Abstract
The development of novel three-dimensional cell culture platforms for the culture of aortic valvular interstitial cells (VICs) has been fraught with many challenges. Although the most tunable, purely synthetic systems have not been successful at promoting cell survivability or function. On the other hand, entirely natural materials lack mechanical integrity. Here we explore a novel hybrid system consisting of gelatin macromers synthetically modified with methacrylate functionalities allowing for photoencapsulation of cells. Scanning electron microscopy observations show a microporous structure induced during polymerization within the hydrogel. This porous structure was tunable with polymerization rate and did not appear to have interconnected pores. Treatment with collagenase caused bulk erosion indicating enzymatic degradation controls the matrix remodeling. VICs, an important cell line for heart valve tissue engineering, were photoencapsulated and examined for cell-directed migration and differentiation. VICs were able to achieve their native morphology within 2 weeks of culture. The addition of the pro-fibrotic growth factor, transforming growth factor-β1, accelerated this process and also was capable of inducing enhanced α-smooth muscle actin and collagen-1 expression, indicating a differentiation from quiescent fibroblasts to active myofibroblasts as demonstrated by quantitative real-time polymerase chain reaction and immunohistochemistry. Although these studies were limited to VICs, this novel hydrogel system may also be useful for studying other fibroblastic cell types.
Introduction
Typically, cell culture experiments are carried out on two-dimensional (2D) surfaces, such as Petri dishes. Although convenient, these flat surfaces are unnaturally stiff, and can create polarity and artificially high surface-to-volume ratios in the cell.1 Three-dimensional (3D) culture matrices are emerging to provide a more tissue-like platform to culture cells. In some cases, these scaffolds can even be tailored to promote or suppress specific cell functions and interactions.2,3 However, the development of 3D culture systems is complex and often requires careful tailoring of the material environment for a given cell type.
For the creation of 3D cell culture platforms there are many material choices, ranging from fully synthetic to naturally derived. Synthetic matrices, such as poly(ethylene glycol) (PEG) and poly(lactic-co-glycolic acid) (PLGA), have been widely characterized for the culture of various cell types, including mesenchymal stem cells,4,5 fibroblasts,6,7 and chondrocytes.8,9 These materials are nontoxic, hydrophilic, and resorbable and have tailorable mechanical properties that make them an attractive option for cell culture.10,11 On the other hand, these materials possess no biological epitopes or signaling capabilities unlike the complex extracellular matrix (ECM) of the native tissues. To maintain cell viability and promote cellular function, bioactive epitopes such as peptides are often incorporated into these materials.12,13
On the other end of the materials' spectrum, scaffolds can also be formed from naturally derived components such as collagen,14 matrigel,15 and fibrin.16 Cells interact with these materials in a complex way by binding and remodeling the matrix; however, it is difficult to engineer the material properties independently. Thus, an emerging paradigm is to consider a synthetic method to modify a natural material to incorporate the control of a synthetic material with the biological compatibility of natural materials.
From this methodology, we explored a chemically modified gelatin material first introduced by Van den Bulcke et al. as a possible cell culture platform.17 Other researchers have also used a similar method of generating methacrylated gelatin for the purposes of generating novel wound dressings18–20 and hepatocyte cell culture.21,22 Gelatin is synthesized upon the partial breakdown of the natural triple-helical structure of collagen as a byproduct of the meat-processing industry and is generally considered a safe material by the U.S. Food and Drug Administration.23 As it is processed from collagen, it maintains many of the bioactive features of collagen, and comes in varying grades quantified by the strength and collagen-like structures still available in the gelatin product.23,24 Unmodified gelatin, nevertheless, has problems with utilization as a culture platform. In particular, the gels formed are mechanically weak, and the physical gelation temperature (under 35°C) is below that of the physiological temperature required for cell culture. As such, without further modification, gelatin cannot be employed as a 3D culture system. To overcome these limitations, gelatin can be modified with standard side group chemistry to alter its physical properties. In this communication, we investigated a methacrylate-modified system that incorporates double bonds as pendent groups on the gelatin chains.17 When exposed to light in the presence of a photoinitiator, the methacrylated gelatin macromer (GelMA) polymerizes to form a covalently and physically crosslinked hydrogel that has more tunable mechanical properties and remains gelled above physiological temperature.
Other strategies have been employed to allow the formation of a crosslinked gelatin hydrogel. The most common method utilizes gluteraldehyde, a common fixative often employed to preserve biological specimens through the nonspecific crosslinking of proteins. Although this method produces a mechanically tunable gelatin hydrogel, gluteraldehyde is toxic to cells and thus prevents cell encapsulation.25,26 Hydrogels can also be created from various enzymes such as transglutimase derived from bacteria; however, studies have shown this enzyme to also be toxic at low concentrations.27,28 Although all of these methods produce gelatin-based hydrogels by forming chemical crosslinks, none allow for the encapsulation of viable cells. For this reason we chose to study a methacrylate-modified gelatin, which can be photopolymerized under cytocompatible conditions. Cell encapsulation allows the formation of evenly distributed cell populations within the gel.
To demonstrate the utility of this material as a tissue engineering and cell culture platform, aortic valvular interstitial cells (VICs) were cultured within the GelMA hydrogels. VICs represent a challenging cell phenotype of great interest to the heart valve tissue engineering community. These heterogeneous cells make up the majority of the cell population residing within valvular leaflets.29,30 They are responsible for maintaining the delicate microstructure of valve tissue to endure the repeated mechanical stresses experienced by the tissue.30 Although 70,000 people a year undergo aortic valve replacement as the result of valve failure, surprisingly little is known about this cell population.31 Most studies in the literature have been performed on 2D surfaces and a few within collagen and fibrin hydrogels.32,33 As a result of the myofibroblastic properties of these cells, collagen hydrogels are easily contracted by these cells and are mechanically inadequate.32 Some synthetic options have also been studied, including PEG and modified PEG platforms.34,35 Although controllable, VICs are unable to degrade and remodel these matrices, which inhibits spreading and attainment of their natural fibroblast-like morphology. Consequently, we studied the behavior of VICs within methacrylate-modified gelatin hydrogels. VIC behavior was also studied within the GelMA hydrogels with and without the addition of the pro-fibrotic growth factor, transforming growth factor-β1 (TGF-β1). This potent cytokine in 2D enhances VIC collagen deposition, and increases their production of α-smooth muscle actin (αSMA) a marker for myofibroblasts (the wound healing phenotype of VICs).36,37 Even though our studies were limited to VICs, we hypothesize that this material will likely afford specific advantages to other cell types—in particular, various fibroblast cells due to its highly bioactive structure and complex porous network allowing for both enzymatic and ameboid movement of cells. This is particularly important for fibroblasts, the main repair cells activated by damage and require quick responses to injury. A similar GelMA network has also been used for hepatocyte culture, illustrating versatility in a variety of cell applications.22
Materials and Methods
Synthesis of methacrylated gelatin
GelMA was synthesized according to the method of Van den Bulcke et. al. Briefly, powdered, type A cell culture–tested gelatin from porcine skin with a Bloom Index of ∼300 was obtained from Sigma-Aldrich (St. Louis, MO). One gram of gelatin was added to 10 mL of phosphate buffered saline (PBS) and heated at 50°C while stirring for approximately 20 min, or until all gelatin was dissolved. One milliliter of 94% methacrylic anhydride (Aldrich, Milwaukee, WI) was added to the stirring mixture at a constant rate of 0.5 mL/min, and the reaction was allowed to proceed for 1 h at 50°C. The reaction was then diluted with 40 mL of 40°C PBS and dialyzed with 12,000–14,000 molecular weight cutoff dialysis tubing (Fisherbrand, Pittsburgh, PA) for 1 week against 40°C diH2O to remove the methacrylic acid and other impurities. At this point, the solution was snap-frozen with liquid nitrogen and lyophilized for 1 week. GelMA product was used without further purification. The amount of lysine groups modified on the gelatin macromer was determined by a method developed by Habeeb using 2,4,6-trinitrobenzenesulfonic acid as previously described.38 This was confirmed with nuclear magnetic resonance spectroscopy in a method similar to that described from modified collagen macromers.39 With this method, the degree of lysine groups modified on the gelatin macromers can be easily controlled through limiting reactant (methacrylic anhydride) available to produce hydrogels with a range of moduli. The range for degree of methacrylation is between 0% and 60%, which ultimately gives rise to moduli between 25 and 45 kPa. For the purposes of these experiments, we selected reaction conditions that produced hydrogels with roughly 57 ± 2% of available lysine residues modified and a compressive modulus in the range of 42 ± 3 kPa. We chose this composition for optimal mechanical properties and ease of handling.
Hydrogel preparation and characterization
Hydrogels were formed by crosslinking the methacrylate groups formed via a photoinitiated chain polymerization. GelMA macromer was dissolved in PBS at 10 wt% concentration and allowed to dissolve at 60°C. Photoinitiator, 1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propanone (Irgacure 2959; Ciba Specialty Chemicals, Tarrytown, NJ), was added to the GelMA solution at various concentrations as indicated in the Results section. Once fully dissolved, the warm macromer and initiator mixture were pipetted between two glass slides separated by 1-mm spacers. Immediately, the mixture was exposed to 365 nm UV light at an intensity of 8 mW/cm2 for 10 min. After gelation, hydrogels were punched using 5-mm round biopsy punches to obtain samples of equal size (diameter, 5 mm; thickness, 1 mm). The hydrogel was subsequently placed into PBS, and the temperature was decreased to 37°C to allow physical gelation to occur.
Porosity of the GelMA hydrogels was analyzed using low vacuum scanning electron microscopy (SEM) (Model JSM-6480LV; JEOL, Tokyo, Japan) in the University of Colorado's Nanomaterials Characterization Facility at 1-0.8 kV. For viewing on SEM, samples were sectioned with a vibratome, flash-frozen in liquid nitrogen, dried under reduced pressure (∼100 mT), and mounted on standard aluminum SEM specimen stubs with double-sided carbon tape. Porosity was calculated from SEM images by utilizing NIH ImageJ software to determine pore size and normalized to the number of pores present in a viewing field.
Bulk macroscopic mechanical properties of GelMA gels were quantified using an MTS Synergie 100 equipped with a 10 N load cell (MTS Systems, Eden Prairie, MN). Swollen gels were subjected to unconfined compression at a constant rate of 1 mm/min up to a strain of 20% at room temperature. Compressive moduli were subsequently calculated from the linear region of the stress–strain curve at low strains.
Characterization of hydrogel degradation with collagenase
GelMA hydrogels (10 wt% macromer) were added to collagenase type II (Worthington Biochemical, Lakewood, NJ) dissolved in PBS at the indicated enzyme concentrations. Samples were then placed on a 37°C shaker (50 rpm). At the indicated time points, hydrogel samples were removed and weighed. Samples were then lyophilized and measured again for dry weight. Mass loss was determined by normalizing dry sample measurements to dry measurements at time zero.
VIC isolation and culture
Aortic leaflets were surgically isolated from porcine hearts purchased from Hormel (Austin, MN) 24 h after sacrifice. VICs were removed from tissue by sequential collagenase digestion as previously described.40 Cells were cultured on tissue culture polystyrene (TCPS) plates in growth medium consisting of Medium 199 supplemented with 15% fetal bovine serum, 2% penicillin/streptomycin (100 U/mL), 0.4% fungizone (0.5 μg/mL), and 0.2% gentamicin (Invitrogen, Carlsbad, CA) in a humid incubator at 37°C and 5% CO2. Cells were successively passaged by trypsinization to expand cultures. Passages 2 and 3 were used for all cell experimentation. Hydrogel–cell constructs were cultured in 1% fetal bovine serum–supplemented medium to minimize cell proliferation and treated with 5 ng/mL porcine TGF-β1 (R & D Systems, Minneapolis, MN).
VIC encapsulation within GelMA hydrogels
VICs were added to warm, sterile, macromer solution of 10 wt% GelMA and 0.05 wt% Igracure 2959 in PBS to balance osmolarity at a final density of 10 million cells/mL. The cell–macromer suspension was transferred to sterile molds constructed of glass slides separated by 1-mm spacers and exposed to UV light (365 nm) at 5 mW/cm2 for 10 min. Cell–gel slabs were then cut with sterile razor blades into smaller hydrogels and transferred to well-plates containing cell culture medium and placed on an orbital shaker for the duration of the experiments. Medium was refreshed every other day over the course of the study.
VIC viability within GelMA hydrogels
VIC viability was determined using Live/Dead® purchased from Invitrogen. Briefly, hydrogels were rinsed in PBS and then placed into phenol red–free medium containing Live/Dead stain. Hydrogels were then incubated in stain for 30 min and subsequently rinsed before imaging. Images of 200 μm stacks were obtained with a Zeiss confocal microscope (model LSM5 Pascal) with pictures taken in 5 μm slices. Projections of each stack were then used to quantify live and dead cells according to staining by manual counting of live (green) versus red (dead) cells. Three hydrogel samples each with three confocal projections were imaged and counted for each condition. Average cell area and circularity were calculated from the Live/Dead image set using NIH ImageJ Software Analyze Particles feature. Circularity ranges from 0 to 1, with 1 indicating a perfect circle.
Isolation of messenger RNA and quantitative real-time polymerase chain reaction
Messenger RNA (mRNA) was isolated from VIC-laden GelMA hydrogels using the SV total RNA isolation kit (Promega, Madison, WI). Reverse transcription was then performed using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). Polymerase chain reaction (PCR) was conducted using an iCycler quantitative real-time PCR (qRT-PCR) machine (Bio-Rad), and primers were designed with the Beacon primer design program. Primers for glyceraldehyde 3-phophate dehydrogenase (GAPDH), αSMA, and collagen-1 are listed in Table 1 (Integrated DNA Technologies, Coralville, IA). Threshold cycle and primer efficiency were analyzed according to the Pfaffl method and normalized to GAPDH41 (Table 1).
Table 1.
Quantitative Real-Time Polymerase Chain Reaction Primer Conditions
| Gene | Sense | Anti-sense | Anneal Temperature (°C) | Efficiency (%) |
|---|---|---|---|---|
| αSMA | AAC AAC CAC AGA ACC ACA AG | TGA CCT GCG ATT AAC C | 55.4 | 110.9 |
| Collagen-1 | GGG CAA GAC AGT GAT TGA ATA CA | GGA TGG AGG GAG TTT ACA GGA A | 55.4 | 109.9 |
| GAPDH | TCG GCA TCG TGG AAG GAC | GGC AGC AGT AGA AGC | 57.6 | 99.6 |
αSMA, α-smooth muscle actin; GAPDH, glyceraldehyde 3-phophate dehydrogenase.
Immunohistochemical staining
VIC GelMA hydrogels were fixed at 4°C in 10% buffered formalin for 24 h. Constructs were then moved to a 30 wt% sucrose solution for an additional 24 h at 4°C. Samples were then frozen and stored at −80°C until sectioning. For sectioning, constructs were mounted in Cryo-Gel embedding medium (Instrumedics, St. Louis, MO) and cryosectioned into 30-μm sections. Slides were then immunostained for αSMA expression. Briefly, slides were blocked in a 3 wt% bovine serum albumin (BSA) solution for 1 h. Slides were then subsequently immunostained with mouse anti-αSMA (Abcam, Cambridge, MA) overnight at 4°C. After primary antibody incubation, slides were rinsed twice and immunostained with goat anti-mouse Alexa® 488 (Invitrogen) for 1 h at room temperature. Slides were then counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) and imaged on a Nikon TE 2000 fluorescent microscope.
Statistical analysis
All analyses were performed using a standard Student's t-test. Statistical significance was considered for p < 0.05. Error bars represent standard deviation of the mean. Unless otherwise indicated, measurements were performed in triplicate.
Results
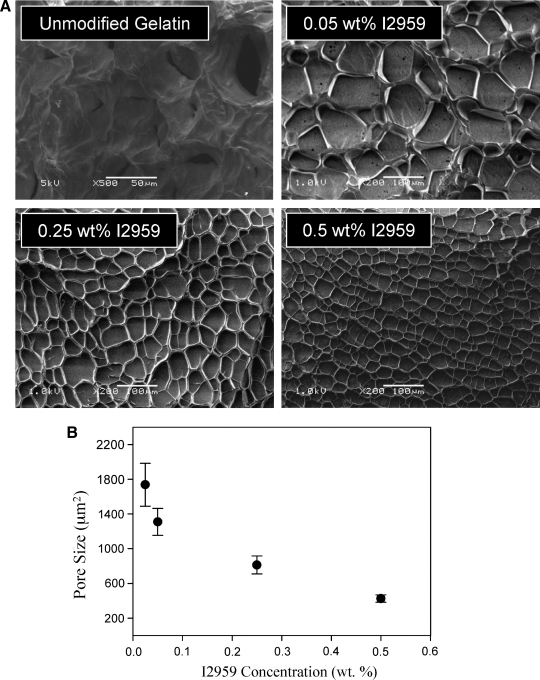
Given that GelMA macromers are composed mostly of proteinaceous material with secondary and tertiary structure, we first investigated the physical structure of GelMA hydrogels formed under differing polymerization conditions before cell encapsulation experiments. Upon examination with SEM, we observed that GelMA hydrogels have a polymerization-induced porosity with diameters on the micron scale (Fig. 1A). This was particularly interesting as most mammalian cells are also on the order of microns in size. The size of the pores was easily tuned by adjusting the rate of polymerization through the photoinitiator concentration (Fig. 1B). Although lyophilization procedures can alter material porosity, this relative difference in pore size using the same drying process indicates a fundamental difference in network structures between prepared samples. The largest pores were formed in the hydrogels with the lowest photoinitiator concentration, while the smallest pores were formed with the highest concentrations. The largest pores were an order of magnitude greater in size (∼1800 μm2, projected area) than the smallest pores (∼400 μm2, projected area). Wall thickness in between the pores was also decreased with the highest photoinitiator concentration. Pores formed were most likely the result of polymerization-induced phase separation, as the same structures were not visible in physically formed natural gelatin gels (Fig. 1A). It did not appear that these pores were interconnected, rather distinct pockets within the hydrogel separated by thin walls. For the subsequent cell encapsulation experiments, the 0.05 wt% photoinitiator concentration was selected, as this commonly used encapsulation composition provided the largest pores while still maintaining no adverse effects of cell viability.
FIG. 1.
(A) Scanning electron microscopic images of 10 wt% lyophilized methacrylated gelatin macromer (GelMA) hydrogel samples with varying photoinitiator concentration and natural gelatin gel. (B) Quantification of GelMA hydrogel porosity from scanning electron microscopic images as a function of initiator concentration. Porosity was determined using the projected area of the pores as determined with NIH ImageJ software. The largest pores were achieved with the least amount of photoinitiator, while the smallest pores were formed with most amount of photoinitiator. Results were calculated with three or more electron micrographs per sample.
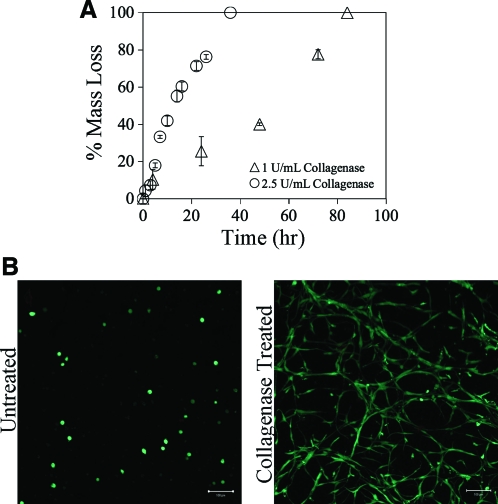
After investigation of the network structure, enzymatic degradation of the GelMA hydrogels was examined to ensure that crosslinking of the macromers did not interfere with enzymatic degradability. Two concentrations of collagenase were applied to hydrogels of uniform size over the time course indicated (Fig. 2A). Increased collagenase enzymatic activity accelerated the rate of degradation, an expected trend, for relatively uniform bulk degradation throughout the hydrogel. To study the effect of exogenous collagenase delivery on VIC morphology within the GelMA hydrogels, cells were encapsulated and treated either with or without (control) the lowest concentration (1 U/mL collagenase) for 4 h and cultured for an additional 2 days. In the non-collagenase-treated samples, VIC spreading was minimal as shown by the observed rounded morphology; this was expected since elongated cell morphology is only apparent in later time points of culture. The cell-laden hydrogels treated with collagenase achieved a more spread morphology throughout the construct, indicating an erosion of the GelMA network (Fig. 2B).
FIG. 2.
(A) GelMA 10 wt% hydrogels of uniform size were exposed to 2.5 U/mL (○) or 1 U/mL (▵) exogenous collagenase until complete degradation. The higher concentration of collagenase increased the rate of degradation as expected. (B) Valvular interstitial cells (VICs) were encapsulated in 10 wt% GelMA hydrogels and incubated with or without 1 U/mL of exogenous collagenase for 4 h. Collagenase was then removed, and the cells were cultured for an additional 2 days. GelMA-VIC hydrogels were then stained with Live/Dead® and imaged with a confocal microscope. No dead cells were observed. Scale bar = 100 μm. VICs had more dramatic spread morphology in treated hydrogels compared with untreated hydrogels. Color images available online at www.liebertonline.com/ten.
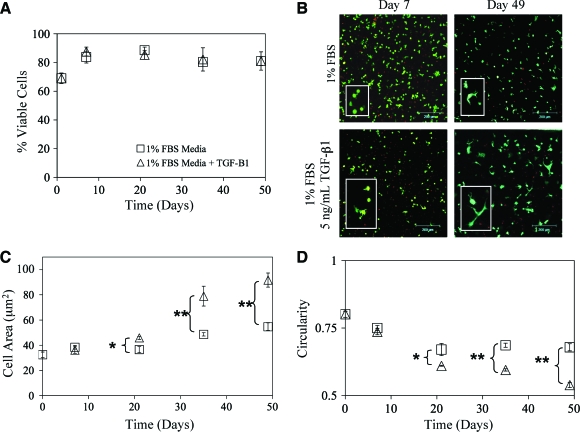
To demonstrate the flexibility and utility of the GelMA hydrogel culture system, VICs were photoencapsulated within 10 wt% GelMA hydrogel networks and cultured for up to 7 weeks with and without supplementation of 5 ng/mL TGF-β1. The cells maintained their viability over the time course of the experiments (Fig. 3A). There was an initial drop in viability, most likely the result of the initial encapsulation stress. This was quickly recovered, however, within the first week of culture. The addition of TGF-β1 had no impact on the viability of VICs (Fig. 3A). The morphology of VICs within 3D culture is also an important indicator of cell activity. VICs are a fibroblastic cell type and should be able to achieve spread morphology through interactions with the gelatin and local degradation of the matrix. Over time in culture, VICs were able to accomplish greater process extension with TGF-β1 treatment over control within GelMA hydrogels as illustrated in Figure 3B. Quantitatively, this was also observed through image analysis of the collected Live/Dead images in Figure 3C and D. Overall, the addition of TGF-β1 increased the amount of VIC process extension and spreading while decreasing circularity, indicating a more active cell type and possibly myofibroblastic activation.42,43
FIG. 3.
(A) VIC viability was measured with Live/Dead staining over a time course of 7 weeks. Constructs were treated with (▵) or without (□) 5 ng/mL transforming growth factor-β1 (TGF-β1) in 1% fetal bovine serum (FBS) medium. Initially, the viability of encapsulated VICs dropped, and then recovered after 1 week in culture. Growth factor treatment had no apparent effect on overall cell viability. (B) Representative Live/Dead projected confocal stacks of VICs encapsulated within GelMA hydrogel networks treated with TGF-β1 as indicated. Greater cell spreading was observed in cells treated with TGF-β1. Scale bar = 200 μm. (C) To quantify differences in cell spreading, average cell area was measured from Live/Dead images with NIH ImageJ software. Significant differences in cell spreading were observed from day 21 onward with TGF-β1 treatment. (D) To confirm differences in cell spreading, circularity was also measured from Live/Dead images with NIH ImageJ software. Significant differences in circularity were observed from day 21 onward with TGF-β1 treatment. n = 3, *p < 0.01, **p < 0.001. Color images available online at www.liebertonline.com/ten.
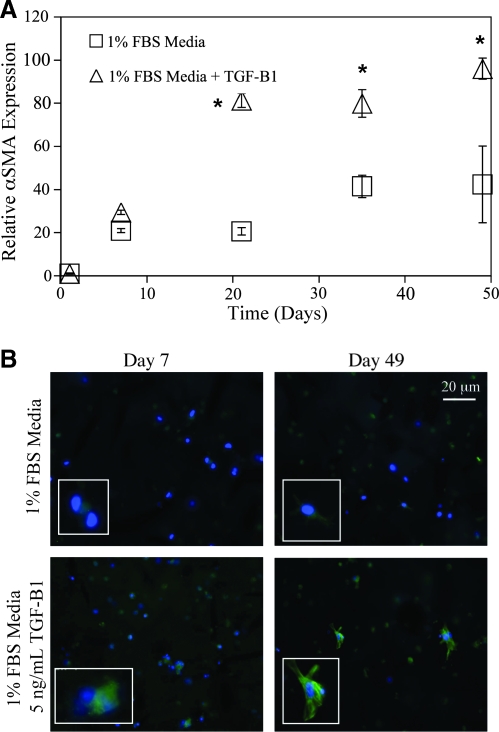
To investigate the myofibroblastic activity, mRNA was collected and analyzed for αSMA, an important myofibroblastic marker. Analysis with qRT-PCR showed a significant doubling of αSMA expression with TGF-β1 treatment from 3 weeks of culture and on (Fig. 4A). Interestingly, there was also an elevation in αSMA expression over day 1 in the untreated VIC-GelMA hydrogels. This may be an innate cellular response to the degraded nature of gelatin itself, as it is derived from processed collagen. To confirm the qRT-PCR results, hydrogels were fixed, sectioned, and immunostained for αSMA protein expression. Cell constructs treated with TGF-β1 had increased positive αSMA staining with clear stress fiber formation, indicating myofibroblastic activity (Fig. 4B). Untreated constructs had much lower positive staining for αSMA mirroring the qRT-PCR results attained.
FIG. 4.
(A) Quantitative real-time polymerase chain reaction analysis of α smooth muscle actin (αSMA) messenger RNA (mRNA) expression treated with (▵) or without (□) 5 ng/mL TGF-β1 in 1% FBS medium. Expression indicated is relative to the day 1 time point and glyceraldehyde 3-phophate dehydrogenase housekeeping gene expression. αSMA mRNA was elevated by twofold with TGF-β1 treatment. (B) Immunohistochemical staining of αSMA protein expression at days 7 and 49 in 30 μm cryosections. Positive αSMA staining is shown in green; 4′,6′-diamidino-2-phenylindole (DAPI) counterstaining for cell nuclei is depicted in blue. Cells treated with TGF-β1 had greater staining for αSMA with distinct stress fiber formation, indicating myofibroblastic differentiation. A blow-up of stress fiber positive cells is also shown as an inset. *p ≤ 0.01 over the untreated VIC-GelMA constructs, n = 4 for all samples.
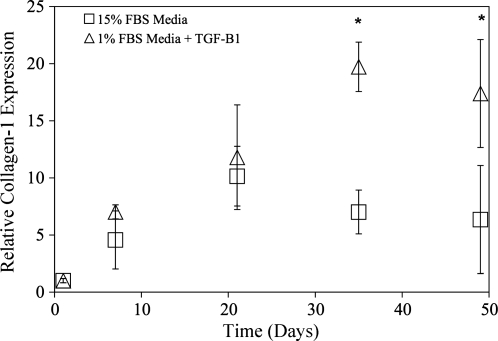
Another important marker for myofibroblastic differentiation of VICs is collagen-1 expression indicating a fibrotic response. Thus, after αSMA experiments, mRNA expression of collagen-1 was explored. Again collagen-1 mRNA expression was increased with TGF-β1 treatment (Fig. 5), although it was not as dramatic as the differences with αSMA expression. Collagen-1 expression was not upregulated until 3 weeks of culture and then was similar to αSMA expression, achieved a 1.5-fold increase over the untreated samples. Unfortunately due to the collagenous nature of gelatin, we were unable to differentiate between GelMA matrix and cellular produced collagen-1 for protein expression analysis.
FIG. 5.
Quantitative real-time polymerase chain reaction analysis of collagen-1 mRNA expression treated with (▵) or without (□) 5 ng/mL TGF-β1 in 1% FBS medium. Expression indicated is relative to the day 1 time point and glyceraldehyde 3-phophate dehydrogenase housekeeping gene expression. Collagen-1 mRNA was elevated by 1.5-fold with TGF-β1 treatment. *p ≤ 0.01 over the untreated VIC-GelMA constructs, n = 4 for all samples.
Discussion
In this communication, we explored the utility of methacrylate-modified gelatin hydrogel scaffolds for the 3D culture of VICs, an important cell type for understanding heart valve function. This material is an attractive cell culture platform for a number of reasons. First, this material can be easily photopolymerized under cytocompatible conditions allowing for the encapsulation of viable cells. Second, this material has tunable and enhanced mechanical properties over natural ECMs as previously shown. Further, the majority of the hydrogel is composed of gelatin chains, which possess many of the bioactive epitopes of collagen promoting cell function such as the classic G-P-Y or G-X-HydroxyP sequence, where X and Y are various other peptides. Interestingly, we observed that GelMA hydrogels also have a unique physical structure, which may promote unique cell behavior such as the ability to migrate through pore walls, remodel the pore structure, and replace gel material with secreted ECM. Photopolymerization of other biosynthetic materials (including methacrylated dextran and chondroitin sulfate), under certain reaction conditions, have been found to give rise to porous network structures as well.44,45 These materials have a polymerization-induced distinct pore structure on the size order of mammalian cells that is tunable with polymerization conditions.46 Here, we found that upon photopolymerization in the presence of excess water, GelMA macromers undergo photopolymerization-induced phase separation via syneresis to form a microporous material. Syneresis occurs when polymerization takes place with a higher amount of water than would be present at equilibrium swelling. As the polymerization proceeds, locally excess water is squeezed out of the formed polymer in an attempt to reach its equilibrium swelling concentration—the thermodynamically favored state of the material. Kinetics prevent the system from forming a fully homogenous material as the features of this two-phase (polymer-rich and water-rich) system are covalently bound as the network continues to form. This phenomenon has been previously observed in the hydrogel community47,48 as well as in liquid crystal–polymer constructs.49,50 Network porosity, pore size, and wall thickness are directly related to the rate of polymerization. Here, varying the rate of initiator concentration altered this reaction rate but a similar effect can also be achieved by altering gelatin methacrylation functionality and photocuring light intensity. Although we did not quantitatively evaluate the connectively of the pores formed, SEM led us to believe that they are not interconnected. This is consistent with the proposed syneresis-mediated, photopolymerization-induced phase separation theory for pore formation as the water-rich phase forms as pockets within the polymer-rich phase.
This porosity may have some interesting implications on directing cell behavior. Since cells are photoencapsulated, they are uniformly dispersed throughout the network, most likely distributed within the walls, as well as the pores of the hydrogel structure. Given that gelatin possesses the same biodegradable epitopes as collagen, the cells should be able to degrade the network to migrate into and through the pores of the network. In contrast to other porous synthetic networks, such as electrospun poly(lactic-co-glycolic acid) (PLGA), hydrolytic degradation controls the rate at which the network and pores disintegrate, which may not be the most desirable rate for the cells. This difficulty can be overcome via the incorporation of enzymatically degradable peptide sequences into the polymer backbone, which allow for cell-mediated material degradation,51 but has not been combined with materials of controlled porosity.
To confirm that the GelMA network is still susceptible to enzymatic degradation, we treated GelMA hydrogels with collagenase. As expected, the networks degraded with collagenase delivery. This result indicates that GelMA materials will also be susceptible to local degradation via cell-secreted enzymes. Unlike hydrolytically controlled degradation, this provides cells with spatial and temporal control of the network structure, not matched in other porous synthetic hydrogels. Encapsulated cells should be capable of degrading GelMA hydrogels through a host of collagenase like enzymes, most notably, the matrix metalloproteinase (MMP) family of enzymes.52,53 Although relatively nonspecific, these enzymes are the main tools used by fibroblasts to remodel ECM. Further, cells were able to attain dramatic spread morphology when treated with collagenase, whereas nondegraded samples produced minimal, to no cell spreading. This observation may indicate that the VICs are possibly encapsulated within the walls of the material, providing support to the hypothesis that porosity is indeed induced during the polymerization process where the VICs would remain in the gelatin-rich phase. The combination of induced porosity on the order of mammalian cell size and the enzymatically degradable network walls provides a unique platform that may enhance cell-dictated migration and differentiation.
To illustrate the function of GelMA hydrogels as cell culture platforms, a unique and highly interesting cell type for the heart valve tissue engineering community was selected. Although VICs specifically reside in heart valves, they share many similarities with other fibroblast like cells important for other tissue engineering applications, such as dermal fibroblasts for skin applications. Natively, VICs reside within the leaflets of aortic heart valves in a spread fibroblast-like morphology. Attaining this cell profile has been difficult in other photopolymerized hydrogel materials without the specific incorporation of bioactive epitopes to promote cell-directed spreading such as degradable peptides.2,34 Given that GelMA hydrogels are entirely composed of cell degradable linkages, VICs were able to achieve a spread morphology reminiscent of native cell profile within the first week of culture, and this was maintained throughout the course of the experiments as shown in Figures 3 and 4. In addition, the polymerization process and GelMA hydrogel platform were very cytocompatible, maintaining a relatively constant percent viability of live cells throughout the experiments. Even though not specifically assayed here, cells should also have the ability to proliferate within these hydrogels under growth conditions. We were interested in cell differentiation, rather than proliferation, and hence performed all of our experiments in low-serum, nonproliferation conditions.
VIC differentiation from the fibroblast to myofibroblast phenotype is critical for proper wound healing in vivo and may also be critical in the production of native ECM during valve tissue engineering.54,55 It has been shown in 2D culture experiments that VICs differentiate to myofibroblasts in the presence of TGF-β1,56 a potent cytokine commonly excreted by inflammatory cells. Since 3D culture environments are clearly different from most 2D culture experiments, we were interested in studying the effect of VIC differentiation in our 3D GelMA hydrogel platforms in response to TGF-β1. Initially, we noticed that cell spreading was enhanced with TGF-β1–treated GelMA hydrogels. It has been shown that activated (myofibroblastic) VICs have increased expression levels of many MMPs (particularly, MMP-2 and 9, the gelatinases)53; enhanced MMP expression would most surely speed the rate of local degradation within the hydrogel and therefore speed cell extension of processes and spreading. In addition, it has been shown in other fibroblastic cells that addition of TGF-β1 increases myofibroblast differentiation and subsequent cell spreading.42,43 By the end of our 7-week experiment, cells were spread within both the TGF-β1–treated and nontreated conditions, indicating that VICs were able to achieve a native morphology even without TGF-β1.
The most widely accepted and important marker for VIC myofibroblastic differentiation is the upregulation and expression of αSMA, a component of stress fibers that bestows contractile properties to the cells. Here, we used qRT-PCR to examine mRNA (gene expression levels) of αSMA, and also immunohistochemistry to look for protein production and incorporation into stress fibers. Within the first week of culture, there was an upregulation in αSMA mRNA in both TGF-β1–treated and –nontreated VIC GelMA hydrogel cultures; there was also diffuse immunostaining for αSMA in both conditions. Since gelatin is in itself degraded collagen, it may trigger a partial wound healing response within the cells promoting the initial upregulation of αSMA.57 Unorganized collagen gel cultures with VICs also have a similar upregulation of αSMA initially.57 Over time, however, the TGF-β1–treated GelMA hydrogels had a much more dramatic induction of αSMA at both the mRNA and protein levels, while the untreated GelMA hydrogel cultures maintained relatively constant levels of αSMA. This result is not unexpected given that TGF-β1 is a potent activator of VICs. Another important marker for VIC myofibroblastic differentiation is increased collagen-1 production. In this study we also used qRT-PCR to follow this marker. Unfortunately, we were unable to specifically immunostain for this protein due to the collagenous nature of the hydrogel. Although not dramatic, an increase in collagen expression is observed with TGF-β1 treatment at later time points.
In summary, GelMA hydrogels possess unique properties that promote cell survival and differentiation in 3D. This synthetically modified natural macromer combines the advantages of synthetic macromers, such as mechanical property control and photopolymerization ability, with the benefits of natural materials, most notably the presence of bioactive epitopes to promote cell survivability, function, and matrix remodeling. Here, unique polymerization-produced pores were observed, providing another distinct feature that may promote cell migration and spreading. To test the suitability of this material as a cell culture platform, VICs were encapsulated within the network and shown to achieve their natural morphology and differentiate in response to TGF-β1.
Acknowledgments
We gratefully acknowledge Ms. Jennifer L. Bruce for assistance with GelMA characterization. We thank support from the National Science Foundation Graduate Research Fellowship Program and the Department of Education Graduate Assistantships in Areas of National Need (GAANN) program for fellowships to J.A.B. We also acknowledge the GAANN program and National Institutes of Health (NIH)/University of Colorado Molecular Biophysics Training Program (NIH T32 GM065103) for support to C.A.D. We also thank the Howard Hughes Medical Institute (HHMI), and NIH (Grant HL089260) for additional funding support.
Disclosure Statement
No competing financial interests exist.
References
- 1.Abbott A. Cell culture: biology's new dimension. Nature. 2003;424:870. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 2.Raeber G.P. Lutolf M.P. Hubbell J.A. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salinas C.N. Anseth K.S. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29:2370. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nuttelman C.R. Tripodi M.C. Anseth K.S. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A. 2004;68A:773. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 5.Nuttelman C.R. Tripodi M.C. Anseth K.S. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24:208. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Zeltinger J. Sherwood J.K. Graham D.A. Mueller R. Griffith L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7:557. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 7.Hern D.L. Hubbell J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Elisseeff J. McIntosh W. Anseth K. Riley S. Ragan P. Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Bryant S.J. Durand K.L. Anseth K.S. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res A. 2003;67A:1430. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 10.Yang S.F. Leong K.F. Du Z.H. Chua C.K. The design of scaffolds for use in tissue engineering. Part 1. Traditional factors. Tissue Eng. 2001;7:679. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 11.Drury J.L. Mooney D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 12.Mann B.K. Gobin A.S. Tsai A.T. Schmedlen R.H. West J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 13.Schmedlen K.H. Masters K.S. West J.L. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials. 2002;23:4325. doi: 10.1016/s0142-9612(02)00177-1. [DOI] [PubMed] [Google Scholar]
- 14.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 15.Cassell O.C.S. Morrison W.A. Messina A. Penington A.J. Thompson E.W. Stevens G.W. Perera J.M. Kleinman H.K. Hurley J.V. Romeo R. Knight K.R. The influence of extracellular matrix on the generation of vascularized, engineered, transplantable tissue. Annals of the New York Academy of Sciences. 2001;944:429. doi: 10.1111/j.1749-6632.2001.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 16.Ye Q. Zund G. Benedikt B. Jockenhoevel S. Hoerstrup S.P. Sakyama S. Hubbell J.A. Turina M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2000;17:587. doi: 10.1016/s1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 17.Van den Bulcke A.I. Bogdanov B. De Rooze N. Schacht E.H. Cornelissen M. Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 18.Peng H.T. Martineau L. Shek P.N. Hydrogel-elastomer composite biomaterials: 1. Preparation of interpenetrating polymer networks and in vitro characterization of swelling stability and mechanical properties. J Mater Sci Mater Med. 2007;18:975. doi: 10.1007/s10856-006-0088-8. [DOI] [PubMed] [Google Scholar]
- 19.Peng H.T. Martineau L. Shek P.N. Hydrogel-elastomer composite biomaterials: 3. Effects of gelatin molecular weight and type on the preparation and physical properties of interpenetrating polymer networks. J Mater Sci Mater Med. 2008;19:997. doi: 10.1007/s10856-007-0167-5. [DOI] [PubMed] [Google Scholar]
- 20.Peng H.T. Mok M. Martineau L. Shek P.N. Hydrogel-elastomer composite biomaterials: 2. Effects of aging methacrylated gelatin solutions on the preparation and physical properties of interpenetrating polymer networks. J Mater Sci Mater Med. 2007;18:1025. doi: 10.1007/s10856-006-0072-3. [DOI] [PubMed] [Google Scholar]
- 21.Barbetta A. Dentini M. Zannoni E.M. De Stefano M.E. Tailoring the porosity and morphology of gelatin-methacrylate polyHIPE scaffolds for tissue engineering applications. Langmuir. 2005;21:12333. doi: 10.1021/la0520233. [DOI] [PubMed] [Google Scholar]
- 22.Barbetta A. Massimi M. Devirgiliis L.C. Dentini M. Enzymatic cross-linking versus radical polymerization in the preparation of gelatin polyHIPEs and their performance as scaffolds in the culture of hepatocytes. Biomacromolecules. 2006;7:3059. doi: 10.1021/bm060533l. [DOI] [PubMed] [Google Scholar]
- 23.Boedtker H. Doty P. A study of gelatin molecules, aggregates and gels. J Phys Chem. 1954;58:968. [Google Scholar]
- 24.Veis A. Cohen J. Reversible transformation of gelatin to the collagen structure. Nature. 1960;186:720. doi: 10.1038/186720a0. [DOI] [PubMed] [Google Scholar]
- 25.Ofner C.M. Bubnis W.A. Chemical and swelling evaluations of amino group crosslinking in gelatin and modified gelatin matrices. Pharm Res. 1996;13:1821. doi: 10.1023/a:1016029023910. [DOI] [PubMed] [Google Scholar]
- 26.Mwangi J.W. Ofner C.M. Crosslinked gelatin matrices: release of a random coil macromolecular solute. Int J Pharm. 2004;278:319. doi: 10.1016/j.ijpharm.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Broderick E.P. O'Halloran D.M. Rochev Y.A. Griffin M. Collighan R.J. Pandit A.S. Enzymatic stabilization of gelatin-based scaffolds. J Biomed Mater Res B Appl Biomater. 2005;72B:37. doi: 10.1002/jbm.b.30119. [DOI] [PubMed] [Google Scholar]
- 28.Crescenzi V. Francescangeli A. Taglienti A. New gelatin-based hydrogels via enzymatic networking. Biomacromolecules. 2002;3:1384. doi: 10.1021/bm025657m. [DOI] [PubMed] [Google Scholar]
- 29.Filip D.A. Radu A. Simionescu M. Interstitial-cells of the heart-valves possess characteristics similar to smooth-muscle cells. Circ Res. 1986;59:310. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- 30.Messier R.H. Bass B.L. Aly H.M. Jones J.L. Domkowski P.W. Wallace R.B. Hopkins R.A. Dual structural and functional phenotypes of the porcine aortic-valve interstitial population—characteristics of the leaflet myofibroblast. J Surg Res. 1994;57:1. doi: 10.1006/jsre.1994.1102. [DOI] [PubMed] [Google Scholar]
- 31.Mohler E.R. Gannon F. Reynolds C. Zimmerman R. Keane M.G. Kaplan F.S. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 32.Taylor P.M. Allen S.P. Dreger S.A. Yacoub M.H. Human cardiac valve interstitial cells in collagen sponge: A biological three-dimensional matrix for tissue engineering. J Heart Valve Dis. 2002;11:298. [PubMed] [Google Scholar]
- 33.Williams C. Johnson S.L. Robinson P.S. Tranquillo R.T. Cell sourcing for fibrin-based heart valve-equivalents. In Vitro Cell Dev Biol Anim. 2006;42:6A. [Google Scholar]
- 34.Shah D.N. Recktenwall-Work S.M. Anseth K.S. The effect of bioactive hydrogels on the secretion of extracellular matrix molecules by valvular interstitial cells. Biomaterials. 2008;29:2060. doi: 10.1016/j.biomaterials.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masters K.S. Shah D.N. Walker G. Leinwand L.A. Anseth K.S. Designing scaffolds for valvular interstitial cells: cell adhesion and function on naturally derived materials. J Biomed Mater Res A. 2004;71A:172. doi: 10.1002/jbm.a.30149. [DOI] [PubMed] [Google Scholar]
- 36.Ronnovjessen L. Petersen O.W. Induction of alpha-smooth muscle actin by transforming growth-factor-beta-1 in quiescent human breast gland fibroblasts—implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696. [PubMed] [Google Scholar]
- 37.Cushing M.C. Liao J.T. Anseth K.S. Activation of valvular interstitial cells is mediated by transforming growth factor-beta 1 interactions with matrix molecules. Matrix Biol. 2005;24:428. doi: 10.1016/j.matbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Habeeb A.F.S. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966;14:328. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 39.Brinkman W.T. Nagapudi K. Thomas B.S. Chaikof E.L. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: mechanical properties, cell viability, and function. Biomacromolecules. 2003;4:890. doi: 10.1021/bm0257412. [DOI] [PubMed] [Google Scholar]
- 40.Johnson C.M. Hanson M.N. Helgeson S.C. Porcine cardiac valvular subendothelial cells in culture—cell isolation and growth-characteristics. J Mol Cell Cardiol. 1987;19:1185. doi: 10.1016/s0022-2828(87)80529-1. [DOI] [PubMed] [Google Scholar]
- 41.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malmstrom J. Lindberg H. Lindberg C. Bratt C. Wieslander E. Delander E.L. Sarnstrand B. Burns J.S. Mose-Larsen P. Fey S. Marko-Varga G. Transforming growth factor-beta(1) specifically induce proteins involved in the myofibroblast contractile apparatus. Mol Cell Proteomics. 2004;3:466. doi: 10.1074/mcp.M300108-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Smith P.C. Caceres M. Martinez J. Induction of the myofibroblastic phenotype in human gingival fibroblasts by transforming growth factor-beta 1: role of RhoA-ROCK and c-Jun N-terminal kinase signaling pathways. J Periodontal Res. 2006;41:418. doi: 10.1111/j.1600-0765.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim S.H. Chu C.C. Synthesis and characterization of dextran-methacrylate hydrogels and structural study by SEM. J Biomed Mater Res. 1999;49:517. doi: 10.1002/(sici)1097-4636(20000315)49:4<517::aid-jbm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Li Q. Williams C.G. Sun D.D.N. Wang J. Leong K. Elisseeff J.H. Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res A. 2004;68A:28. doi: 10.1002/jbm.a.20007. [DOI] [PubMed] [Google Scholar]
- 46.Kim S.H. Chu C.C. Pore structure analysis of swollen dextran-methacrylate hydrogels by SEM and mercury intrusion porosimetry. J Biomed Mater Res. 2000;53:258. doi: 10.1002/(sici)1097-4636(2000)53:3<258::aid-jbm11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 47.Bell C.L. Peppas N.A. Swelling/syneresis phenomena in gel-forming interpolymer complexes. J Biomater Sci Polym Ed. 1996;7:671. doi: 10.1163/156856296x00444. [DOI] [PubMed] [Google Scholar]
- 48.Kwok A.Y. Prime E.L. Qiao G.G. Solomon D.H. Synthetic hydrogels 2. Polymerization induced phase separation in acrylamide systems. Polymer. 2003;44:7335. [Google Scholar]
- 49.Nwabunma D. Chin H.W. Kyu T. Morphology development and dynamics of photopolymerization-induced phase separation in mixtures of a nematic liquid crystal and photocuratives. Macromolecules. 2000;33:1416. [Google Scholar]
- 50.Roussel F. Buisine J.M. Maschke U. Coqueret X. Photopolymerization kinetics and phase behaviour of acrylate based polymer dispersed liquid crystals. Liquid Crystals. 1998;24:555. [Google Scholar]
- 51.Lutolf M.P. Raeber G.P. Zisch A.H. Tirelli N. Hubbell J.A. Cell-responsive synthetic hydrogels. Adv Mater. 2003;15:888. [Google Scholar]
- 52.Edep M.E. Shirani J. Wolf P. Brown D.L. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc Pathol. 2000;9:281. doi: 10.1016/s1054-8807(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 53.Rabkin E. Aikawa M. Stone J.R. Fukumoto Y. Libby P. Schoen F.J. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 54.Durbin A.D. Gotlieb A.I. Advances towards understanding heart valve response to in injury. Cardiovasc Pathol. 2002;11:69. doi: 10.1016/s1054-8807(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 55.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker G.A. Masters K.S. Shah D.N. Anseth K.S. Leinwand L.A. Valvular myofibroblast activation by transforming growth factor-beta—implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 2004;95:253. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 57.Butcher J.T. Nerem R.M. Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. 2004;13:478. [PubMed] [Google Scholar]