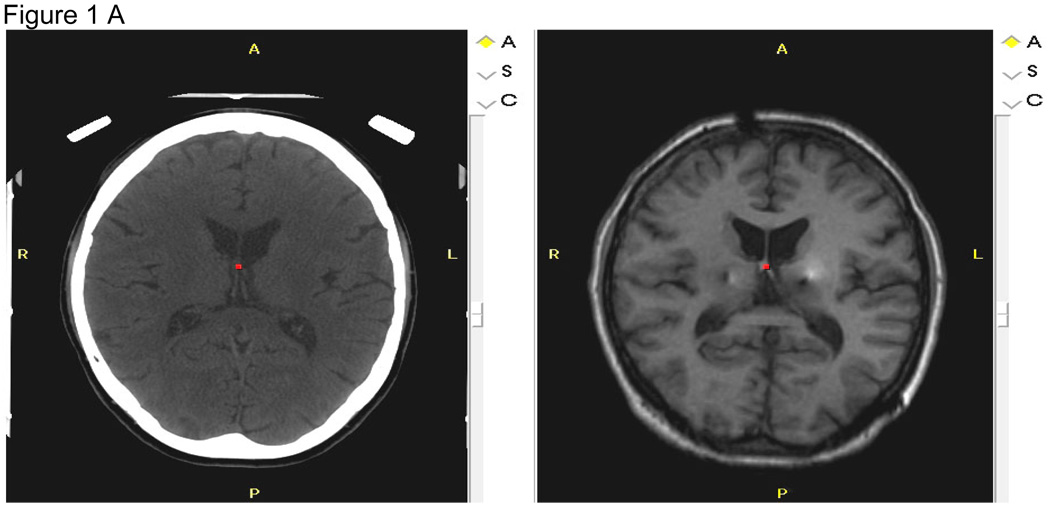
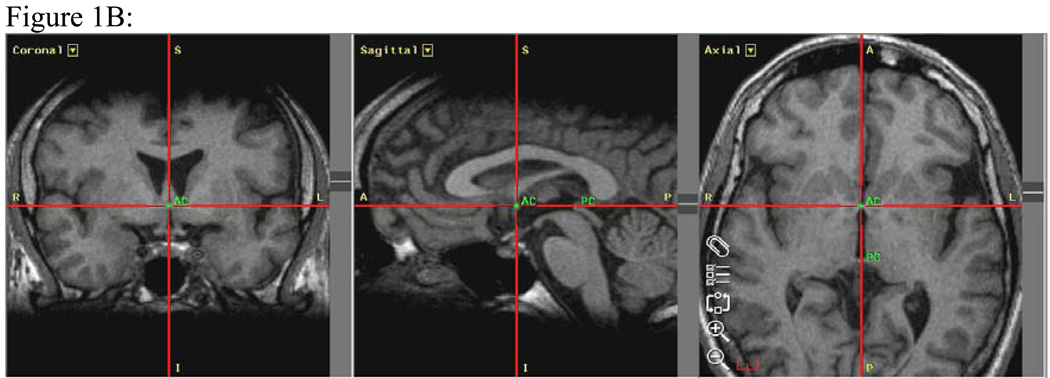
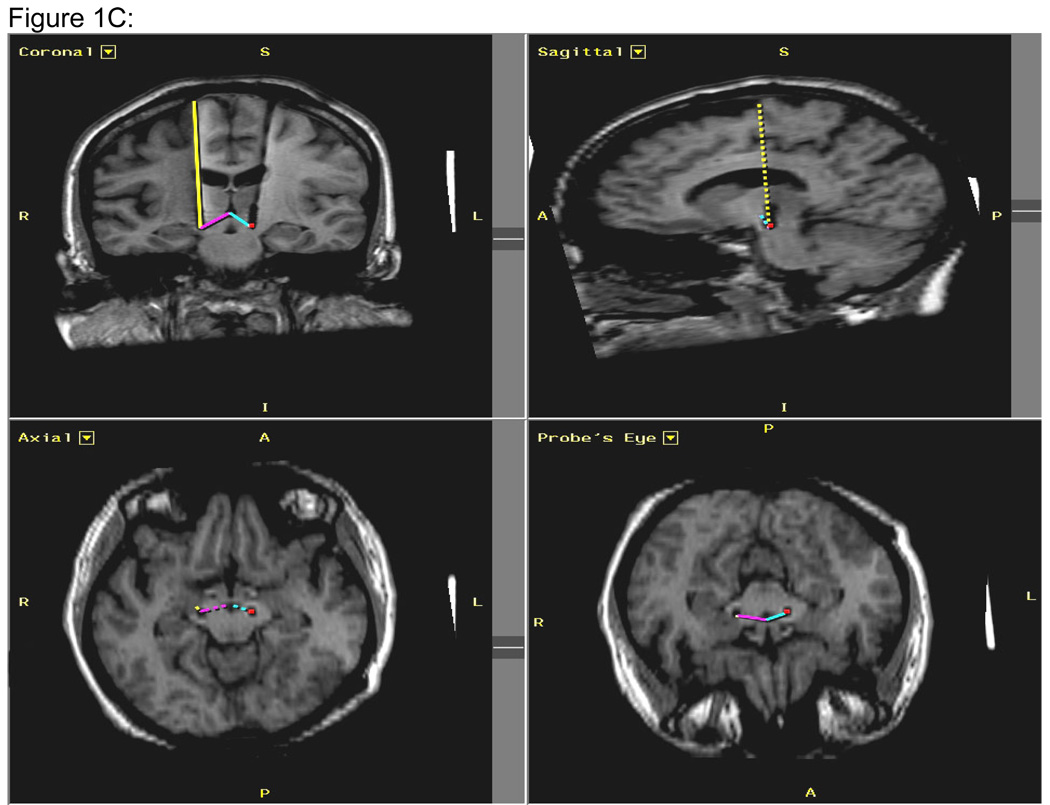
Figure 1. Figures 1 A–C: MRI Analyses.
Figure 1A: Co-registration of the 6-month post-operative MRI in the axial plane (right) to the pre-operative CT (left) of a subject. This co-registration process places the post-operative MRI in the same spatial position as the CT and enables determination of stereotactic coordinates based upon the stereotactic surgical frame. R=right, L=left, A=anterior, P=posterior.
Figure 1B: Realignment of the scans to the anterior commissure-posterior commissure (AC-PC) line shown in three planes. Images are also aligned to at least three other points in the coronal plane to correct for twist and tilt. The AC-PC midpoint is a point equidistant between the anterior commissure (labeled AC) and the posterior commissure (labeled PC). R=right, L=left, A=anterior, P=posterior.
Figure 1C: Measurement of electrode placement on post-surgical MRI. The yellow-colored line indicates the surgical trajectory from the brain surface to the DBS electrode tip near the STN. Tracking of this pathway was facilitated by the simultaneous visualization of the lead in all three planes from the most inferior axial slice where the lead tip was evident to the most superior slice at the surface of the brain. The DBS lead pathway is also evident on the left side in the coronal view. The measurement of linear distance between the AC-PC-midpoint and the electrode tip is reflected in the pink-colored line for the right hemisphere and the blue-colored line for the left hemisphere.
Surgical Trajectory Intersection
Using all three planes as well as a “probe’s eye view” tool, we determined whether the lead trajectory intersected the body of the lateral ventricles, the head of the caudate or the thalamus on each hemisphere in each patient. This was recorded as the presence or absence of intersection with the structure for each hemisphere, and was aided by visual inspection in all three planes simultaneously.
Electrode Location
Distance to Electrode Tip (Length of Surgical Trajectory)
The length of the surgical trajectory (in mm) in each case was linear between the point of entry at the brain surface and the final placement of the tip of the DBS lead in the STN region; this pathway was visible on post-surgical MRI due to the presence of the DBS lead device (Figure 1C).
Distance of Electrode Tip from Midpoint of AC-PC midline
The linear distance (in mm) from the AC-PC midpoint to the DBS lead tip was also measured for the right and the left hemispheres (Figure 1C). In all cases, the DBS lead tip was inferior and posterior to the AC-PC midpoint, though the angle of this measure differed for each patient (discussed below).
Distance to the Active Electrode
The active electrode was calculated using the positive and negative electrode information gathered from the patients’ stimulator adjustment prior to the 6 month MRI scan for the right and left hemispheres. Using the quadripolar lead electrode specifications, with four 1.5 mm electrodes spaced 1.5 mm apart and 1.5 mm from the tip to the first electrode, we calculated the distance to the negative electrode, which exerts the therapeutic effect. This length (in mm) was measured as the distance from the brain surface to the active electrode (Figure 1C).
Distance of Active Electrode from Midpoint of AC-PC midline
The linear distance (in mm) from the AC-PC midpoint to the active electrode as visualized on the MRI was measured for the right and the left hemispheres (Figure 1C).
Angle Measurement
Because linear distance could not fully capture three-dimensional differences in surgical trajectories, angles in relation to the axial plane and to the midsagittal plane were calculated using Framelink 5.1™ software (Figures 2A and 2B). Angle measures were recorded for each hemisphere for the trajectory of the surgical pathway from the brain surface to the electrode tip and to the active electrode. In relation to the axial plane (range from 0 to 90 degrees), angles closer to 0 degrees were more laterally located, while angles closer to 90 degrees were more medially located. Additionally, these angles were calculated in relation to the midsagittal plane (range from 0 to 90 degrees), such that angles that were closer to 0 degrees were more anterior and medial and angles that were closer to 90 degrees were more posterior and lateral. All of these angles are located within the frontal quadrant of the brain for each hemisphere.
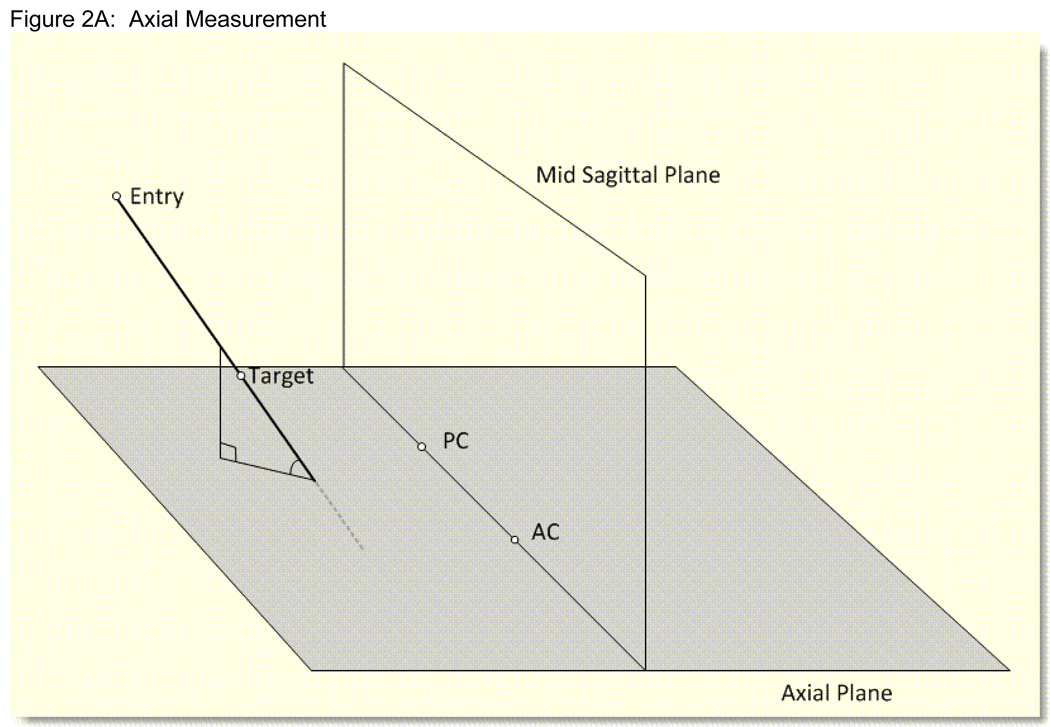
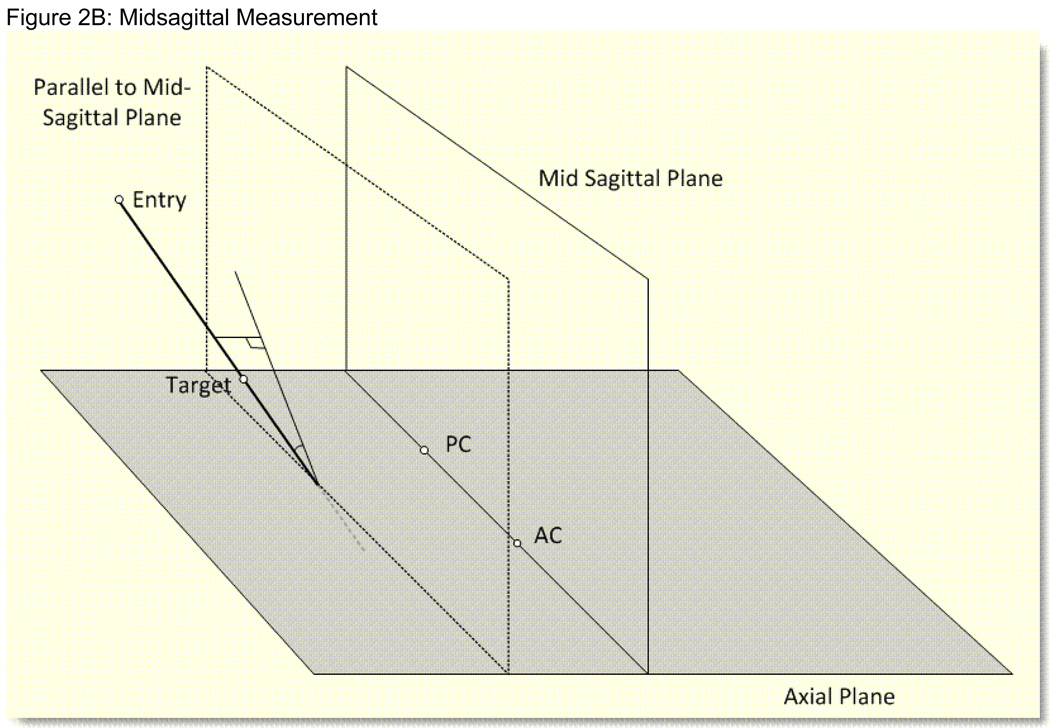
Figure 2. Figures 2A, 2B: Stealth Angle Measurements.
Figure 2A: Schematic diagram of the Stealth angle measurement for the surgical trajectory in relation to the axial plane. The angle ranges from −90 to +90 degrees. 0 degrees means the surgical trajectory is parallel to the axial plane. 90 degrees means the surgical trajectory is perpendicular to the axial plane. Positive numbers mean the entry point is superior to the target point. All surgical entry points for the surgical trajectory were superior to the target. For the AC-PC midline (entry) to target calculations, the positive numbers mean that the entry point is inferior to the target and the negative numbers mean the entry point is superior to the target.
Figure 2B: Schematic diagram of the Stealth angle measurement for the surgical trajectory in relation to the midsagittal plane. The angles range between 0 and 90 degrees. 0 degrees means the surgical trajectory is parallel to the midsagittal plane. 90 degrees means the surgical trajectory is perpendicular to the midsagittal plane.
Next, the trajectory from the midpoint of the AC-PC line (entry) to the electrode tip and to the active electrode for each hemisphere in relation to the midsagittal and axial planes were calculated. In relation to the axial plane, the AC-PC to target angle (range from −90 to +90 degrees) revealed that angles that were closer to 0 degrees were more medial, while angles closer to −90 degrees were more superior, and angles closer to +90 degrees were more inferiorally located. These angles in relation to the midsagittal plane (range 0 to 90 degrees) were defined as angles closer to 0 degrees being more anterior-medially located, while angles closer to 90 degrees were more posterior laterally located within the frontal quadrant. A total of 16 angles were calculated for each patient.
Distance Off Plan and Distance To/Past Target
Because the anatomical STN was often difficult to visualize on images, we utilized a software-generated approximation of the location of the STN. The Framelink 5.1™ software approximated the location of the STN, and the shortest distance (in mm) was calculated between the actual placement of the DBS lead tip, the actual placement of the active electrode, and the approximated location of the STN for both the right and the left hemispheres. The distance off the plan is based upon the shortest distance to the line of the surgical plan in any direction, which does not take into account the location of the target. The distance to or past the target is based on the known general anatomic position of the STN relative to the AC-PC midpoint, which is perpendicular to the surgical plan. For the surgical trajectory plan, this measurement is the shortest distance to the target in the superior (to target) and inferior (past target) directions, while for the measurements from the midpoint of the AC-PC line, this distance is the shortest distance to the target in the medial (to target) and lateral (past target) directions.
Statistical Analysis
The alpha level was set at p=0.05 for all analyses. First, a series of ANOVAs were conducted to investigate differences in cognitive decline following surgery (performance at baseline subtracted from performance at 6 months post-operative follow-up) based on whether or not the ventricles, caudate, or thalamus were intersected by the surgical trajectory (intersected, not intersected). Second, to investigate the relationship between neuropsychological performance and DBS electrode placement, a series of correlational analyses were run using the imaging variables (described above) and difference scores on the neuropsychological measures (performance at the 6 month follow-up evaluation minus the baseline score). Third, we categorized DBS patients into two groups based on their neuropsychological outcome: 1) patients who remained stable or improved cognitively (Stable: no declines on RAVLT indices, verbal fluency or semantic fluency) versus 2) patients who declined on at least 2 of these neuropsychological measures at the 6 month follow-up evaluation (Decliners). No patients declined on only one measure and no patient improved on one measure and declined on the other two measures. We then ran a series of ANOVAs to investigate if the patients who had declined cognitively following surgery differed from those who had not declined cognitively on the location of their electrodes and the angle of their surgical trajectory.
Results
Participants
Of the 18 patients whose MRI scans were evaluated, one patient’s electrode in the left hemisphere had migrated outside the radiologically defined STN. Her quantitative scores were assessed as statistical outliers; thus, her data were excluded from further analyses. Briefly, she is a 65 year-old, white female who had PD for 19 years prior to DBS. Prior to surgery, she was an H&Y 2.5 “on” her medications and a 4.0 “off” her medications. Her UPDRS motor scores went from a 15 “on” medications to 51 “off” medications. She did not receive good motor benefit following the surgery and her left hemisphere stimulator was eventually turned off following the MRI results. She elected not to have a revision of the DBS electrodes.
Motor Outcome
The DBS patients as a group demonstrated significant improvement in their UPDRS motor scores 6 months following surgery (Mean change = −12.1; t(13)=3.12, p=0.008). The improvement in motor functioning was correlated with the angle of the surgical trajectory to the electrode tip and the active electrode in relation to the midsagittal plane in the right hemisphere (electrode tip: r=0.66, p=0.01; active electrode: r=0.58, p=0.04; Figure 3A and 3B). No other significant correlations were found between the motor functioning and the electrode tip placement or active electrode location.
Figure 3. Figures 3A, 3B: Motor Outcome.
Figure 3A: Scatterplot with regression line demonstrating the relationship between the angle of the surgical trajectory to the electrode tip in relation to the midsagittal plane in the right hemisphere with motor outcome. (p=0.01)
Figure 3B: Motor Outcome. Scatterplot with regression line demonstrating the relationship between the angle of the surgical trajectory to the active electrode in relation to the midsagittal plane in the right hemisphere with motor outcome. (p=0.04)
Neuropsychological Outcome
These 17 patients are a subset of DBS patients who had a preoperative CT scan with stereotactic frame and a post-operative MRI and whose neuropsychological and motor functioning has been reported on previously by York et al.(19) To describe the sample in terms of their neuropsychological functioning both before and after surgery, we present a subset of their neuropsychological scores (see Table 2).
Table 2.
Neuropsychological Scores at baseline and 6 months post DBS surgery
| Measure | Baseline | 6 months post | p |
|---|---|---|---|
| MMSE | 28.1 (2.88) | 26.9 (4.43) | 0.04 |
| DRS | 134.8 (8.74) | 134.8 (9.09) | 0.44 |
| RAVLT Total | 39.5 (12.2) | 37.0 (14.4) | 0.05 |
| RAVLT STM | 8.12 (3.80) | 7.75 (4.04) | 0.30 |
| RAVLT LTM | 7.94 (3.91) | 6.88 (4.16) | 0.02 |
| Verbal Fluency | 31.5 (14.5) | 28.3 (11.5) | 0.05 |
| Semantic Fluency | 17.9 (5.84) | 14.3 (4.13) | 0.001 |
| BDI | 15.1 (7.40) | 12.9 (9.23) | 0.02 |
| STAI State (%ile) | 58.6 (28.3) | 54.1 (32.1) | 0.73 |
| STAI Trait (%ile) | 63.5 (27.1) | 59.3 (33.3) | 0.23 |
Stimulation Parameters
The stimulation parameters differed between the left and the right hemispheres for amplitude, rate and current, with these three indices being higher in the left versus the right hemisphere. The pulse width and voltage did not differ between the right and the left hemispheres (see Table 3). We stratified the patients based on the type of stimulation (monopolar versus bipolar) and conducted t-tests for each of the MRI indices. The groups differed significantly on the angle from the AC-PC midpoint to the electrode tip in relation to the midsaggital plane and the active electrodes distance off the approximated STN in the left hemisphere, with the bipolar stimulation group’s electrodes being closer to the approximated STN and more posterior-laterally located (p=0.02, p=0.02, respectively).
Table 3.
Electrode Placement Descriptives
| Measure | Right M(SD) |
Left M(SD) |
p |
|---|---|---|---|
| Amplitude (volts) | 3.31 (0.92) | 3.54 (0.95) | 0.01 |
| Pulse Width (µsec) | 63.5 (9.96) | 65.3 (15.9) | 0.63 |
| Rate (Hz) | 165.0 (19.0) | 169.4 (26.6) | 0.04 |
| Current (mA) | 50.7 (27.7) | 75.6 (73.9) | 0.001 |
| Voltage (volts) | 3.72 (0.01) | 3.72 (0.01) | 0.87 |
Surgical Trajectory Intersection
We evaluated whether the surgical trajectory intersected with either one or both of the ventricles, the caudate, and the thalamus for each of the DBS patients. The surgical trajectory intersected with at least one ventricle in 67% of the patients, with 17% of these patients having both ventricles traversed. Similarly, the trajectory intersected with the caudate on one side in 56% of patients, and on both sides in 38% of patients. At least one side of the thalamus was intersected in all patients, and both thalami were intersected in 89% of the sample.
Surgical trajectory intersection correlation with neuropsychological measures
A trend was found for the patients in whom the ventricle(s) were intersected to demonstrate a decline on verbal long-term memory and verbal fluency following surgery as compared to those patients who did not have either ventricle intersected (p=0.06). Whether or not the surgical trajectory intersected the caudate was not significantly related to changes in verbal fluency, verbal memory, or psychological measures following surgery. This analysis could not be conducted for the thalamus as 100% of the patients had at least one side of the thalamus intersected.
Electrode Location
Electrode Tip
The length of the track from entry to the electrode tip (Length of Surgical Trajectory) and from the midpoint of the AC-PC line to the electrode tip did not differ significantly between the left and the right hemispheres (p=0.94, p=0.14, respectively; Table 4).
Table 4.
Electrode Tip and Active Electrode MRI Measurements for the Left and Right Hemispheres
| Measure | Electrode Tip | Active Electrode | ||
|---|---|---|---|---|
| Right M(SD) |
Left M(SD) |
Right M(SD) |
Left M(SD) |
|
| Location | ||||
| Entry to target (mm) | 78.4 (5.12) | 78.4 (3.90) | 70.2 (4.47) | 70.7 (5.29) |
| AC-PC midpoint to target (mm) | 4.85 (1.64) | 4.16 (1.38) | 12.1 (1.59) | 13.4 (1.54) |
| Angle Measurements | ||||
| Entry to target | ||||
| Midsagittal | 10.8 (4.61) | 8.61 (4.69) | 10.5 (4.36) | 8.72 (5.04) |
| Axial | 76.6 (8.08) | 73.8 (8.20) | 76.1 (8.37) | 74.1 (8.20) |
| AC-PC midpoint to target | ||||
| Midsagittal | 48.9 (7.820 | 53.8 (6.80) | 79.3 (10.9) | 75.2 (9.81) |
| Axial | 66.6 (7.50) | 59.2 (10.3) | 13.8 (39.5) | 31.5 (42.1) |
| Distance off Approximated STN | ||||
| Entry to Target (mm) | 2.21 (1.02) | 1.54 (0.79) | 2.30 (0.98) | 1.51 (0.84) |
| AC-PC midpoint to target (mm) | 4.59 (1.86) | 4.08 (1.48) | 4.22 (2.26) | 3.32 (2.15) |
| Distance Past Approximated STN | ||||
| Entry to Target (mm) | 5.02 (2.41) | 5.07 (1.93) | −2.38 (4.66) | −0.82 (4.24) |
| AC-PC midpoint to target (mm) | 1.61 (5.48) | 5.03 (3.43) | 3.01(8.16) | 3.09 (5.87) |
Correlation of Electrode tip distances and neuropsychological functioning
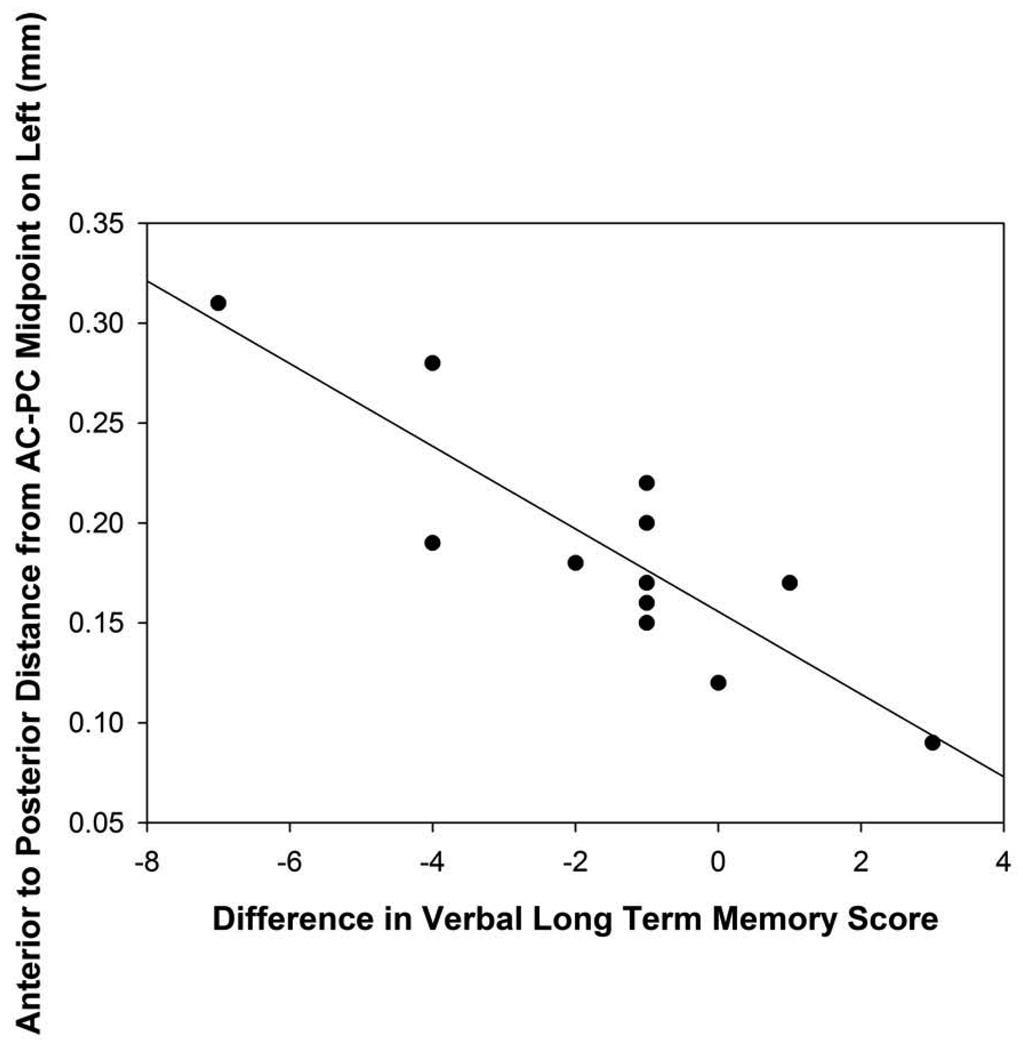
The distance of the electrode tip from the midpoint of the AC-PC in the left hemisphere was correlated with changes on verbal long-term recall (r=−0.70, p=0.005; Figure 4A), with the patients whose electrode tips are in a more posterior position demonstrating the largest declines in verbal long-term recall following surgery. The length of the track in the right and left hemispheres and the distance of the electrode tip from the midpoint of the AC-PC in the right hemisphere were not correlated significantly with neuropsychological or psychological changes following surgery.
Figure 4. Figures 4A, 4B: Electrode Location.
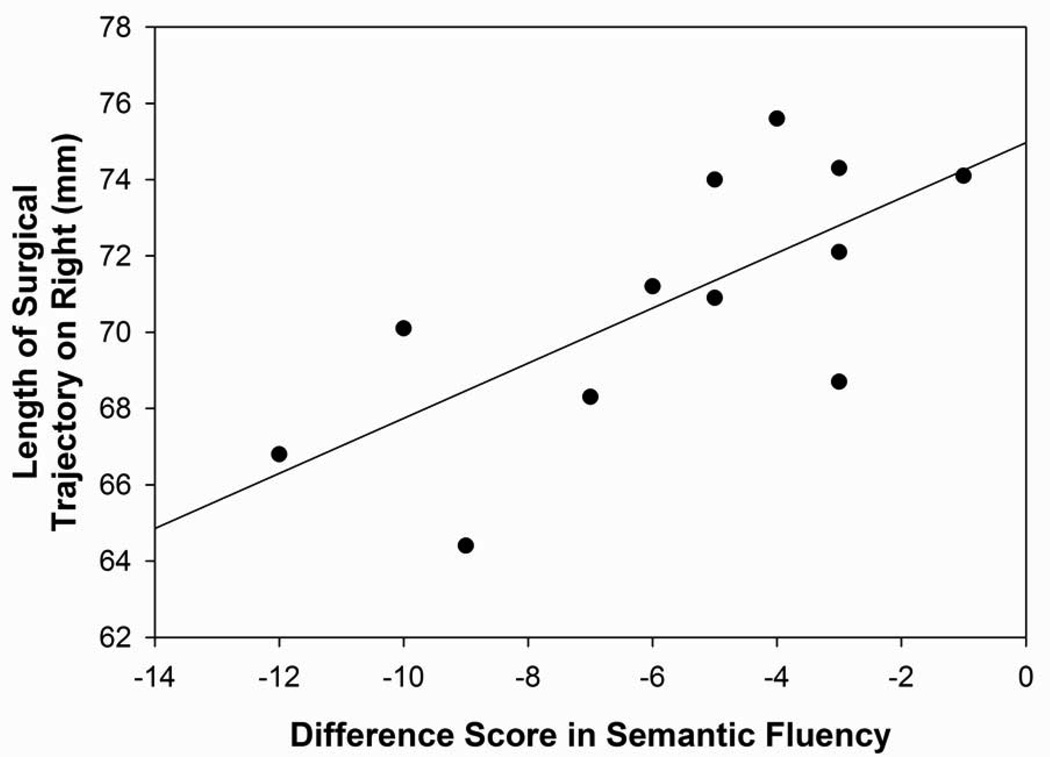
Figure 4A: Scatterplot with regression line demonstrating the relation between the length of the track to the active electrode (in mm) in the right hemisphere and the difference score for semantic fluency (6-month score minus baseline score). The lower the number (in mm) reflects a shorter track in the right hemisphere and a lower difference score reflects a greater decline in semantic fluency performance following surgery. (p=0.01)
Figure 4B: Scatterplot with regression line demonstrating the relation between the distance (in mm) of the active electrode from the midpoint of the AC-PC line in the right hemisphere with verbal short-term memory difference scores (6-month score minus baseline score). The lower distance is in a more anterior direction while a higher distance is in a more posterior direction. A lower difference score reflects worse verbal short-term memory performance following surgery. (p=0.003)
Active Electrode
The length of the track from entry to the active electrode did not differ significantly between the hemispheres (p=0.68; Table 4). The distance from the midpoint of the AC-PC line to the active electrode was significantly longer in the left hemisphere as compared to the right hemisphere (p=0.03; Table 4).
Correlation of Active Electrode Distances and Neuropsychological Functioning
In the right hemisphere, the length of the track from entry to the active electrode was significantly correlated with declines in semantic fluency following surgery (r=0.70; p=0.01; Figure 4A). The patients with shorter right hemisphere tracks to the active electrode demonstrated the largest declines in semantic fluency following surgery. The distance of the active electrode from the midpoint of the AC-PC in the right hemisphere was correlated significantly with changes in short-term verbal memory (r=−0.73; p=0.003; Figure 4B). Patients whose active electrodes were more lateral demonstrated the largest declines on verbal short-term recall following surgery. No other significant correlations were found between the active electrode location and the cognitive and emotional variables.
Angle Measurements
Electrode Tip
The angles for the surgical trajectory for the electrode tip differed significantly for the right and left hemispheres in relation to the midsagittal and to the axial planes (midsagittal: p=0.05; axial: p=0.002; Table 4). The angles for the surgical trajectory from the midpoint of the AC-PC line to the electrode tip differed significantly for the right and left hemispheres (midsagittal: p=0.02; axial: p=0.002; Table 4).
Correlation of Electrode Tip Angle Measurements with Neuropsychological Functioning
The angle of the surgical trajectory to the electrode tip in relation to the midsagittal plane in the left hemisphere was correlated significantly with MMSE (r=−0.57; p=0.03), verbal long-term memory (r=−0.57, p=0.03), and depression change scores (r=0.63; p=0.04). These findings suggest that the surgical trajectories that were more lateral in the left hemisphere were associated with a greater decline on mental status and verbal long-term memory scores and were associated with more depressive symptoms following surgery. In the right hemisphere, the angle of the surgical trajectory in relation to the midsagittal plane was correlated significantly with MMSE outcome (r=−0.59; p=0.02), with the more lateral angles related to declines in MMSE scores.
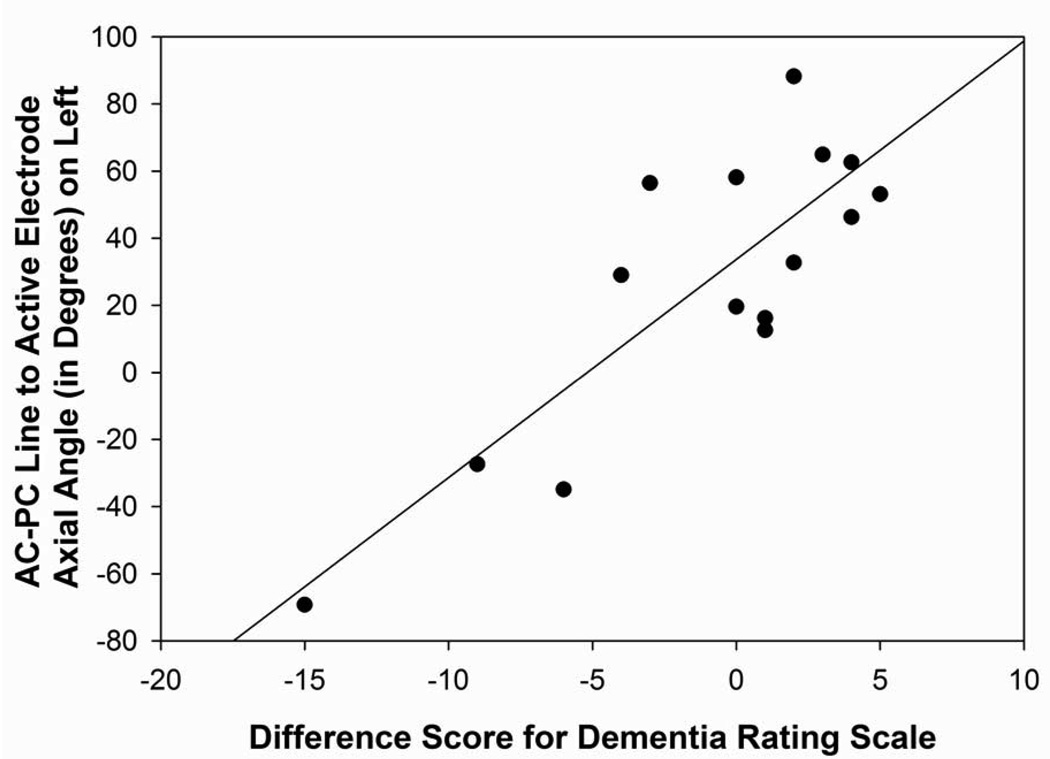
The angle from the midpoint of the AC-PC to the electrode tip in relation to the midsagittal plane in the left hemisphere was correlated significantly with MMSE change scores (r=−0.52, p=0.05) and DRS outcome (r=−0.67, p=0.007; Figure 5A), suggesting that the surgical trajectories that were more posterior-lateral within in the frontal quadrant of the brain in the left hemisphere were associated with a greater decline in mental status scores following surgery. In relation to the axial plane, the angle from the midpoint of the AC-PC line to the electrode tip in the left hemisphere correlated significantly with difference scores for the DRS (r=0.72; p=0.002), RAVLT short-term memory (r=0.71; p=0.005; Figure 5B), and RAVLT total (r=0.52; p=0.05) scores. In the left hemisphere, surgical trajectories that were more superior were related to declines in mental status, verbal learning, and verbal short-term memory scores. No significant correlations were found between the angles from the midpoint of the AC-PC line to the electrode tip in the right hemisphere.
Figure 5. Figures 5A–D: Angle Measurements.
Figure 5A: Scatterplot with regression line demonstrating the relation between the angle from the midpoint of the AC-PC line to the electrode tip in relation to the midsagittal plane in the left hemisphere and Dementia Rating Scale difference scores. The lower the angle reflects a more posterior-lateral direction in the frontal quadrant and a lower difference score reflects a greater decline in mental status performance following surgery (p=0.007).
Figure 5B: Scatterplot with regression line demonstrating the relation between the angle from the midpoint of the AC-PC line to the electrode tip in relation to the axial plane in the left hemisphere and verbal short-term memory difference scores. The lower the angle reflects a more superior direction and a lower difference score reflects a greater decline in verbal short-term memory performance following surgery (p=0.005).
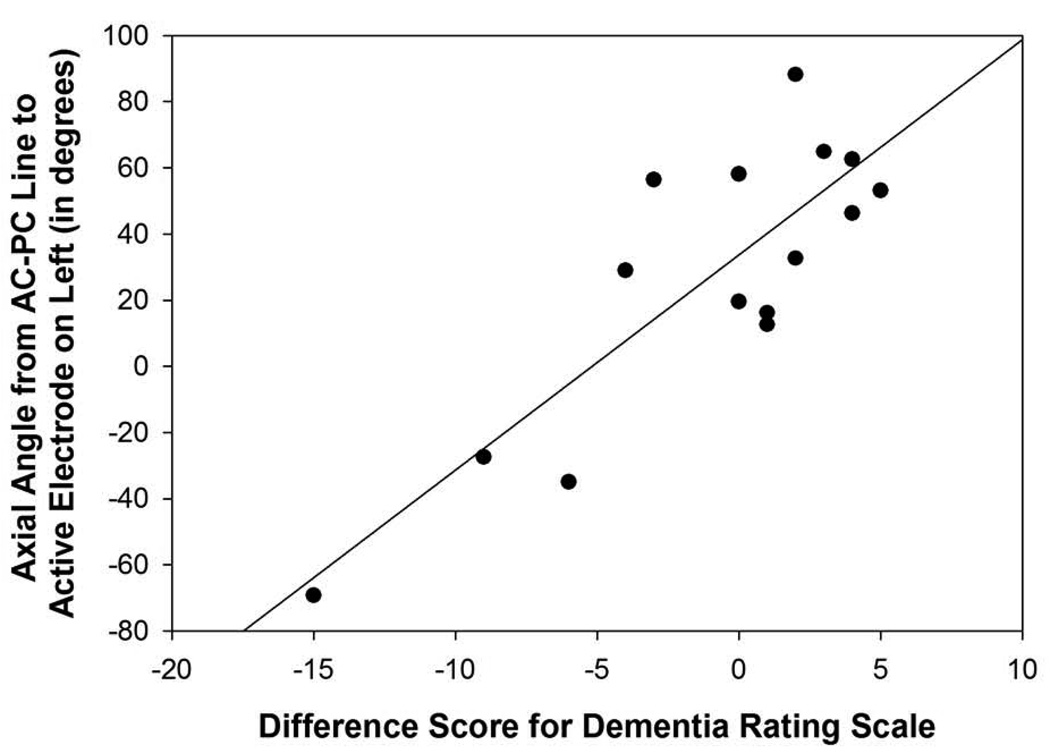
Figure 5C: Scatterplot with regression line demonstrating the relation between the angle from the midpoint of the AC-PC line to the active electrode in relation to the axial plane in the left hemisphere and Dementia Rating Scale difference scores. The lower the angle reflects a more superior direction and a lower difference score reflects a greater decline in mental status performance following surgery (p=0.001).
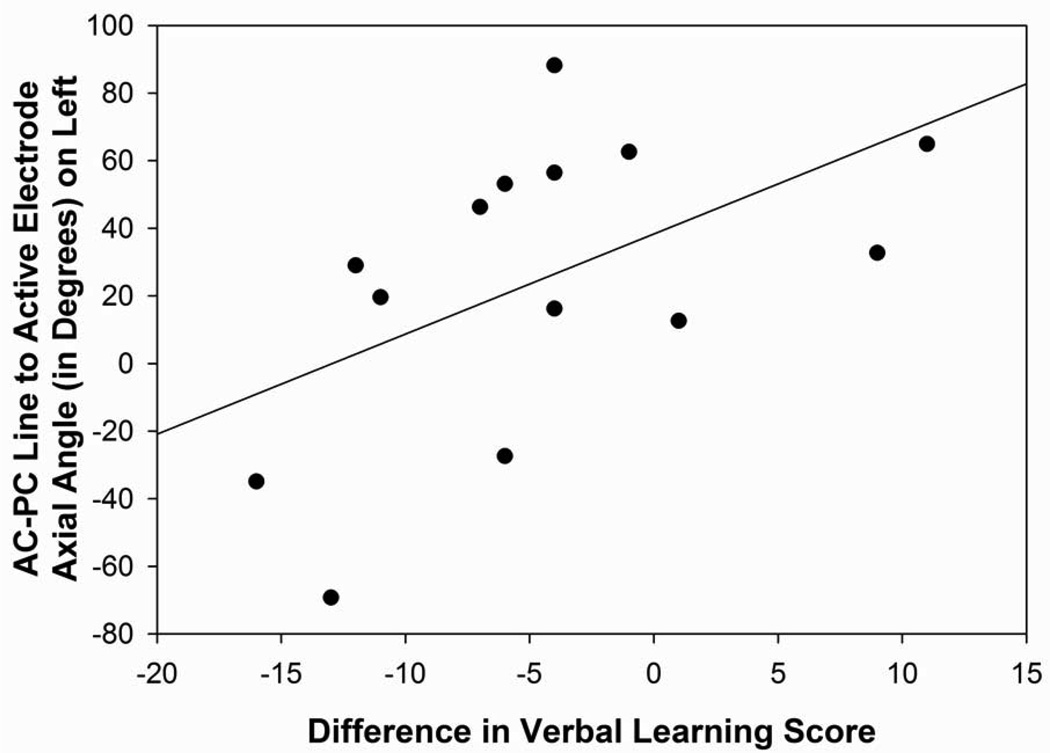
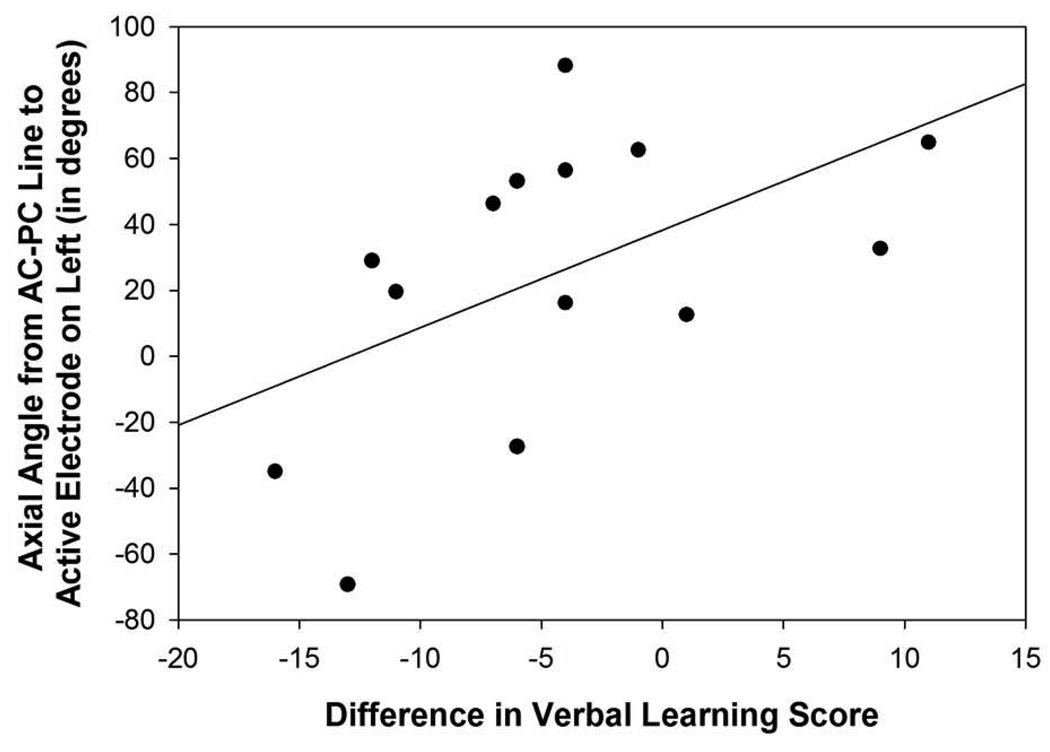
Figure 5D: Scatterplot with regression line demonstrating the relation between the angle from the midpoint of the AC-PC line to the active electrode in relation to the axial plane in the left hemisphere and verbal learning difference scores. The lower the angle reflects a more superior direction and a lower difference score reflects a greater decline in verbal learning (p=0.05).
Active Electrode
The angles for the surgical trajectory for the active electrode did not differ significantly for the right and left hemispheres in relation to the midsagittal and to the axial planes (midsagittal: p=0.27; axial: p=0.12; Table 4). The angle for the surgical trajectory from the midpoint of the AC-PC line to the active electrode differed significantly between the right and left hemispheres in relation to the axial plane (p=0.03), but not in relation to the midsagittal plane (p=0.12; Table 4).
Correlation of Active Electrode Angle Measurements with Neuropsychological Functioning
For the active electrode, the angle of the surgical trajectory in relation to the midsagittal plane in the left hemisphere was correlated significantly with MMSE (r=−0.56; p=0.03) and RAVLT long-term memory (r=−0.53, p=0.05) difference scores. The surgical trajectories that were more posterior-lateral in the frontal quadrant in the left hemisphere were associated with a greater decline on mental status and verbal long-term memory scores following surgery. In the right hemisphere, the angle of the surgical trajectory in relation to the midsagittal plane was correlated significantly with MMSE outcome scores (r=−0.62; p=0.01), suggesting that the surgical trajectories that were more posterior-lateral in the frontal quadrant of the brain in the right hemisphere were related to declines in mental status following the surgery.
The angle from the midpoint of the AC-PC to the active electrode in relation to the axial plane in the left hemisphere was correlated significantly with difference scores for the DRS (r=0.84, p=0.001; Figure 5C), RAVLT short-term memory (r=0.56, p=0.04), RAVLT total (r=0.53, p=0.05; Figure 5D), and verbal fluency (r=0.56, p=0.04), suggesting that the surgical trajectories that were more superior in the left hemisphere were associated with a greater decline on mental status, verbal learning and short-term memory, and verbal fluency measures following surgery. In the right hemisphere, the angles from the midpoint of the AC-PC line to the active electrode in relation to the axial and midsagittal planes were correlated significantly with verbal fluency outcome scores (axial: 0.54, p=0.05; midsagittal: r=−0.60, p=0.03). These findings suggest that angles that were more superior and more posterior lateral in the frontal quadrant of the brain were related to declines in verbal fluency performance following surgery.
Distance Off Approximated STN
Electrode Tip
The distance of the electrode tip off the approximated STN for the left versus right hemispheres did not differ (Table 4).
Correlation of Electrode Tip Distance Off Approximated STN with Neuropsychological Functioning
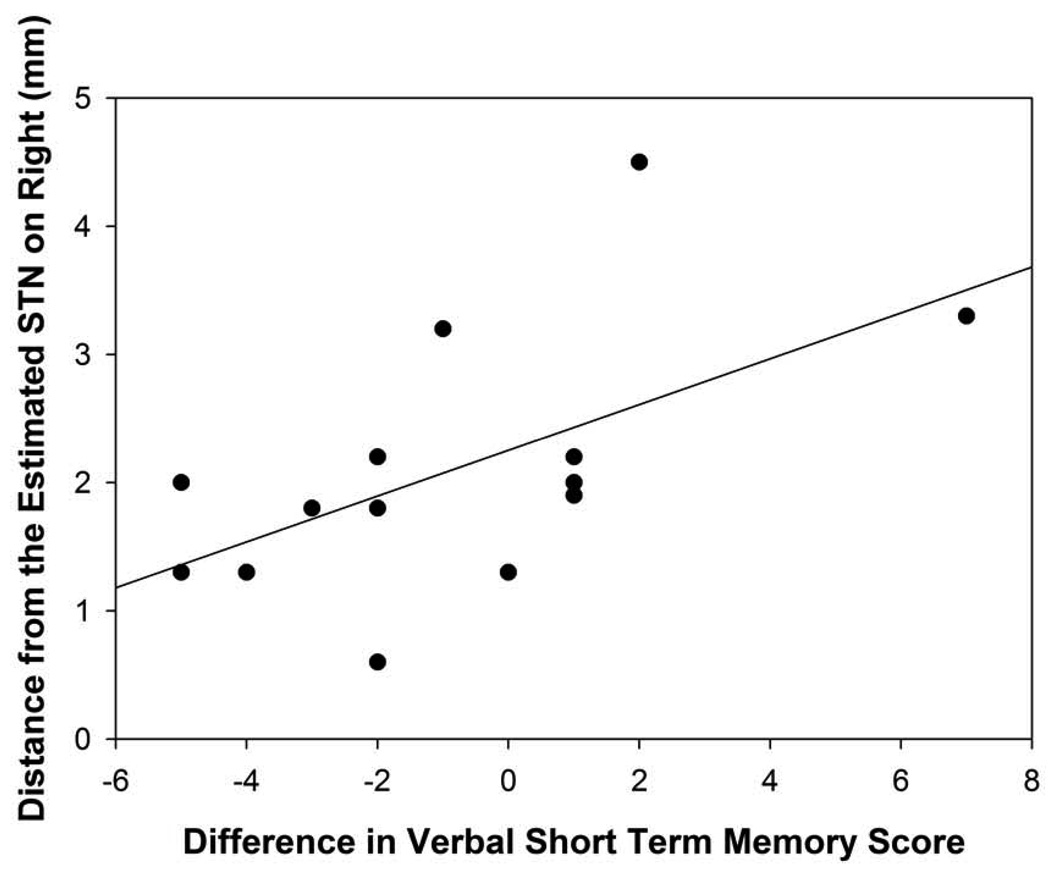
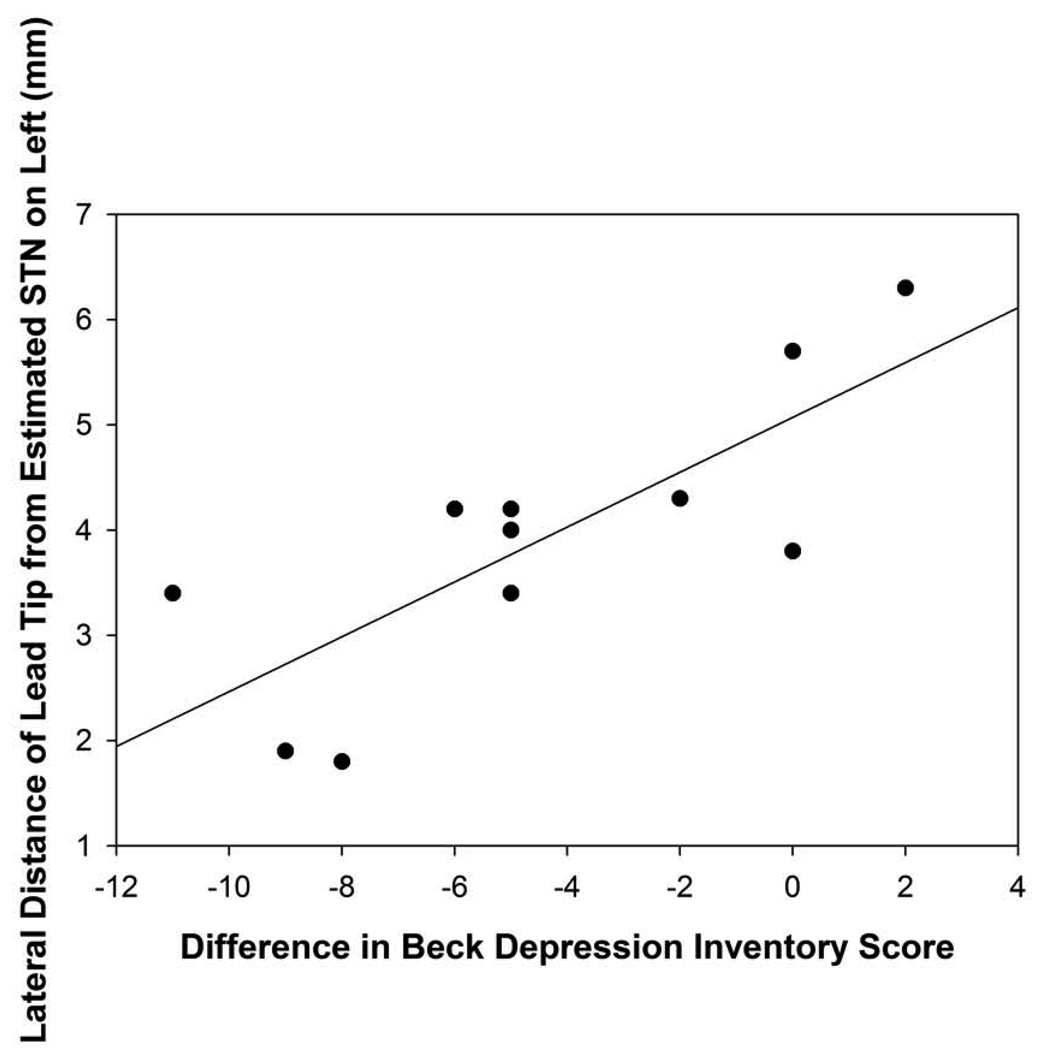
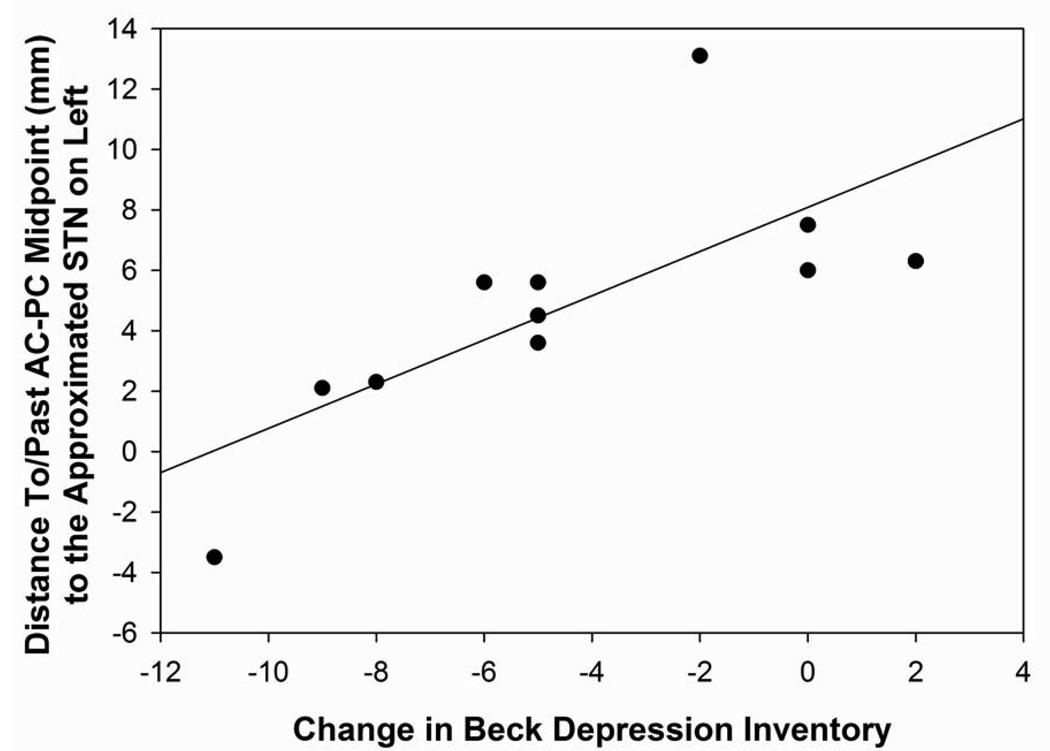
The distance off the approximated STN in the right hemisphere was positively correlated with verbal short-term memory outcome (r=0.58, p=0.03; Figure 6A). The closer the electrode tip was to the approximated STN in the right hemisphere, the greater the decline was demonstrated on verbal short-term memory scores relative to baseline. The distance off the approximated STN in the lateral direction in the right hemisphere was positively correlated with verbal fluency changes (r=0.70, p=0.006). The closer the electrode tip was to the approximated STN, the greater the decline in verbal fluency performance was found following surgery. The distance off the approximated STN in the lateral direction in the left hemisphere was positively correlated with depression difference scores (r=0.79, p=0.004; Figure 6B). The further the electrodes were from the approximated STN target, the greater the decline in depression scores for the DBS patients following surgery. No other statistically significant differences were found between the distance off the approximated STN measurement and neuropsychological or psychological measures.
Figure 6. Figures 6A–6D: Distances Off/To/From Approximated STN.
Figure 6A: Scatterplot with regression line demonstrating the relation between the distance (in mm) of the electrode tip off the approximated STN in the right hemisphere and verbal short-term memory difference scores. The lower distance numbers reflect electrodes closer to the approximated STN and a lower difference score reflects a greater decline in verbal short-term recall. (p=0.03)
Figure 6B: Scatterplot with regression line demonstrating the relation between the distance (in mm) o the electrode tip off the approximated STN in the left hemisphere and depression difference scores. The lower distance numbers reflect electrodes closer to the approximated STN and a lower depression difference score reflects an improvement in depression scores following surgery. (p=0.004)
Figure 6C: Scatterplot with regression line demonstrating the relation between the distance of the electrode tip past the approximated STN in the left hemisphere and depression difference scores. The lower the distance past reflects electrodes located in a more posterior direction and a lower depression difference score reflects an improvement in depression scores following surgery. (p=0.003).
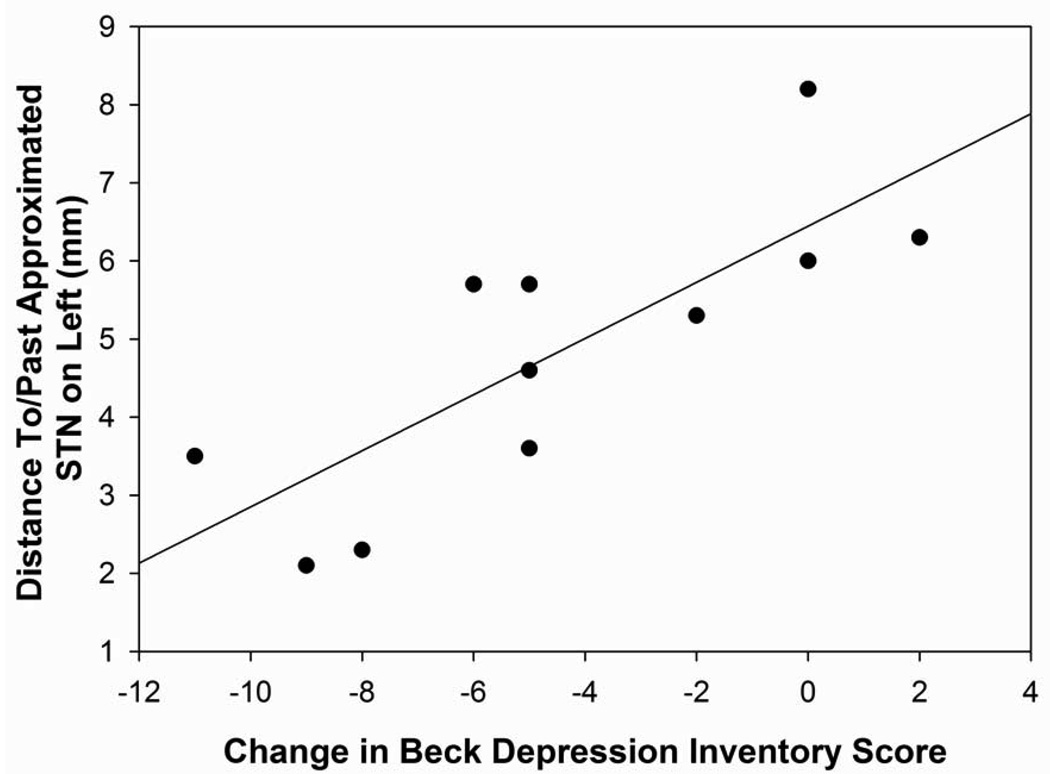
Figure 6D: Scatterplot with regression line demonstrating the relation between the distance past the approximated STN for the midpoint of the AC-PC line to the electrode tip in the left hemisphere and depression difference scores. The negative numbers reflect a distance to the approximated STN while positive numbers reflect a distance past the approximated STN and a lower depression difference score reflects an improvement in depression scores following surgery. (p=0.01)
Active Electrode
The distance of the active electrode off the approximated STN for the left versus right hemispheres differed significantly (p=0.01; Table 4). No other significant differences between the hemispheres were found for the distance off the STN for the active electrode.
Correlation of Active Electrode Distance Off Approximated STN with Neuropsychological Functioning
The distance off the approximated STN for the active electrode in the inferior to superior direction in the left hemisphere was positively correlated with verbal learning total and short-term recall change scores (r=0.55, p=0.04; r=0.58, p=0.03, respectively). The closer the active electrode was to the approximated STN, the greater the decline was demonstrated on verbal memory scores relative to baseline. No other statistically significant differences were found between the distance off the approximated STN measurement and neuropsychological or psychological measures.
Distance To/Past Approximated STN
Electrode Tip
The distance of the electrode tip to/past the approximated STN for the left versus right hemispheres differed significantly between the hemispheres for the midpoint of the AC-PC to target measure (p=0.05; Table 4), with the larger difference in the left hemisphere. This distance did not differ for the other measurements.
Correlation of Electrode Tip Distance To/Past Approximated STN with Neuropsychological Functioning
The distance to/past the approximated STN in the inferior to superior direction in the right hemisphere was positively correlated with verbal fluency changes following surgery (r=0.54, p=0.05). In the right hemisphere, the further past (inferior) the approximated STN the electrode tip, the greater the decline was on verbal fluency scores relative to baseline. In the left hemisphere, the distance to/past the approximated STN for the surgical trajectory and from the midpoint of the AC-PC line to the electrode tip was positively correlated with depression changes (r=0.80, p=0.003; r=0.74, p=0.01, respectively; Figures 6C and 6D). The more inferior (past target) and lateral (past target) the electrode tip was from the approximated STN, the greater the depression symptoms the patients reported following surgery. The distance to/past the approximated STN in the left hemisphere for the AC-PC midpoint measurement was also significantly correlated with verbal fluency changes following surgery (r=0.54, p=0.05). The more lateral the electrode tip was from the approximated STN in the left hemisphere, the larger the decline in verbal fluency scores were found following surgery. No other statistically significant differences were found between the distance to/past the approximated STN for the electrode tip measurements and neuropsychological or psychological measures.
Active Electrode
The distance of the active electrode to/past the approximated STN for the left versus right hemispheres did not differ significantly between the hemispheres. (Table 4)
Correlation of Active Electrode Distance To/Past Approximated STN with Neuropsychological Functioning
The distance to/past the approximated STN from the AC-PC midpoint to the active electrode in the right hemisphere was negatively correlated with verbal fluency changes following surgery (r=−0.59, p=0.03). The more lateral the active electrode was from the approximated STN, the greater decline that was demonstrated on verbal fluency scores relative to baseline. No other statistically significant differences were found between the distance past the approximated STN for the electrode tip measurements and neuropsychological or psychological measures.
MRI Differences for Cognitive Stable versus Decline groups
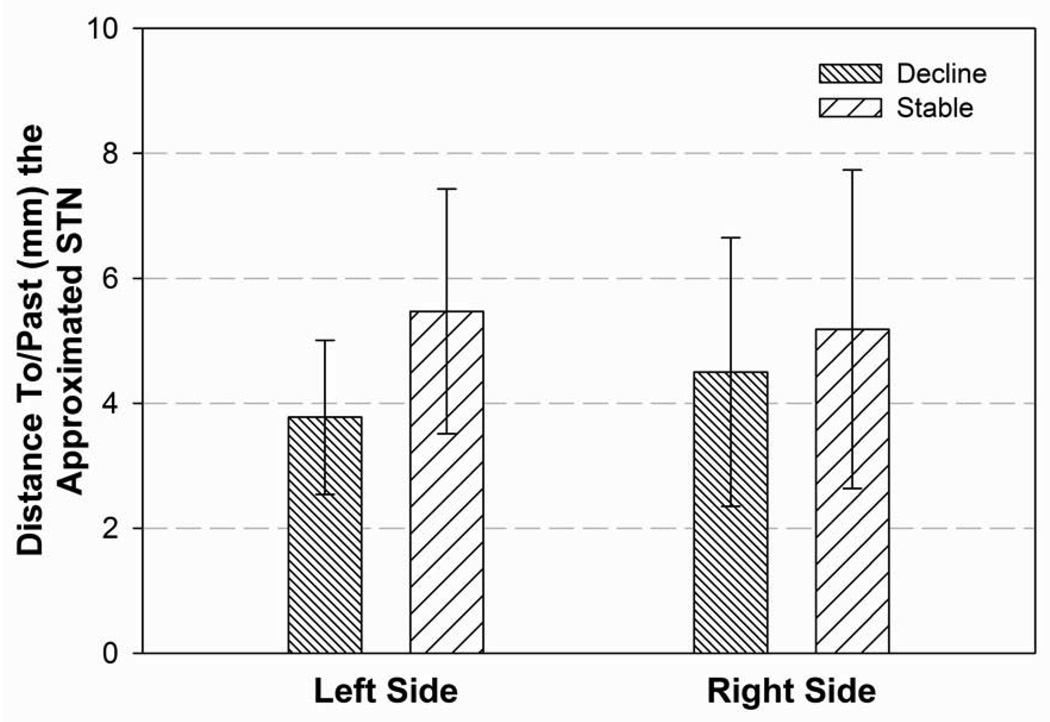
In the left hemisphere, the electrode tip for those DBS patients who declined cognitively following surgery were significantly closer to the approximated STN target than those DBS patients who remained stable cognitively following surgery (p=0.05; Figure 7A). No other statistically significant differences were found between the two groups for the electrode tip locations.
Figure 7. Figures 7A and 7B: Cognitively Stable versus Cognitive Declines Following DBS.
Figure 7A: Difference between those patients who remained stable cognitively and those patients who declined on at least 2 neuropsychological measures at the 6 month follow-up evaluation (no patients declined on only one measure) for the distance of the electrode tip to/past the approximated STN target for the left and right hemispheres. The lower the number (in mm) reflects a closer position to the approximated STN in the left hemisphere. (p=0.05)
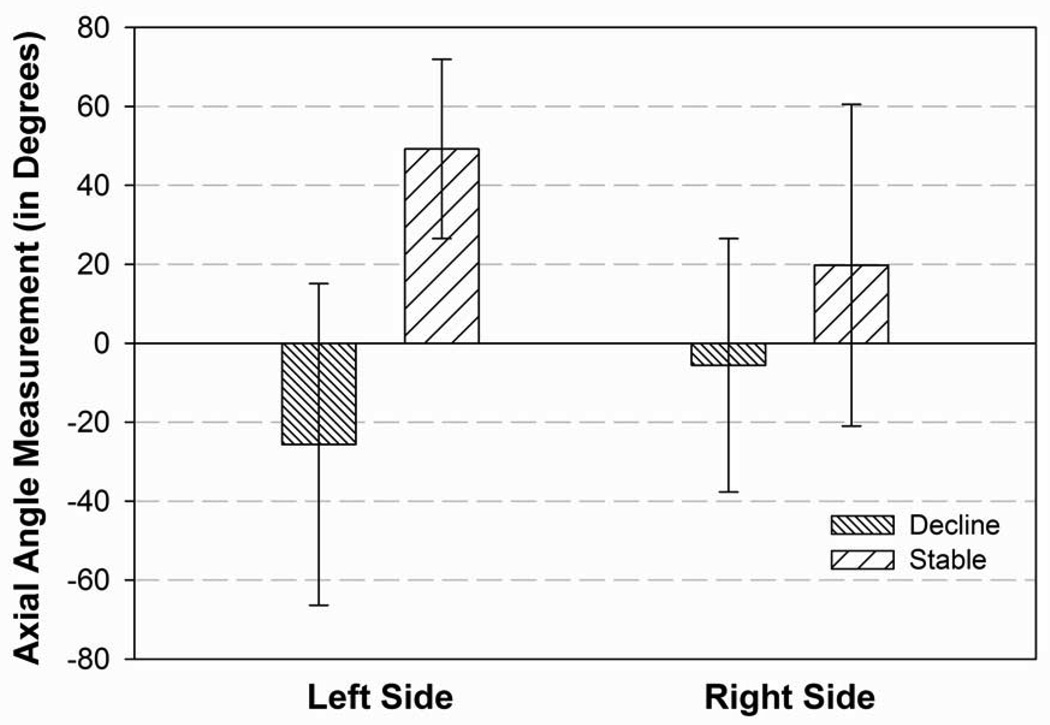
Figure 7B: Difference between those patients who remained stable cognitively and those patients who declined on at least 2 neuropsychological measures at the 6 month follow-up evaluation (no patients declined on only one measure) for the angle from the midpoint of the AC-PC line to the active electrode in the right and left hemispheres in relation to the axial plane. (p=0.03)
For the active electrodes in the left hemisphere, the angle from the midpoint of the AC-PC midpoint to the target in relation to the axial plane differed between the cognitive groups (t=5.65, p=0.03; Figure 7B). The DBS patients who declined cognitively had electrodes which were significantly more superior than those patients whose cognition remained stable or improved following surgery. In the right hemisphere, the angle from the AC-PC midpoint to the target in relation to the midsagittal plane differed between the cognitive groups (t=4.84, p=0.04). The patients who declined cognitively had active electrodes which were significantly more posterior and lateral within the frontal quadrant as compared to the patients whose cognition remained stable or improved following DBS. Additionally, the distance to/past the active electrode in the right hemisphere differed between the cognitively groups (t=5.31, p=0.04), with those patients who declined cognitively having active electrodes located more laterally than the cognitively stable patients.
Conclusions
We investigated the relationship between the intersection and the location of the surgical trajectory and the active electrodes and neuropsychological outcome 6 months following bilateral STN DBS. Declines in MMSE scores were related to electrodes that were more laterally placed in either hemisphere, particularly when the active electrodes were posterior-lateral within the frontal quadrant. A similar association was found for decline in DRS scores in the left hemisphere, which additionally demonstrated a relationship with electrodes which were more superiorally located. In regard to declines in verbal learning, the electrodes that were closer to the approximated STN and more superiorally located in the left hemisphere were associated with declines at 6 months following surgery. However, in the right hemisphere the electrodes that were located more in the lateral direction were related to verbal short-term memory declines. Verbal long-term memory declines were found for electrodes located more posterior-laterally in the left hemisphere. Declines in verbal fluency scores were more variable with associations found between change scores and electrodes in the lateral and superior directions in the left hemisphere and those electrodes closer to the approximated STN and more superiorally and posteriorally located in the left hemisphere. In contrast, semantic fluency declines were only related to right hemisphere electrodes located more superiorally. Declines in mood were related to those electrodes located further away from the approximated STN, particularly those located more inferiorally and laterally in the left hemisphere. Anxiety change scores were not associated with the location of the electrodes. The results provide preliminary evidence that 6 months following bilateral STN DBS for PD, cognitive and emotional changes may be related to the surgical trajectory and electrode placement.
By using difference scores from baseline cognitive functions, we demonstrated that the relationship between cognitive functioning and electrode placement was not due to pre-existing cognitive dysfunction but represents the decline in cognitive functioning over 6-months. These cognitive declines following DBS are commonly reported in the literature for bilateral STN DBS as a potential side effect of the surgical intervention. However, the factors that are associated with these declines are poorly understood.
The basal ganglia are richly connected to other subcortical structures, and also specific cortical regions, including motor, occulomotor, prefrontal associative, and limbic areas.(23) In conjunction with the globus pallidus, the STN has been labeled as the “central pacemaker of the basal ganglia,” which is responsible for action selection. Some neurons in the GPi, the STN, and the thalamus have abnormal phasic activity, which supports the belief that these sites are involved in bradykinesia and rigidity in PD.(8) However, the STN also projects to associative and limbic areas of the basal ganglia and the substantia nigra pars retcularis.(24) The STN is structured into 3 components: the dorsolateral component, the ventromedial component, and the medial tip. The dorsolateral component (2/3 of the STN) is involved in somatomotor function; and the ventromedial component (1/3 of the STN) is involved in associative processing, while the medial tip of the STN projects to limbic structures. The current study supports the theory that the STN is intimately involved in associative cognitive processing and potentially affective functioning.
In a preliminary study correlating electrode location with motor outcomes of STN DBS, Tintner et al.(25) found that some electrode contacts located within the radiologically-definable STN failed to improve parkinsonism motor symptoms while others induced dyskinesias and produced anti-parkinsonian effects. In contrast, Kumar et al.(5) argued that only DBS contacts located in the radiologically-definable STN produce optimal motor improvements. Moreover, Rizzone et al.(26) reported that the best clinical effect on Parkinson’s disease-related motor symptoms was obtained when the STN electrodes were placed in the dorsolateral portion of the STN. While it is generally recognized that the 4 electrodes allow for versatility to compensate for final stereotactically placed electrode tips that are within 2–3 mm of the optimal target(27), deviations in the placement of electrodes may provide us insight into the mechanism of action of DBS. Clinical evidence from microstimulation has revealed that if the DBS electrodes are positioned posterior laterally from the STN, the internal capsule will be stimulated and facial and hemi-body pulling can result. If the electrodes are located more medial to the STN, the stimulation may impinge on the red nucleus, the medial longitudinal fasciculus or the zona inserta leading to motor and/or eye deviations. Stimulation occurring more superior to the STN in the thalamus would result in tremor management without other PD symptom relief. None of the patients in the current sample exhibited any of these motoric side effects following DBS.
Although the role of the STN in motor functioning has been clarified, the investigations into the role of the STN in cognitive functioning remains in its infancy. As reported previously, Tsai and colleagues(20) were the first to report that the neuropsychological effects of chronic STN-DBS was related to the more anteriorly located electrodes within the ventral STN. However, their two groups (cognitive side effect versus no cognitive side effect) were based on postoperative changes on the MMSE only, which is not an overly sensitive measure of subcortical cognitive functioning. Their study highlights the importance of investigating the cause of differential cognitive outcome following STN DBS and was the first to suggest the potential role of the electrode location.
STN DBS outcome studies have highlighted the role of the STN in verbal memory, verbal fluency, executive functioning, attention, working memory, and response inhibition. Animal models have also demonstrated evidence for a role of the STN in attention and response inhibition.(24) Taken together, this literature has revealed that the STN has a regulatory function in processing associative and limbic information. Although the precise mechanism is unknown, two hypotheses have been proposed as to the mechanism of cognitive change following STN DBS. The first hypothesis proposes that the stimulation is not confined only to the motor part of the STN; that it spreads to other areas of the STN or neighboring subcortical structures. The second hypothesis, which is currently favored, proposes that the STN itself is responsible for the cognitive changes. The current research adds supportive evidence to the second hypothesis that it is the STN itself and not neighboring structures, that is responsible for cognitive and behavioral change following stimulation. Those patients whose active electrodes were closer to the approximated STN were more likely to show verbal learning and memory and verbal fluency declines six months following surgery. Further research will be needed to clarify the role of the STN in cognitive functioning. However, current literature suggests that the STN is not only the central pacemaker for motor functioning but also for cognitive and behavioral functioning. Future DBS research using microelectrode recordings and higher resolution imaging with controlled and selective targeting will aid in clarifying the role of the STN in cognitive and behavioral functioning.
The STN has also been implicated in the processing of emotional information. Using direct recordings from macroelectrodes during the presentation of emotionally laden pictures, Brucke and colleagues(28) found a significant relationship between a desynchronization of STN alpha activity with pleasantly rated stimuli. This finding suggests that because the STN is involved in the processing of valence-related emotional processing, stimulation in this area may lead to emotional changes. Mallet and colleagues(29) further investigated the role of the STN in cognition and emotions in two patients who demonstrated hypomanic symptoms during electrical stimulation. The hypomanic state was localized to stimulation of the electrode in the anteromedial STN. These authors proposed a model in which the STN integrates motor, cognitive, and emotional components of behavior, and our current research findings supports this model of the STN, particularly in the left hemisphere.
This study has several limitations. First, the small sample size limits the conclusions that can be drawn. We chose to focus on verbal memory and verbal fluency changes to limit the number of statistical analyses; however, an evaluation of a comprehensive neuropsychological evaluation may reveal further information regarding the role of the STN in cognitive and behavioral functioning. The statistical power of this study is not sufficient to make clinical decisions regarding this neurosurgical procedure. Second, the majority of the analyses were correlational. Consequently, we are limited to discussions of the relationships between the electrode location and cognitive and behavioral functioning, and causation cannot be assumed. Third, the electrode artifact found on the MRI images limits the ability to precisely locate the electrodes and the exact path of the surgical trajectory. Fourth, due to the difficulty in visualizing the STN on the MRIs, particularly with the artifact, we are unable to say with precise certainty that the electrodes are located within the STN. We used computational models to estimate the location of the active electrodes, these results can be misleading as the exact location of the tip of the electrode within the MRI artifact is unknown. We also used a proxy measure of the approximated STN which was verified on the patients’ scans. Additionally, future research should investigate the relationship between the volume of tissue being stimulated and the neuropsychological outcome. While this study is preliminary, it highlights the need for future neuropsychological research to investigate the role of the STN in the relationship between motor, cognitive, and behavioral outcome following STN DBS.
Conclusion
The angle of the surgical trajectory and the proximity of the electrodes to the approximated STN were related to changes in cognitive and emotional functioning following DBS. The current findings extend the literature on the role of the STN behavioral functioning and add support to the hypothesis that the STN plays an integrative role not only in motor function but also in cognitive and emotional functioning.
Table 1.
Demographic and Medical Variables for DBS patients (n=17)
| N | Mean (SD) | |
|---|---|---|
| Gender (M/F) | 12/5 | |
| Race (White/Hispanic) | 15/2 | |
| Handedness (R/L) | 17/0 | |
| Age at Testing (yrs) | 58.2 (11.2) | |
| Education (yrs) | 14.5 (2.8) | |
| Age of PD Onset (yrs) | 45.8 (8.1) | |
| UPDRS motor score “on” | 34.6 (13.4) |
Acknowledgments
Research supported by Medtronic, Inc., NIH / NINDS — 5K23NS041254, and the Department of Veterans Affairs, Office of Research and Development. We gratefully acknowledge the assistance of Stephen McCauley, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Burchiel KJ, Anderson VC, Favre J, Hammerstad JP. Comparison of pallidal and subthalamic nucleus deep brain stimulation for advanced Parkinson's disease: results of a randomized, blinded pilot study. Neurosurgery. 1999;45(6):1375–1382. doi: 10.1097/00006123-199912000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Kishore A, Turnbull IM, Snow BJ, Fuente-Fernandez R, Schulzer M, Mak E, et al. Efficacy, stability and predictors of outcome of pallidotomy for Parkinson's disease. Six-month follow-up with additional 1-year observations. Brain. 1997;120(Pt 5):729–737. doi: 10.1093/brain/120.5.729. [DOI] [PubMed] [Google Scholar]
- 3.Krauss JK, Desaloms JM, Lai EC, King DE, Jankovic J, Grossman RG. Microelectrode-guided posteroventral pallidotomy for treatment of Parkinson's disease: postoperative magnetic resonance imaging analysis. J Neurosurg. 1997;87(3):358–367. doi: 10.3171/jns.1997.87.3.0358. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R, Lozano AM, Montgomery E, Lang AE. Pallidotomy and deep brain stimulation of the pallidum and subthalamic nucleus in advanced Parkinson's disease. Mov Disord. 1998;13 Suppl 1:73–82. [PubMed] [Google Scholar]
- 5.Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999;53(3):561–566. doi: 10.1212/wnl.53.3.561. [DOI] [PubMed] [Google Scholar]
- 6.Lang AE, Lozano AM, Montgomery E, Duff J, Tasker R, Hutchinson W. Posteroventral medial pallidotomy in advanced Parkinson's disease. N Engl J Med. 1997;337(15):1036–1042. doi: 10.1056/NEJM199710093371503. [DOI] [PubMed] [Google Scholar]
- 7.Limousin-Dowsey P, Pollak P, Van Blercom N, Krack P, Benazzouz A, Benabid A. Thalamic, subthalamic nucleus and internal pallidum stimulation in Parkinson's disease. J Neurol. 1999;246 Suppl 2:II42–II45. doi: 10.1007/BF03161080. [DOI] [PubMed] [Google Scholar]
- 8.Starr PA, Vitek JL, Bakay RA. Ablative surgery and deep brain stimulation for Parkinson's disease. Neurosurgery. 1998;43(5):989–1013. doi: 10.1097/00006123-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Pahwa R, Wilkinson SB, Overman J, Lyons KE. Bilateral subthalamic stimulation in patients with Parkinson disease: long-term follow up. J Neurosurg. 2003;99(1):71–77. doi: 10.3171/jns.2003.99.1.0071. [DOI] [PubMed] [Google Scholar]
- 10.Rettig G, Lai E, Krauss J, Grossman R, Jankovic J. Neuropsychological Evaluation of Patients with Parkinson's Disease Before and After Pallidal Surgery. In: Krauss J, Grossman R, Jankovic J, editors. Pallidal Surgery for the Treatment of Parkinson's Disease and Movement Disorders. 1998. pp. 211–240. [Google Scholar]
- 11.Trepanier LL, Saint-Cyr JA, Lozano AM, Lang AE. Neuropsychological consequences of posteroventral pallidotomy for the treatment of Parkinson's disease. Neurology. 1998;51(1):207–215. doi: 10.1212/wnl.51.1.207. [DOI] [PubMed] [Google Scholar]
- 12.Ardouin C, Pillon B, Peiffer E, Bejjani P, Limousin P, Damier P, et al. Bilateral subthalamic or pallidal stimulation for Parkinson's disease affects neither memory nor executive functions: a consecutive series of 62 patients. Ann Neurol. 1999;46(2):217–223. doi: 10.1002/1531-8249(199908)46:2<217::aid-ana11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Fields JA, Troster AI, Wilkinson SB, Pahwa R, Koller WC. Cognitive outcome following staged bilateral pallidal stimulation for the treatment of Parkinson's disease. Clin Neurol Neurosurg. 1999;101(3):182–188. doi: 10.1016/s0303-8467(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 14.Fields JA, Troster AI. Cognitive outcomes after deep brain stimulation for Parkinson's disease: a review of initial studies and recommendations for future research. Brain Cogn. 2000;42(2):268–293. doi: 10.1006/brcg.1999.1104. [DOI] [PubMed] [Google Scholar]
- 15.Pillon B, Ardouin C, Damier P, Krack P, Houeto JL, Klinger H, et al. Neuropsychological changes between "off" and "on" STN or GPi stimulation in Parkinson's disease. Neurology. 2000;55(3):411–418. doi: 10.1212/wnl.55.3.411. [DOI] [PubMed] [Google Scholar]
- 16.Vingerhoets G, van der LC, Lannoo E, Vandewalle V, Caemaert J, Wolters M, et al. Cognitive outcome after unilateral pallidal stimulation in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;66(3):297–304. doi: 10.1136/jnnp.66.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trepanier, Lisa L, Kumar, Rajeev, Lozano, Andres M, et al. Neuropsychological outcome of GPi pallidotomy and GPi or STN deep brain stimulation in Parkinson's disease. not sure. 2000;(3):347. doi: 10.1006/brcg.1999.1108. [DOI] [PubMed] [Google Scholar]
- 18.Morrison CE, Borod JC, Brin MF, Raskin SA, Germano IM, Weisz DJ, et al. A program for neuropsychological investigation of deep brain stimulation (PNIDBS) in movement disorder patients: development, feasibility, and preliminary data. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13(3):204–219. [PubMed] [Google Scholar]
- 19.York MK, Dulay M, Macias A, Levin HS, Grossman R, Simpson R, et al. Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson's disease. J Neurol Neurosurg Psychiatry. 2008;79(7):789–795. doi: 10.1136/jnnp.2007.118786. [DOI] [PubMed] [Google Scholar]
- 20.Tsai ST, Lin SH, Lin SZ, Chen JY, Lee CW, Chen SY. Neuropsychological effects after chronic subthalamic stimulation and the topography of the nucleus in Parkinson's disease. Neurosurgery. 2007;61(5):E1024–E1029. doi: 10.1227/01.neu.0000303198.95296.6f. [DOI] [PubMed] [Google Scholar]
- 21.Benabid AL, Vercucil L, Benazzouz A, Koudsie A, Chabardes S, Minotti L, et al. Deep brain stimulation: what does it offer? Adv Neurol. 2003;91:293–302. [PubMed] [Google Scholar]
- 22.Brown RG, Marsden CD. Cognitive function in Parkinson's disease: from description to theory. Trends Neurosci. 1990;13(1):21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- 23.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64(1):20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 24.Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005;76(6):393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Tintner R, Jankovic J. Treatment options for Parkinson's disease. Curr Opin Neurol. 2002;15(4):467–476. doi: 10.1097/00019052-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Rizzone M, Lanotte M, Bergamasco B, Tavella A, Torre E, Faccani G, et al. Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: effects of variation in stimulation parameters. J Neurol Neurosurg Psychiatry. 2001;71(2):215–219. doi: 10.1136/jnnp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mobin F, De Salles AA, Behnke EJ, Frysinger R. Correlation between MRI-based stereotactic thalamic deep brain stimulation electrode placement, macroelectrode stimulation and clinical response to tremor control. Stereotact Funct Neurosurg. 1999;72(2–4):225–232. doi: 10.1159/000029730. [DOI] [PubMed] [Google Scholar]
- 28.Brucke C, Kupsch A, Schneider GH, Hariz MI, Nuttin B, Kopp U, et al. The subthalamic region is activated during valence-related emotional processing in patients with Parkinson's disease. Eur J Neurosci. 2007;26(3):767–774. doi: 10.1111/j.1460-9568.2007.05683.x. [DOI] [PubMed] [Google Scholar]
- 29.Mallet L, Schupbach M, N'Diaye K, Remy P, Bardinet E, Czernecki V, et al. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci U S A. 2007;104(25):10661–10666. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]