Abstract
Activation of neurons in the anterolateral bed nucleus of the stria terminalis (BNSTALG) plays an important role in mediating the behavioral response to stressful and anxiogenic stimuli. Application of 5-HT elicits complex postsynaptic responses in BNSTALG neurons, which includes 1) membrane hyperpolarization (5-HTHyp), 2) hyperpolarization followed by depolarization (5-HTHyp-Dep), 3) depolarization (5-HTDep) or 4) no response (5-HTNR). We have shown that the inhibitory response is mediated by activation of postsynaptic 5-HT1A receptors. Here, we used a combination of in vitro whole-cell patch-clamp recording and single cell reverse transcriptase polymerase chain reaction (RT-PCR) to determine the pharmacological properties and molecular profile of 5-HT receptor subtypes mediating the excitatory response to 5-HT in BNSTALG neurons. We show that the depolarizing component of both the 5-HTHyp/Dep and the 5-HTDep response was mediated by activation of 5-HT2A, 5-HT2C and/or 5-HT7 receptors. Single cell RT-PCR data revealed that 5-HT7 receptors (46%) and 5-HT1A receptors (41%) are the most prevalent receptor subtypes expressed in BNSTALG neurons. Moreover, 5-HT receptor subtypes are differentially expressed in Type I – III BNSTALG neurons. Hence, 5-HT2C receptors are almost exclusively expressed by Type III neurons, whereas 5-HT7 receptors are expressed by Type I and II neurons, but not Type III neurons. Conversely, 5-HT2A receptors are found predominantly in Type II neurons. Finally, bi-directional modulation of individual neurons occurs only in Type I and II neurons. Significantly the distribution of 5-HT receptor subtypes in BNSTALG neurons predicted the observed expression pattern of 5-HT responses determined pharmacologically. Together, these results suggest that 5-HT can differentially modulate the excitability of Type I – III neurons, and further suggest that bi-directional modulation of BNSTALG neurons occurs primarily through an interplay between 5-HT1A and 5-HT7 receptors. Hence, modulation of 5-HT7 receptor activity in the BNSTALG may offer a novel avenue for the design of anxiolytic medications.
Keywords: extended amygdala, anxiety disorders, whole cell patch clamp recording, reverse transcriptase polymerase chain reaction
Introduction
Although a large body of evidence suggests that serotonin (5-HT) systems play an important role in modulating behaviors associated with affect, the nature of this function remains perplexing. Indeed, the therapeutic benefit of increasing brain 5-HT with chronic selective serotonin reuptake inhibitors (SSRI) for the treatment of mood disorders is well documented. Paradoxically, however, acute SSRI treatment can also increase behavioral measures of fear and anxiety in both humans and rodents (Pohl et al., 1988, Bagdy et al., 2001, Burghardt et al., 2004). Similarly, 5-HT depletion studies have shown that decreasing brain 5-HT levels can either increase (Hohmann et al., 2007), or decrease (Soderpalm and Engel, 1990, Bechtholt et al., 2007) anxiety-like behavior. In sum, these data suggest that the activation of brain 5-HT systems can be either anxiolytic or anxiogenic depending on the context in which that activation occurs (see (Handley, 1995) for review).
The opposing actions of 5-HT can be explained, in part, by the large number of cognate receptors that mediate the effects of 5-HT. At least 14 subtypes of 5-HT receptors have been identified that are classified into seven major categories (5-HT1–7; for review see (Hoyer et al., 2002)), and are differentially distributed throughout the central nervous system. Moreover, in many brain regions individual neurons can express multiple 5-HT receptors (Pazos et al., 1985), further increasing the complexity of the neuronal response to 5-HT (see (Uphouse, 1997) for review).
One such region, the bed nucleus of the stria terminalis (BNST), is believed to play a critical role in mediating the anxiety-like behavioral response to physiological and psychological stressors (Walker and Davis, 1997, Erb and Stewart, 1999, Leri et al., 2002, Schulz et al., 2002, Fendt et al., 2003, Aston-Jones and Harris, 2004, Hammack et al., 2004, Jasnow et al., 2004, Sullivan et al., 2004). Consistent with this role, lesion or transient inactivation of the BNST reduces many of the behavioral indices of stressor exposure (Brutus et al., 1988, Walker et al., 1997, Erb and Stewart, 1999, Fendt et al., 2003, Aston-Jones and Harris, 2004, Hammack et al., 2004). Moreover, exposure to stressors increases neural activation in the BNST (Duncan et al., 1993, Martinez et al., 1998, Chung et al., 1999, Lino-de-Oliveira et al., 2001), and electrical stimulation of the BNST evokes cardiovascular and behavioral responses similar to those initiated by stressful stimuli (Shaikh et al., 1986, Casada and Dafny, 1991, Dunn and Williams, 1995). Hence, BNST-dependent behaviors may result from a stress-induced increase in the activity of BNST neurons.
Significantly, stressors that activate the BNST also activate central 5-HT systems (Dilts and Boadle-Biber, 1995, Grahn et al., 1999, Funada and Hara, 2001, Lowry, 2002, Summers et al., 2003, Takase et al., 2004), and maladaptive changes in 5-HT release and/or 5-HT receptor function have been proposed to play a major role in the etiology of many stress-related affective disorders, including anxiety disorders (Chaouloff et al., 1993, Lucki, 1996, Deakin, 1998, Ressler and Nemeroff, 2000, Charney, 2003). Critically, many stressors activate a subset of 5-HT neurons in the caudal dorsal raphé nucleus (Grahn et al., 1999, Lowry et al., 2000, Singewald et al., 2003) that preferentially target limbic forebrain regions including the BNST (Phelix et al., 1992, Commons et al., 2003), suggesting that 5-HT modulation of neural activity in the BNST may play a fundamental role in regulating the behavioral response to stressors.
In both rat and non-human primate, 5-HT fibers preferentially target neurons in the anterolateral cell group of the BNST (BNSTALG; (Phelix et al., 1992, Dong et al., 2001, Freedman and Shi, 2001, Commons et al., 2003)), an area that has been show to express multiple 5-HT receptors including moderate to high levels of 5-HT1A, 5-HT2A, 5-HT2C, 5-HT4, and 5-HT7 receptors (Mengod et al., 1990, Pompeiano et al., 1994, Waeber et al., 1994, Wright et al., 1995, Heidmann et al., 1998, Cornea-Hebert et al., 1999, Xu and Pandey, 2000, Vilaro et al., 2005). The diversity of 5-HT receptor subtypes in this region would suggest a complex response pattern to local 5-HT release.
Recently, we reported that activation of postsynaptic 5-HT receptors could differentially modulate the excitability of BNSTALG neurons (Rainnie, 1999; Levita et al., 2004). Significantly, a substantial number of neurons exhibited both an inhibitory and an excitatory component of their response to 5-HT. The inhibitory response was mediated by activation of postsynaptic 5-HT1A receptors coupled via Gαi to inwardly-rectifying potassium channels. Importantly, local infusion of the non-selective 5-HT1A,7 agonist 5-carboxyamidotryptamine (5-CT) into the BNSTALG elicits an anxiolytic-like behavioral response (Levita et al., 2004), suggesting that 5-HT1A receptor-mediated inhibition of BNSTALG neurons may modulate anxiety-like behaviors.
However, these same neurons have the capacity to respond to 5-HT with excitation, providing a mechanism by which 5-HT release might differentially elicit anxiolytic or anxiogenic behavior depending on the balance of inhibition and excitation within individual BNSTALG neurons. Significantly, four out of the five 5-HT receptors reportedly expressed in the BNSTALG (5-HT2A, 5-HT2C, 5-HT4 and 5-HT7) have been shown to mediate postsynaptic excitatory responses elsewhere in the central nervous system (Araneda and Andrade, 1991, McCormick and Wang, 1991, Stevens et al., 1992, Chapin and Andrade, 2001, Craven et al., 2001, Hoyer et al., 2002, Beique et al., 2004). The present study was designed to fully characterize the 5-HT receptor subtype/s mediating the excitatory response of BNSTALG neurons to 5-HT.
Here, we have used whole-cell patch clamp recording from BNSTALG neurons in conjunction with reverse transcriptase polymerase chain reaction (RT-PCR) to demonstrate that the 5-HT-evoked excitation results from activation of either 5-HT2A/2C receptors, or activation of 5-HT7 receptors. Moreover, the data suggest that subpopulations BNSTALG neurons express different combinations of 5-HT receptor subtypes, and the heterogeneous distribution of 5-HT receptor transcripts predicts the heterogeneous 5-HT response profile in BNSTALG neurons.
Experimental Procedures
Subjects
Brain slices for electrophysiology were obtained from male Sprague-Dawley rats 24–40 days old (Charles River, Raleigh, NC). Prior to tissue preparation, rats had unrestricted access to food and water, and were housed four per cage with an alternating 12-hr light-dark cycle (light on at 7:30 am). Effort was taken to minimize the number and suffering of animals, and all procedures were conducted in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Care and Use Committee at Emory University. Because BNST 5-HT responses can be altered by stressor exposure (Hammack et al., 2009), care was taken to minimally handle/stress rats prior to sacrifice.
Tissue Preparation and Patch Clamp Recording
Slices (350 µM) containing the anterolateral BNST cell group were obtained as previously described (Levita et al., 2004). Brains were sectioned in an ice-cold kynurenic-based artificial cerebrospinal fluid (ACSFKA), which contained (in mM): NaCl (130), KCl (3.5), KH2PO4 (1.1), MgCl2 (6.0), CaCl2 (1.0), NaHCO3 (30), glucose (10), and kynurenic acid (2). The ACSFKA was adjusted to minimize the excitotoxicity associated with glutamate release during tissue slicing, and included the addition of the glutamate antagonist, kynurenic acid, decreased calcium levels to minimize neurotransmitter release, and increased magnesium levels. Immediately after slicing, slices were hemisected, trimmed, and placed in a holding chamber containing oxygenated ACSFKA at room temperature for 30 min. Slices were then transferred to oxygenated ACSF maintained at room temperature for at least 30 min before recording. For recording, individual slices were transferred to a Warner Series 20 recording chamber (0.5 ml volume) mounted on the fixed stage of a Leica DM-LFS microscope. The slices were maintained fully submerged and continuously perfused with ACSF heated to 32°C, and gassed with a 95%-5% oxygen/carbon dioxide mixture. Individual neurons were identified within the slice using a 40X water immersion objective in conjunction with differential interference microscopy and infra-red illumination (IR-DIC).
Standard whole-cell recordings in current- and voltage-clamp mode were obtained from BNSTALG neurons using techniques described elsewhere (Levita et al., 2004). Patch pipettes were fabricated from borosilicate glass (resistance 4–7 MΩ) and filled with a recording solution of the following composition (in mM): 130 K-Gluconate, 2 KCl, 10 HEPES, 3 MgCl2, and 5 phosphocreatine, 2 K-ATP, 0.2 NaGTP. Data acquisition and analysis were performed using an Axpoatch-1D amplifier (Molecular Devices, Burlingame, CA) in conjunction with pClamp 9.0 software, and a continuous record of each experiment was captured on a chart recorder (Kipp & Zonen, Bohemia, NY). All voltage clamp data were low pass filtered at 1 kHz, and sampled at a frequency determined by the speed of the response that was to be measured.
Drug application
Known concentrations of drugs were applied to the slice dissolved in the ACSF using a continuous gravity fed bath application. Previously, we demonstrated that the response of BNSTALG neurons to 5-HT is unaffected by repeat application of 5-HT (Levita et al., 2004). Consequently, the effects of 5-HT receptor antagonists and second messenger blockers were examined after the initial response to 5-HT had first been determined in control ACSF. The slice was then perfused with the drug of choice for approximately 10–20 min, and the neuronal response to reapplication of 5-HT determined. We typically used 50 µM 5-HT because we have previously shown that this concentration produces stable responses on BNSTALG neurons, and would be expected to activate multiple 5-HT receptor subtypes expressed by a given BNSTALG neuron including those with lower affinity to 5-HT (Rainnie, 1999b, Levita et al., 2004). Most of the experiments described here were conducted using a within-subject design.
Drugs applied to the tissue were obtained from the following sources: 5-hydroxytryptamine (5-HT, 50 µM); 5-carboxyamidotrypatmine (5-CT, 10 µM); 5-methoxytryptamine (50–250 µM); (−)1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI; 5 – 50 µM); m-chlorophenylbiguanide (mCPBG, 50 µM); pirenperone (10 µM); and WAY 100635 (200 nM), SB-269970 (1 µM) were all purchased from Sigma-Aldrich (Saint Louis, MO). m-chlorophenylpiperazine (mCPP, 5–75 µM); Cisapride (10 µM); CP 93129 (10 µM); BRL 54443 (5–75 µM); LY 344864 (10 µM); -methyl-5-HT (25–250 µM); TCB 2 (10 µM); WAY 161503 (10 µM) from purchased from Tocris Bioscience (Ellisville, MO). MDL100907 (10 mM) was a gift from Aventis Pharmaceuticals and Sumatriptan (50–250 µM) was a gift from Glaxo-Wellcome. All drugs were made as concentrated stock solutions in distilled H2O, except MDL100907, which was made in 30% acetic acid, cisapride and BRL 54443, which were made in 50% DMSO, and pirenperone, which was made in 0.1N HCl. The diluted concentration of each vehicle in the bath application was 0.03% acetic acid, 0.05% DMSO, and 0.0001 N HCl. These vehicle solutions did not affect any biophysical properties in BNSTALG neurons. Stock solutions were kept as frozen aliquots at −20°C until required.
Agonist and antagonist concentrations were determined both from pilot studies and from effective concentrations reported in published studies investigating similar responses in other brain regions. Although the selectivity of these agents is concentration-dependent, many of the agents were only reported effective in brain slice preparations at concentrations that likely exceeded the 5-HT receptor subtype selectivity based on receptor binding studies. Most likely this results from penetration issues, diffusional barriers, and the relative lipid solubility of these agents. Hence, higher drug concentrations are often required to produce effective drug levels at the synaptic cleft. In similar studies (Egli and Winder, 2003, Dumont and Williams, 2004), a similar concentration of norepinephrine (100 µM) was used to produce pre- or postsynaptic actions in BNST.
Analysis
The effects of 5-HT application on BNSTALG neurons were determined in current-or voltage-clamp mode. Each response to 5-HT (or 5-HT agonist) was defined as a pure hyperpolarization/outward current (5-HTHyp), pure depolarization/inward current (5-HTDep), or mixed response (5-HTHyp-Dep) based on visual inspection of the response and its calculated reversal potential. For antagonist studies, reductions in the 5-HT response in the presence of antagonist are reported as a percentage of the initial 5-HT response determined before antagonist treatment. All comparisons were made using two-tailed paired t-tests unless otherwise noted. All statistics were performed using GraphPad Prism version 4.02 (GraphPad Software, San Diego, CA).
RT-PCR from isolated BNST sections
For these experiments 8 rats were used to isolate the mRNA from the BNSTALG. Here, 500 µm rat brain slices were prepared as outlined above and the BNSTALG isolated using a dissecting microscope. BNST tissue was then homogenized in Trizol and total RNA was isolated. Both the quality and quantity of each RNA sample was assessed by gel electrophoresis and optical density measurements. PCR conditions were optimized using total RNA isolated from rat BNSTALG so that a PCR product could be detected from (250 pg–1 ng) of total RNA. The isolated RNA was then reverse transcribed using a cocktail containing 5–8 µl diethylpyrocarbonate (DEPC) treated water, RNase inhibitor (40 U/µl), dithiothreitol (DTT, 0.1 M), and random hexanucleotides (50 ng/µl) primers. The mixture was rapidly heated to 70 °C and then incubated on ice for 2 min. Single strand cDNA was synthesized from the cellular mRNA by addition of 8 µl of an RT master mix consisting of superscript RT III (200 U/µl), 5X first strand buffer, RNasin (40 U/µl), DTT (0.1 M), and mixed nucleotide triphosphates (10 mM dNTPs), which was then incubated at 42 °C for 50 min. The reaction was terminated by heating the mixture to 70°C for 15 min and then chilling on ice. The resultant cDNA was isolated by adding 1 µl of RNase H (2 U/µl) and incubating for 20 min at 37°C. The cDNA was amplified using 10X PCR buffer, 3.0 mM MgCl2, 10mM dNTPs, 2.5 U of Taq DNA Polymerase (Qiagen) and 100 nM primers. The reactions were duplicated for each cDNA sample for each target gene in order to obtain more convincing results. Specific PCR primers used for each of the 5-HT receptor mRNAs were developed from GenBank sequences with commercially available Oligo software (IDT Tools). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used in all experiments as a positive control. The oligonucleotide primers used for PCR reactions are summarized in Table 1. All reagents were obtained from Invitrogen, unless otherwise stated. Standard PCR was performed on a PTC-200 Peltier thermal cycler (MJ Research) using the following program: 94 °C for 40 s, 56 °C for 40 s and 72°C for 1min for 40 cycles.
Table 1.
PCR Primers for 5-HT Receptors
| Genes | Accession No. |
PCR Product size (bp) |
Genes | Accession No. | PCR Product size (bp) |
|---|---|---|---|---|---|
| GAPDH | M17701 | 315 | 5-HT2C | M21410 | 545 |
| 5-HT1A | NM_012585 | 336 | 5-HT3 | U59672 | 415 |
| 5-HT1B | M89954 | 200 | 5-HT4 | AJ011370 | 610 |
| 5-HT1D | M89953 | 367 | 5-HT5 | NM_013148.1 | 637 |
| 5-HT1F | NM_021857 | 618 | 5-HT6 | NM_024365 | 370 |
| 5-HT2A | M30705 | 465 | 5-HT7 | L19654 | 383 |
Single-Cell RT-PCR
At the end of each recording session, the cell cytoplasm was aspirated into the patch recording pipette containing ~ 5 µl of RNase-free patch solution under visual control, by applying gentle negative pressure. The contents of the patch pipette were then expelled into an eppendorf tube containing 7 µl of the reverse transcription cocktail (ibid) by applying positive pressure. mRNA was reverse transcribed using 200 U of Superscript RT III and RT was performed in a final volume of 21 µl as described earlier. The cDNA was stored at −20 °C before further processing. The cDNA from each cell was then amplified and screened for the expression of four specific 5-HT receptor mRNAs (5-HT1A, 5-HT2A, 5-HT2C and 5-HT7) and GAPDH as a positive control marker using a two step multiplex-PCR reaction (Toledo-Rodriguez et al., 2004).
Initially all four genes were simultaneously amplified in a single tube using 2 µl of cDNA from each cell as a template and 100 nM of each of the four primers. The PCR master mix was comprised of 10X PCR Buffer, 3 mM MgCl2, 10 mM dNTPs, 2.5 U of Taq DNA Polymerase in a final volume of 100 µl. PCR was performed using a 10 min hot start at 95°C followed by a 25 cycle program (94 °C for 40 s, 56°C for 40 s and 72°C for 1 min). Subsequently, 2 µl of the amplified cDNA was used as the template for the second amplification step. Here, each gene was individually amplified in a separate tube and a 40 cycle program using the same PCR master mix and primer concentrations as mentioned above, in a final volume of 20 µl. The products of the second PCR were analyzed by electrophoresis in 1 % agarose gels using ethidium bromide.
Results
Expression of 5-HT receptor mRNA in BNSTALG tissue
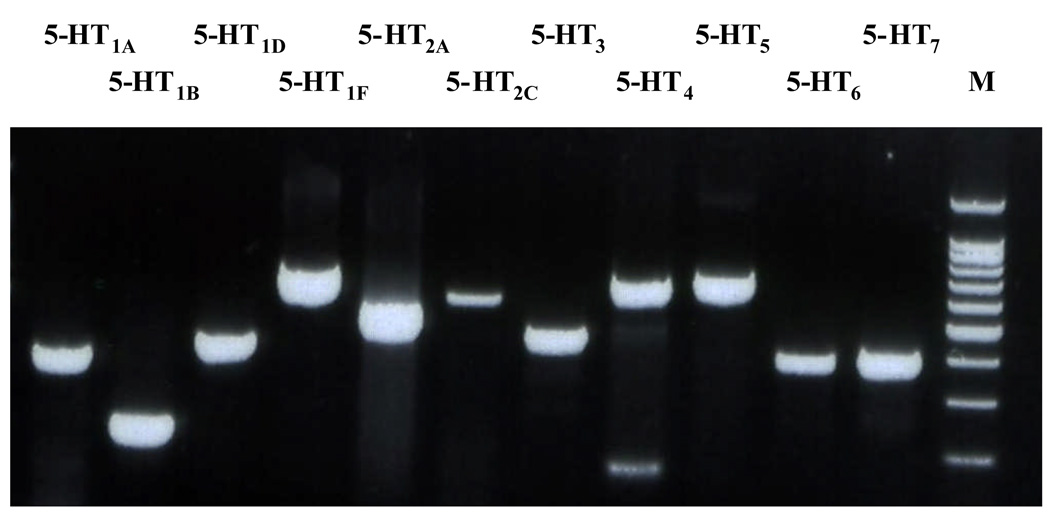
Before determining the likely candidates for the 5-HT receptors mediating the excitatory response of BNSTALG neurons to 5-HT, it was first necessary to determine the profile of 5-HT receptor expression in this region. Previous studies had reported moderate to high levels of expression for 5-HT1, 5-HT2A, 5-HT2C, 5-HT4, and 5-HT7 receptors within the BNST as a whole (Mengod et al., 1990, Waeber et al., 1994, To et al., 1995, Waeber and Moskowitz, 1995, Wright et al., 1995, Kia et al., 1996, Heidmann et al., 1998, Sari et al., 1998, Cornea-Hebert et al., 1999); however, these studies did not specifically examine the expression of 5-HT receptor subtypes within the anterolateral region of the BNST. Hence, we micro-dissected the BNSTALG from coronal brain sections, extracted tissue RNA, and then probed the samples for specific 5-HT receptor subtype mRNA using a RT-PCR protocol (n=8 rats). The BNSTALG mRNA expression pattern for multiple 5-HT receptor subtypes was qualitatively similar across all animals, and consistently included transcripts for 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1F, 5-HT2A, 5-HT2C, 5-HT3, 5-HT4 5-HT5A, 5-HT6, and 5-HT7 receptors (Fig. 1). These results confirmed the observations of previous in situ studies (ibid) and also acted as an expression control for our subsequent single-cell RT-PCR studies (see below). Moreover, these data suggest that 5-HT receptor expression in the BNSTALG is heterogeneous, which supports our electrophysiological data describing a complex response profile to 5-HT (Rainnie, 1999; Levita et al., 2004). In order to determine the 5-HT receptor subtype(s) that mediate the excitatory response of BNSTALG neurons to 5-HT application, we used whole-cell patch-clamp recording and subtype selective antagonists to block the excitatory response to exogenous 5-HT application, and subtype selective agonists to mimic 5-HT induced excitation, followed by single cell RT-PCR screening of the mRNA expression of these receptor subtypes.
Figure 1.
Photograph of an agarose gel showing the expression of 5-HT receptor subtypes mRNA in the BNSTALG tissue. Whole BNSTALG tissue was dissected from 500 µm BNST slices. M denotes a molecular weight marker.
Excitation in 5-HTHyp-Dep neurons
In this study, the response to 5-HT application (50 µM) was examined in a total of 148 BNSTALG neurons. Consistent with our previous studies (Rainnie, 1999; Levita, et al 2004), BNSTALG neurons respond to 5-HT with 5-HTHyp-Dep (28%), 5-HTDep (34%), or 5-HTHyp (16%). On contrast to 5-HTDep, HTHyp-Dep has two components, that is, a membrane hyperpolarization immediately followed by a depolarization deflection. The properties of the 5-HTHyp-Dep and 5-HTDep responses are summarized in Table 2.
Table 2.
Properties of 5-HT-induced 5-HTHyp-Dep and 5-HTDep responses in the BNSTALGneurons
| 5-HT response | membrane response (mV) |
membrane current (pA) |
reversal potential (mV) |
Conductance (nS) | |
|---|---|---|---|---|---|
| Baseline | 5-HT | ||||
| 5-HTHyp-Dep peak | −5.79 ± 0.63 (n=26) |
−17.0 ± 2.2 (n=26) |
−79.1 ± 1.3 (n=15) |
3.38 ± 0.57 (n=10) |
4.67 ± 0.89* (n=10) |
| 5-HTHyp-Dep Steady state |
2.83 ± 0.24# (n=26) |
9.0 ± 1.5# (n=26) |
−66.5 ± 1.7 (n=15) |
3.15 ± 0.42 (n=12) |
4.97 ± 0.78** (n=12) |
| 5-HTDep | 4.0 ± 0.7 (n=19) |
13.2 ± 1.8 (n=28) |
−48.5 ± 1.4 (n=32) |
2.83 ± 0.94 (n=28) |
3.52 ± 1.02** (n=28) |
5-HT (50 µM) induced membrane potential or current changes were recorded in current clamp or voltage clamp mode respectively.
*, ** p<0.05, 0.01 respectively, paired t-test.
#, these values are determined as relative to the peak of the 5-HTHyp-Dep.
In 5-HTHyp-Dep neurons, we conducted voltage ramp protocols at the peak of the outward deflection and then again when the inward deflection had reached steady state, the E5-HT was found to shift right significantly from the peak to the steady state (t(14) = 5.2, p <0.001, Table 2). At the peak of the outward deflection, the membrane conductance significantly increased by 42 ± 11% compared to baseline (t(9) = 3.1, p <0.05). Importantly, the membrane conductance was 55 ± 6% greater than baseline when the response reached a steady state level (t(11) = 4.7, p < 0.001), suggesting that the mixed 5-HTHyp-Dep response was due to the slow onset of an overlapping inward current, and not due to the gradual decay of an outward current.
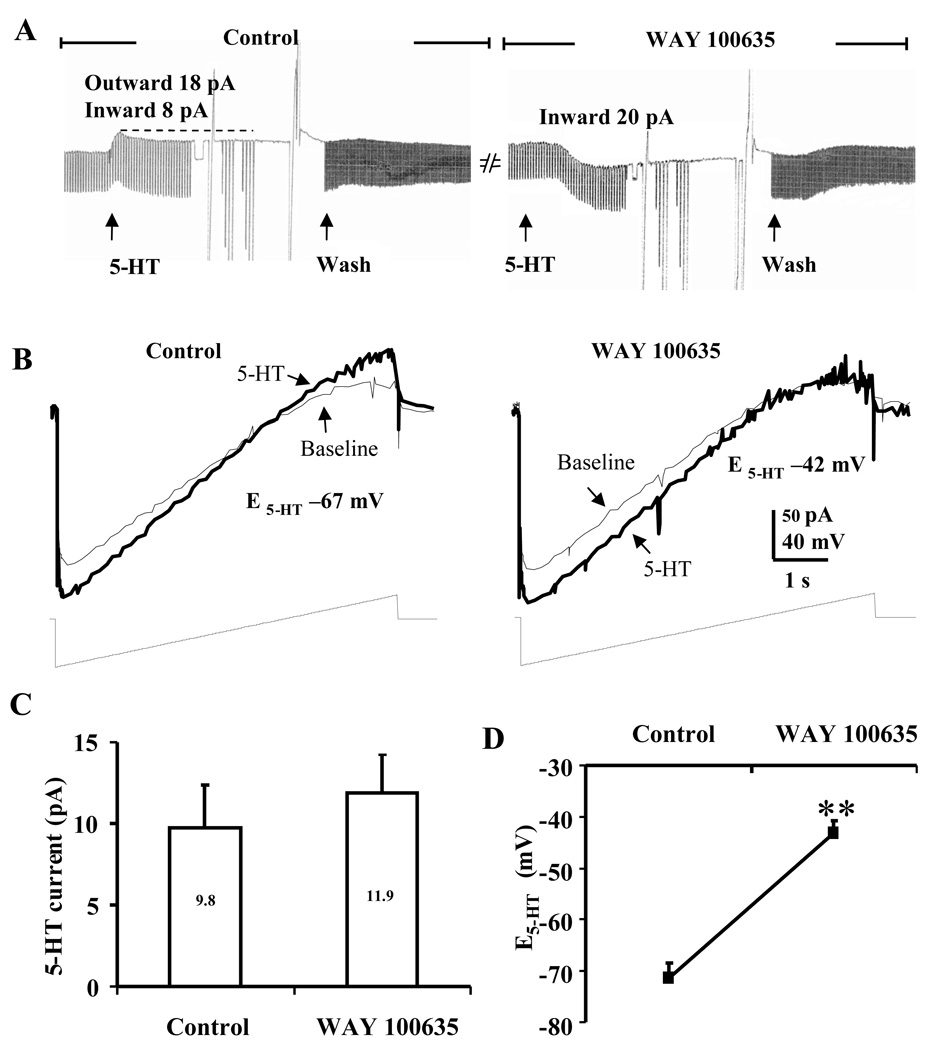
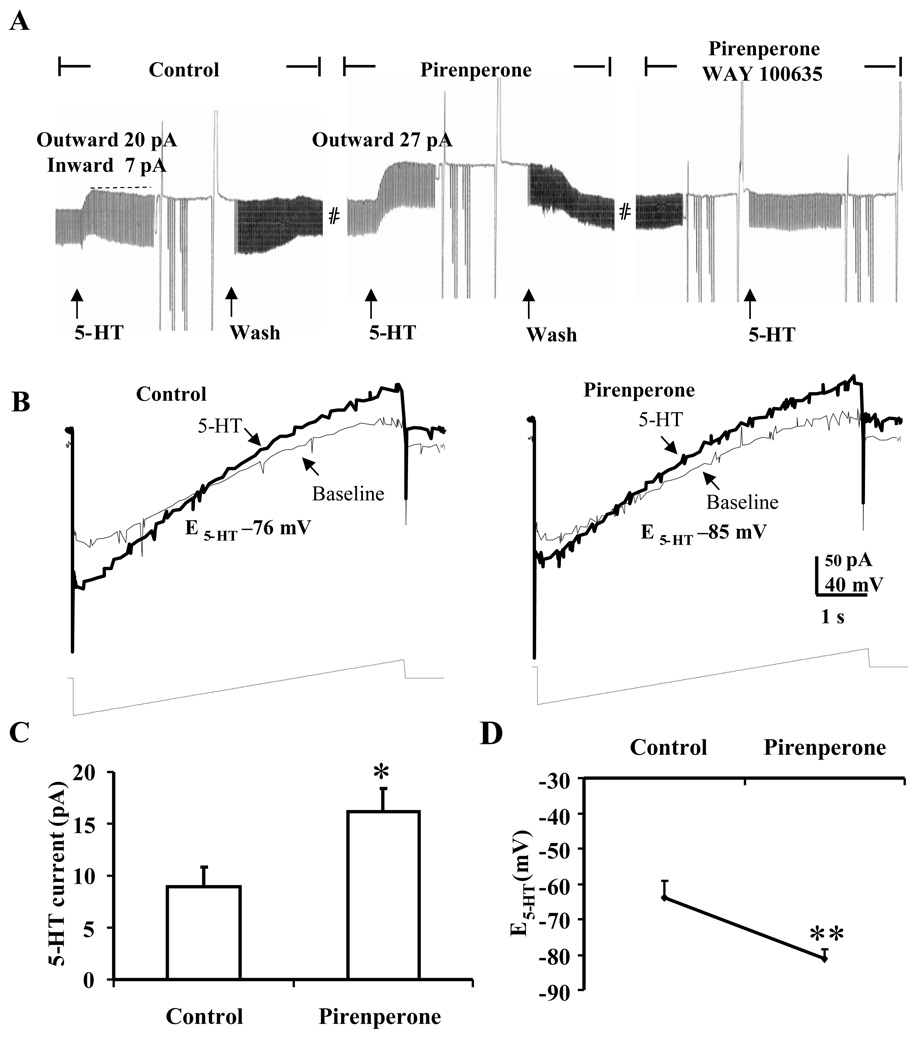
Consistent with this hypothesis, in those neurons that exhibited a 5-HTHyp-Dep response, application of 5-HT (50 µM) in the presence of the 5-HT1A receptor antagonist, WAY 100635 (200 nM) evoked only a monophasic inward current (Figure 2A) with a reversal potential that was significantly more depolarized than that observed in control ACSF (t(8) = 7.2, p < 0.001, see Fig. 2B, D). Moreover, application of 5-HT in the presence of the non-selective 5-HT2 receptor antagonist, pirenperone (10 µM), evoked only a monophasic outward current in 5-HTHyp-Dep neurons (Fig. 3A). Importantly, pirenperone application caused a significant increase in the amplitude of the peak outward current (t(6) = 2.8, p < 0.05; Fig. 3C), and caused a −17.3 ± 3.6 mV leftward shift of the 5-HT reversal potential (t(6) = 4.8, p < 0.01; Fig. 3B,D). Finally, co-application of WAY 100635 and pirenperone fully blocked the response to 5-HT in these neurons (n = 3, Fig. 3A right). These data were consistent with our previous study, in which we showed that the inhibitory component of the 5-HT response in these neurons was mediated by 5-HT1A receptor activation (Levita et al., 2004), and suggested that the 5-HT2 family of receptors may mediate the excitatory component of the mixed 5-HTHyp-Dep response. However, pirenperone is not a selective 5-HT2 receptor antagonist and has affinity for other 5-HT receptor subtypes, including 5-HT7 receptors. Consequently, we next examined the ability of more selective 5-HT receptor subtype antagonists to block the 5-HT evoked depolarizing component in 5-HTHyp-Dep BNSTALG neurons.
Figure 2.
Monophasic inward current to 5-HT unmasked by the 5-HT1A antagonist, WAY 100635 in BNSTALG neurons that initially exhibited a 5-HTHyp-Dep response to 5-HT. A, Application of 5-HT1A antagonist WAY 100635 (200 nM) unmasked an inward current. B, Ramp traces showing the shift of E5-HT to more depolarized level of −42 mV when 5-HT was applied in the presence of WAY 100635, which suggested the activation of a non-selective cation current. C, Histographs showing the inward currents elicited by 5-HT in the control condition (9.8 ± 2.6 pA, n = 9) and in the presence of WAY 100635 (11.9 ± 2.3 pA, n = 9). D, In the presence of WAY 100635, E5-HT was significantly shifted from the control at −71.4 ± 2.9 mV to −43.2 ± 2.4 mV, n=9. **, paired t-test, p<0.01.
Figure 3.
Monophasic outward current to 5-HT in the presence of the nonselective 5-HT2 antagonist pirenperone in BNSTALG neurons that initially exhibited a 5-HTHyp-Dep response to 5-HT. A, In a BNSTALG neuron exhibited initial 5-HTHyp-Dep response, 5-HT (50 µM) induced a monophasic outward current in the presence of pirenperone (10 µM); B, Ramp traces showing the shift of E5-HT to more hyperpolarized level of −85 mV when 5-HT was applied in the presence of pirenperone, suggesting the activation of a potassium current; C, Histographs showing pirenperone pre-application increased the amplitude of 5-HT-induced outward currents in BNSTALG neurons (ACSF 9.0± 1.9; pirenperone 16.1 ± 2.2 pA, n=7); D, E5-HT was shifted to the more hyperpolarized level in the presence of pirenperone (ACSF −63.8 ± 4.8 mV, pirenperone −81.1 ± 2.7 mV; n=7. *, ** paired t-test, p<0.05 and 0.01 respectively.
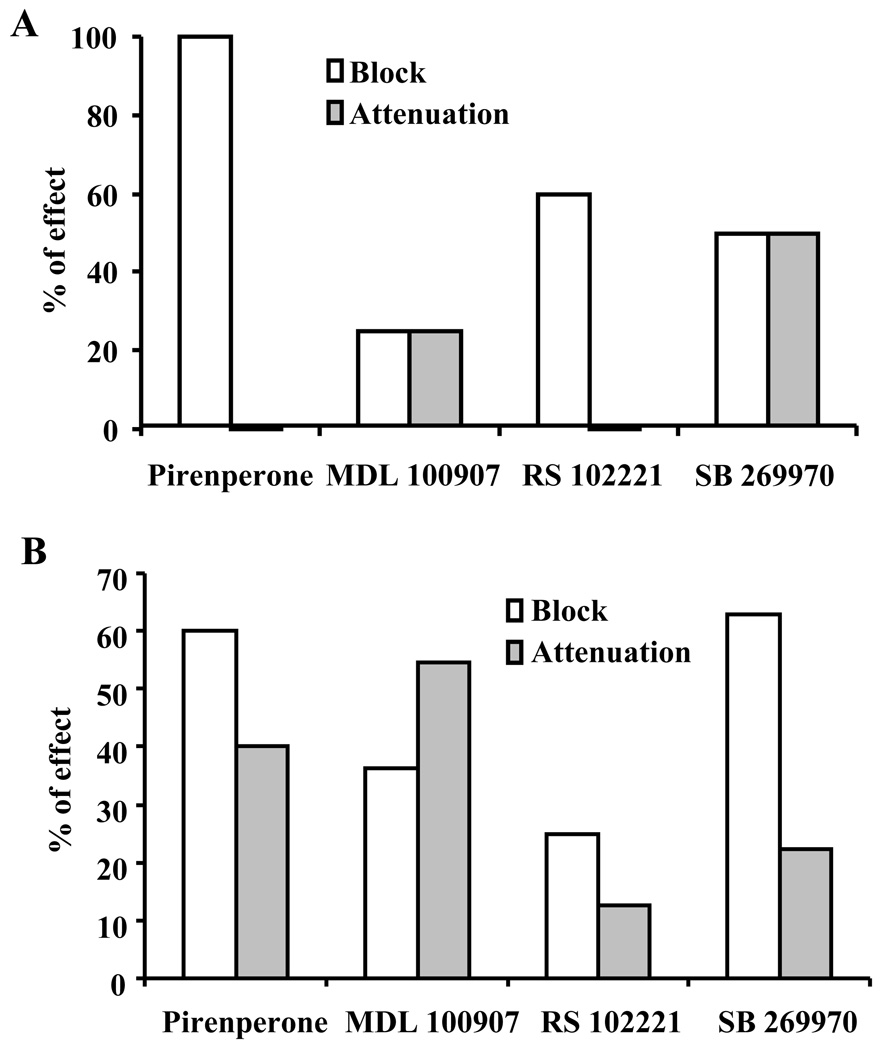
We first determined whether prior application of the 5-HT2A receptor antagonist MDL 100907 would attenuate the excitatory component of 5-HTHyp-Dep response. In 8 neurons that exhibited an initial mixed 5-HTHyp-Dep response, prior application of MDL 100907 (10 µM) blocked the inward current in 2 neurons, attenuated the 5-HT-induced inward current in 2 neurons (58 ± 8% of initial 5-HT current), and had no effect in the remaining 4 neurons, suggesting that 5-HT2A receptors mediate excitation in some 5-HTHyp-Dep neurons. We subsequently used a similar strategy with the 5-HT2C receptor antagonist RS 102221 (10 µM). Here, RS 102221 blocked the inward current in 3 of 5 neurons and had no effect in the remaining 2 neurons, suggesting that 5-HT2C receptors also contribute to the 5-HT-induced excitation in some 5-HTHyp-Dep neurons. Importantly, these antagonists had no observable membrane effects when administered alone. Hence, activation of 5-HT2A and 5-HT2C receptors can mediate an excitatory response in some 5-HTHyp-Dep neurons. However, since pirenperone also has affinity at 5-HT7 receptors, we next tested whether a 5-HT7 receptor antagonist could also block the excitatory response in some 5-HTHyp-Dep neurons.
Prior application of the 5-HT7 receptor antagonist, SB 269970 (1 µM) alone had no effect on the membrane properties of BNSTALG neurons, but fully blocked the depolarizing response to 5-HT or its associated inward current in 5 of the 10 neurons tested. In the remaining 5 neurons, SB 269970 attenuated the 5-HT-induced inward current to 41 ± 4% of baseline. Significantly, in 3 of 5 neurons that exhibited an attenuated excitatory response to 5-HT in the presence of SB 269970, co-application of SB 269970 and MDL 100907 fully blocked the residual 5-HT response. The inward current in the remaining 2 neurons was not changed by co-application of SB 269970 and MDL 100907. These data are summarized in Fig. 4A. While the number of neurons tested in these studies was not sufficient to determine conclusively the relative ability of the antagonists to block 5-HT excitation in BNSTALG neurons, or whether the pharmacology of these responses differed according to BNSTALG physiological subtype (see below, Hammack et al., 2007), they give clear evidence that the 5-HT-induced excitation in mixed 5-HTHyp-Dep BNSTALG neurons can be mediated by 5-HT2A, 5-HT2C, and/or 5-HT7 receptors. Moreover, in some 5-HTHyp-Dep neurons multiple receptors contribute to the excitatory response to 5-HT. Since 5-HT1A receptors mediate the inhibitory response to 5-HT in these neurons, these data imply that 5-HTHyp-Dep neurons can express at least 3 functional 5-HT receptor subtypes that mediate their response to 5-HT.
Figure 4.
Blockade of excitatory response to 5-HT by 5-HT2A, 5-HT2C and 5-HT7 receptor antagonists in BNSTALG neurons. A, in 5-HTHyp-Dep neurons, the relative efficacies of 5-HT receptor antagonists in blocking or attenuating the excitatory component of 5-HT response; Pirenperone 10 µM, n=7; MDL 100907, 10 µM, n=8; RS 102221, 10 µM, n=5; SB 269970, 1 µM, n=10. B, in 5-HTDep neurons, the relative efficacies of 5-HT receptor antagonists in blocking or attenuating the excitatory responses to 5-HT; Pirenperone, 10 µM, n=5; MDL 100907, 10 µM, n=11; RS 102221, 10 µM, n=8; SB 269970, 1 µM, n=27.
Excitation in 5-HTDep neurons
As mentioned above, 34% of recorded BNSTALG neurons exhibited a pure excitatory response to exogenous 5-HT (5-HTDep). Hence, we next examined whether the pure excitatory response was mediated by the activation of the same receptor population that mediated excitation in 5-HTHyp-Dep neurons. In 5-HTDep neurons, 5-HT induced an inward current, which associated with a significant increase of membrane conductance (23.4 ± 1.7%, t(27) = 5.0, p < 0.001), and had a reversal potential closing to that of non-selective cation channels (Table 2).
Similar to the strategy described for 5-HTHyp-Dep neurons (see above), we first examined whether pretreatment with pirenperone could block 5-HT-induced excitation in 5-HTDep neurons. In 3/5 neurons tested, 10 µM pireperone fully blocked the 5-HT-evoked inward current. In the remaining 2 neurons, pirenperone attenuated the 5-HT-induced inward current by 69 ± 6%.
We next examined the effect of subtype-selective 5-HT receptor antagonists on 5-HT-induced inward current in 5-HTDep neurons. Prior application of the 5-HT2A antagonist MDL 100907 (10 µM) fully blocked the 5-HT induced inward current in 4/11 neurons tested, and attenuated the 5-HT evoked current in 6/11 neurons (57.4 ± 6.8% of the initial 5-HT current). These data suggest that 5-HT2A receptors mediate at least part of the 5-HT-induced excitatory response in the majority of 5-HTDep neurons.
We next determined the contribution of 5-HT2C receptor activation to the excitatory response in 5-HTDep neurons. Pretreatment with the 5-HT2C receptor antagonist RS 102221 (10 µM) fully blocked the inward current in 2 of 8 neurons tested, attenuated the inward current in 1 neuron (30% of control), and had no noticeable effect in 5 neurons.
In contrast, prior application of the 5-HT7 antagonist SB 269970 (1 µM) fully blocked the 5-HT-evoked inward current in 17 of 27 5-HTDep neurons, and attenuated the inward current to 44 ± 11% of the amplitude of the initial 5-HTDep response in 6/27 neurons (t(5) = 5.3, p < 0.01). Prior application of SB 269970 had no noticeable effect in the remaining 4 neurons. Hence, activation of 5-HT7 receptors mediated 5-HT-induced excitation in a significant population of 5-HTDep neurons.
Finally, we examined the effects of co-application of SB 269970 and MDL 100907 in 5-HTDep neurons that exhibited an attenuated 5-HT response in the presence of SB 269970. Here, co-application of the two antagonists fully blocked the residual 5-HT-induced inward current in all 5-HTDep neurons tested (n = 4). Hence, similar to 5-HTHyp-Dep BNSTALG neurons, these data suggest that the 5-HT-induced excitation in 5-HTDep neurons is predominantly mediated by 5-HT2A, 5-HT2C, and/or 5-HT7, receptors. Moreover, in some 5-HTDep neurons multiple receptors contribute to the excitatory response to 5-HT. The relative efficacies of the three 5-HT receptor antagonists in blocking or attenuating the 5-HTDep response are illustrated in Fig. 4B.
Relative efficacy of 5-HT receptor subtype-selective agonists
As outlined above, both our PCR data and our antagonist studies showed that multiple 5-HT receptors are expressed in the BNSTALG, and more than one receptor subtype can mediate the excitatory response to 5-HT application. Consequently, we next examined the ability of a selection of relatively selective 5-HT receptor agonists to mimic the 5-HT-induced excitation in BNSTALG neurons.
We have previously shown that BNSTALG inhibition by 5-HT was mediated by 5-HT1A receptors, and is always blocked in the presence of a 5-HT1A antagonist (Levita et al., 2004). Consistent with this report, none of the following 5-HT1 receptor-selective agonists evoked an observable response in any BNSTALG neuron tested: the selective 5-HT1B/D agonist, sumatriptan (10 µM; n = 11), the selective 5-HT1B agonist, CP 93129 (10 µM, n = 10), the selective 5-HT1E/F receptor agonist, BRL54443 (5 – 75 µM, n = 4), or the selective 5-HT1F agonist LY 344864 (10 µM, n = 4).
Conversely, application of the putative broad spectrum 5-HT2 receptor agonist, α-methyl 5-HT (25–250 µM) mimicked both the inhibitory and the excitatory response to 5-HT, irrespective of the concentration applied (n = 7). Similar results were observed for the 5-HT derivative 5-methoxytryptamine (n = 3, 50–250 µM). The inhibitory action of these agonists were likely mediated by an action at 5-HT1A receptors, and consistent with this interpretation, inhibition was blocked in the presence of the 5-HT1A antagonist WAY 100635. Hence, we next tested the response of BNSTALG neurons to exogenous application of more selective 5-HT2 receptor subtype agonists. Surprisingly, the selective 5-HT2A agonists, DOI (5 – 50 µM; n = 5), which has been shown to increase c-fos expression in BNSTALG neurons (Van de Kar et al., 2001), and TCB 2 (10 µM, n = 6) failed to mimic the excitatory response to 5-HT. Similarly, the potent and selective 5-HT2C agonist, WAY 161503 (10 µM, n = 7) failed to mimic the excitatory response in any BNSTALG neuron tested. In contrast, application of the relatively selective 5-HT2B/2C receptor agonist, mCPP (5–75 µM), elicited a depolarizing response in 6/14 neurons tested, had no effect in 6/14 neurons, and elicited a hyperpolarizing response in the remaining 2 neurons.
We reasoned that the 5-HT3 receptor was unlikely to mediate the slow onset and long duration excitatory response due to its rapid activation and desensitization kinetics (Yang et al., 1992, van Hooft and Vijverberg, 1997). As expected, the 5-HT3 receptor agonist, mCPBG (50 µM), which can induce a transient inward current in cortical slices (Zhou and Hablitz, 1999), had no effect on either the resting membrane potential or membrane input resistance in 9/10 BNSTALG neuron tested. Similarly, the selective 5-HT4 receptor agonist, cisapride (10 µM) (Roychowdhury et al., 1994, Xiang et al., 2005), failed to induce a membrane depolarization in any BNSTALG neuron tested (n =12); however, it did induce a small hyperpolarization in 2 neurons that was accompanied by a decrease of input resistance.
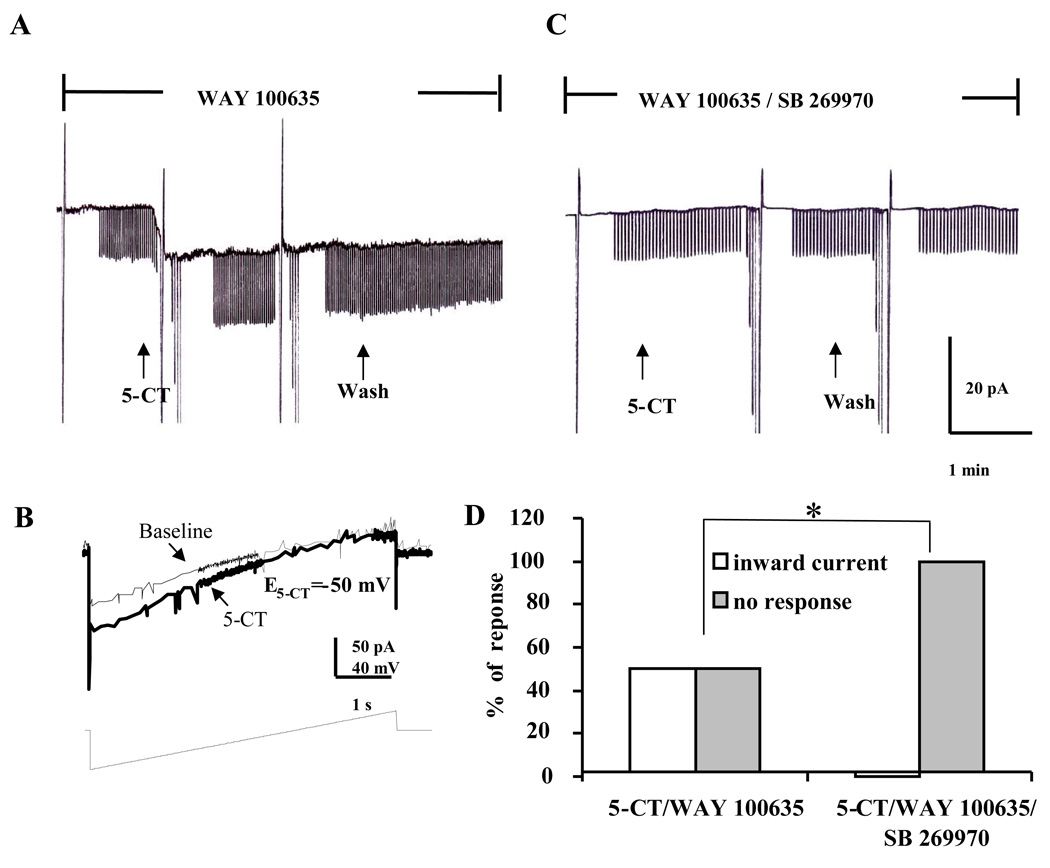
Finally, we examined whether the mixed 5-HT1/7 agonist, 5-CT (10 µM), could mimic the depolarizing response of 5-HT in the presence of the 5-HT1A receptor antagonist WAY 100635 (200 nM). In 50% (9/18) of BNSTALG neurons, 5-CT evoked an inward current that was associated with an 18.9 ± 4.4% increase in conductance (t(8) = 3.8, p < 0.01), and a reversal potential of −48.0 ± 3.1 mV (Fig. 5A,B). Moreover, the 5-CT reversal potential did not differ significantly from the E5-HT (−48.9 ± 1.3 mV, t(33) = 0.31, p = 0.76) for the 5-HTDep response, suggesting that 5-CT evoked excitation might be mediated by the same cellular mechanisms as the pure depolarizing response to 5-HT.
Figure 5.
The 5-CT induced inward current in BNSTALG neurons. A, In the presence of the 5-HT1A antagonist, WAY 100635 (200 nM), 5-CT (10 µM) induced inward current (8.9 ± 1.4 pA, n = 9), which associated with an increase in membrane conductance (baseline 2.16 ± 0.30 nS, 5-CT 2.60 ± 0.40 nS; t(8) = 3.8, p < 0.01); B, 5-CT induced inward current had a reversal potential of −42 mV, which close to that of non-selective cation channels; C, In the presence of the 5-HT7 antagonist SB 269970 (1 µM) and WAY 100635 (200 nM), 5-CT failed to induce any change in steady state current; D, Group data showing 5-CT in the presence of WAY 100635 induced an inward current in 50% (9/18) of the BNSTALG neurons tested, but failed to induce any inward current in neurons pretreated with both WAY 100635 and SB 269970 (n = 7). *, Chi-Square test, χ2 = 5.459, df = 1, p<0.05.
Because the effects of 5-CT were not readily reversible on washout with ACSF, we were prevented from blocking the 5-CT-evoked depolarization using a within-subjects design. Hence, we next examined the effects of co-application of WAY 100635 and the 5-HT7 antagonist SB269970 (1 µM) on the 5-CT induced excitatory response in BNSTALG neurons. In the presence of SB 269970 and WAY 100635, 5-CT failed to induce an inward or outward current in any BNSTALG neuron tested (n = 7, see Fig. 5C; χ2 = 5.459, df = 1, p < 0.05; Fig. 5D). These results suggest that 5-CT induced an excitatory response in the presence of WAY 100635 that was mediated by 5-HT7 receptors. Significantly, in 3 of 4 BNSTALG neurons that exhibited an initial 5-HTHyp-Dep response, subsequent application of 5-CT in the presence of WAY 100635 induced an inward current (5.3 ± 0.9 pA) that was accompanied by an 14.2 ± 6.3% increase in membrane conductance (baseline 4.03 ± 1.20 nS, 5-CT 4.74 ± 1.54 nS), which had a reversal potential of −52.5 ± 4.1 mV. These data confirmed the results of our antagonist study and support the contention that 5-HT7 receptor activation plays a significant role in the excitatory response to 5-HT in 5-HTHyp-Dep neurons. However, the lack of response of BNSTALG neurons to several selective 5-HT receptor agonists and the questionable selectivity of some of the commercially available antagonists hindered definitive statements about the true expression pattern of 5-HT receptors in BNSTALG.
Expression of 5-HT receptor mRNA in individual BNSTALG neurons
In an attempt to further clarify the expression pattern of 5-HT receptors in individual BNSTALG neurons we extracted cytosolic mRNA from physiologically identified neurons and then used single-cell RT-PCR to examine their 5-HT receptor mRNA expression profile.
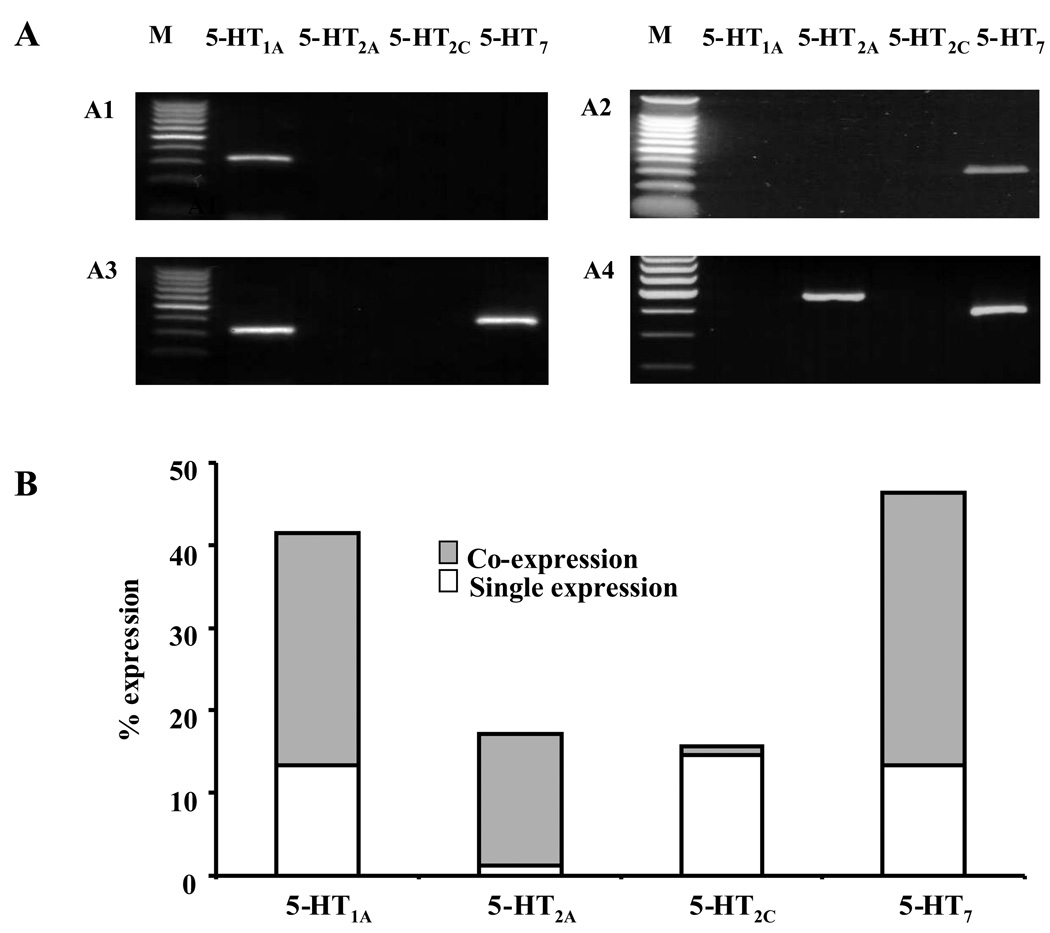
Signal for the mRNA transcripts of at least one 5-HT receptor subtype (Fig 6A) was detected in 67/82 BNSTALG neurons sampled (82%). Of the four 5-HT receptor subtypes initially screened, the percentage of the 82 BNSTALG neurons expressing each receptor was: 5-HT7 receptor (46%), > 5-HT1A receptor (41%) > 5-HT2A receptor (17%) > 5-HT2C receptor (16%), and transcripts for each 5-HT receptor subtype were expressed either alone (43%) or in combination (39%; Fig 6A, B). For example, of those neurons that express 5-HT7 mRNA, 33% co-express mRNA for other 5-HT receptor subtypes (5-HT1A/7, 23%; 5-HT2A/7 10%;), whereas only 13% expressed 5-HT7 receptor mRNA alone. Similarly, while 41% of sampled BNSTALG neurons expressed 5-HT1A mRNA, 28% co-expressed 5-HT1A mRNA with either 5-HT2A and/or 5-HT7 receptor mRNA and only 13% of these neurons express 5-HT1A mRNA alone. Significantly, 5-HT2A receptor transcripts were almost always detected with other 5-HT receptor subtypes (92% of sample). In contrast, 5-HT2C transcripts were rarely detected with the mRNA for other 5-HT receptor subtypes. Of the 13 BNSTALG neurons in which 5-HT2C mRNA was detected (16%), 12 expressed only 5-HT2C mRNA (see Fig. 6B).
Figure 6.
5-HT receptor mRNA expression in single BNSTALG neurons detected by single cell RT-PCR. A, Representative photographs showing the expression of 5-HT receptor subtype mRNA in single BNSTALG neurons: 5-HT1A mRNA single expression (A1); 5-HT7 mRNA single expression (A2); 5-HT1A/5-HT7 mRNA co-expression (A3); 5-HT2A/5-HT7 mRNA co-expression (A4). B, Summary of 5-HT receptor subtypes mRNA expression in a population of 82 BNSTALG neurons. 5-HT2A, 5-HT1A,5-HT7 mRNA tend to be co-expressed with each other whereas 5-HT2C mRNA tend to be expressed alone.
Comparison of the single-cell 5-HT receptor mRNA expression profile and the observed response to 5-HT in BNSTALG neurons
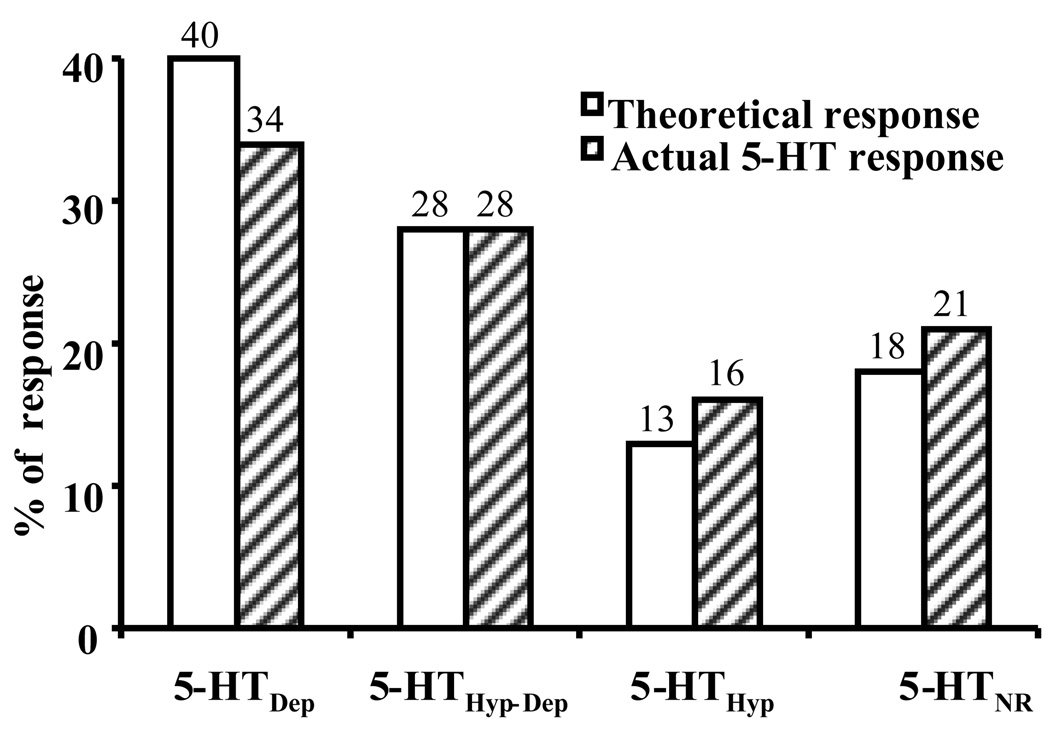
In the present study, we examined the 5-HT response from a total of 148 BNSTALG neurons. In agreement with our previous study (Levita et al., 2004), BNSTALG neurons responded to the application of 5-HT in any one of four distinct ways: 5-HTHyp-Dep (28%), 5-HTDep (35%), 5-HTHyp (16%), and 5-HTNR (21%). As illustrated in Fig. 8, the 5-HT response profile predicted by BNSTALG 5-HT receptor mRNA expression (5-HTHyp-Dep 28%, 5-HTDep 40%, 5-HTHyp 13%, and 5-HTNR 18%) was not statistically different from the observed 5-HT response profile of BNSTALG neurons, (Chi Square test, χ2 = 1.03, df = 3, p = 0.80), suggesting that the mRNA expression profile in individual BNSTALG neurons is a true reflection of their relative 5-HT receptor distribution. Consistent with this hypothesis, the relative expression of 5-HT7 mRNA (46%) was similar to the percentage of excitatory responses induced by 5-CT in BNSTALG neurons (50%) (Chi Square test, χ2 = 0.08, df = 1, p = 0.78).
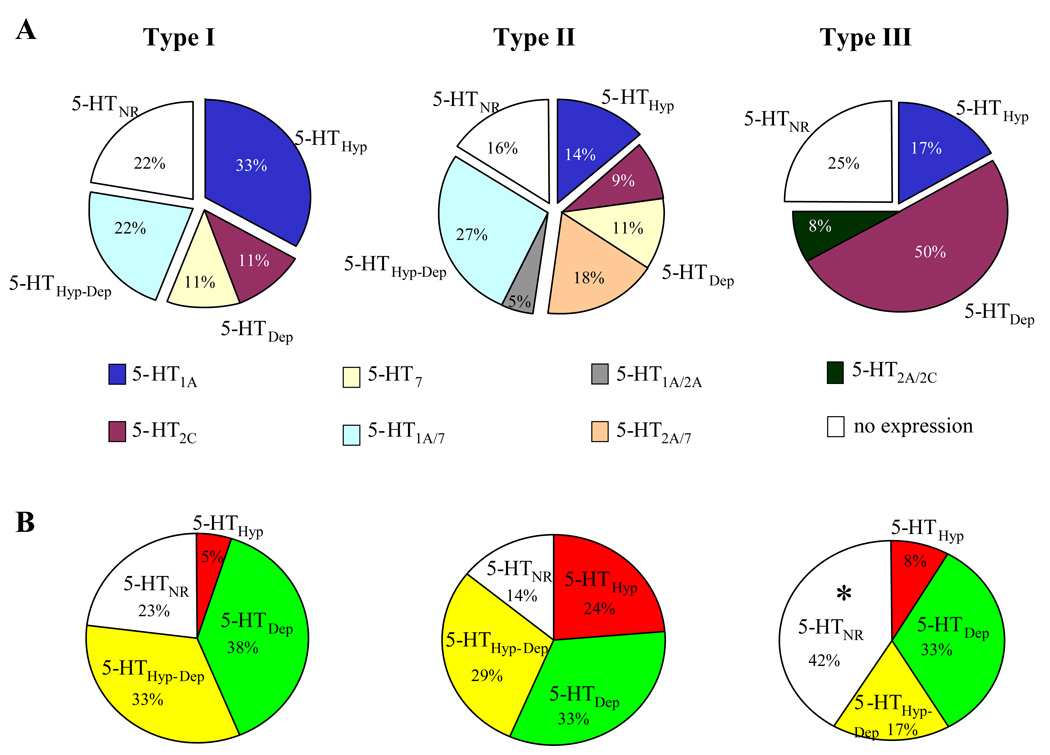
Figure 8.
5-HT receptor mRNA expressions and 5-HT population responses in 3 types of physiology distinct BNSTALG neurons. BNSTALG neurons were divided into 3 cell types (Type I – III) based on their physiological response to depolarizing and hyperpolarizing current injection (Hammack et al., 2007). A, The expression of 5-HT1A,2A,2C,7 receptor mRNAs in three types of BNSTALG neurons, Type I, n=9; Type II, n=44; Type III, n=12. B, 5-HT responses examined in 39 type I, 85 type II and 24 type III BNSTALG neurons; Compared to type II, Type III neurons has higher percentage of 5-HTNR. *, Chi Square test followed by post-hoc analysis, R=2.22, p < 0.05.
Cell type-specific 5-HT receptor subtype expression
In the study above, we initially sampled mRNA from some cells (~30) without determining the physiological properties of the cell. In the BNSTALG, three physiologically distinct cell types (Type I–III) have been identified based on their differential expression of sub-threshold membrane currents (Hammack et al., 2007). Briefly, Type I neurons were characterized by a depolarizing sag in response to hyperpolarizing current injection that was mediated by the hyperpolarization-activated cation current, Ih and a regular firing pattern in response to depolarization current injection. Type II neurons also expressed Ih, and exhibited a burst-firing pattern that was mediated by activation of the low-threshold calcium current, IT. Type III neurons did not exhibit characteristics of Ih or IT expression, but instead exhibited a pronounced inwardly rectifying potassium current, IK(IR). Hence, we next explored the relative expression patterns of 5-HT receptor subtype mRNA in Type I–III neurons. Transcripts for 5-HT receptor subtypes were detected in 7/9 Type I, 37/44 Type II, and 9/12 Type III BNSTALG neurons. The relative expression of each of the 5-HT receptor transcripts are summarized in Fig. 8A. Thus Type I neurons express high levels of 5-HT1A mRNA (56%), Type II neurons express high levels of 5-HT7 (57%) and 5-HT1A (45%) mRNA, and Type III neurons express high levels of 5-HT2C (58%) mRNA. Significantly, no 5-HT2A and 5-HT7 mRNA was detected in Type I and Type III neurons, respectively. These results suggest that the three physiological subtypes of BNSTALG neurons not only differ in their physiological properties (Hammack et al., 2007), but also in their 5-HT receptor subtype expression.
Consequently we wanted to determine if the response to 5-HT was cell type specific. Hence, we mapped the 5-HT response profile across the three cell types. The relative distribution of 5-HT responses among 39 Type I, 85 Type II, and 24 Type III neurons is summarized in Fig. 8B. A Chi square analysis revealed a significant difference in the response of the three cell types to 5-HT (χ2 = 15.35, df = 6, p = 0.018). To find out the difference between groups, we next compared two groups at a time. No significant difference was observed between the response of Type I and Type II neurons, although a trend was suggested (χ2 = 6.74, df = 3, p = 0.08). No difference was found between Type I and Type III neurons (χ2 = 3.58, df = 3, p = 0.31). However, the 5-HT response of Type II neurons was significantly different from that of Type III neurons (χ2 = 10.32, df = 3, p = 0.016). We then calculated the standardized residuals (R) to determine which response caused the difference between the Type II and Type III neurons. The standardized residual for the 5-HTNR of Type III neurons has a higher magnitude (R=2.22) than the criteria value corresponding to α of 0.05 (1.96), suggesting that Type III neurons had a higher percentage of 5-HTNR than Type II neurons. This analysis was consistent with our observation that Type III neurons had a higher percentage of 5-HTNR (42%) than Type II neurons (14%, Fig. 8).
Discussion
The results outlined above strongly suggest that neuronal activity in the BNSTALG is bidirectionally regulated by 5-HT through complex interactions between multiple 5-HT receptor subtypes. We previously demonstrated that the hyperpolarizing response of BNSTALG neurons to 5-HT was mediated by activation of 5-HT1A receptors coupled to G protein-gated inwardly-rectifying potassium channels, and that in vivo activation of BNSTALG 5-HT1A receptors reduced anxiety-like behavior (Levita et al., 2004). In the present report, we used RT-PCR to detect the expression of mRNA transcripts for multiple 5-HT receptor subtypes in BNSTALG tissue and also single BNSTALG neurons. Our PCR data clearly suggest that at least three 5-HT receptor subtypes mediate the excitatory effects of 5-HT on BNSTALG neurons, including 5-HT2A, 5-HT2C, and/or 5-HT7 receptors. Moreover, our PCR data confirmed our pharmacological and physiological observations that individual BNSTALG neurons express distinct combinations of 5-HT receptor subtypes. Lastly, we showed that the 5-HT receptor mRNA expression and 5-HT response pattern was cell type specific, suggesting that Type I – III BNSTALG neurons would respond differently to local 5-HT release, which raises the possibility that 5-HT receptor activation may also differentially alter the intrinsic membrane properties of discrete populations of BNSTALG neurons.
5-HT receptor subtypes in the BNSTALG
5-HT neurons in the caudal regions of the dorsal raphe nucleus (DRN) selectively innervate the BNSTALG (Phelix et al., 1992, Commons et al., 2003) where 5-HT fibers have been observed to make contact with BNST neurons (Phelix et al, 1992). In response to unpredictable stress, 5-HT levels dramatically increase in caudal DRN projection regions (Amat et al., 1998a, 1998b), and would be expected to increase in the BNSTALG. Hence, the current study was designed to determine the response of BNSTALG neurons to stress-induced 5-HT release. While several studies have demonstrated the presence of multiple 5-HT receptor subtypes within the BNST (Mengod et al., 1990, Waeber et al., 1994, To et al., 1995, Waeber and Moskowitz, 1995, Wright et al., 1995, Kia et al., 1996, Heidmann et al., 1998, Sari et al., 1998, Cornea-Hebert et al., 1999), none have specifically investigated the distribution of these receptors within the BNSTALG. Consistent with these earlier studies we have shown whole tissue mRNA expression for multiple 5-HT receptor subtypes in the BNSTALG, including 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1F, 5-HT2A, 5-HT2C, 5-HT3, 5-HT4, 5-HT5A, 5-HT6, and 5-HT7 receptors. The cellular localization of these receptors is currently unknown, although it is likely that many of these receptors are somatodendritic, while others, such as the 5-HT1B receptor, may be located presynaptically in axon terminals. However a glial localization for some of these receptor subtypes cannot be ruled out (Fillion et al., 1983, Huang et al., 2004, Patel and Zhou, 2005). Nevertheless, our single-cell RT-PCR data clearly show that at least 4 of these receptors are functionally expressed in the neuronal population of the BNSTALG.
The diversity of 5-HT receptor subtype expression together with the high degree of heterogeneity in BNST neuronal cytoarchitecture (McDonald, 1983, Ju and Swanson, 1989, Larriva-Sahd, 2004), chemoarchitecture (Ju et al., 1989, Walter et al., 1991, Gray and Magnuson, 1992, Day et al., 2004), and physiology (Rainnie, 1999, Egli and Winder, 2003, Hammack et al., 2007), suggest that 5-HT release would induce a complex response profile depending on the neuronal population affected, which may account for the complex relationship between 5-HT receptor activation and anxiety-like behavior (Handley et al., 1993, Handley, 1995, Graeff et al., 1996, Lowry et al., 2005, Lowry et al., 2008).
5-HT receptor subtypes mediating the excitatory response to 5-HT
Previously we have demonstrated that a single receptor population mediates the 5-HTHyp response in BNSTALG neurons, namely 5-HT1A receptors (Levita et al., 2004). However, the nature of the 5-HTHyp-Dep response was less clear. Preliminary studies suggested that it resulted from the activation of two separate currents, rather than the activation and passive decay of a 5-HT1A receptor-mediated outward current. This notion was supported by the observation that the reversal potential for 5-HT in 5-HTHyp-Dep neurons was significantly more depolarized at steady-state as compared to the peak of the response, and that the steady-state reversal potential was always associated with an increase in membrane conductance. These data were consistent with an activation of multiple currents and suggested that in 5-HTHyp-Dep neurons, the initial membrane hyperpolarization and associated outward current were followed by a slower overlapping membrane depolarization and associated inward current. As expected, when 5-HT was applied in the presence of the 5-HT1A receptor antagonist, WAY 100635, a monophasic inward current was unmasked. The characteristics of the inward current (i.e. amplitude and reversal potential) were similar to those observed in 5-HTDep neurons; hence, it was likely that the depolarization response in these two populations of BNSTALG neurons was mediated by the same mechanism(s). Using subtype-selective antagonists, we further demonstrated that the 5-HT-induced membrane depolarization and associated inward current in 5-HTHyp-Dep and 5-HTDep neurons were mediated by three 5-HT receptor subtypes, 5-HT2A, 5-HT2C, and 5-HT7.
5-HT2A receptors
Here we have shown that approximately 17% of BNSTALG neurons express 5-HT2A receptors. Significantly, 5-HT2A receptor transcripts were almost always detected with other 5-HT receptor subtypes, most notably with 5-HT7 receptors in Type II BNSTALG neurons. Activation of 5-HT2A receptors in the BNSTALG elicits a membrane depolarization that was associated with an inward current, an increase in membrane conductance, and a reversal potential of ~ −48 mV. These data are consistent with the activation of cation channels rather closure of potassium channels, which is consistent with reports from other brain regions which suggest that 5-HT2A receptor activation may be linked through Gq/11 (Hoyer et al., 2002) to activation of a non-selective cation current (Hardy et al., 2005).
Interestingly, in some 5-HTHyp-Dep and some 5-HTDep neurons, the 5-HT-induced membrane depolarization or associated inward current was attenuated in the presence of MDL 100907, but not blocked, and the residual 5-HT depolarization was only blocked completely only when MDL 100907 was combined with another subtype selective 5-HT antagonist (see below), suggesting that in subpopulations of BNSTALG neurons the excitatory postsynaptic response to 5-HT was mediated by activation of at least two 5-HT receptor subtypes.
The broad spectrum 5-HT2 receptor agonists α-methyl 5-HT and 5-methoxytryptamine mimicked both the inhibitory and excitatory response produced by 5-HT; however, the inhibitory response was blocked in the presence of the 5-HT1A antagonist WAY 100635, suggesting that the concentrations applied, these agonists were activating multiple 5-HT receptor subtypes. Interestingly, more selective 5-HT2 receptor subtype agonists such as DOI, TCB 2 and WAY 161503 failed to mimic the excitatory response to 5-HT. While it is unknown why these agonists did not mimic the excitatory response to 5-HT, these results were particularly surprising given that systemically administrated DOI has been shown to produce robust c-fos expression in the BNST (Leslie et al., 1993, Van de Kar et al., 2001). However, DOI induced cortical c-fos expression is thought to be an indirect process, such that c-fos expression was only observed in neurons that do not express 5-HT2A receptors (Mackowiak et al., 1999). Indeed, Scruggs et al (2000) found that DOI-induced activation of the cortex was dependent on activation of 5-HT2A receptors on thalamocortical neurons (Scruggs et al., 2000). Hence, DOI-induced c-fos expression in the BNST may be mediated by similar indirect pathways.
5-HT2C receptors
In contrast to the expression of 5-HT2A receptors, the expression of 5-HT2C receptors appeared to be mutually exclusive with the expression of any other 5-HT receptor subtype. Hence, ~ 16 % of BNSTALG neurons express only 5-HT2C transcripts. Significantly, 58% of Type III neurons express 5-HT2C receptor transcripts suggesting that this receptor may be expressed in a unique subpopulation of Type III neurons. Consistent with this hypothesis, 5-HT2C receptors are preferentially expressed by dynorphin containing neurons in the striatal patch compartment (Ward and Dorsa, 1996). Moreover, a recent report by Dumont and colleagues showed that the properties of BNSTALG neurons projecting to the ventral tegmental area were similar to that of Type III neurons (Dumont et al., 2008). If these neurons were to preferentially express 5-HT2C receptors it could provide mechanism by which 5-HT could fine-tune the output of select cell types. However, a larger sample population of Type III neurons will be required before the cell-type selective expression can be confirmed.
Nonetheless, activation of 5-HT2C receptors has been demonstrated to mediate a similar membrane depolarization or an inward current to 5-HT in several brain regions, including the pontine reticular formation (Weber et al., 2008), striatum, and subthalamic nuclei (Rick et al., 1995, Hardy et al., 2005, Xiang et al., 2005, Bonsi et al., 2007). Significantly, in several of these studies the depolarization was reported to result from a PLC-dependent reduction in potassium conductance. However, in the present study, the 5-HT2B/2C agonist mCPP induced a membrane depolarization, that was associated with an increase membrane conductance in most neurons, suggesting that 5-HT2C receptors in the BNSTALG may couple through different receptor-effector mechanisms than elsewhere in the brain.
It should be noted that the 5-HT2C receptor antagonist RS102221 also has a low affinity at noradrenergic receptors (pKi <5 for α1, <4 for β receptors) (Bonhaus et al., 1997). Dumont and Williams have shown that noradrenergic agonists can excite neurons of the lateral BNST (Dumont and Williams, 2004). Hence, it cannot be discounted that some of the action of this compound may be due to the blockade of an indirect release of norepinephrine within the BNSTALG. However, high concentrations of norepinephrine are required for postsynaptic excitation of BNSTALG neurons (Dumont and Williams, 2004), and the low affinity of RS102221 to noradrenergic receptors suggests that this action of RS102221 is unlikely to block any indirect excitatory actions of norepinephrine.
Consistent with a direct excitatory response to 5-HT2C receptor activation in the BNST, systemic injection of mCPP produced an anxiogenic response in several animal models of anxiety, including the social interaction test, light/dark box and zero maze (Kennett et al., 1989, Shepherd et al., 1994). Moreover, mCPP has also been shown to increase anxiety in humans (Silverstone et al., 1994, Broocks et al., 2001), and increases Fos expression in the BNSTALG (Singewald et al., 2003). Together, these data suggest that activation of 5-HT2C receptors on Type III BNSTALG neurons might be an important locus for mediating the anxiogenic effects of mCPP.
5-HT7 receptors
It is noteworthy that transcripts for 5-HT7 receptors were found in 46% of all BNSTALG neurons examined, and yet no Type III neuron examined to date has shown any 5-HT7 receptor mRNA expression. Moreover, 5-HT7 receptor expression was often found in association with 5-HT1A receptor expression suggesting that the bidirectional 5-HTHyp-Dep response in BNSTALG neurons is most likely mediated by activation of these two receptors, respectively. Moreover, because a combination of MDL 100907 and SB269970 was typically required to fully block 5-HT-depolarization, it is likely that many BNSTALG neurons co-express both 5-HT2A and 5-HT7 receptors.
Consistent with an excitatory response to 5-HT7 receptor activation, these receptors have been shown to link through Gs (Hoyer et al., 2002), and mediate depolarization in other brain regions, including the paraventricular nucleus of the hypothalamus (Ho et al., 2007), thalamus (Chapin and Andrade, 2001), hippocampus (Tokarski et al., 2003), and cholinergic interneurons of the striatum (Bonsi et al., 2007). As mentioned above, in the BNSTALG the 5-HT-induced depolarization was associated with an inward current, an increase in membrane conductance, and a reversal potential of ~ −48 mV. In the thalamus and dorsal root ganglia the depolarizing response to 5-HT7 receptor activation has been reported to be mediated by an increase in membrane conductance that resulted from a rightward shift in the voltage dependence of Ih channel activation (Cardenas et al., 1999, Chapin and Andrade, 2001). Future studies will examine the effects of 5-HT on Ih channel activation in BNSTALG neurons.
In vivo studies with 5-HT7 receptor antagonists, such as SB 269970, have revealed a potential role for 5-HT7 receptor activation in novel object recognition (Ballaz et al., 2007), stereotypic behavior (Hedlund and Sutcliffe, 2007), as well as anxiogenic and depressive-like behavior (Wesolowska et al., 2006, Nandam et al., 2007). Moreover, 5-HT7 knockout mice exhibit impaired contextual fear conditioning (Roberts et al., 2004), and decreased immobility in the Porsolt swim test (Guscott et al., 2005). Both of these effects were consistent with a naturally anxiogenic or depressive effect of 5-HT7 receptor activation. The high expression of 5-HT7 receptors in Type I and II BNSTALG neurons suggest cell-specific activation of these receptors likely play a role in modulating these behavioral effects.
Other 5-HT receptor subtypes
Given our previous data (Levita et al., 2004), it was not surprising that receptors of the 5-HT1 family do not appear to mediate the excitatory response to 5-HT in BNSTALG neurons. Moreover, although we demonstrated the presence of 5-HT3 receptor transcript in BNSTALG tissue, the response elicited by this ionotropic receptor is rapid and desensitizes rapidly (van Hooft and Vijverberg, 1997), and hence was unlikely to mediate the slow onset depolarizing response to 5-HT. Consistent with this hypothesis the 5-HT3 receptor agonist mCPBG did not mimic the excitatory response in any neuron tested. We also confirmed that moderate levels of 5-HT4 receptors are expressed in the BNSTALG (Waeber et al., 1994). However, no effect of the 5-HT4 agonist, cisapride, was observed in the present study. Because some combination of 5-HT2A, 5-HT2C, and/or 5-HT7 antagonists were able to block the entire excitatory response to 5-HT in all BNSTALG neurons, it is likely that only these three 5-HT receptor subtypes mediate the slow membrane depolarization in BNSTALG neurons.
Expression of 5-HT receptor subtypes in single neurons
First, no 5-HT receptor mRNA was detected in 18% of the BNSTALG neurons tested, which was similar to the percentage of neurons that failed to respond to 5-HT in our electrophysiological studies (21%). Second, the expression patterns of 5-HT receptor subtypes in single BNSTALG neurons was consistent with the pharmacological response profiles that we observed in our electrophysiological studies, such that some neurons only expressed 5-HT1A mRNA (consistent with 5-HTHyp), some neurons co-expressed 5-HT1A mRNA with 5-HT2A or 5-HT7 (consistent with 5-HTHyp-Dep), and some neurons expressed only 5-HT2A, 5-HT2C, or 5-HT7 mRNA, or co-expressed 5-HT2A/2C, 5-HT2A/7 mRNA (consistent with 5-HTDep). Third, the distribution of 5-HT responses predicted by the receptor mRNA expression profile did not statistically differ from the observed distribution of 5-HT responses in BNSTALG neurons. Hence, converging evidence suggested that most BNSTALG neurons expressed more than one 5-HT receptor subtype, with 5-HT7 receptors and 5-HT1A receptors being the most prevalent.
Neither the observed 5-HT response distributions, nor the expression of 5-HT receptor subtypes were uniformly distributed across the three BNSTALG cell types. For example, Type III neurons expressed a higher percentage of 5-HTNR, which corresponded to a higher percentage of cells that did not express any 5-HT receptor subtype. While the observed 5-HT receptor subtype mRNA distribution was consistent with the observed 5-HT response in the Type II neurons, some discrepancy was observed between these two measures in type I and type III neurons (Figure 8). This inconsistency in type I and type III was likely due to the smaller sample size obtained for these BNSTALG cell types. Type II neurons are the most prevalent cell type in BNSTALG (Hammack et al. 2007); hence, more observations were made in this cell type‥
While there are limitations to all of the techniques used in this study, the convergence of pharmacological, electrophysiological and molecular data strongly support the conclusion that the complex interactions of multiple 5-HT receptor subtypes, including 5-HT1A, 5-HT2A, 5-HT2C, and 5-HT7 receptors, modulate BNSTALG activity, and likely play an important role in modulating anxiety-like behavior.
The significance of opposing 5-HT actions
Multiple 5-HT receptor subtypes that have functionally opposing responses have been shown to colocalize on single neurons in other regions of the central nervous system (Roychowdhury et al., 1994, Martin-Ruiz et al., 2001, Zhang et al., 2004). The function of having multiple opposing 5-HT responses in single neurons has been postulated by several investigators. Araneda and Andrade (1991) have suggested that 1) multiple receptors on the same neuron could be targeted by different populations of serotonergic fibers and 2) multiple responses could act to “fine tune” the ability of 5-HT to potentiate excitatory stimulation, perhaps by increasing the signal-to-noise ratio of small and large input (Araneda and Andrade, 1991). Uphouse (1997) proposed that multiple 5-HT receptors on the same neurons increases the variety of signals that can be produced by 5-HT, so that in multiple receptor systems the pattern of the signal, and not just signal strength, are critical in determining the effect of transmission (Uphouse, 1997). Lastly, interactions between the effector systems of different 5-HT receptor subtypes can interact (Uphouse, 1997, Berg et al., 1998) and change the response of each receptor to subsequent agonist application.
We have proposed that the capacity for BNSTALG neurons to respond to 5-HT with both inhibition and excitation might help explain some of the inconsistencies regarding the role of 5-HT in anxiety-like responding (Hammack et al., 2009). Many treatments that increase 5-HT levels have been shown to both increase and decrease anxiety and anxiety-like behaviors (see (Handley et al., 1993, Handley, 1995, Graeff et al., 1996, Lowry et al., 2005, Lowry et al., 2008) for review), and we have suggested that these effects are mediated, in part, by the excitation and/or inhibition of BNSTALG neurons by 5-HT. If BNSTALG activity mediates anxiety-like behavior, then the net effect of 5-HT release within this region will depend on multiple factors, including the extracellular concentration, the affinity of each 5-HT receptor subtype, the balance of receptor subtypes, and the potential interactions between receptor subtypes. Because we have shown that the inhibitory response to 5-HT predominates in naïve animals in most BNSTALG neurons, we have proposed that the normal function of 5-HT release in the BNSTALG is to dampen neuronal activity following exposure to threatening stimuli to prevent an over-activation of this anxiety-related circuitry (Hammack et al., 2009).
However, treatments such as chronic stress or the chronic administration of stress hormones have been shown to decrease 5-HT1A and/or increase 5-HT2A receptors in other brain regions (Kuroda et al., 1992, Ferretti et al., 1995, McKittrick et al., 1995, Crayton et al., 1996, Fernandes et al., 1997, Takao et al., 1997, Lopez et al., 1998, Lanfumey et al., 1999, Lopez et al., 1999, Maines et al., 1999, Katagiri et al., 2001, Ossowska et al., 2001). A reduction in BNSTALG 5-HT1A receptor activity and/or and increase 5-HT2A receptor activity would favor an anxiogenic role of BNSTALG 5-HT release, and could disrupt the normally anxiolytic role of 5-HT and produce a behavioral state characterized by pathological anxiety. Further studies are necessary to clarify the specific function of 5-HT receptors in subpopulations of BNSTALG neurons, as well as its influence on the network properties of the BNSTALG.
Summary
In summary, BNSTALG neurons can respond to 5-HT with a complex response pattern. 5-HT1A receptors mediate the inhibitory component of 5-HTHyp-Dep response, whereas the excitatory component of 5-HTHyp-Dep and 5-HTDep response can be mediated by 5-HT2A, 5-HT2C, or 5-HT7 receptors. The presence of multiple 5-HT receptors on single BNSTALG neurons permits 5-HT to exhibit a much greater variety of signaling than one receptor would allow; furthermore, the net effect on anxiety-like behaviors might depend on the net interaction between the inhibitory and excitatory responses, and exposure to stress or stress hormones or therapeutic pharmacological agents could alter the balance of BNSTALG 5-HT receptors to favor excitation or inhibition, respectively, and this change could underlie the mechanisms by which chronic stress produces anxiety-like behavioral pathologies, as well as the mechanism for therapeutic action of many anxiolytic drugs.
Figure 7.
Comparison of 5-HT receptor mRNA expression and actual 5-HT responses in BNSTALG neurons. Clear bars showed the theoretical percentage of 5-HT response predicted by the expression of 5-HT receptor subtype mRNA in 82 neurons screened. Grey bar is the distribution of actual 5-HT responses from 148 neurons recorded through whole cell patch clamp. There is no statistical difference between theoretical percentages and actual response percentages (Chi Square test, χ2 = 0.08, df = 1, p = 0.78).
Acknowledgements
This work was supported by National Institute of Mental Health Grants MH-072908 to D. G. Rainnie and MH-072088 to S. E. Hammack; Science and Technology Centers Integrative Partnership Program of the National Science Foundation (The Center for Behavioral Neuroscience) Grant IBN-987675, Yerkes National Primate Research Center Base Grant RR-00165 awarded by the Animal Resources Program of National Institutes of Health.
Abbreviations
- 5-CT
5-carboxamidotryptamine
- 5-HT
5-hydroxytryptamine, serotonin
- 5-HT1A
serotonin 1A receptor
- 5-HT1B
serotonin 1B receptor
- 5-HT1D
serotonin 1D receptor
- 5-HT1E
serotonin 1E receptor
- 5-HT1F
serotonin 1F receptor
- 5-HT2A
serotonin 2A receptor
- 5-HT2B
serotonin 2B receptor
- 5-HT2C
serotonin 2C receptor
- 5-HT3
serotonin 3 receptor
- 5-HT4
serotonin 4 receptor
- 5-HT5
serotonin 5 receptor
- 5-HT6
serotonin 6 receptor
- 5-HT7
serotonin 7 receptor
- 5-HTDep
5-HT induced depolarization
- 5-HTHyp
5-HT induced hyperpolarization
- 5-HTHyp-Dep
5-HT induced hyperpolarization followed by depolarization
- 5-HTNR
no response to 5-HT
- ACSF
artificial cerebrospinal fluid
- BNST
bed nucleus of stria terminalis
- BNSTALG
anterolateral bed nucleus of the stria terminalis
- E5-CT
reversal potential of 5-CT response
- E5-HT
reversal potential of 5-HT response
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- PCR
polymerase chain reaction
- RT-PCR
reverse transcriptase polymerase chain reaction
- SSRI
selective serotonin reuptake inhibitors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998a;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Research. 1998b;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47 Suppl 1:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. International Journal of Neuropsychopharmacology. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Ballaz SJ, Akil H, Watson SJ. The 5-HT7 receptor: role in novel object discrimination and relation to novelty-seeking behavior. Neuroscience. 2007;149:192–202. doi: 10.1016/j.neuroscience.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Hill TE, Lucki I. Anxiolytic effect of serotonin depletion in the novelty-induced hypophagia test. Psychopharmacology. 2007;190:531–540. doi: 10.1007/s00213-006-0615-9. [DOI] [PubMed] [Google Scholar]
- Beique JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. Journal of Neuroscience. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Clarke WP. Interactions between effectors linked to serotonin receptors. Annals of the New York Academy of Sciences. 1998;861:111–120. doi: 10.1111/j.1749-6632.1998.tb10181.x. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM. RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Ding J, Sciamanna G, Ulrich S, Tscherter A, Bernardi G, Surmeier DJ, Pisani A. Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT 2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology. 2007;32:1840–1854. doi: 10.1038/sj.npp.1301294. [DOI] [PubMed] [Google Scholar]
- Broocks A, Meyer T, Gleiter CH, Hillmer-Vogel U, George A, Bartmann U, Bandelow B. Effect of aerobic exercise on behavioral and neuroendocrine responses to meta-chlorophenylpiperazine and to ipsapirone in untrained healthy subjects. Psychopharmacology. 2001;155:234–241. doi: 10.1007/s002130100706. [DOI] [PubMed] [Google Scholar]
- Brutus M, Zuabi S, Siegel A. Effects of D-Ala2-Met5-enkephalinamide microinjections placed into the bed nucleus of the stria terminalis upon affective defense behavior in the cat. Brain Research. 1988;473:147–152. doi: 10.1016/0006-8993(88)90326-5. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Mar LP, Vysokanov AV, Arnold PB, Cardenas LM, Surmeier DJ, Scroggs RS. Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. Journal of Physiology. 1999;518:507–523. doi: 10.1111/j.1469-7793.1999.0507p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27:207–212. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Baudrie V, Coupry I. Behavioural and biochemical evidence that glucocorticoids are not involved in DOI-elicited 5-HT2 receptor down-regulation. European Journal of Pharmacology. 1993;249:117–120. doi: 10.1016/0014-2999(93)90670-d. [DOI] [PubMed] [Google Scholar]
- Chapin EM, Andrade R. A 5-HT(7) receptor-mediated depolarization in the anterodorsal thalamus. I. Pharmacological characterization. Journal of Pharmacology & Experimental Therapeutics. 2001;297:395–402. [PubMed] [Google Scholar]
- Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- Chung KK, Martinez M, Herbert J. Central serotonin depletion modulates the behavioural, endocrine and physiological responses to repeated social stress and subsequent c-fos expression in the brains of male rats. Neuroscience. 1999;92:613–625. doi: 10.1016/s0306-4522(99)00028-7. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphelimbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Craven RM, Grahame-Smith DG, Newberry NR. 5-HT1A and 5-HT2 receptors differentially regulate the excitability of 5-HT-containing neurones of the guinea pig dorsal raphe nucleus in vitro. Brain Research. 2001;899:159–168. doi: 10.1016/s0006-8993(01)02221-1. [DOI] [PubMed] [Google Scholar]
- Crayton JW, Joshi I, Gulati A, Arora RC, Wolf WA. Effect of corticosterone on serotonin and catecholamine receptors and uptake sites in rat frontal cortex. Brain Research. 1996;728:260–262. doi: 10.1016/0006-8993(96)00189-8. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. Journal of Comparative Neurology. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JF. The role of serotonin in panic, anxiety and depression. Int Clin Psychopharmacol. 1998;13 Suppl 4:S1–S5. doi: 10.1097/00004850-199804004-00001. [DOI] [PubMed] [Google Scholar]
- Dilts RP, Boadle-Biber MC. Differential activation of the 5-hydroxytryptamine-containing neurons of the midbrain raphe of the rat in response to randomly presented inescapable sound. Neuroscience Letters. 1995;199:78–80. doi: 10.1016/0304-3940(95)12027-2. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, Maiz J, Williams JT. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153:232–239. doi: 10.1016/j.neuroscience.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Johnson KB, Breese GR. Topographic patterns of brain activity in response to swim stress: assessment by 2-deoxyglucose uptake and expression of Fos-like immunoreactivity. Journal of Neuroscience. 1993;13:3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JD, Williams TJ. Cardiovascular responses to electrical stimulation of the bed nucleus of the stria terminalis. Journal of Comparative Neurology. 1995;352:227–234. doi: 10.1002/cne.903520206. [DOI] [PubMed] [Google Scholar]
- Egli RE, Winder DG. Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. Journal of Neurophysiology. 2003;90:405–414. doi: 10.1152/jn.00228.2003. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. Journal of Neuroscience. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, McKittrick CR, File SE, McEwen BS. Decreased 5-HT1A and increased 5-HT2A receptor binding after chronic corticosterone associated with a behavioural indication of depression but not anxiety. Psychoneuroendocrinology. 1997;22:477–491. doi: 10.1016/s0306-4530(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Ferretti C, Blengio M, Gamalero SR, Ghi P. Biochemical and behavioral changes induced by acute stress in a chronic variate stress model of depressioin: the effect of amitriptyline. Eur J Pharmacol. 1995;280:19–26. doi: 10.1016/0014-2999(95)00172-h. [DOI] [PubMed] [Google Scholar]
- Fillion G, Beaudoin D, Fillion MP, Rousselle JC, Robaut C, Netter Y. 5-Hydroxytryptamine receptors in neurones and glia. J Neural Transm Suppl. 1983;18:307–317. [PubMed] [Google Scholar]
- Freedman LJ, Shi C. Monoaminergic innervation of the macaque extended amygdala. Neuroscience. 2001;104:1067–1084. doi: 10.1016/s0306-4522(01)00157-9. [DOI] [PubMed] [Google Scholar]
- Funada M, Hara C. Differential effects of psychological stress on activation of the 5-hydroxytryptamine- and dopamine-containing neurons in the brain of freely moving rats. Brain Research. 2001;901:247–251. doi: 10.1016/s0006-8993(01)02160-6. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacology, Biochemistry & Behavior. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, Bromidge F, Owens AP, Huscroft I, Myers J, Rupniak NM, Patel S, Whiting PJ, Hutson PH, Fone KC, Biello SM, Kulagowski JJ, McAllister G. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Guo J, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: Implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009 doi: 10.1016/j.pnpbp.2009.05.013. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98:638–656. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behavioral Neuroscience. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Handley SL. 5-Hydroxytryptamine pathways in anxiety and its treatment. Pharmacol Ther. 1995;66:103–148. doi: 10.1016/0163-7258(95)00004-z. [DOI] [PubMed] [Google Scholar]
- Handley SL, McBlane JW, Critchley MA, Njung'e K. Multiple serotonin mechanisms in animal models of anxiety: environmental, emotional and cognitive factors. Behavioural Brain Research. 1993;58:203–210. doi: 10.1016/0166-4328(93)90104-x. [DOI] [PubMed] [Google Scholar]
- Hardy A, Palouzier-Paulignan B, Duchamp A, Royet JP, Duchamp-Viret P. 5-Hydroxytryptamine action in the rat olfactory bulb: in vitro electrophysiological patch-clamp recordings of juxtaglomerular and mitral cells. Neuroscience. 2005;131:717–731. doi: 10.1016/j.neuroscience.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Sutcliffe JG. The 5-HT7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neuroscience Letters. 2007;414:247–251. doi: 10.1016/j.neulet.2006.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann DE, Szot P, Kohen R, Hamblin MW. Function and distribution of three rat 5-hydroxytryptamine7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology. 1998;37:1621–1632. doi: 10.1016/s0028-3908(98)00070-7. [DOI] [PubMed] [Google Scholar]
- Ho SS, Chow BK, Yung WH. Serotonin increases the excitability of the hypothalamic paraventricular nucleus magnocellular neurons. European Journal of Neuroscience. 2007;25:2991–3000. doi: 10.1111/j.1460-9568.2007.05547.x. [DOI] [PubMed] [Google Scholar]
- Hohmann CF, Walker EM, Boylan CB, Blue ME. Neonatal serotonin depletion alters behavioral responses to spatial change and novelty. Brain Research. 2007;1139:163–177. doi: 10.1016/j.brainres.2006.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacology, Biochemistry & Behavior. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Huang J, Spier AD, Pickel VM. 5-HT3A receptor subunits in the rat medial nucleus of the solitary tract: subcellular distribution and relation to the serotonin transporter. Brain Research. 2004;1028:156–169. doi: 10.1016/j.brainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behavioral Neuroscience. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. Journal of Comparative Neurology. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. Journal of Comparative Neurology. 1989;280:603–621. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Katagiri H, Kagaya A, Nakae S, Morinobu S, Yamawaki S. Modulation of serotonin2A receptor function in rats after repeated treatment with dexamethasone and L-type calcium channel antagonist nimodipine. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1269–1281. doi: 10.1016/s0278-5846(01)00179-8. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Whitton P, Shah K, Curzon G. Anxiogenic-like effects of mCPP and TFMPP in animal models are opposed by 5-HT1C receptor antagonists. European Journal of Pharmacology. 1989;164:445–454. doi: 10.1016/0014-2999(89)90252-5. [DOI] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Mikuni M, Ogawa T, Takahashi K. Effect of ACTH, adrenalectomy and the combination treatment on the density of 5-HT2 receptor binding sites in neocortex of rat forebrain and 5-HT2 receptor-mediated wet-dog shake behaviors. Psychopharmacology (Berl) 1992;108:27–32. doi: 10.1007/BF02245281. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Pardon MC, Laaris N, Joubert C, Hanoun N, Hamon M, Cohen-Salmon C. 5-HT1A autoreceptor desensitization by chronic ultramild stress in mice. Neuroreport. 1999;10:3369–3374. doi: 10.1097/00001756-199911080-00021. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. Juxtacapsular nucleus of the stria terminalis of the adult rat: extrinsic inputs, cell types, and neuronal modules: a combined Golgi and electron microscopic study. Journal of Comparative Neurology. 2004;475:220–237. doi: 10.1002/cne.20185. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. Journal of Neuroscience. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie RA, Moorman JM, Grahame-Smith DG. Lithium enhances 5-HT2A receptor-mediated c-fos expression in rat cerebral cortex. Neuroreport. 1993;5:241–244. doi: 10.1097/00001756-199312000-00014. [DOI] [PubMed] [Google Scholar]
- Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG. 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience. 2004;128:583–596. doi: 10.1016/j.neuroscience.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Lino-de-Oliveira C, Sales AJ, Del Bel EA, Silveira MC, Guimaraes FS. Effects of acute and chronic fluoxetine treatments on restraint stress-induced Fos expression. Brain Research Bulletin. 2001;55:747–754. doi: 10.1016/s0361-9230(01)00566-4. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. Regulation of 5-HT1a receptor, glucocorticoid and mineralocorticoid receptor in rat and human hippocampus: Implications fo the neurobiology of depression. Biol Psychiatry. 1998;43:543–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Liberzon I, Vazquez DM, Young EA, Watson SJ. Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biological Psychiatry. 1999;45:934–937. doi: 10.1016/s0006-3223(98)00224-8. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. Journal of Neuroendocrinology. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Annals of the New York Academy of Sciences. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. Journal of Neuroscience. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. Serotonin receptor specificity in anxiety disorders. J Clin Psychiatry. 1996;57 Suppl 6:5–10. [PubMed] [Google Scholar]
- Mackowiak M, Chocyk A, Fijal K, Czyrak A, Wedzony K. c-Fos proteins, induced by the serotonin receptor agonist DOI, are not expressed in 5-HT2A positive cortical neurons. Brain Research Molecular Brain Research. 1999;71:358–363. doi: 10.1016/s0169-328x(99)00195-3. [DOI] [PubMed] [Google Scholar]
- Maines LW, Keck BJ, Smith JE, Lakoski JM. Corticosterone regulation of serotonin transporter and 5-HT1A receptor expression in the aging brain. Synapse. 1999;32:58–66. doi: 10.1002/(SICI)1098-2396(199904)32:1<58::AID-SYN8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. Journal of Neuroscience. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. European Journal of Neuroscience. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Wang Z. Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. Journal of Physiology. 1991;442:235–255. doi: 10.1113/jphysiol.1991.sp018791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the bed nucleus of the stria terminalis: a golgi study in the rat. Brain Res Bull. 1983;10:111–120. doi: 10.1016/0361-9230(83)90082-5. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biological Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- Mengod G, Nguyen H, Le H, Waeber C, Lubbert H, Palacios JM. The distribution and cellular localization of the serotonin 1C receptor mRNA in the rodent brain examined by in situ hybridization histochemistry. Comparison with receptor binding distribution. Neuroscience. 1990;35:577–591. doi: 10.1016/0306-4522(90)90330-7. [DOI] [PubMed] [Google Scholar]
- Nandam LS, Jhaveri D, Bartlett P. 5-HT7, neurogenesis and antidepressants: a promising therapeutic axis for treating depression. Clin Exp Pharmacol Physiol. 2007;34:546–551. doi: 10.1111/j.1440-1681.2007.04608.x. [DOI] [PubMed] [Google Scholar]
- Ossowska G, Nowa G, Kata R, Klenk-Majewska B, Danilczuk Z, Zebrowska-Lupina I. Brain monoamine receptors in a chronic unpredictable stress model in rats. J Neural Transm. 2001;108:311–319. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- Patel TD, Zhou FC. Ontogeny of 5-HT1A receptor expression in the developing hippocampus. Brain Research Developmental Brain Research. 2005;157:42–57. doi: 10.1016/j.devbrainres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Research. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Serotonin-CRF interaction in the bed nucleus of the stria terminalis: a light microscopic double-label immunocytochemical analysis. Brain Research Bulletin. 1992;28:943–948. doi: 10.1016/0361-9230(92)90217-l. [DOI] [PubMed] [Google Scholar]
- Pohl R, Yeragani VK, Balon R, Lycaki H. The jitteriness syndrome in panic disorder patients treated with antidepressants.[see comment] J Clin Psychiatry. 1988;49:100–104. [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Research Molecular Brain Research. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Neurons of the bed nucleus of the stria terminalis (BNST). Electrophysiological properties and their response to serotonin. Annals of the New York Academy of Sciences. 1999;877:695–699. doi: 10.1111/j.1749-6632.1999.tb09304.x. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depression & Anxiety. 2000;12 Suppl 1:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rick CE, Stanford IM, Lacey MG. Excitation of rat substantia nigra pars reticulata neurons by 5-hydroxytryptamine in vitro: evidence for a direct action mediated by 5-hydroxytryptamine2C receptors. Neuroscience. 1995;69:903–913. doi: 10.1016/0306-4522(95)00283-o. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, Hedlund PB. Mice lacking 5-HT receptors show specific impairments in contextual learning. European Journal of Neuroscience. 2004;19:1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, Haas H, Anderson EG. 5-HT1A and 5-HT4 receptor colocalization on hippocampal pyramidal cells. Neuropharmacology. 1994;33:551–557. doi: 10.1016/0028-3908(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Verge D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: Immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1998;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- Schulz D, Aksoy A, Canbeyli R. Lesion of the bed nucleus of the stria terminalis enhances learned despair. Pharmacology, Biochemistry & Behavior. 2002;71:341–344. [Google Scholar]
- Scruggs JL, Patel S, Bubser M, Deutch AY. DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. Journal of Neuroscience. 2000;20:8846–8852. doi: 10.1523/JNEUROSCI.20-23-08846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh MB, Brutus M, Siegel HE, Siegel A. Regulation of feline aggression by the bed nucleus of stria terminalis. Brain Research Bulletin. 1986;16:179–182. doi: 10.1016/0361-9230(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Rue JE, Franklin M, Hallis K, Camplin G, Laver D, Cowen PJ. The effects of administration of mCPP on psychological, cognitive, cardiovascular, hormonal and MHPG measurements in human volunteers. Int Clin Psychopharmacol. 1994;9:173–178. doi: 10.1097/00004850-199409000-00005. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biological Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Engel JA. Serotonergic involvement in conflict behaviour. Eur Neuropsychopharmacol. 1990;1:7–13. doi: 10.1016/0924-977x(90)90004-t. [DOI] [PubMed] [Google Scholar]
- Stevens DR, McCarley RW, Greene RW. Serotonin1 and serotonin2 receptors hyperpolarize and depolarize separate populations of medial pontine reticular formation neurons in vitro. Neuroscience. 1992;47:545–553. doi: 10.1016/0306-4522(92)90164-w. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Summers CH, Kampshoff JL, Ronan PJ, Lowry CA, Prestbo AA, Korzan WJ, Renner KJ. Monoaminergic activity in subregions of raphe nuclei elicited by prior stress and the neuropeptide corticotropin-releasing factor. Journal of Neuroendocrinology. 2003;15:1122–1133. doi: 10.1111/j.1365-2826.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- Takao K, Nagatani T, Kitamura Y, Yamawaki S. Effects of corticosterone on 5-HT1A and 5-HT2 receptor binding and on the receptor-mediated behavioral responses of rats. Eur J Pharmacol. 1997;333:123–128. doi: 10.1016/s0014-2999(97)01126-6. [DOI] [PubMed] [Google Scholar]
- Takase LF, Nogueira MI, Baratta M, Bland ST, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Inescapable shock activates serotonergic neurons in all raphe nuclei of rat. Behavioural Brain Research. 2004;153:233–239. doi: 10.1016/j.bbr.2003.12.020. [DOI] [PubMed] [Google Scholar]
- To ZP, Bonhaus DW, Eglen RM, Jakeman LB. Characterization and distribution of putative 5-HT7 receptors in guinea-pig brain. Br J Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski K, Zahorodna A, Bobula B, Hess G. 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Research. 2003;993:230–234. doi: 10.1016/j.brainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Blumenfeld B, Wu C, Luo J, Attali B, Goodman P, Markram H. Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb Cortex. 2004;14:1310–1327. doi: 10.1093/cercor/bhh092. [DOI] [PubMed] [Google Scholar]
- Uphouse L. Multiple serotonin receptors: too many, not enough, or just the right number? Neurosci Biobehav Rev. 1997;21:679–698. doi: 10.1016/s0149-7634(96)00022-x. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Javed A, Zhang Y, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. Journal of Neuroscience. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooft JA, Vijverberg HP. Full and partial agonists induce distinct desensitized states of the 5-HT3 receptor. J Recept Signal Transduct Res. 1997;17:233–239. doi: 10.3109/10799899709036608. [DOI] [PubMed] [Google Scholar]
- Vilaro MT, Cortes R, Mengod G. Serotonin 5-HT4 receptors and their mRNAs in rat and guinea pig brain: distribution and effects of neurotoxic lesions. Journal of Comparative Neurology. 2005;484:418–439. doi: 10.1002/cne.20447. [DOI] [PubMed] [Google Scholar]
- Waeber C, Moskowitz MA. [3H]sumatriptan labels both 5-HT1D and 5-HT1F receptor binding sites in the guinea pig brain: an autoradiographic study. Naunyn-Schmiedeberg's Arch Pharmacol. 1995;352:263–275. doi: 10.1007/BF00168556. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Nieoullon A, Bockaert J, Dumuis A. Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology. 1994;33:527–541. doi: 10.1016/0028-3908(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Walker DL, Cassella JV, Lee Y, De Lima TC, Davis M. Opposing roles of the amygdala and dorsolateral periaqueductal gray in fear-potentiated startle. Neuroscience & Biobehavioral Reviews. 1997;21:743–753. doi: 10.1016/s0149-7634(96)00061-9. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Mai JK, Lanta L, Gorcs T. Differential distribution of immunohistochemical markers in the bed nucleus of the stria terminalis in the human brain. Journal of Chemical Neuroanatomy. 1991;4:281–298. doi: 10.1016/0891-0618(91)90019-9. [DOI] [PubMed] [Google Scholar]
- Ward RP, Dorsa DM. Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. Journal of Comparative Neurology. 1996;370:405–414. doi: 10.1002/(SICI)1096-9861(19960701)370:3<405::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Weber M, Schmitt A, Wischmeyer E, Doring F. Excitability of pontine startle processing neurones is regulated by the two-pore-domain K+ channel TASK-3 coupled to 5-HT2C receptors. European Journal of Neuroscience. 2008;28:931–940. doi: 10.1111/j.1460-9568.2008.06400.x. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. 2006;51:578–586. doi: 10.1016/j.neuropharm.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Wang L, Kitai ST. Modulation of spontaneous firing in rat subthalamic neurons by 5-HT receptor subtypes. Journal of Neurophysiology. 2005;93:1145–1157. doi: 10.1152/jn.00561.2004. [DOI] [PubMed] [Google Scholar]
- Xu T, Pandey SC. Cellular localization of serotonin(2A) (5HT(2A)) receptors in the rat brain. Brain Research Bulletin. 2000;51:499–505. doi: 10.1016/s0361-9230(99)00278-6. [DOI] [PubMed] [Google Scholar]
- Yang J, Mathie A, Hille B. 5-HT3 receptor channels in dissociated rat superior cervical ganglion neurons. Journal of Physiology. 1992;448:237–256. doi: 10.1113/jphysiol.1992.sp019039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gray TS, D'Souza DN, Carrasco GA, Damjanoska KJ, Dudas B, Garcia F, Zainelli GM, Sullivan Hanley NR, Battaglia G, Muma NA, Van de Kar LD. Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. Journal of Pharmacology & Experimental Therapeutics. 2004;310:59–66. doi: 10.1124/jpet.103.062224. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. Journal of Neurophysiology. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]