Abstract
CD14+ peripheral blood monocytes can differentiate into fibroblast-like cells called fibrocytes. Fibrocytes are associated with, and are at least partially responsible for, wound healing and fibrosis in multiple organ systems. In a variety of lesions in vivo, monocytes appear to differentiate into fibrocytes within days. However, in vitro culture conditions can take up to two weeks to generate fibrocytes. In this study, we describe enhanced serum-free conditions that support the rapid differentiation of human and murine fibrocytes. We compared the effect on fibrocyte differentiation of different anti-coagulants used when collecting blood, and for culturing cells, the effects of different commercial media formulations, the addition of a variety of supplements, cell density, conditioned medium, and glass and plastic substrates. We found that both heparin and EDTA were suitable anti-coagulants, but that blood treated with citrate-phosphate dextrose led to a reduced number of fibrocytes. Fibrocyte differentiation was enhanced when the serum-free medium was based on either FibroLife or StemPro formulations. We also found that only positively charged or hydrophilic glass and plastic surfaces provide adequate support for fibrocyte differentiation. Finally, the optimal cell density was 2.5 × 105 cells/ml (approximately 800 cells per mm2). These results indicate that blood collection, substrates, media, and cell density all influence in vitro fibrocyte differentiation.
Keywords: Fibrocytes, Monocytes, serum-free culture, anti-coagulants, cell density, substrates
1. Introduction
Fibrocytes are spindle-shaped fibroblast-like cells that are involved in both tissue repair and fibrosis (Bucala et al., 1994; Abe et al., 2001; Quan et al., 2004; Bellini and Mattoli, 2007; Gomperts and Strieter, 2007; Wang et al., 2007b). Fibrocytes appear to differentiate from a subpopulation of CD14 positive peripheral blood monocytes, and express markers of both hematopoietic cells (CD34, CD45, LSP-1, and MHC class II) and stromal cells (collagen and proly-4-hydroxylase) (Bucala et al., 1994; Abe et al., 2001; Yang et al., 2002; Pilling et al., 2003; Pilling et al., 2006). Fibrocytes appear to have both beneficial and detrimental effects on health. Fibrocytes can stimulate innate and adaptive immune responses (Chesney et al., 1997; Chesney et al., 1998; Abe et al., 2001; Balmelli et al., 2005; Balmelli et al., 2007), and also promote wound healing both directly and also by secreting factors that activate fibroblasts (Yang et al., 2005; Wang et al., 2007b). Fibrocytes are also implicated in chronic inflammation and fibrosis, and have been detected in tumors, hypertrophic scars, bronchial asthma, pulmonary fibrosis, and nephrogenic systemic fibrosis (Schmidt et al., 2003; Quan et al., 2004; Mori et al., 2005; Yang et al., 2005; Mehrad et al., 2007). Fibrocyte differentiation is regulated by a number of factors, including leukotrienes, IFN-α, TGF-β and PPAR-γ agonists (Abe et al., 2001; Hong et al., 2007; Vannella et al., 2007; Wang et al., 2007a). We have shown that fibrocyte differentiation is inhibited by serum amyloid P (SAP) and aggregated IgG, and that cytokines, glucose, insulin, and extracellular matrix proteins can also regulate fibrocyte differentiation (Pilling et al., 2003; Haudek et al., 2006; Pilling et al., 2006; Pilling and Gomer, 2007; Pilling et al., 2007; Haudek et al., 2008; Shao et al., 2008).
A variety of in vitro culture conditions have been used to generate human, murine, and porcine fibrocytes. Many groups follow the original protocols developed by Bucala and colleagues using RPMI- or DMEM-based culture media, supplemented with 10-20% fetal bovine serum (Bucala et al., 1994; Chesney et al., 1997; Abe et al., 2001; Balmelli et al., 2005; Quan and Bucala, 2007). This system involves incubating PBMC for 1-3 days before the removal of the non-adherent cells, and then culturing the remaining adherent cells (Schmidt et al., 2003; Mori et al., 2005; Bellini and Mattoli, 2007; Quan and Bucala, 2007). After 10-14 days, fibrocytes then appear in the cultures.
We observed that human PBMC cultured in serum-free medium gave rise to fibrocytes within 5 days (Pilling et al., 2003; Pilling et al., 2006; Shao et al., 2008). This time-period is closer to the 3-7 days that fibrocytes take to appear in wounds, inflammatory sites, and fibrotic lesions (Bucala et al., 1994; Chesney et al., 1998; Abe et al., 2001; Schmidt et al., 2003; Phillips et al., 2004; Mori et al., 2005; Yang et al., 2005; Haudek et al., 2006; Naik-Mathuria et al., 2008). In this study, we have assessed factors that affect fibrocyte differentiation in serum-free culture.
2. Materials and methods
2.1. Cell culture conditions and fibrocyte differentiation assay
Human peripheral blood was collected from healthy adult volunteers with specific approval of Rice University's Institutional Review Board. Heparin (H1027, 10 units/ml, Sigma-Aldrich, St. Louis, MO), EDTA (2.5 mM, Sigma-Aldrich), or citrate-phosphate dextrose (CPD, C7165, 140 μl/ml, Sigma-Aldrich) were used as anti-coagulants. In some experiments, blood was collected directly into commercially available vacutainer tubes (Heparin #367874, 150 units per tube, 15 units per ml; EDTA # 366643, 18 mg K2 EDTA per tube, 1.8 mg per ml; BD Bioscience; San Diego, CA). Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque Plus (GE Healthcare Biosciences, Piscataway, NJ), following the manufacturers' protocol (Pilling et al., 2003; Pilling et al., 2006). Briefly, blood was diluted with an equal volume of l× PBS (137 mM NaCl, 2.7 mM KC1, 10 mM Na2HPO4, 1.8 mM KH2PO4 pH 7.4; Mediatech, Manassas, VA) and then 12 ml was gently layered over 3 ml of Ficoll-Paque Plus stored at room temperature, and the PBMC were then separated by centrifugation at 400 × g for 40 minutes at room temperature. PBMC were collected from the Ficoll-plasma interface by carefully removing only 1-2 ml of liquid from the interface to reduce contamination with platelets, mixed with 10 ml of PBS, and collected by centrifugation at 300 × g for 10 minutes. The PBMC pellets were then resuspended in 14 ml of PBS, and the cells were again collected by centrifugation. This process was repeated five times. PBMC were then resuspended in either RPMI-1640 (R0883, Sigma-Aldrich,), FibroLife (LM-0001, Lifeline Cell Technology, Walkersville, MD), or StemPro-34 (10640, Invitrogen, Carlsbad, CA) basal media. All three basal media were supplemented with 10 mM HEPES (Sigma-Aldrich), 1 × non-essential amino acids (NEAA, Sigma-Aldrich), 1 mM sodium pyruvate (Sigma-Aldrich), 2 mM glutamine (Invitrogen), 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma-Aldrich), and 1 × ITS-3 (500 μg/ml bovine serum albumin, 10 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenite, 5 μg/ml linoleic acid, and 5 μg/ml oleic acid, Sigma-Aldrich). PBMC were cultured in flat-bottomed 96 well tissue culture plates (type 353072, BD Biosciences) in 200 μl volumes at 2.5 × 105 cells per ml in a humidified incubator containing 5% CO2 at 37°C as described previously (Pilling et al., 2003; Pilling et al., 2006). PBMC were also cultured in eight well “standard” soda lime glass microscope slides (177402, Lab-Tek, Nalge Nunc International, Naperville, IL), Permanox plastic slides (177445, Nalge-Nunc), or CC2 glass slides (154941, Nalge-Nunc). Eight well slides are sterilized using ethylene oxide gas as provided by the manufacturer and have a detachable upper medium chamber which is removed after cell culture, and before staining. Fibrocytes were defined as adherent spindle-shaped cells with an oval nucleus, as described previously (Pilling et al., 2003; Pilling et al., 2006; Shao et al., 2008). After 5 days, PBMC were air dried, fixed in methanol, and stained with eosin and methylene blue (Hema 3 Stain, Fisher Scientific, Middletown, VA). Fibrocytes from duplicate wells were counted in five different 900 μm diameter fields per well. All cultures were counted by at least two independent observers blinded to the experimental design. Intra-observed error has a coefficient of variation of 10.5 ± 1.9%, and inter-observer variation has a coefficient of variation of 16.5 ± 2.4%.
Glucose levels in the basal cell culture media were analyzed using a diabetic glucose meter (Accu-Check Active, Roche Diagnostics, Indianapolis, IN) following the manufacturers instructions.
Murine heparinized blood was collected from C57BL/6 mice by cardiac puncture, with specific approval of Rice University's Institutional Animal Use and Care Committee. Between 500 and 800 μl of blood was collected per mouse, diluted with an equal volume of PBS, and the diluted blood of three mice were combined before isolation. Murine PBMC were isolated by Lympholyte-Mammal (Cedarlane, Burlington, NC) at 800 × g for 20 minutes at room temperature, following the manufacturers' protocol. PBMC were collected from the interface, mixed with 10 ml of PBS, and collected by centrifugation at 300 × g for 10 minutes. PBMC were then resuspended in PBS to 14 ml and collected by centrifugation. This was repeated five times. PBMC were then resuspended in either RPMI or FibroLife basal media, in the presence or absence of 50 μM 2-mercaptoethanol (EMD, San Diego, CA) supplemented with 20 mM HEPES, 2× NEAA, 2 mM sodium pyruvate, 4 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2× ITS-3. PBMC were cultured in 200 μl volumes at 2.5 × 10 cells per ml as described above in a humidified incubator containing 5% CO2 at 37°C for 5 days. Fibrocytes were counted as described above.
2.2. Co-culture conditions and preparation of conditioned medium
PBMC from high and low yield donors were cultured in flat-bottomed 96 well tissue culture plates in 200 μl volumes at the indicated cell density, in the presence of 10% PBMC of low and high yield donors respectively. As an example, 90 μl of 5 × 10 cells per ml of a high-yield donor were mixed with 10 μl of 5 × 10 cells per ml of a low yield donor, and the cells were then cultured in flat-bottomed 96 well tissue culture plates in 200 μl volumes at 2.5 × 105 cells per ml. To prepare conditioned medium, PBMC from high and low yield donors were cultured at 2.5 × 105 cells per ml in FibroLife-based serum-free medium at 37°C for 5 days. Supernatants were then collected, clarified by centrifugation at 10,000 × g for 10 minutes to remove cell debris, and then added to cell cultures at either 20% or 50% final volume.
2.3. Flow Cytometry
Human PBMC in 1.5 ml polypropylene micro-centrifuge tubes were incubated on ice for 30 minutes in 100 μl of ice cold PBS containing 4% BSA (Fraction V, Sigma-Aldrich) (PBS-BSA) with 5 μg/ml primary antibodies. Cells were stained for CD3 (UCHT1, mouse IgG1, BD-Biosciences) to detect T cells, CD14 (clone 61D3, mouse IgG1, Southern Biotechnology, Birmingham, AL) to detect monocytes, CD16 (clone GRM1, mouse IgG2a, Southern Biotechnology) to detect NK cells and monocytes, CD19 (HIB19, mouse IgG1, eBioscience, San Diego, CA) to detect B cells, or 5 μg/ml isotype-matched irrelevant mouse IgG1 or IgG2a monoclonal antibodies (BD-Biosciences). Cells were then washed by the addition of 1.4 ml ice cold PBS and collected by centrifugation at 300 × g for 5 minutes. The cells were then resuspended in 1.5 ml ice cold PBS and collected by centrifugation. Cell pellets were then resuspended in 100 μl in PBS-BSA containing 2.5 μg/ml secondary FITC-conjugated F(ab′)2 goat anti-mouse IgG antibodies (cross-adsorbed against human Ig, Southern Biotechnology) and incubated on ice for 30 minutes. Cells were then washed twice in ice cold PBS, and then resuspended in 200 μl of ice cold PBS-BSA and analyzed by flow cytometry (FACScan, BD-Biosciences), as described previously (Pilling et al., 1989; Akbar et al., 1991; Salmon et al., 1994; Pilling et al., 1996; Salmon et al., 1997; Faint et al., 1999).
2.4. Immunohistochemistry
PBMC cultured on eight well glass microscope slides were air dried for at least 60 minutes before fixation and permeabilization in acetone for 15 minutes. Non-specific binding was blocked by incubation in PBS-BSA for 60 minutes. Slides were incubated with 5 μg/ml primary antibodies in PBS-BSA for 60 minutes. Slides were stained for CD13 (clone WM15, mouse IgG1, BD-Biosciences), CD14 (clone 61D3, mouse IgG1, Southern Biotechnology), CD34 (clone QBend10, mouse IgG1, Beckman Coulter, Fullerton, CA), pan CD45 (clone HI30, mouse IgG1, BD-Biosciences), CD68 (clone Y1/82A, mouse IgG2b, BioLegend, San Diego, CA), CD164 (clone 67D2, mouse IgG1, BioLegend), or prolyl-4-hydroxylase (clone 3-2B12, mouse IgG41, Millipore, Temecula, CA). Collagen-I was stained with rabbit polyclonal antibody (600-401-103, Rockland Inc, Gilbertsville, PA). Isotype-matched irrelevant mouse monoclonal antibodies (BD-Biosciences) or irrelevant rabbit polyclonal antibodies (Jackson ImmunoResearch, West Grove, PA) were used as controls. Slides were then washed in six changes of PBS over 30 minutes and incubated for 30 minutes in PBS-BSA with 2.5 μg/ml biotinylated goat F(ab′)2 anti-mouse IgG or biotinylated goat F(ab′)2 anti-rabbit IgG (both cross-adsorbed against human Ig, Southern Biotechnology). After washing, the biotinylated antibodies were detected by a 1/300 dilution of ExtrAvidin alkaline phosphatase (Sigma-Aldrich) in PBS-BSA. Staining was developed with the Vector Red Alkaline Phosphatase Kit (Vector Laboratories, Burlingame, CA) for 7 minutes. Sections were then counterstained for 10 seconds with Gill's hematoxylin #3 (Sigma-Aldrich) diluted 1:5 with water, rinsed in water, and then treated with Scott's tap water substitute (20 g/L MgSO47H2O; 2 g/L NaHCO3) for 30 seconds. Slides were then dehydrated through 70%, 95%, and 100% ethanol, cleared with xylene, and mounted with VectaMount (Vector Laboratories). All procedures were performed at room temperature.
2.5. Statistics
Statistical analysis was performed using GraphPad Prism software v4.03 (GraphPad Software, San Diego, CA). Differences between two groups were assessed by Student's t-test. Differences between multiple groups were assessed by ANOVA using Tukey's post-test. Significance was defined as p < 0.05. In the figures, * indicates p < 0.05, ** indicates p < 0.01.
3. Results
3.1. Effect of anti-coagulants on fibrocyte differentiation
To assess which reagents and conditions may influence fibrocyte differentiation, we first evaluated whether any of the common anti-coagulants used to prepare human PBMC had an effect on fibrocyte differentiation. Human peripheral blood was collected and then immediately separated into tubes containing either heparin, EDTA, or CPD. Heparin and EDTA are commonly used in laboratories as anti-coagulants, and CPD is in regular use in blood banks to prepare leukopacks. PBMC were isolated and then cultured for 5 days in RPMI-based serum-free medium (SFM). We found that PBMC cultures prepared from CPD-treated blood had significantly fewer fibrocytes, compared to EDTA-treated blood (Figure 1A). We also found that compared to heparin-treated blood, there was an increased number of fibrocytes cultured from EDTA-treated blood, but this was not significant as assessed by ANOVA or t-test (Figure 1 A).
Figure 1. Effect of different anti-coagulants on fibrocyte differentiation.
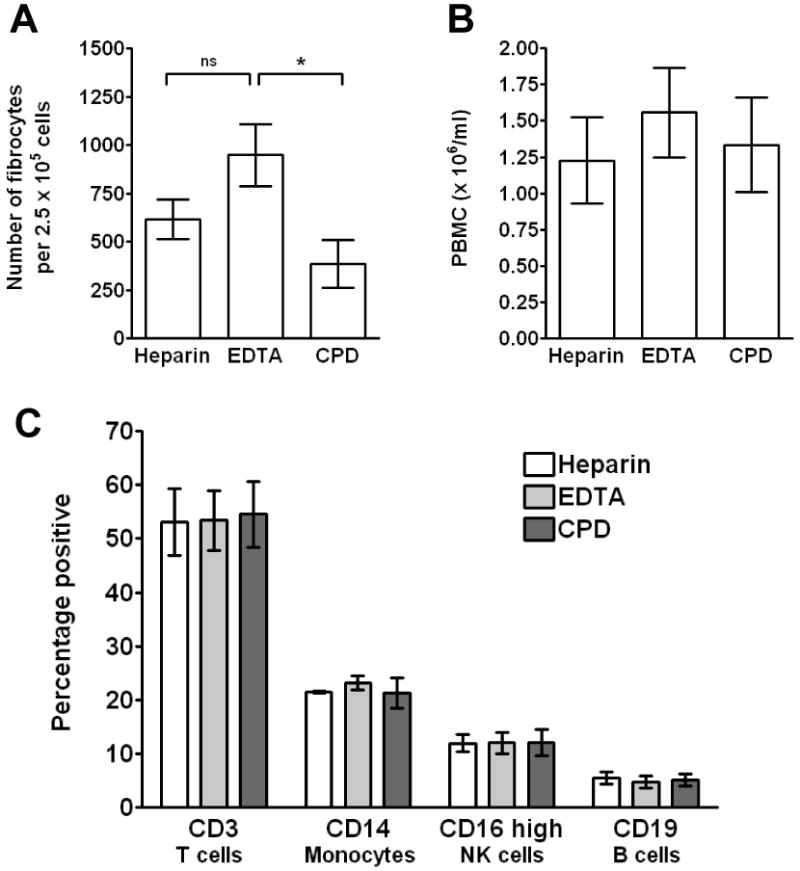
A) PBMC separated from heparin, EDTA, or CPD-treated blood were cultured in RPMI-based serum-free medium for 5 days at 2.5 × 105 cells per ml. Cells were then air-dried, fixed, stained, and fibrocytes were enumerated by morphology. Results are mean ± SEM (n=10 separate donors). B) Total number of PBMC per ml of blood from heparin, EDTA, or CPD treated blood. Results are mean ± SEM (n=11 separate donors) C) PBMC were labeled with monoclonal antibodies for CD3, CD14, CD16, and CD19. Results are mean ± SEM (n=3 separate donors). Statistical significance was determined by ANOVA.
The numbers of fibrocytes differentiating in the three treated blood samples could be due to a loss of cells following treatment with anti-coagulant, or a difference in the number of monocytes or other cell populations isolated from peripheral blood. Following separation over Ficoll there was no significant difference in the total number of PBMC isolated from either heparin, EDTA, or CPD-treated blood (Figure 1B). There was also no apparent difference in the percentage of PBMC subpopulations isolated from either heparin, EDTA, or CPD-treated blood as assessed by flow cytometry (Figure 1C). These data suggest that CPD has an unknown deleterious effect on the ability of monocytes to differentiate into fibrocytes. It is unclear if the effect of CPD is related to the known problem of lactate production by platelets, rather than the sequestration of extracellular calcium, with would also occur with EDTA-treated blood (Gulliksson, 2000). However, heparin and EDTA are satisfactory anti-coagulants to prepare cells for fibrocyte cultures.
3.2. Comparison of basal media on fibrocyte differentiation
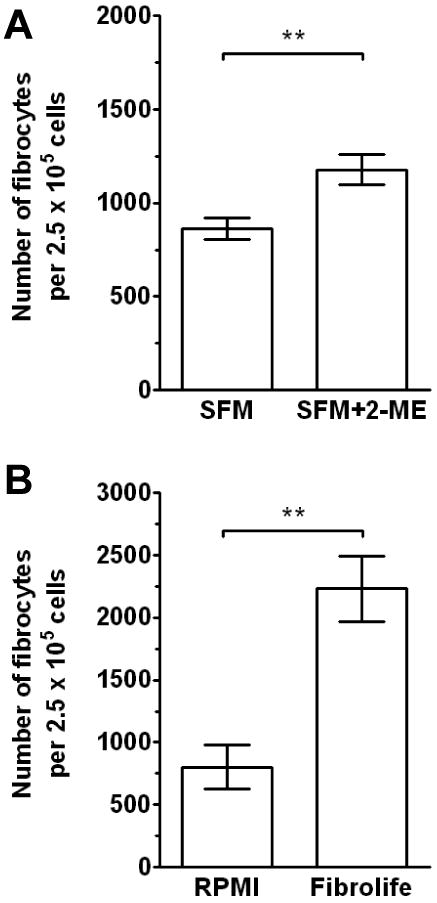
RPMI-1640 and DMEM are the standard basal media used to culture fibrocytes (Bucala et al., 1994; Abe et al., 2001; Quan and Bucala, 2007). Therefore, we next assessed if different basal media would have an effect on fibrocyte differentiation. We compared RPMI with basal media designed for fibroblast (FibroLife) or stem cell (StemPro34) growth. PBMC were cultured for 5 days in RPMI, FibroLife, or StemPro34-based SFM. We found that compared to RPMI, there were increased numbers of fibrocytes in FibroLife and StemPro-based SFM (Figure 2A). We also found that when either FibroLife or StemPro were used as the basal media, the previously observed, but not statistically significant effect of the increased numbers of fibrocytes cultured from EDTA-treated blood was not apparent (Figure 2A). These data indicate that both FibroLife and StemPro are more suitable as basal media for fibrocyte differentiation, although at present, FibroLife is less expensive.
Figure 2. Effect of different basal media and supplements on fibrocyte differentiation.
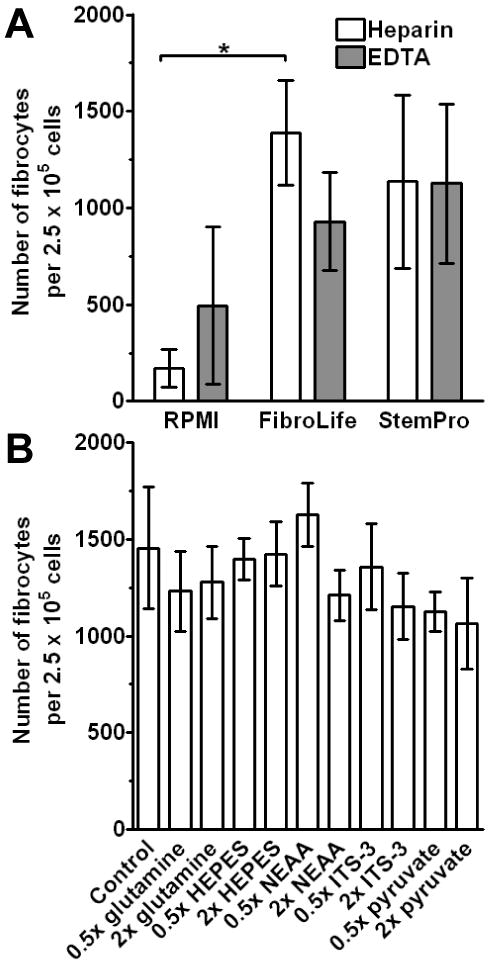
A) PBMC separated from heparin or EDTA-treated blood were cultured in RPMI, FibroLife, or StemPro-based serum-free media for 5 days at 2.5 × 105 cells per ml. Cells were then air-dried, fixed, stained, and fibrocytes were enumerated by morphology. Results are mean ± SEM (n=4 separate donors). B) PBMC were cultured in FibroLife SFM with standard amounts of glutamine, HEPES, NEAA, ITS-3, or pyruvate (control) or 0.5× or 2× concentrations of the indicated supplements. For each experimental condition, the concentration of all the other supplements was the standard concentration. Results are mean ± SEM (n=5 separate donors). Statistical significance was determined by ANOVA.
We have previously shown that glucose, pyruvate, and insulin all affect fibrocyte differentiation (Pilling and Gomer, 2007). To check if FibroLife and StemPro were more potent than RPMI simply due to increased levels of glucose, we assessed glucose levels using a diabetic glucose meter. We found that FibroLife had lower levels of glucose compared to RPMI, and StemPro had higher levels (data not shown). These data indicate that the reason FibroLife and StemPro are more potent than RPMI is not simply due to glucose levels. We also found that the supplements that are supplied with FibroLife medium, especially hydrocortisone, also inhibited fibrocyte differentiation (data not shown). We also halved (0.5×) or doubled (2×) the amounts of glutamine, HEPES, NEAA, pyruvate, and ITS-3 in cultures containing FibroLife as the basal media. We found no significant differences compared to the standard concentrations of these reagents (Figure 2B). This suggests that our standard concentrations of these supplements may be optimal.
We then stained PBMC cultured in RPMI and FibroLife based SFM to assess if these media altered the expression of markers expressed by fibrocytes. Many groups have identified fibrocytes from humans, mice, pigs, quail, rats, and sheep, using general leukocyte markers such as LSP-1 and CD45; myeloid markers such as CD13 and CD172 (SWC3); sialomucins such as CD34, CD68, and CD164; and matrix biosynthesis markers such as collagen-I and prolyl-4-hydroxlase, a key enzyme in collagen synthesis (Bucala et al., 1994; Pilling et al., 2003; Quan et al., 2004; Balmelli et al., 2005; Nagy et al., 2005; Yang et al., 2005; Varcoe et al., 2006; Shao et al., 2008). PBMC were cultured for 5 days on 8-well “standard” soda lime glass microscope slides in either RPMI or FibroLife-based SFM. Cells were then air-dried, fixed and permeabilized in acetone, and labeled with antibodies. Fibrocytes cultured in both RPMI and FibroLife basal media expressed CD13, CD34, CD45, CD68, CD164, prolyl-4-hydroxylase (P-4-H) and collagen-I, and did not express CD14 (Figure 3 and data not shown). There was no apparent effect of the media on the intensity of the antibody staining. These data suggest that the spindle-shaped cells observed in the FibroLife-based media are fibrocytes.
Figure 3. Expression of markers by fibrocytes cultured in RPMI or FibroLife SFM.
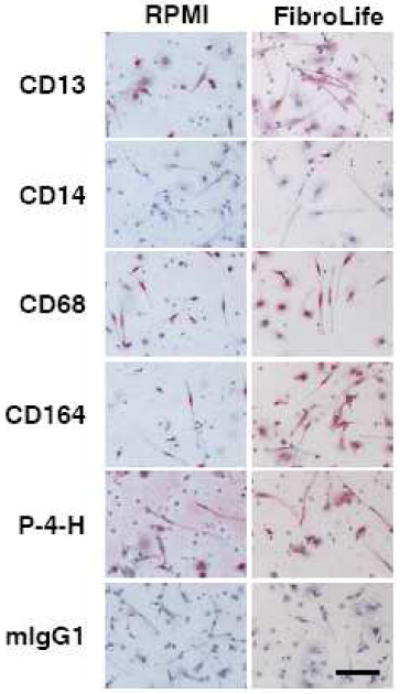
PBMC isolated from heparin treated blood were cultured in RPMI- or FibroLife-based serum-free medium for 5 days at 2.5 × 105 cells per ml on 8 well glass slides. Cells were then air-dried, fixed, and stained with antibodies. Cells were counterstained with hematoxylin to identify nuclei. Positive staining was identified by red staining, with nuclei counterstained blue. Bar is 100 μm. The images are representative of three independent experiments.
3.3. Comparison of glass and plastic substrata on fibrocyte differentiation
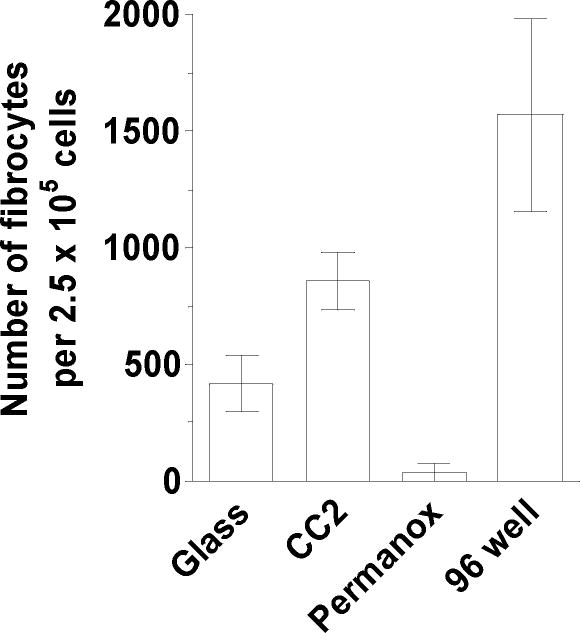
Fibrocytes are generally cultured on tissue culture grade plastic or plastic pre-coated with fibronectin (Quan and Bucala, 2007). Although fibronectin-coated tissue culture plastic does not appear to increase the number of fibrocytes, we and others have observed that fibrocytes cultured on fibronectin are easier to detach than fibrocytes cultured on plastic (Pilling and Gomer, 2007; Quan and Bucala, 2007). We have also previously cultured fibrocytes on soda lime glass and borosilicate glass microscope slides for analysis by immunohistochemistry and video-microscopy (Pilling et al., 2003; Pilling et al., 2006; Shao et al., 2008). Therefore, we assessed whether plastic and glass substrates have a differential effect on fibrocyte differentiation. PBMC from heparin treated blood were cultured for 5 days in FibroLife serum-free medium on 8 well slides made from soda lime glass, plastic slides with increased gaseous exchange (Permanox), or chemically modified glass with a positive charge to promote cell attachment (CC2). We also cultured PBMC in 96 well tissue culture plates. We found that compared to soda lime glass microscope slides, PBMC cultured on Permanox slides had fewer fibrocytes, and CC2 glass slides had more fibrocytes (Figure 4). However, compared to tissue culture plastic, all three microscope slide substrates had less fibrocytes. These data indicate that CC2 glass slides may be a more suitable substrate for fibrocyte differentiation than other microscope slide types, but they are still suboptimal compared to tissue culture plastic. In addition, CC2 glass slides are currently more expensive than soda lime glass microscope slides.
Figure 4. Effect of different substrates on fibrocyte differentiation.
PBMC separated from heparin treated blood were cultured in FibroLife SFM for 5 days at 2.5 × 105 cells per ml on 8-well slides composed of “standard” soda lime glass (glass), positively charged glass (CC2), or plastic (Permanox) substrates. PBMC were similarly cultured in 96 well tissue culture plates (96 well). Results are mean ± SEM (n=3 separate donors).
3.4 Effect of cell density on fibrocyte differentiation
Previously, high cell density has been shown to promote cell survival and differentiation of many cell types (Mehdy and Firtel, 1985; Gomer et al., 1991; Barres et al., 1992; Ishizaki et al., 1995; Pilling et al., 2000). We therefore assessed whether cell density had an effect on fibrocyte differentiation. We cultured human PBMC in FibroLife-based SFM at a range of cell densities from 7.8 × 103 to 1 × 106 cells per ml. We found that PBMC cultured at 2.5 to 5 × 105 cells/ml gave the highest number of PBMC differentiating into fibrocytes (Figure 5). We also observed that when PBMC were cultured at 1 × 106 cells/ml and above the ability to discriminate fibrocytes accurately was difficult due to the large numbers of cells in the wells (data not shown).
Figure 5. Effect of cell density on fibrocyte differentiation.
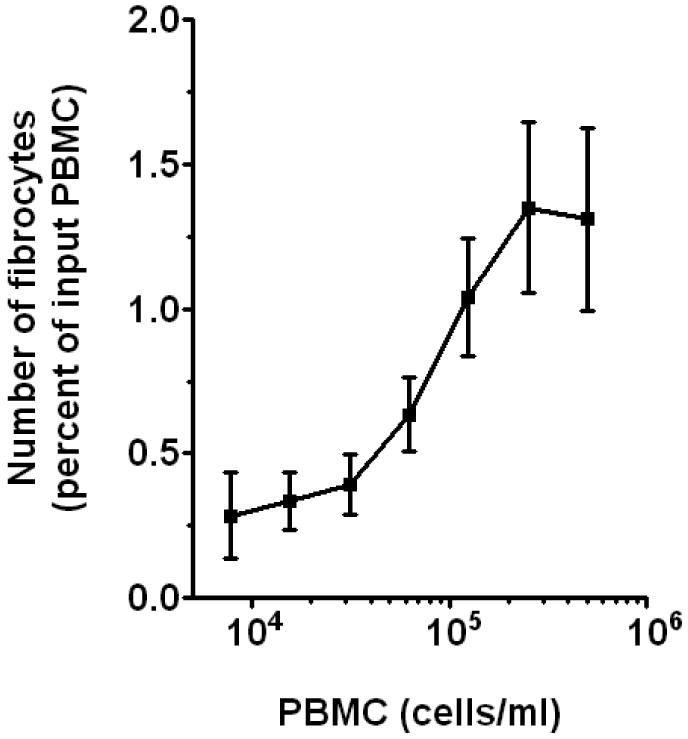
PBMC separated from heparin treated blood were cultured in FibroLife-based SFM for 5 days at a range of cell densities. Cells were then air-dried, fixed, stained, and fibrocytes were enumerated by morphology. Results are mean ± SEM (n=4 separate donors).
3.5 Effect of conditioned medium on fibrocyte differentiation
There is a wide variation in the number of fibrocytes that differentiate from PBMC from healthy individuals, burn victims, and patients with fibrotic or autoimmune diseases (Abe et al., 2001; Postlethwaite et al., 2004; Yang et al., 2005; Pilling et al., 2006; Moeller et al., 2009). In healthy individuals, this variation appears to be in part due to a population of blood donors whose PBMC generate low numbers of fibrocytes when cultured in vitro, and we have observed that this population does not appear to correlate with age, ethnicity, or sex of the donor (data not shown). We defined this population as “low yield donors” if their PBMC generated fibrocytes below the 90% confidence interval of the mean of a population of healthy individuals (data not shown). Conversely, PBMC from “high yield donors” generate fibrocytes above the 90% confidence interval of the mean of a population of healthy individuals (data not shown). To assess if the low yield donors have an intrinsic defect in fibrocyte differentiation, or had reduced levels of soluble factors from other cells within the PBMC population that promote fibrocyte differentiation (Abe et al., 2001; Yang et al., 2002), we cultured PBMC from high and low yield donors with conditioned medium (CM) from high and low yield donor PBMC cultured for 5 days. We found that when PBMC from high donors were cultured in FibroLife-based SFM at a range of cell densities in the presence or absence of 20% CM produced by PBMC from either high or low yield donors, there was no effect on fibrocyte differentiation (Figure 6A). We also found that when PBMC from low yield donors were similarly cultured in the presence or absence of 20% CM produced by PBMC from either high or low yield donors there was also no effect on fibrocyte differentiation (Figure 6B). We also cultured PBMC from high and low yield donors in the presence or absence of 50% CM from high or low yield donors, and again no effect was seen on fibrocyte differentiation (data not shown). These data suggest that the low level of differentiation of fibrocytes observed in PBMC from low yield donors does not appear to be due to either a lack of fibrocyte-differentiation helper factors or the presence of inhibitory factors in these cultures. We also assessed if helper factors were labile or cell contact dependent by culturing PBMC from low yield donors in the presence of 10% PBMC from high yield donors. We found that at a range of cell densities, the presence of 10% PBMC from high yield donors did not affect fibrocyte differentiation from low yield donors (data not shown). We also found that the presence of 10% PBMC from low yield donors did not significantly affect the differentiation of fibrocytes from high yield donors (data not shown). These data suggest that the low numbers of fibrocytes produced by PBMC from some donors may be due to intrinsic factors.
Figure 6. Effect of conditioned medium on fibrocyte differentiation.
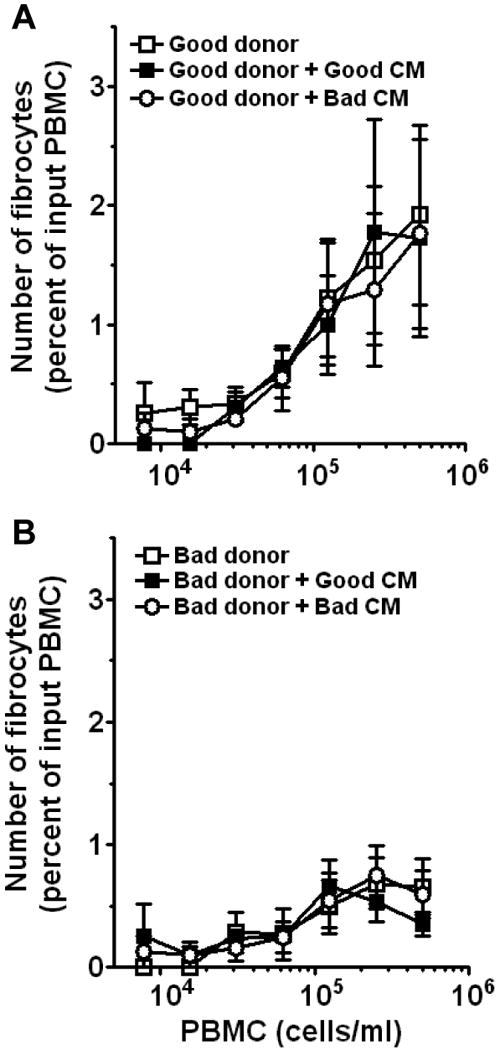
A) PBMC from high yield donors were cultured in FibroLife-based SFM for 5 days at the indicated cell densities in the presence or absence of 20% (v:v) conditioned medium (CM) collected from PBMC cultured for 5 days from high or low yield donors. B) PBMC from low yield donors were cultured in FibroLife-based SFM for 5 days at a range of cell densities in the presence or absence of 20% (v:v) CM collected from PBMC cultured for 5 days from high or low yield donors. Results are mean ± SEM (n=4 separate high and low yield donors). The absence of error bars indicates that the error was smaller than the plot symbol.
3.6 Effect of culture conditions on murine fibrocytes
To assess whether the conditions described above for human fibrocytes were also applicable for murine fibrocyte differentiation, we compared murine PBMC cultured in a variety of SFM culture media. We also assessed whether the presence or absence of 2-mercaptoethanol, which is commonly used for murine leukocyte cell cultures, also affected fibrocyte differentiation (Garland and Owen, 1978; Nordin, 1978). Compared to murine PBMC cultured in RPMI-SFM in the absence of 2-mercaptoethanol, cells cultured in the presence of 2-mercaptoethanol had increased numbers of fibrocytes (Figure 7A). We also found that, as with human fibrocytes, there was an increased number of murine fibrocytes when cultured in FibroLife-SFM compared to RPMI-SFM (Figure 7B). These data suggest that as seen with human fibrocytes, murine fibrocytes differentiate more efficiently in FibroLife-SFM, and that the presence of 2-mercaptoethanol is a useful supplement.
Figure 7. Effect of different basal media on murine fibrocyte differentiation.
A) Murine PBMC separated from heparin treated blood of five mice were cultured in RPMI-based serum-free medium for 5 days at 2.5 × 105 cells per ml in the presence or absence of 2-mercaptoethanol. Cells were then air-dried, fixed, stained, and fibrocytes were enumerated by morphology. Representative data of three independent experiments. Results are mean ± SD. Statistical significance was determined by t-test. B) Murine PBMC separated from heparin treated blood of three mice were cultured in different 2-mercaptoethanol-containing SFM formulations for 5 days at 2.5 × 105 cells per ml. Results are mean ± SD. Statistical significance was determined by t-test. Representative data of three independent experiments.
4. Discussion
We previously found serum-free culture conditions that support the differentiation of monocytes into fibrocytes from both human PBMC (Pilling et al., 2003; Pilling et al., 2006; Shao et al., 2008). In this report, we identified conditions that affect this differentiation, including the choice of anti-coagulant, basal media, media supplements, culture substrates, and cell density. For optimal fibrocyte differentiation, we suggest using heparin as an anti-coagulant, and culturing cells at 2.5 × 105 cells/ml in FibroLife basal medium with glutamine, FIEPES, non-essential amino acids, sodium pyruvate, albumin, insulin, transferrin, sodium selenite, linoleic acid, and oleic acid, on tissue culture plastic. Under these conditions, approximately 1.5% of the PBMC become fibrocytes. Approximately 20% of the PBMC are monocytes, and since fibrocytes appear to differentiate from monocytes, this suggests that under these optimized conditions approximately 8% of the monocytes differentiate into fibrocytes. These conditions thus potentiate fibrocyte differentiation compared to serum-containing culture conditions, where approximately 0.1-1% of the PBMC differentiate into fibrocytes (Bucala et al., 1994; Yang et al., 2002; Wang et al., 2007a). We also observed that as fibrocytes are highly adherent, after five days in culture the remaining non-adherent cells can be removed from cell culture by pipetting, leading to populations of fibrocytes in excess of 50% of the adherent cells.
In many of the experiments, we observed that in two different conditions, aliquots of the same batch of PBMC gave rise to different numbers of fibrocytes. This indicates that some monocytes were able to differentiate into fibrocytes in both conditions, while other monocytes would become fibrocytes only in one of the two conditions. Similar results have been reported for the effect of cytokines and other growth factors that increase fibrocyte differentiation (Abe et al., 2001; Hong et al., 2007; Vannella et al., 2007; Wang et al., 2007a; Shao et al., 2008). Whether the ability of monocyte to differentiate into fibrocytes is affected by the degree of maturity of the cells as they leave the bone-marrow is unclear. These data indicate that there is heterogeneity, with respect to ability to differentiate into fibrocytes, in the population of monocytes in the culture.
The probability that a monocyte will differentiate into a fibrocyte increases with cell density in the culture. This suggests that cell-cell contact or a factor secreted by some cells in the PBMC population potentiate fibrocyte differentiation. For PBMC cultured in the presence of serum, fibrocyte differentiation can be regulated by soluble factors such as TGF-β released from cells in the PBMC (Abe et al., 2001; Yang et al., 2002). We have also shown that in serum-free media other factors, such as IL-4 and IL-13 promote fibrocyte differentiation, whereas IL-12 and IFN-γ inhibit fibrocyte differentiation (Shao et al., 2008). Therefore, in an inflammatory or fibrotic lesion, the presence of activated T cells or other cells present in the peripheral blood, as well as the concentration and ratio of these pro- and anti-fibrocyte factors, could affect whether monocytes will differentiate into fibrocytes.
No increase in the number of fibrocytes was seen when we added 5-day conditioned medium from relatively high cell density PBMC (where ∼1% of the PBMC differentiate into fibrocytes) to PBMC at low cell density (where ∼ 0.2% of the PBMC differentiate into fibrocytes). This indicates that the increased number of fibrocytes observed at high cell density is not due to a factor in the 5-day conditioned medium. This then suggests that the effect of cell density on fibrocyte differentiation is due to either cell-cell contact or a labile factor that is no longer present at an effective concentration in the 5-day conditioned medium.
Some fibrosis patients have elevated numbers of circulating fibrocytes, and PBMC cultures from some fibrosis patients have increased numbers of fibrocytes in vitro (Yang et al., 2002; Mori et al., 2005; Kisseleva et al., 2006; Sakai et al., 2006; Mehrad et al., 2007; Moeller et al., 2009). We identified some individuals whose PBMC did not generate many fibrocytes. These “low yield” donors appear to have an intrinsic property that leads to reduced numbers of monocytes differentiating into fibrocytes. Whether this donor population is less likely to develop a fibrotic disease is unknown.
Acknowledgments
This work was supported by NIH grant HL083029. We would also like to thank Anu Maharjan for enumeration of fibrocytes and Nancy Tucker for help with preliminary experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. Journal of Immunology. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Akbar AN, Salmon M, Ivory K, Taki S, Pilling D, Janossy G. Human CD4+ CD45RO+ and CD4+ CD45RA+ T cells synergize in response to alloantigens. European Journal Of Immunology. 1991;21:2517–2522. doi: 10.1002/eji.1830211031. [DOI] [PubMed] [Google Scholar]
- Balmelli C, Alves MP, Steiner E, Zingg D, Peduto N, Ruggli N, Gerber H, McCullough K, Summerfield A. Responsiveness of fibrocytes to toll-like receptor danger signals. Immunobiology. 2007;212:693–699. doi: 10.1016/j.imbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Balmelli C, Ruggli N, McCullough K, Summerfield A. Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. Journal Of Leukocyte Biology. 2005;77:923–933. doi: 10.1189/jlb.1204701. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HSR, Burne JF, Voyyodic JT, Richardson WD, Raff MC. Cell-death and control of cell-survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Molecular Medicine. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- Faint JM, Pilling D, Akbar AN, Kitas GD, Bacon PA, Salmon M. Quantitative flow cytometry for the analysis of T cell receptor Valpha chain expression. Journal Of Immunological Methods. 1999;27:53–60. doi: 10.1016/s0022-1759(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Garland JM, Owen JJ. Macrophage-lymphocyte association in in vitro mouse spleen cultures; the formation of B-cell colonies. Immunology. 1978;34:707–713. [PMC free article] [PubMed] [Google Scholar]
- Gomer RH, Yuen IS, Firtel RA. A secreted 80 × 10(3) Mr protein mediates sensing of cell density and the onset of development in Dictyostelium. Development. 1991;112:269–278. doi: 10.1242/dev.112.1.269. [DOI] [PubMed] [Google Scholar]
- Gomperts BN, Strieter RM. Fibrocytes in lung disease. Journal Of Leukocyte Biology. 2007;82:449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- Gulliksson H. Additive solutions for the storage of platelets for transfusion. Transfusion Medicine. 2000;10:257–264. doi: 10.1046/j.1365-3148.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- Haudek SB, Trial J, Xia Y, Gupta D, Pilling D, Entman ML. Fc Receptor Engagement Mediates Differentiation of Cardiac Fibroblast Precursor Cells. Proceedings of the National Academy of Sciences. 2008;105:10179–10184. doi: 10.1073/pnas.0804910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone Marrow-derived Fibroblast Precursors Mediate Ischemic Cardiomyopathy in Mice. Proceedings of the National Academy of Sciences. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y, Cheng L, Mudge AW, Raff MC. Programmed cell-death by default in embryonic-cells, fibroblasts, and cancer-cells. Molecular Biology Of The Cell. 1995;6:1443–1458. doi: 10.1091/mbc.6.11.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Mehdy MC, Firtel RA. A secreted factor and cyclic AMP jointly regulate cell-type-specific gene expression in Dictyostelium discoideum. Mol Cell Biol. 1985;5:705–713. doi: 10.1128/mcb.5.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, O'Byrne PM, Strieter RM, Kolb M. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Experimental Cell Research. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Nagy N, Biro E, Takacs A, Polos M, Magyar A, Olah I. Peripheral blood fibrocytes contribute to the formation of the avian spleen. Dev Dyn. 2005;232:55–66. doi: 10.1002/dvdy.20212. [DOI] [PubMed] [Google Scholar]
- Naik-Mathuna B, Pilling D, Crawford JR, Gay AN, Smith CW, Gomer RH, Olutoye OO. Serum amyloid P inhibits dermal wound healing. Wound Repair and Regeneration. 2008;16:266–273. doi: 10.1111/j.1524-475X.2008.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin AA. The in vitro immune response to a T-independent antigen. I. The effect of macrophages and 2-mercaptoethanol. European Journal of Immunology. 1978;8:776–781. doi: 10.1002/eji.1830081105. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Akbar AN, Bacon PA, Salmon M. CD4+ CD45RA+ T Cells from Adults Respond to Recall Antigens after CD28 Ligation. International Immunology. 1996;8:1737–1742. doi: 10.1093/intimm/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Pilling D, Akbar AN, Shamsadeen N, Scheel-Toellner D, Buckley C, Salmon M. High cell density provides potent survival signals for resting T-cells. Cell Mol Biol. 2000;46:163–174. [PubMed] [Google Scholar]
- Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. Journal Of Immunology. 2003;17:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Gomer RH. Regulatory Pathways for Fibrocyte Differentiation. In: Bucala R, editor. Fibrocytes-New Insights into Tissue Repair and Systemic Fibroses. 2007. pp. 37–60. [Google Scholar]
- Pilling D, Kitas GD, Salmon M, Bacon PA. The kinetics of interaction between lymphocytes and magnetic polymer particles. Journal Of Immunological Methods. 1989;122:235–241. doi: 10.1016/0022-1759(89)90269-x. [DOI] [PubMed] [Google Scholar]
- Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. Journal Of Leukocyte Biology. 2006;79:1242–1251. doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite AE, Shigemitsu H, Kanangat S. Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol. 2004;16:733–738. doi: 10.1097/01.bor.0000139310.77347.9c. [DOI] [PubMed] [Google Scholar]
- Quan TE, Bucala R. Culture and analysis of circulating fibrocytes. Methods in molecular medicine. 2007;135:423–434. doi: 10.1007/978-1-59745-401-8_28. [DOI] [PubMed] [Google Scholar]
- Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. The International Journal of Biochemistry & Cell Biology. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proceedings of the National Academy of Sciences. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon M, Pilling D, Borthwick NJ, Viner N, Janossy G, Bacon PA, Akbar AN. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. European Journal Of Immunology. 1994;24:892–899. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- Salmon M, Scheel-Toellner D, Huissoon AP, Pilling D, Shamsadeen N, Hyde H, D'Angeac AD, Bacon PA, Emery P, Akbar AN. Inhibition of T cell apoptosis in the rheumatoid synovium. Journal Of Clinical Investigation. 1997;99:439–446. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of Circulating Fibrocytes as Precursors of Bronchial Myofibroblasts in Asthma. The Journal of Immunology. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. Journal of Leukocyte Biology. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannella KM, McMillan TR, Charbeneau RP, Wilke CA, Thomas PE, Toews GB, Peters-Golden M, Moore BB. Cysteinyl leukotrienes are autocrine and paracrine regulators of fibrocyte function. J Immunol. 2007;179:7883–7890. doi: 10.4049/jimmunol.179.11.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varcoe RL, Mikhail M, Guiffre AK, Pennings G, Vicaretti M, Hawthorne WJ, Fletcher JP, Medbury HJ. The role of the fibrocyte in intimal hyperplasia. J Thromb Haemost. 2006;4:1125–1133. doi: 10.1111/j.1538-7836.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Improvement in postburn hypertrophic scar after treatment with IFN-alpha2b is associated with decreased fibrocytes. J Interferon Cytokine Res. 2007a;27:921–930. doi: 10.1089/jir.2007.0008. [DOI] [PubMed] [Google Scholar]
- Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007b;15:113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, Ghahary A, Tredget EE. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair and Regeneration. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral Blood Fibrocytes from Burn Patients: Identification and Quantification of Fibrocytes in Adherent Cells Cultured from Peripheral Blood Mononuclear Cells. Laboratory Investigation. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]